Abstract
The t(14;18) translocation, which involves the bcl-2oncogene, occurs in follicular lymphomas (FL) at two common sites: the major breakpoint region (MBR) and the minor cluster region (mcr). The biological and clinical significance of these breakpoints is unknown. The bcl-2 breakpoint site was determined in 247 previously untreated patients (49% men; median age 52 years) with indolent FL (155 grade I, 83 grade II, and 8 grade III) to correlate it with pretreatment characteristics, response, and outcome. The bcl-2 breakpoint site was determined by a polymerase chain reaction method of peripheral blood (all cases), bone marrows (149 cases), and fresh lymph node biopsy specimens (68 cases). The breakpoint site occurred at MBR in 175 cases (71%) and atmcr in 27 (11%). In 45 cases (18%), no breakpoint was detected (germline). No significant relationship was found between the rearrangements and the expression of BLC-2 and BAXproteins. Patients’ germline for MBR and mcr tended to present more frequently with stage IV disease and higher β2-microglobulin (β2M) levels, whereas mcr-rearranged patients presented more frequently with early stage and normal β2M. The complete response rate of germline patients was significantly lower than that of MBR and mcr patients. An estimated 3-year failure-free survival (FFS) for mcr, MBR, and germline cases was 95%, 76%, and 57%, respectively (P < .001). Thebcl-2 breakpoint site was independent of serum β2M and lactate dehydrogenase in its correlation with FFS. In conclusion, thebcl-2 rearrangement site is an important prognostic factor in indolent FL, useful to identify patients who may require different treatment.
FOLLICULAR LYMPHOMAS (FLs) are characterized by the presence of the t(14;18)(q32;q21) translocation, which causes a rearrangement of the bcl-2 oncogene. This rearrangement normally occurs in chromosome 18, with the immunoglobulin heavy-chain gene (IgH) in chromosome 14.1,2 The consequence is an overexpression of a chimericalbcl-2/IgH mRNA.3 Because the breakpoint is usually located outside the translated portion of the bcl-2gene, the protein product is identical to the normal BCL-2protein.4 The function of the BCL-2 protein is to block apoptosis, probably by means of its interaction with otherbcl-2-family proteins such as bax.5-8 The inhibition of apoptosis leads to accumulation of B lymphocytes, which might later acquire additional mutations that eventually result in the development of FL.
Approximately 70% of bcl-2 rearrangements in FL occur at the major breakpoint region (MBR) located in the untranslated 3′ end of the last exon,9-11 and in approximately 10% of cases, the rearrangement occurs in the minor cluster region (mcr) located approximately 30 kb downstream of thebcl-2 gene.12 In a few cases, rearrangements occur at other sites such as the variant cluster region at the 5′ end of thebcl-2 gene. In approximately 15% of patients, no t(14;18) translocation can be detected by either cytogenetic, Southern blot analysis, or polymerase chain reaction (PCR) techniques13(germline configuration). Tight clustering of the breakpoints atMBR and mcr, as well as the availability of consensus sequences of the JH segments of the IgH make this a particularly favorable target for PCR amplification.9-12 In fact, it is possible to use two universal primers (one for each breakpoint) along with a primer derived from JH region to amplify the majority of the translocations at MBR andmcr,9,14 18 with less than 5% of translocations failing to be amplified.
The biological and clinical significance of these differences inbcl-2 rearrangement sites in FL remain unclear. In fact, it has been suggested that the presence of the t(14;18) translocation in FL correlates with better,16 worse,17 and similar18-21 clinical outcomes. However, these studies are based on a small number of cases and frequently included high-grade and intermediate-grade lymphomas. On the other hand, BCL-2-protein expression recently has been associated with poor prognosis in patients with diffuse large-cell lymphoma.22-24 Currently, the treatment to indolent FL is based largely on the Ann Arbor stage of the disease at the time of diagnosis.25 Management of patients with advanced stage includes observation and single agent or combination chemotherapy. A therapeutic approach based on the patient’s prognostic risk could be of practical help in the decision-making process.26 Furthermore, knowledge of these prognostic factors could lead to an improvement in the analysis of clinical trials and in the understanding of the biology of these disorders. Consequently, it is important to ascertain whether or not the bcl-2 rearrangements are useful in predicting the outcome of FL patients.
MATERIALS AND METHODS
Patients.
The presence of bcl-2 rearrangements was analyzed in the pretreatment peripheral blood (PB) and/or bone marrow (BM) aspirates of 247 patients diagnosed with indolent FL during a 7-year period. The median age was 52 years (range, 18 to 84 years); 120 patients (49%) were men and 127 (51%) were women. The distribution, according to the REAL classification, was follicular center cell lymphoma grade I, 155 cases; grade II, 83 cases; grade III, 8 cases, and unclassified, 1 case. Only eight patients had grade III histology and were included in the study because their tumors predominantly contained cleaved cells and for that reason were considered indolent. Twenty-two patients (9%) presented with Ann Arbor stage I disease, 28 (11%) stage II, 44 (18%) stage III, and 153 (62%) stage IV. Bulky disease was present in 21% of the cases, extranodal involvement in 68%, and BM infiltration in 56%. The proportion of patients with elevated serum lactate dehydrogenase (LDH) (top normal: 618 IU/L) and high β2-microglobulin (β2M) (top normal: 2 mg/L) was 16% and 42%, respectively.
Patients with stage IV disease received anthracycline-containing chemotherapy regimens depending on the protocol being used at the time they were diagnosed. The treatment consisted of an intensive alternating triple therapy (ATT) regimen27 in 106 cases, FND (fludarabine, Novantrone, and dexamethasone)28 in 34 cases, and standard cyclophosphamide doxorubicin, vincristine (oncovin), prednisone (CHOP) in 13 cases. Patients with stage III disease were treated with ATT chemotherapy in 16 cases, with CHOP in 13, and with total nodal radiotherapy in 15. Patients with early stage received either radiotherapy alone (9 cases) or a combined modality of chemotherapy plus radiotherapy to the involved fields (41 cases).
Histologic and immunophenotypic studies.
The diagnosis of indolent FL was based on conventional examination of paraffin-embedded slides and whenever possible on immunohistologic staining of frozen sections, according to currently accepted criteria.29 In 47 cases, the expression of BCL-2and BAX proteins was analyzed by an immunostaining method. The results were expressed in a semiquantitative manner by comparingBCL-2 or BAX-tumor expression with that of contiguous interfollicular areas. Four patterns were distinguished: negative (0), no tumor BCL-2 expression; weak (+), BCL-2-tumor expression present but weaker than in interfollicular areas; positive (++), positivity similar to interfollicular areas; and strongly positive (+++), BCL-2-tumor expression stronger than in the interfollicular areas.
PCR methods for detecting bcl-2 rearrangements.
Baseline pretreatment PB samples were collected in 242 patients as part of a study aimed at assessing minimal residual disease in FL (in five cases, the PCR result was not assessable). In addition, a BM aspirate for PCR analysis was obtained in 154 cases. Finally, fresh material from the original lymph node biopsy specimen was available forbcl-2 rearrangement studies in 68 patients.
DNA was isolated from PB, BM, and/or lymph node material by using conventional methods. A PCR technique was used to detect thebcl-2 of MBR and mcr. PCR amplification was performed with 1 μg of purified DNA that was subjected to a 45-cycle PCR amplification.14,15 The primersMBR+, mcr+, and JH− have been previously described.12,14,15Twenty percent of the PCR products were size fractionated in a 2% NuSieve gel (FMC Bioproducts, Rockland, ME) and then transferred to a nylon membrane. Membranes were hybridized with 5′ end radiolabeled oligonucleotide probes MBR ormcr.15
To ensure the reliability of the PCR assay, we routinely included a weak, positive control (100 pg of positive DNA), a negative control (normal DNA), a reagent control, and an internal control. These controls helped us detect contamination, avoid false negativity caused by suboptimal PCR efficiency, and standardize the variation in PCR efficiency.
Statistical considerations.
FFS was the outcome variable of major interest. FFS was defined as the time interval from the start of initial therapy to the first evidence of relapse or death from toxicity. Response to therapy and associations between bcl-2-breakpoint site and other pretreatment patient characteristics were also reported. Differences in patient characteristics and response were tested with the chi-square or Fisher’s exact tests. Survival curves were estimated by using the Kaplan and Meier methods30 and differences among curves were tested by using the Wilcoxon test.31 A multivariate analysis was performed by the Cox’s stepwise proportional hazard regression method.32 However, because there was an apparent lack of proportionality of hazard rates, a stratified test was used to assess the independent prognostic value of the bcl-2-breakpoint site. For this purpose, because a standard score system for FLs does not exist, the patients were divided into three sets according to the most important prognostic variables in MDACC series, serum LDH, and β2M levels. We analyzed the prognostic value of thebcl-2-breakpoint sites in each group.
RESULTS
Distribution of the bcl-2 rearrangement site.
The breakpoint site ocurred at MBR in 175 patients (71%), at the mcr in 27 patients (11%), and no rearrangement was detected for both MBR and mcr in the remaining 45 patients (18%). These results were obtained in PB and BM samples from the same patients in 149 cases and in PB only in 98 cases. In those patients in whom the bcl-2 rearrangement was observed in blood or BM samples only, they were graded as positive.
Assessment of bcl-2 rearrangement in lymph node, PB, and BM samples.
To ascertain if the results obtained from PB and BM samples were representative of those obtained from the tumor itself, we studied by PCR a subset of 68 samples (from the 247 patients) obtained from the lymph nodes or masses involved by lymphoma. The results showed that 46 patients (68%) had MBR rearrangements, 4 (6%) had mcrrearrangements, and 18 (27%) were germline for both MBR andmcr. These results are similar to those obtained from blood and marrow analyses. When comparing tumor samples with blood or marrow, the only discrepancy found was in 4 of the 18 germline lymph node biopsy samples in which MBR (1) or mcr (3) rearrangements were found in PB, the BM, or both. In the remaining 64 cases (94.2% of all cases), there was agreement between the results of lymph node and PB/BM analyses. Of particular note, all patients germline for MBR ormcr in PB or BM also showed a germline pattern in lymph node tissue. Therefore, the rate of agreement between blood/marrow and tumor tissue was 94%.
On the other hand, we also compared the results obtained in PB and BM in 149 patients in whom paired blood and marrow were available (Table1). MBR or mcrrearrangements were observed in 87 paired samples of blood and marrow, and no rearrangement was seen in another 31 paired samples. In 23 cases (15%), a bcl-2 rearrangement was found in the PB sample and not in BM, whereas in 8 cases (5%) it was observed in BM but not in blood.
Results of the PCR Analysis for Bcl-2Rearrangement Performed in 149 Patients With Both PB and BM Pretreatment
| . | Overall Series (n = 149) . | MBR (n = 105) . | mcr (n = 13) . | Germline (n = 31) . |
|---|---|---|---|---|
| PB+ BM+ | 87 (59%) | 77 | 10 | — |
| PB− BM− | 31 (21%) | — | — | 31 |
| PB+ BM− | 23 (15%) | 21 | 2 | — |
| PB− BM+ | 8 (5%) | 7 | 1 | — |
| . | Overall Series (n = 149) . | MBR (n = 105) . | mcr (n = 13) . | Germline (n = 31) . |
|---|---|---|---|---|
| PB+ BM+ | 87 (59%) | 77 | 10 | — |
| PB− BM− | 31 (21%) | — | — | 31 |
| PB+ BM− | 23 (15%) | 21 | 2 | — |
| PB− BM+ | 8 (5%) | 7 | 1 | — |
Expression of BCL-2 and BAX proteins.
In an attempt to determine if there was any correlation between thebcl-2-breakpoint site and the expression of BCL-2 andBAX proteins, we analyzed such expression in a subset of 47 patients for whom tumor tissue was available. In 40 of 47 patients,BCL-2-protein expression was found in tumor tissue, whereas no expression was observed in 7 patients. Although not statistically significant, the proportion of cases expressing positive or strongly positive BCL-2 protein was higher among germline cases (89%) than among MBR (73%) and mcr cases (60%). No differences were found regarding BAX-protein expression.
Bcl-2 breakpoints and their correlation with pretreatment features.
The correlation of the bcl-2-breakpoint site with pretreatment features is summarized in Table 2. Germline patients tended to be older, to have higher LDH and β2M values, and to have a higher incidence of advanced stage. Patients with anmcr breakpoint tended to be younger, to have lower LDH and β2M values, and to have a lower rate of advanced stage. Differences in distribution of patient characteristics among breakpoint groups were not statistically significant at the .05 level with the exception of Ann Arbor stage (P = .03) and β2M values (P = .02). Differences between mcr and MBRpatients did not reach statistical significance.
Characteristics of the 247 Patients With FL According to the Site of the Bcl-2 Rearrangement
| . | Percent of Patients . | ||
|---|---|---|---|
| mcr (n = 27) . | MBR (n = 175) . | G-MBR/mcr (n = 45) . | |
| Male gender (%) | 44 | 48 | 55 |
| Age ≥60 yr (%) | 30 | 30 | 41 |
| Histologic subtype (%) | |||
| Grade I | 67 | 63 | 60 |
| Grade II | 30 | 34 | 36 |
| Grade III | 4 | 3 | 4 |
| Bulky disease (%) | 23 | 22 | 16 |
| Ann Arbor stage (%) | |||
| I-II | 33 | 20 | 11 |
| III | 11 | 17 | 27 |
| IV | 56 | 63 | 62 |
| Extranodal involvement ≥1 site (%) | 67 | 69 | 66 |
| Bone marrow (+) (%) | 52 | 57 | 53 |
| Serum LDH level (median) | 431 | 472 | 491 |
| Serum β2M level (median) | 1.7 | 1.9 | 2.2 |
| . | Percent of Patients . | ||
|---|---|---|---|
| mcr (n = 27) . | MBR (n = 175) . | G-MBR/mcr (n = 45) . | |
| Male gender (%) | 44 | 48 | 55 |
| Age ≥60 yr (%) | 30 | 30 | 41 |
| Histologic subtype (%) | |||
| Grade I | 67 | 63 | 60 |
| Grade II | 30 | 34 | 36 |
| Grade III | 4 | 3 | 4 |
| Bulky disease (%) | 23 | 22 | 16 |
| Ann Arbor stage (%) | |||
| I-II | 33 | 20 | 11 |
| III | 11 | 17 | 27 |
| IV | 56 | 63 | 62 |
| Extranodal involvement ≥1 site (%) | 67 | 69 | 66 |
| Bone marrow (+) (%) | 52 | 57 | 53 |
| Serum LDH level (median) | 431 | 472 | 491 |
| Serum β2M level (median) | 1.7 | 1.9 | 2.2 |
Abbreviation: G-MBR/mcr, germline for MBRand mcr.
Response to therapy.
Among the 228 patients with evaluable response, 200 (88%) achieved complete response (CR) and 26 (11%) achieved partial response CR rates for mcr, MBR, and germline groups were 96%, 90%, and 71%, respectively (P < .01). The trend for fewer responses among germline cases held true when consideration was restricted to patients with stage IV disease (Table 3). Differences between mcr and MBR patients did not reach statistical significance.
Clinical Response to Therapy in 228 Assessable Patients According to the Bcl-2 Breakpoint Site
| . | mcr (n = 27) . | MBR (n = 163) . | Germline (n = 38) . |
|---|---|---|---|
| Whole series | |||
| CR | 26 (96%) | 147 (90.5%) | 27 (71%) |
| PR | 1 (4%) | 15 (9%) | 10 (26%) |
| Failure | 0 | 1 (0.5%) | 1 (3%) |
| Stage IV disease | (n = 15) | (n = 104) | (n = 24) |
| CR | 14 (93%) | 92 (89%) | 17 (71%) |
| PR | 1 (7%) | 11 (10%) | 7 (29%) |
| Failure | 0 | 1 (1%) | 0 |
| . | mcr (n = 27) . | MBR (n = 163) . | Germline (n = 38) . |
|---|---|---|---|
| Whole series | |||
| CR | 26 (96%) | 147 (90.5%) | 27 (71%) |
| PR | 1 (4%) | 15 (9%) | 10 (26%) |
| Failure | 0 | 1 (0.5%) | 1 (3%) |
| Stage IV disease | (n = 15) | (n = 104) | (n = 24) |
| CR | 14 (93%) | 92 (89%) | 17 (71%) |
| PR | 1 (7%) | 11 (10%) | 7 (29%) |
| Failure | 0 | 1 (1%) | 0 |
Failure-free survival.
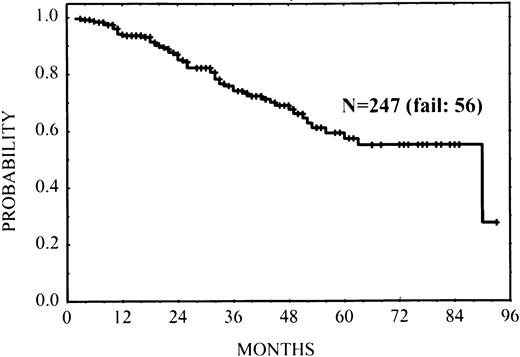
At the time of analysis, 56 patients (23%) had experienced relapse or progression; 7 patients experienced histologic transformation into large-cell lymphoma at relapse (5 cases MBR, 2 cases germline). Estimated FFS at 3 years after initiation of therapy was 0.74 (standard error: 0.04) (Fig 1).
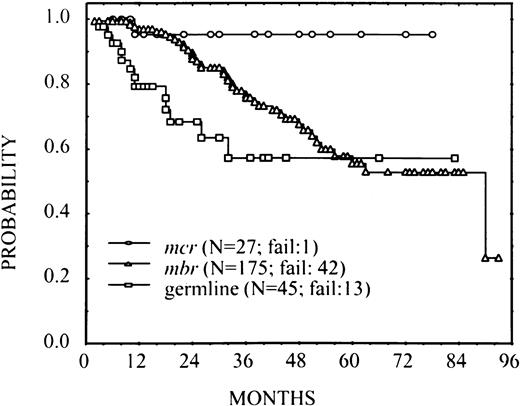
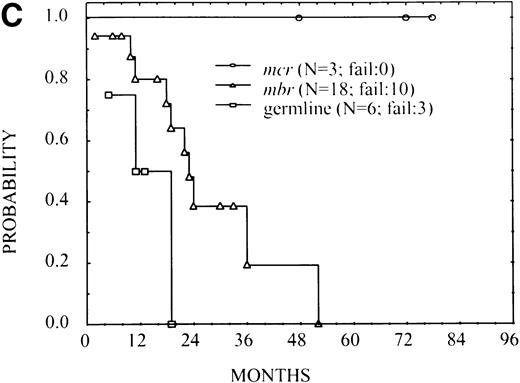
There was one relapse among 27 patients with mcr breakpoint, 42 among 175 patients with MBR breakpoint and 13 among 45 germline patients. An estimated 3-year FFS for these groups was 0.95 (standard error = 0.05), 0.76 (standard error = 0.04), and 0.57 (standard error = 0.10), respectively. The overall difference among FFS curves was statistically significant (P < .001; Fig2). These results suggested a trend for superior FFS among patients with mcr breakpoint and unfavorable outcomes for germline cases. The large group of MBR-breakpoint cases was of particular interest because of the apparent low risk of failure in early follow-up, with a subsequent increase in the rate of failure. This observation was verified in an analysis of smoothed hazard rates (not shown), which indicated for theMBR-breakpoint group a gradually increasing risk of failure during the first 24 months after treatment. Although treatment failure among patients in the germline group was higher, there was no real evidence that the rate changed over time, at least through the first 36 months. The single failure in the mcr group did not allow characterization of their risk of failure over time.
FFS in 247 patients with FL according to thebcl-2-breakpoint site MBR, mcr, and germline for bothMBR and mcr (P < .001).
FFS in 247 patients with FL according to thebcl-2-breakpoint site MBR, mcr, and germline for bothMBR and mcr (P < .001).
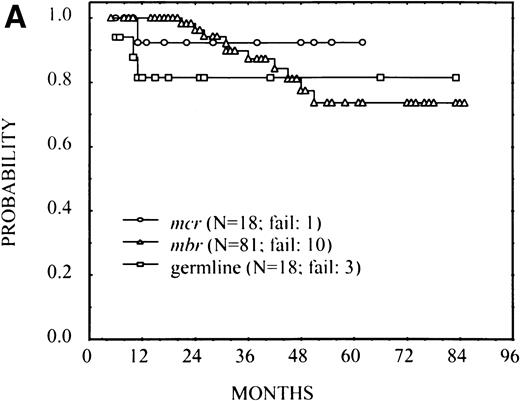
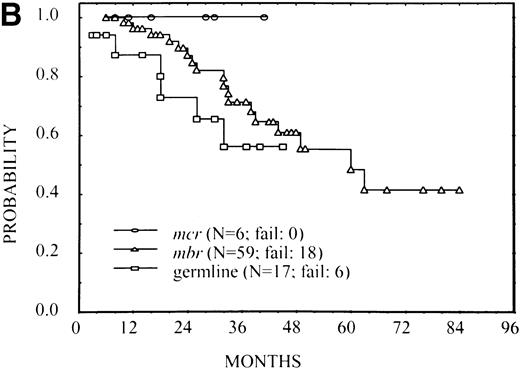
Abnormal pretreatment LDH and β2M levels have been reported as poor prognostic factors in FL, and their association with FFS was verified in this patient group (results not shown). Because these poor features also occurred more commonly in the MBR group and particularly in the germline group, it was possible that these factors accounted for differences in FFS among the breakpoint groups. To investigate this question, patients were divided into three risk groups: those whose LDH and β2M were both normal, those in whom only one of these factors was normal, and those in whom both factors were abnormal. FFS for patients with mcr and MBR breakpoints and those with germline rearrangements are plotted in Fig 3separately within the three risk groups. Although the number of patients was small, the same pattern of FFS differences by breakpoint was noted within each risk group as observed in the overall group. A Wilcoxon test stratified by risk group resulted in a P value of .08. These results suggest that the breakpoint site is an independent prognostic variable from LDH and β2M values in predicting FFS. Although there was an apparent lack of proportionality of hazard rates, we performed a proportional hazard model analysis32that confirmed the same results as the stratified test previously reported; the presence of bcl-2 rearrangement (at mcr orMBR) maintained its prognostic importance along with serum β2M and LDH levels.
FFS according to the bcl-2-breakpoint site (mcr, MBR, and germline) in three subsets of patients with different risks: (A) patients with normal serum LDH and β2M (no significant difference among breakpoints), (B) patients with high LDH or β2M (P = .02), and (C) patients with high LDH and β2M (P = .03).
FFS according to the bcl-2-breakpoint site (mcr, MBR, and germline) in three subsets of patients with different risks: (A) patients with normal serum LDH and β2M (no significant difference among breakpoints), (B) patients with high LDH or β2M (P = .02), and (C) patients with high LDH and β2M (P = .03).
Patients with stage IV disease treated with the intensive ATT regimen fared somewhat better than those who received the FND regimen. In an attempt to consider the possibility that a treatment effect could have influenced the FFS as related to the breakpoint site, FFS was compared for those patients with stage IV disease treated with ATT and FND. Although the number of patients was small, the same ordering of FFS curves was preserved within the homogeneously treated subsets of ATT and FND.
Overall survival.
There have been 17 deaths so far in this group of patients. The cause of death was directly or indirectly related to lymphoma in 11 cases (progressive disease in 9 cases, toxicity in 2). The remaining six patients died in CR from other causes such as concurrent tumors in two cases (glioblastoma multiforme, disseminated breast cancer), myocardial infarction in one case, gunshot wound in one case, and unknown (probably cardiovascular disease) in two cases.
DISCUSSION
Most patients with FL, even those in early stages, have circulating cells that carry the bcl-2 rearrangement providing a unique opportunity to identify and classify them according to theirbcl-2 rearrangement without necessarily having to directly study their tumor tissue. However, in a considerable number of cases (one third of the whole series) we performed the same analysis directly on tissue involved by the malignant lymphoma to ascertain if the results obtained from PB and BM samples were representative of those obtained from the tumor. The agreement between the results from PB/BM and lymph node was 94.2%. No patient with a bcl-2rearrangement assessed in lymph-node tissue failed to be detected when combining PB and BM determinations. Of note is the fact that in 5.8% of the cases, a bcl-2 rearrangement was detected in PB or BM by PCR but was not observed in tumor tissue. Possible explanations for this discrepancy are (1) the tumor sample studied was only partially infiltrated by lymphoma, (2) some technical problems could have occurred in extracting DNA from tumor tissue, (3) the blood/marrow results could be spurious and could perhaps represent contamination, or (4) the bcl-2 rearrangement detected was real but did not correspond to the tumor tissue. This latter alternative cannot be completely ruled out in these four cases, because bcl-2rearrangements have been described in PB of healthy individuals.33-35 However, when only 1 μg of DNA is loaded, as we have done in this study, the bcl-2 rearrangement in blood is only detected in 6% of normal individuals.33When whole PB white cells are tested and not B lymphocytes, as in our study, this proportion is expected to be much lower.
Relatively good concordance between the detection of lymphoma cells in blood and BM at diagnosis has been previously observed by different groups.36-38 However, in one fifth of the patients tested, the bcl-2 rearrangement was detected only in the PB or in BM but not in both. Two thirds of these cases were positive in blood, but negative in BM. Because BM infiltration in FL is characteristically patchy and frequently not observed in marrow aspirates, this finding is not viewed as unusual. In fact, Gribben et al39 previously observed that the results of PCR from BM biopsy specimens varied according to the site where the biopsy was performed.
The most significant and interesting observation in this study is the intriguing correlation between the bcl-2 breakpoint site and the clinical outcome. As a group, the mcr+ patients so far have shown an excellent prognosis. Their pretreatment prognostic variables were more favorable than those of the other groups (earlier Ann Arbor stage and lower serum β2M levels); in addition, they also had a higher CR rate and superior FFS. So far, only 1 of 27 patients have relapsed at a median follow-up of 27 months. On the other hand, those patients with germline bcl-2 had a poor prognosis. Their response to therapy was suboptimal (<70% of CR rate), and their relapse rate was highest of all. It is interesting to note that the relapse pattern of MBR+ cases appears different when compared with germline cases. The MBR+ cases display the typical FFS curve of FL, which consists of a slow but relentless relapse pattern, without any hint of a plateau. Interestingly, the germline cases have a FFS curve that mimics that of aggressive lymphomas: early and frequent relapses for the first 3 years but no more relapses. A longer follow-up will be necessary to confirm this observation.
The type of treatment delivered to patients with advanced stage was associated with some differences in FFS. Those who received the intensive ATT regimen experienced a better FFS than those treated with FND. However, the two groups of patients might not be strictly comparable because the median follow-up is different. This is because of the fact that FND recently has been introduced for advanced, previously untreated FL in our institution.40 In fact, in a current prospective randomized study comparing FND with ATT, no statistically significant differences have been found between these two regimens.40
Table 4 summarizes previous studies16-21 on the prognostic importance of bcl-2rearrangements in FL. In most of these series, the number of patients was small. Furthermore, most authors included all FL-cell types, whereas Yunis et al17 only considered mixed and large cell FL, and one half of Johnson’s cases16 were of “high-grade” histology. This indicates a predominance of large cell as well as a diffuse pattern on the biopsy specimen. These subgroups are not comparable with typical indolent FL and probably a large number of FL transformed to large cell lymphoma have been included in these series. In large cell lymphomas, the presence of abcl-2 rearrangement has been related to a poor outcome,41 supposedly because these patients correspond to transformed FL, which traditionally have had a poor outcome. On the other hand, Johnson et al16 found bcl-2rearrangement to be a favorable factor for survival, but only for patients with the indolent cell types.
Previous Series Regarding the Prognostic Importance ofBcl-2 Rearrangement in FL
| Reference . | No. of Patients . | Histology . | Technique to Assess t(14;18) . | Prognostic Value of bcl-2 Rearrangement . | ||
|---|---|---|---|---|---|---|
| CR Rate . | DFS . | OS . | ||||
| Levine,18 1988 | 30 | FL | Cytogenetics | NS | NS | NS |
| Yunis,17 1989 | 20 | FL mixed/large cell | SB | MBR(+) > others | NA | MBR(+) > others |
| Pezzella,19 1992 | 70 | FL | SB/PCR | NA | NA | NS |
| Tilly,20 1994 | 66 | FL | Cytogenetics | NA | NA | NS |
| Johnson,16 1995 | 102 | FL (52 high-grade) | PCR | NA | NA | MBR(+) > others (low-grade) |
| Louie,21 1996 | 79 | FL | SB/cytogenetics | NA | NA | NS |
| This study, 1998 | 247 | Indolent FL | PCR | mcr(+)/MBR(+) > Others | mcr(+)/MBR(+) > Others | NS |
| Reference . | No. of Patients . | Histology . | Technique to Assess t(14;18) . | Prognostic Value of bcl-2 Rearrangement . | ||
|---|---|---|---|---|---|---|
| CR Rate . | DFS . | OS . | ||||
| Levine,18 1988 | 30 | FL | Cytogenetics | NS | NS | NS |
| Yunis,17 1989 | 20 | FL mixed/large cell | SB | MBR(+) > others | NA | MBR(+) > others |
| Pezzella,19 1992 | 70 | FL | SB/PCR | NA | NA | NS |
| Tilly,20 1994 | 66 | FL | Cytogenetics | NA | NA | NS |
| Johnson,16 1995 | 102 | FL (52 high-grade) | PCR | NA | NA | MBR(+) > others (low-grade) |
| Louie,21 1996 | 79 | FL | SB/cytogenetics | NA | NA | NS |
| This study, 1998 | 247 | Indolent FL | PCR | mcr(+)/MBR(+) > Others | mcr(+)/MBR(+) > Others | NS |
Abbreviations: DFS, disease-free survival; OS, overall survival; SB, Southern blot; NS, not significant; NA, not assessed; PCR, polymerase chain reaction.
Finally, it is difficult to explain the differences in outcome of the patients according to the bcl-2 rearrangement. In fact, the expression of BCL-2 and BAX proteins, assessed by a semiquantitative immunostaining technique in a significant number of patients, did not show significant differences according to thebcl-2 rearrangement, although the proportion of germline cases with positive or strongly positive bcl-2 expression was higher than that of MBR and mcr cases. Because the number of patients tested is small, we have to be cautious in the interpretation of these results. On the other hand, p53 mutations,42 c-myc rearrangements43 or, more recently, different p16 alterations44 have been related to aggressive behavior in FL, mostly by histologic transformation of FL. However, there is no proved link between such alterations and the bcl-2rearrangement so far.
In conclusion, the type of bcl-2 rearrangement appears to be an important biological feature that correlates well with the outcome in patients with FL, especially when combined with other classical prognostic factors such LDH and β2M. The biological explanation for the clinical differences we have identified remains unclear. More studies on bcl-2, bax, and other related molecules, including a more detailed analysis of such proteins and their mRNA would be useful to better understand the pathogenesis of FL.
Supported in part by National Cancer Institute, Grants No. CA62518 and No. CA62597 and by the Herschel and Hilda Rich Fund for Lymphoma Research. A.L.G. was supported by the Hospital Clı́nic of Barcelona and the Spanish Ministry of Education (EX 95/43390399).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Fernando Cabanillas, MD, Chairman, Department of Myeloma/Lymphoma, Box 68, The University of Texas, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.