Voltage-gated K+ channels have been shown to be required for proliferation of various types of cells. Much evidence indicates that K+-channel activity is required for G1 progression of the cell cycle in different cell backgrounds, suggesting that K+-channel activity is required for early-stage cell proliferation in these cells. However, little is known about the molecular mechanisms that underlie this phenomenon. We have shown in human myeloblastic leukemia ML-1 cells that K+ channels are activated by epidermal growth factor (EGF), whereas serum starvation deprivation suppressed their activity. In addition, voltage-gated K+ channels are required for G1/S-phase transition of the cell cycle. We report here that suppression of K+ channels prevented the activation of extracellular signal-regulated protein kinase 2 (ERK-2) in response to EGF and serum. However, blockade of K+ channels did not prevent ERK-2 activation induced by 12-O-tetradecanoyl-phorbol 13-acetate (TPA). Elimination of extracellular Ca2+ did not alter either ERK-2 activation or the effect of K+-channel blockade on ERK-2 activation. Our data demonstrate that the K+ channel is a part of the EGF-mediated mitogenic signal-transduction process and is required for initiation of the EGF-mediated mitogen-activated protein kinase (MAPK) pathways. Our findings may thus explain why an increase in K+-channel activity is associated with cell proliferation in many types of cells, including ML-1 cells.
GROWTH FACTORS STIMULATE the entry of cells into the cell cycle. They stimulate cell proliferation by initiating G1 progression to S phase of the cell cycle.1 Growth factors transmit their mitogenic signals via mitogen-activated protein kinase (MAPK) pathways,2,3 kinase cascades that eventually activate MAPKs. The MAPKs that primarily respond to growth factor stimulation are extracellular signal-regulated kinases 1 and 2 (ERK-1 and ERK-2).2-4 Growth factor–mediated mechanisms regulate voltage-gated K+channels, with the activity and/or expression level of K+channels generally increasing following mitogenic stimulation.5-7 Voltage-gated K+ channels are required for proliferation of a variety of cells, including T and B lymphocytes; blockade of these K+ channels usually inhibits cell proliferation.8,9 Substantial evidence indicates that voltage-gated K+ channels are required for cells to progress through the G1 phase of the cell cycle.8 10 However, the cellular mechanisms that underlie this phenomenon are still largely unknown.
Potassium channels are the most diverse membrane ion channels, being essential for a variety of physiologic functions. Voltage-gated K+ channels function to modulate electrical excitability in excitable cells.11-14 In nonexcitable cells, their main function is to maintain proper cell membrane potential and cell volume.15-17 Alteration of K+-channel activity in the cell membrane can mediate functional adaptation to a variety of chemical and physical stimulation through membrane potential stabilization and maintenance of salt and water balance. We found that cytokine-mediated stimulation of proliferation in myeloblastic ML-1 cells is associated with increases in K+ channel activity.
A voltage-gated delayed-rectifier K+ channel has been identified in ML-1 human myeloblastic leukemia cells.10,18,19 The K+ channel has a conductance of 31 pS and is very sensitive to the traditional K+-channel blocker 4-aminopyridine (4-AP).10,18,19 K+-channel activity is less sensitive to inhibitions by Ba2+ and tetraethylene ammonium (TEA).18 K+-channel activity, which has been shown to be strongly activated by serum growth factors,19is high in proliferating ML-1 cells and diminished in quiescent and differentiated cells.18,19 Suppression of the K+ channels with K+-channel blockers inhibits proliferation of ML-1 cells by preventing the G1/S transition of the cell cycle.10 Blockade of K+channels also prevents phosphorylation of the retinoblastoma protein.10 These findings indicate a possible association of K+-channel activity with the early signaling events of mitogenic processes in ML-1 cells. In the present study, we demonstrate that activity of voltage-gated K+ channels is required for activation of ERKs, represented by ERK-2, in ML-1 cells in response to growth factors, indicating that the K+ channel is one of the early components in EGF-mediated MAPK pathway. These results may provide an explanation for the association of K+-channel activity with cell proliferation in other types of cells.
MATERALS AND METHODS
Cell culture.
ML-1 cells were cultured in RPMI 1640 containing 7.5% heat-inactivated fetal bovine serum (FBS; GIBCO, Grand Island, NY) in a humidified incubator supplied with 5% CO2 at 37°C. Cells were passed at 3 × 105 cells/mL seeding density. Serum starvation of the cells was achieved by maintaining cells in medium containing 0.3% FBS for 36 hours. Blockade of K+ channel was performed by adding K+-channel blockers in the medium 30 minutes before the treatment with EGF or FBS.
Patch clamp experiments.
Patch pipettes, with a resistance of 3 to 4 MW when filled with 150 mmol/L KCl solution, were manufactured with a two-stage puller (PP-83; Narishige, Japan). For whole-cell K+-current recording, the nystatin perforated-patch technique was used to provide measurements of stable whole-cell currents without disrupting cytoplasmic concentration of divalent ions or metabolites. The pipette tip was filled with a solution containing (in mmol/L): KCl 140, CaCl2 0.05, MgCl2 2, adenosine triphosphate (ATP) 2, guanosine triphosphate (GTP) 0.05, ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA) 1, and HEPES 10 (titrated with KOH to pH 7.2). The remainder of the pipette was backfilled with the same pipette solution with the addition of 200 mg/mL nystatin. The bath solution was composed of (in mmol/L): NaCl 140, KCl 2, CaCl2 1, HEPES 10, pH 7.4. Voltage-clamp experiments were performed using an Axopatch 200A patch-clamp amplifier; voltage stimulation pulses were controlled with a pCLAMP program (Axon Instruments, Inc, Foster City, CA). Whole-cell currents were prefiltered at 1 kHz through a 4-pole Bessel low-pass filter and digitized at 22 kHz by an A/D converter interface and video recorder (A.R. Vetter, Rebersburg, PA). Data were collected and analyzed with pCLAMP software. For the cell-attached single-channel patch clamp, solutions were as follows: (1) KCl-bath solution containing (in mmol/L): KCl 140, MgCl2 2, CaCl20.5, EGTA 1, and HEPES 10, pH 7.4; and (2) pipette solution containing KCl 140, MgCl2 2, CaCl2 1, EGTA 1, HEPES 10, pH 7.4. Single-channel currents were recorded with an Axonpatch 200A amplifier and filtered with a 4-pole Bessel low-pass filter at 2 kHz and digitized at 22 kHz. The pCLAMP program was used to analyze the single-channel data. Channel activity was determined as NPo, where N represents the number of channels in the patch and Po represents the probability of an open channel. All experiments were performed at room temperature (21°C to 23°C).
Kinase assay and Western blot.
ML-1 cells (1 × 107/treatment at cell density of 5 × 105 cells/mL) were washed once with ice-cold phosphate-buffered saline (PBS) and lysed with 1 mL of lysis buffer (20 mmol/L Tris, pH 7.5, 137 mmol/L NaCl, 1.5 mmol/L MgCl2, 2 mmol/L EDTA, 10 mmol/L sodium pyrophosphate, 25 mmol/L β-glycerophosphate, 10% glycerol, 1% Triton X-100, 1 mmol/L Na-orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 mg/mL leupeptin). Cell lysates were maintained on ice for 10 minutes and then precleared by centrifugation at 13,000g for 25 minutes. ERK-2 proteins were immunoprecipitated with 0.5 μg rabbit polyclonal antibody against ERK-2 (Santa Cruz Biotechnology, Santa Cruz, CA) and Protein A-Sepharose beads (Sigma, St Louis, MO). The immunocomplex was washed three times with lysis buffer and twice with kinase buffer (20 mmol/L HEPES, pH 7.6, 20 mmol/L MgCl2, 25 mmol/L β-glycerophosphate, 100 mmol/L sodium orthovanadate, and 2 mmol/L dithiothreitol [DTT]) and resuspended in 90 μL of kinase buffer. Myelin basic protein (MBP; 2.5 μg) was added to 30 μL of the immunocomplex. Kinase reactions were initiated by adding 2 μL of ATP cocktail (20 mmol/L ATP and 10 μCi [γ-32P]ATP; Amersham, Arlington Heights, IL). Reactions were allowed to proceed at room temperature for 15 minutes before termination by the addition of 30 μL of 2X Laemmli buffer. Phosphorylation of MBP was visualized by autoradiography after sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). MBP phosphorylation levels were quantified by densitometry.
ERK-2 protein levels were determined by Western blotting. Briefly, an equal volume of 2X Laemmli buffer was added to 20 mL of immunocomplex and boiled for 5 minutes. After resolution by 12% SDS-PAGE, proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA) and incubated with the anti–ERK-2 antibody. The membranes were then incubated with goat anti-rabbit IgG conjugated with alkaline phosphatase (Santa Cruz Biotechnology). Secondary antibodies were detected with a Phototope-Star Western Blot Detection Kit (New England Biolabs, Beverly, MA). Data shown are from three independent experiments.
RESULTS
Using the nystatin-perforated whole-cell technique, the whole-cell current in ML-1 cells was activated by depolarization of the membrane potential from a holding potential of −60 to +80 mV in 20-mV increments. Upon exposure of ML-1 cells to 50 ng/mL of EGF, the amplitude of the K+ current increased markedly (Fig1A and B). The time course showed that the amplitude of the K+ current doubled within 2 to 3 minutes after exposure to EGF stimulation and reached the maximum amplitude within 5 to 10 minutes. EGF-evoked K+ current was sensitive to 4-AP, being completely blocked by 2 mmol/L 4-AP (Fig 1A and B). The time course for 4-AP blockade of EGF-stimulated K+ channel activity is shown in Fig 1C.
Activation of a 4-AP–sensitive K+ channel by EGF stimulation. (A) Effect of EGF on the 4-AP–sensitive K+ current. The membrane potential was depolarized from a holding potential of −60 mV to +80 mV at 20-mV increments with a pulse protocol showing in the top panel. Whole-cell currents were recorded from (1) ML-1 cells in the absence (control) and presence of 2 mmol/L 4-AP (as indicated); and (2) ML-1 cells simulated with 10 ng/mL EGF in the absence (EGF) and presence of 4-AP (EGF + 4-AP). (B) Current-voltage relationship of the 4-AP–sensitive K+current activated by EGF in the absence and presence of 4-AP. (C) Time course of EGF-activated K+ current in the absence and presence of 4-AP. Currents were normalized as IEGF/IC , where IEGF represents amplitudes of the EGF-induced K+ current and IC represents the K+ current measured from control ML-1 cells. (D) Single-channel recording of K+ channel in ML-1 cells. Inward current recorded as a downward deflection was obtained from cell-attached patches at a membrane potential of −60 mV in the symmetrical 140/140 mmol/L KCl condition. EGF (50 ng/mL) was directly applied to the patch chamber to activate K+ channels in the same patch. (E) Current trace demonstrates that application of 100 μmol/L 4-AP in the patch pipette prevented the EGF-induced increase of K+-channel activity. Channel activity (NPo) was plotted as a function of time in the lower portion of D and E. (F) Statistics of K+-channel activity stimulated by EGF and FBS in the absence and presence of 100 μmol/L 4-AP. Vertical bars represent mean K+-channel activity (horizontal bars represent standard error of the mean [SE]). *Significant difference (statistical tests: ANOVA and Tukey, P < .001). Data were collected from five independent experiments.
Activation of a 4-AP–sensitive K+ channel by EGF stimulation. (A) Effect of EGF on the 4-AP–sensitive K+ current. The membrane potential was depolarized from a holding potential of −60 mV to +80 mV at 20-mV increments with a pulse protocol showing in the top panel. Whole-cell currents were recorded from (1) ML-1 cells in the absence (control) and presence of 2 mmol/L 4-AP (as indicated); and (2) ML-1 cells simulated with 10 ng/mL EGF in the absence (EGF) and presence of 4-AP (EGF + 4-AP). (B) Current-voltage relationship of the 4-AP–sensitive K+current activated by EGF in the absence and presence of 4-AP. (C) Time course of EGF-activated K+ current in the absence and presence of 4-AP. Currents were normalized as IEGF/IC , where IEGF represents amplitudes of the EGF-induced K+ current and IC represents the K+ current measured from control ML-1 cells. (D) Single-channel recording of K+ channel in ML-1 cells. Inward current recorded as a downward deflection was obtained from cell-attached patches at a membrane potential of −60 mV in the symmetrical 140/140 mmol/L KCl condition. EGF (50 ng/mL) was directly applied to the patch chamber to activate K+ channels in the same patch. (E) Current trace demonstrates that application of 100 μmol/L 4-AP in the patch pipette prevented the EGF-induced increase of K+-channel activity. Channel activity (NPo) was plotted as a function of time in the lower portion of D and E. (F) Statistics of K+-channel activity stimulated by EGF and FBS in the absence and presence of 100 μmol/L 4-AP. Vertical bars represent mean K+-channel activity (horizontal bars represent standard error of the mean [SE]). *Significant difference (statistical tests: ANOVA and Tukey, P < .001). Data were collected from five independent experiments.
To further confirm the effect of EGF stimulation on single K+-channel activity, the cell-attached patch clamp technique was used in our study. The single-channel current was recorded at a membrane potential of −60 mV in vivo (Fig 1D). Exposure to 50 ng/mL EGF or 10% FBS (data not shown) stimulation strongly activated K+-channel activity (NPo). Activity increased from 9.6% ± 1.6% to 36.0% ± 3.6% for EGF stimulation and from 9.6% ± 1.6% to 52% ± 9.8% for FBS stimulation within 5 minutes (Fig 1F). In the presence of 100 μmol/L 4-AP in the patch pipette, EGF and FBS stimulation failed, in seven independent patches, to activate K+-channel activity. NPo remained unchanged at 12.2% ± 3.5% (Fig 1E and F). These results indicate that an early effect of EGF or FBS is to activate cell-membrane K+-channel activity in ML-1 cells.
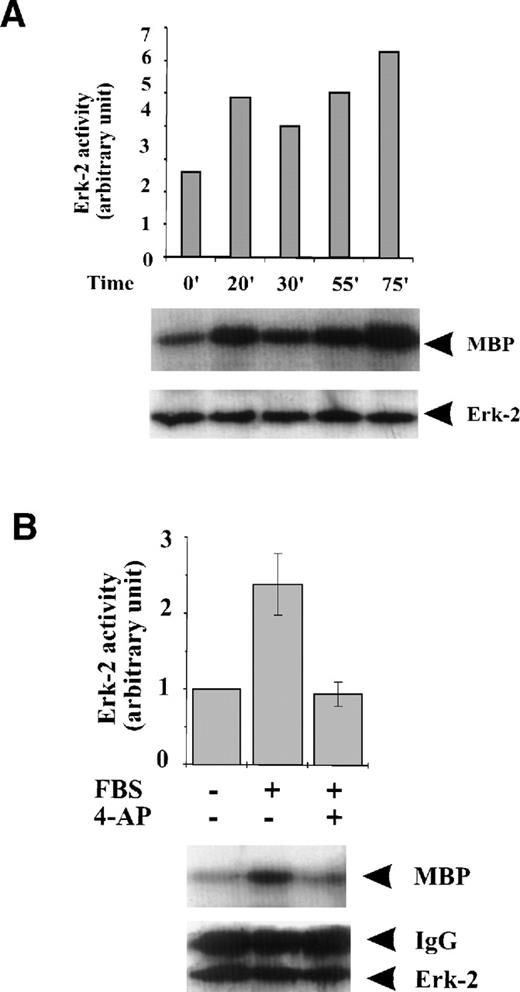
To examine if K+-channel activity is associated with the activation of MAPKs in response to serum growth factors, we studied the effect of K+-channel blockade on MAPK activation. FBS caused persistent, although variable, activation of ERK-2 during the 75-minute period following the addition of FBS to the culture medium (Fig 2A). Blockade of the K+ channel with 4-AP prevented the induction of ERK-2 activation by FBS (Fig 2B). Because ERK-2 is a component of the MAPK pathway, these results indicate that K+-channel activity is required for activation of the MAPK pathway when induced by serum growth factors in ML-1 cells.
Effect of K+-channel blockade on ERK-2 activation. (A) Time-dependent activation of ERK-2 by FBS. Serum-starved ML-1 cells were left untreated or stimulated with FBS (10%). At the indicated time points, cells were collected and measured for ERK-2 activity and ERK-2 protein levels. (B) Effect of K+-channel blockade on FBS-induced ERK-2 activation in ML-1 cells. ML-1 cells were serum-starved and either untreated or stimulated with FBS (10%) for 15 minutes in the presence or absence of 2 mmol/L 4-AP. Cells were collected at the end of the treatment. ERK-2 activities protein levels were measured. (C) Time-dependent activation of ERK-2 by EGF in ML-1 cells. Serum-starved ML-1 cells were either untreated or stimulated with EGF (50 ng/mL). At the indicated time points, cells were collected and measured for ERK-2 activity and ERK-2 protein levels. (D) Effect of K+-channel blockade on EGF-induced ERK-2 activation in ML-1 cells. Serum-starved ML-1 cells were untreated or stimulated with EGF (50 ng/mL) for 15 minutes in the presence or absence of 2 mmol/L 4-AP. The cells were collected at the end of the treatment, and ERK-2 activities were measured. (E) Dose-dependent inhibitions of EGF-induced ERK-2 activation by K+-channel blockade with 4-AP. Serum-starved ML-1 cells were either untreated or stimulated with EGF (50 ng/mL) for 15 minutes in the presence of the indicated dosages of 4-AP. The cells were collected at the end of the treatment, and ERK-2 activities were measured.
Effect of K+-channel blockade on ERK-2 activation. (A) Time-dependent activation of ERK-2 by FBS. Serum-starved ML-1 cells were left untreated or stimulated with FBS (10%). At the indicated time points, cells were collected and measured for ERK-2 activity and ERK-2 protein levels. (B) Effect of K+-channel blockade on FBS-induced ERK-2 activation in ML-1 cells. ML-1 cells were serum-starved and either untreated or stimulated with FBS (10%) for 15 minutes in the presence or absence of 2 mmol/L 4-AP. Cells were collected at the end of the treatment. ERK-2 activities protein levels were measured. (C) Time-dependent activation of ERK-2 by EGF in ML-1 cells. Serum-starved ML-1 cells were either untreated or stimulated with EGF (50 ng/mL). At the indicated time points, cells were collected and measured for ERK-2 activity and ERK-2 protein levels. (D) Effect of K+-channel blockade on EGF-induced ERK-2 activation in ML-1 cells. Serum-starved ML-1 cells were untreated or stimulated with EGF (50 ng/mL) for 15 minutes in the presence or absence of 2 mmol/L 4-AP. The cells were collected at the end of the treatment, and ERK-2 activities were measured. (E) Dose-dependent inhibitions of EGF-induced ERK-2 activation by K+-channel blockade with 4-AP. Serum-starved ML-1 cells were either untreated or stimulated with EGF (50 ng/mL) for 15 minutes in the presence of the indicated dosages of 4-AP. The cells were collected at the end of the treatment, and ERK-2 activities were measured.
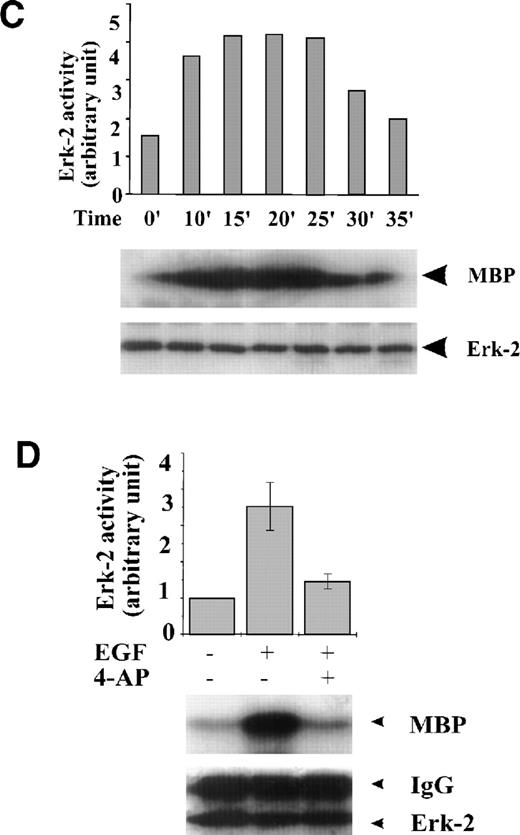
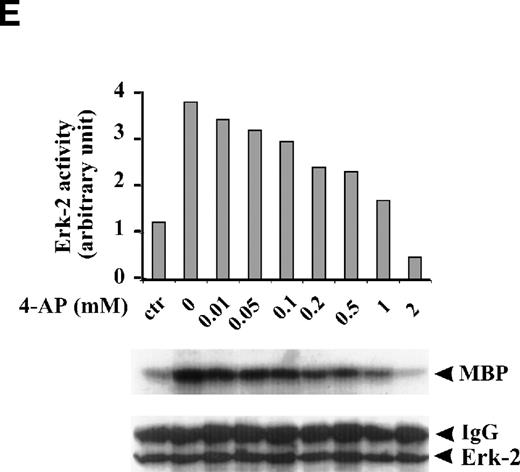
FBS contains multiple growth factors, cytokines, and hormones, any one of which may be responsible for the observed ERK-2 activation. To isolate the possible components in growth factor–mediated MAPK pathways affected by the K+-channel activity, we examined the role of the K+ channels in MAPK pathways mediated by a single growth factor, that is, EGF-mediated MAPK pathways. EGF (50 ng/mL) induced a transient ERK-2 activation that lasted approximately 25 minutes (Fig 2C). Suppression of the K+ channels with 4-AP (2 mmol/L) prevented the ERK-2 activation (Fig 2D). The inhibition of ERK-2 activation by 4-AP was dose-dependent, reaching a maximum at 2 mmol/L (Fig 2E). These results, indicating that K+-channel activity was required for EGF-induced ERK-2 activation, indicate that K+ channels may interact with receptor tyrosine kinase-mediated MAPK pathways in ML-1 cells.
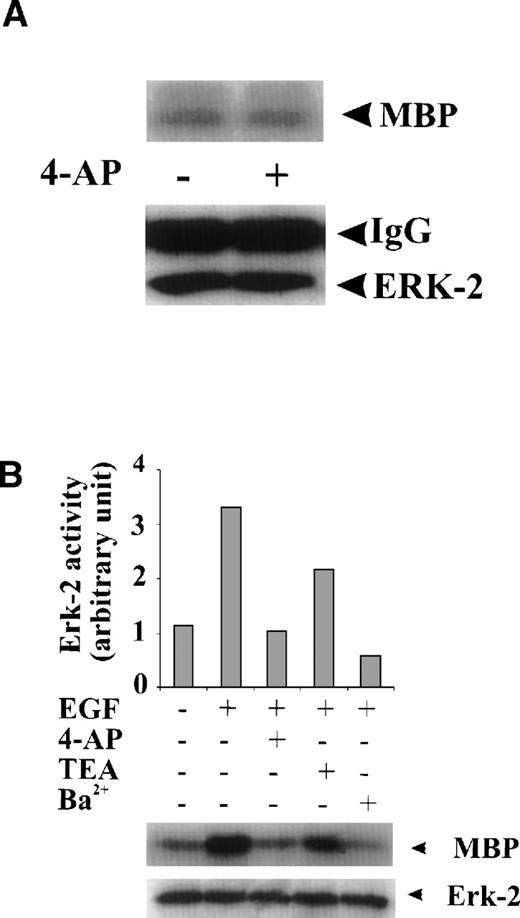
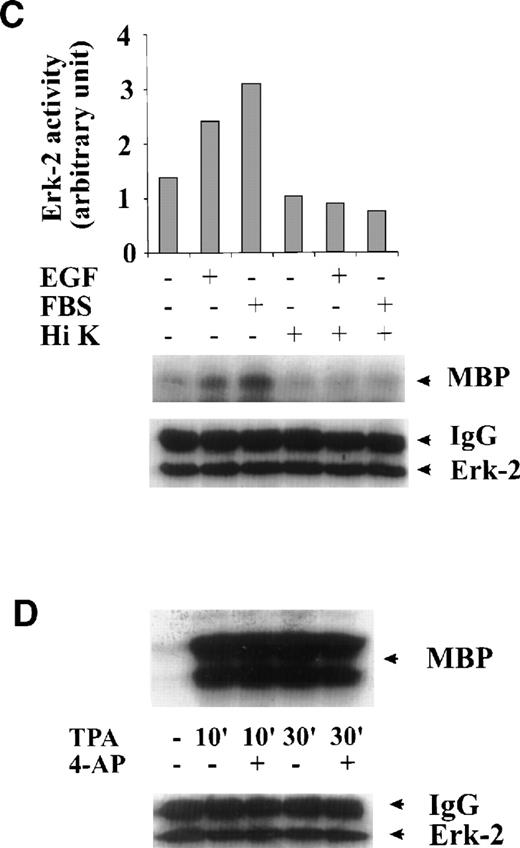
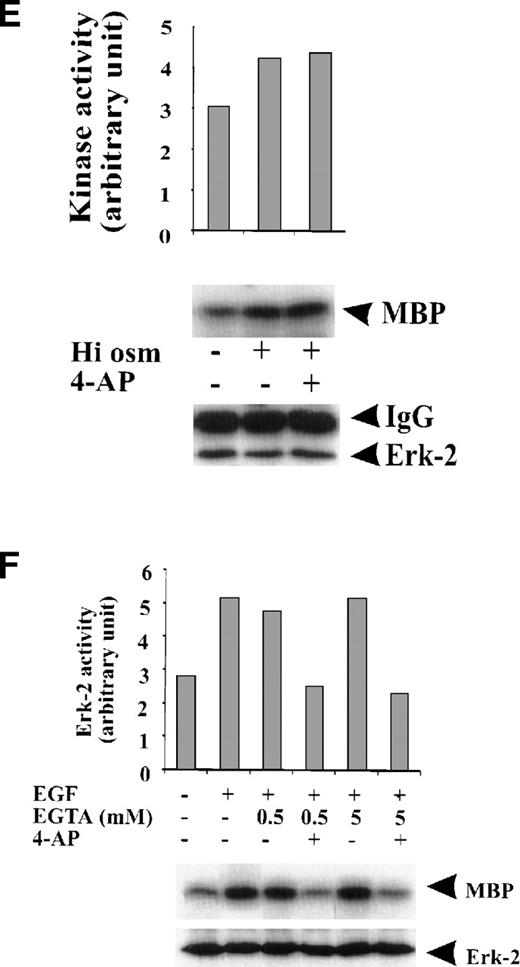
To test whether the inhibition of ERK-2 activation by 4-AP was by suppression of the K+ channel rather than by nonspecific inhibition of the EGF-mediated MAPK pathway, we tested the direct effect of 4-AP on ERK-2 activity. No direct effect of 4-AP was found on ERK-2 activity, which was measured by directly adding 3 mmol/L 4-AP to the kinase reaction (Fig 3A), indicating that 4-AP inhibits the activation of components that are upstream of ERK-2. We further tested effects of other classic K+-channel blockers on ERK-2 activation, reasoning that, if different K+ channel blockers have similar effects on ERK-2 activation, then the effect of 4-AP, more likely, would be specific for the K+ channel. Indeed, TEA (10 mmol/L) and Ba2+ (5 mmol/L) significantly inhibited ERK-2 activation (Fig 3B). Inhibition of ERK-2 activation also was achieved by increasing the concentration of extracellular K+ (Fig 3C).
Specificity of the effect of K+-channel blockade on ERK-2 activation. (A) Direct effect of 4-AP on ERK-2 activity. ERK-2 was immunoprecipitated and measured for activity in the presence and absence of 3 mmol/L 4-AP in kinase buffer. (B) Effects of different K+-channel blockers on EGF-induced ERK-2 activation. Serum-starved ML-1 cells were either untreated or were stimulated with EGF (50 ng/mL) for 15 minutes in the presence or absence of 4-AP (2 mmol/L), TEA (10 mmol/L), or Ba2+ (5 mmol/L). The cells were collected at the end of the treatment, and ERK-2 activities were measured. (C) Effect of high extracellular K+ on EGF- and FBS-induced ERK-2 activation. Serum-starved ML-1 cells were either untreated or were stimulated with EGF (50 ng/mL) or FBS (10%) for 15 minutes in normal medium or in medium containing high K+ (60 mmol/L). The cells were then collected and assayed for ERK-2 activity. (D) Effect of 4-AP on TPA-induced ERK-2 activation. Serum-starved ML-1 cells were untreated or treated with TPA (1 nmol/L) for 10 or 30 minutes in the presence or absence of 4-AP. At the end of the treatment, cells were collected and measured for ERK-2 activity. (E) Effect of 4-AP on high-osmolarity–induced ERK-2 activation. Serum-starved ML-1 cells were untreated or treated with high-osmolarity medium (600 mmol/L sorbitol) in the presence or absence of 2 mmol/L 4-AP. The cells were then collected and measured for ERK-2 activity. (F) Effect of extracellular Ca2+ on EGF-induced ERK-2 activation and on the inhibition of ERK-2 activation by K+-channel blockade. Serum-starved ML-1 cells were untreated, treated with EGTA alone, or treated with EGTA plus 4-AP (2 mmol/L) for 30 minutes. Cells were then left untreated or stimulated with EGF (50 ng/mL) for 15 minutes. The cells were collected at the end of the treatment and measured for ERK-2 activity and protein levels.
Specificity of the effect of K+-channel blockade on ERK-2 activation. (A) Direct effect of 4-AP on ERK-2 activity. ERK-2 was immunoprecipitated and measured for activity in the presence and absence of 3 mmol/L 4-AP in kinase buffer. (B) Effects of different K+-channel blockers on EGF-induced ERK-2 activation. Serum-starved ML-1 cells were either untreated or were stimulated with EGF (50 ng/mL) for 15 minutes in the presence or absence of 4-AP (2 mmol/L), TEA (10 mmol/L), or Ba2+ (5 mmol/L). The cells were collected at the end of the treatment, and ERK-2 activities were measured. (C) Effect of high extracellular K+ on EGF- and FBS-induced ERK-2 activation. Serum-starved ML-1 cells were either untreated or were stimulated with EGF (50 ng/mL) or FBS (10%) for 15 minutes in normal medium or in medium containing high K+ (60 mmol/L). The cells were then collected and assayed for ERK-2 activity. (D) Effect of 4-AP on TPA-induced ERK-2 activation. Serum-starved ML-1 cells were untreated or treated with TPA (1 nmol/L) for 10 or 30 minutes in the presence or absence of 4-AP. At the end of the treatment, cells were collected and measured for ERK-2 activity. (E) Effect of 4-AP on high-osmolarity–induced ERK-2 activation. Serum-starved ML-1 cells were untreated or treated with high-osmolarity medium (600 mmol/L sorbitol) in the presence or absence of 2 mmol/L 4-AP. The cells were then collected and measured for ERK-2 activity. (F) Effect of extracellular Ca2+ on EGF-induced ERK-2 activation and on the inhibition of ERK-2 activation by K+-channel blockade. Serum-starved ML-1 cells were untreated, treated with EGTA alone, or treated with EGTA plus 4-AP (2 mmol/L) for 30 minutes. Cells were then left untreated or stimulated with EGF (50 ng/mL) for 15 minutes. The cells were collected at the end of the treatment and measured for ERK-2 activity and protein levels.
The effect of 4-AP on TPA-induced ERK-2 activation was also tested. TPA (1 nmol/L) induced a robust increase in ERK-2 activity 10 minutes after treatment and 30 minutes after treatment (Fig 3D). 4-AP (2 mmol/L) had no effect on this ERK-2 activation (Fig 3D). Because TPA is a PKC activator, which can activate ERKs by activating MAPK kinase kinase, Raf-1,20-23 these results indicate that 4-AP is not an inhibitor of Raf-1 or of the components below Raf-1 in the ERK-signaling cascade. The results lend further support to the hypothesis that K+-channel activity may be required for the upstream components of an EGF-mediated ERK pathway.
We also measured the effect of 4-AP on high-osmolarity stress-induced ERK-2 activation. High osmolarity (600 mmol/L sorbitol) induced a slight increase in ERK-2 activity (Fig 3E). No inhibitory effect of 4-AP was found on this ERK-2 activation (Fig 3E), which again indicates that 4-AP does not nonspecifically inhibit the ERK-2 pathway and/or high osmolarity may overcome the effects of K+-channel blockade.
It has shown that elevation of intracellular Ca2+ is required for T-cell activation.27 A similar increase in Ca2+ influx may happen in ML-1 cells and, therefore, may contribute to ERK-2 activation. This mechanism may contribute to the effect of K+-channel blockade on ERK-2 activation. To test this possibility, we examined whether EGF could induce ERK-2 activation under conditions of diminished extracellular Ca2+. In culture media from which free Ca2+ was chelated with either 0.5 or 5 mmol/L EGTA, we found that ERK-2 activation in response to EGF was unaffected, compared with the response of the cells in normal medium (Fig 3F). Suppression of K+-channel activity with 4-AP inhibited EGF-induced ERK-2 activation of the cells equally in media that contained different concentrations of EGTA (Fig 3F). These results, therefore, rule out the possible involvement of Ca2+ influx in the EGF-induced ERK-2 activation in ML-1 cells and show that prevention of ERK-2 activation was not due to inhibition of Ca2+ influx.
DISCUSSION
Other researchers have suggested a few possibilities regarding how the K+-channel activity is involved in cell proliferation. These include Ca2+ influx as a result of hyperpolarization induced by K-channel activation, cell volume change caused by loss of K+ as a result of K+-channel activation, and expression of cell cycle–regulator proteins such as cyclin D1.8 Hyperpolarization could also facilitate Na+-dependent transport of metabolic substrates by increasing the electrochemical gradient for Na+.8 Our finding that K+-channel activity is required for MAPK activation indicates that K+channels are involved in the initiation stage of the cell cycle in ML-1 cells instead of a later stage of the G1 phase. Our data demonstrate that the K+ channels are part of the growth factor–mediated mitogenic signal transduction processes and are required for initiation of the growth factor–mediated MAPK pathways. We propose that K+-channel activity is required for early, membrane-associated events of the growth factor signal-transduction pathways.
In the present study, we have also examined the possibility that Ca2+ influx may play a role in the EGF-induced mitogenic effect in ML-1 cells. Calcium may activate the MAPK pathway by activating CAM kinase in some cells.24,25 One of the early events of the EGF-mediated signaling pathway is the transient elevation of intracellular Ca2+.26 An increase in intracellular calcium may arise from either the release of stored intracellular Ca2+ or from an influx of extracellular Ca2+.24,25 Intracellular Ca2+release from storage is induced by second-messenger inositol trisphosphate3 (IP3), produced by hydrolysis of polyphosphoinositides as a result of activation of phospholipase C-γ (PLC-γ) by activated EGF receptors.24,25 Extracellular Ca2+ influx may be caused by EGF-induced membrane hyperpolarization as a result of activation of Ca2+-activated K+ channels, which is caused by intracellular Ca2+ release.27 In other studies, voltage-gated K+ channels have been shown to be a component in the activation of T cells27 and of T-cell receptors.28 The elevation of cell membrane potential as a result of increased K+-channel activity increases the driving force for Ca2+ influx thereby increasing intracellular Ca2+. Our results shown in Fig 3F clearly demonstrate that extracellular Ca2+ did not have any effect on either EGF-induced mitogenic pathway or the interaction between K+-channel activity and the EGF-mediated pathway in ML-1 cells.
Because our study ruled out the possible involvement of Ca2+ influx in the activation of MAPK by EGF in ML-1 cells, mechanisms other than alteration of Ca2+ influx may be involved. Change in cell volume, induced by loss of K+ as a result of K+-channel activation, is one possible explanation. The findings that high osmolarity alone can induce EGF receptor clustering and activation in HeLa cells give support to this hypothesis.29 The transient cell shrinkage caused by high-osmolarity shock could greatly facilitate, or be required for, activation of EGF receptors in ML-1 cells. This hypothesis is supported by the finding that K+-channel blockade did not inhibit ERK-2 activation induced by high-osmolarity shock in ML-1 cells (Fig3E), because the strong force of cell shrinkage caused by high osmolarity may far overcome the swelling effect of K+-channel blockade. The present results could explain our previous findings that K+-channel blockade inhibits cell proliferation in ML-1 cells and may provide an answer to why K+-channel activity is required for proliferation of other cells.
Supported by National Institutes of Health (NIH) Grants No. GM46834 and EY11653 to L.L. and supported in part by NIH Grant No. CA59985 to W.D.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Luo Lu, MD, PhD, Department of Physiology and Biophysics, School of Medicine, Wright State University, Dayton, OH 45435; e-mail: LLU@WRIGHT.EDU.

![Fig. 1. Activation of a 4-AP–sensitive K+ channel by EGF stimulation. (A) Effect of EGF on the 4-AP–sensitive K+ current. The membrane potential was depolarized from a holding potential of −60 mV to +80 mV at 20-mV increments with a pulse protocol showing in the top panel. Whole-cell currents were recorded from (1) ML-1 cells in the absence (control) and presence of 2 mmol/L 4-AP (as indicated); and (2) ML-1 cells simulated with 10 ng/mL EGF in the absence (EGF) and presence of 4-AP (EGF + 4-AP). (B) Current-voltage relationship of the 4-AP–sensitive K+current activated by EGF in the absence and presence of 4-AP. (C) Time course of EGF-activated K+ current in the absence and presence of 4-AP. Currents were normalized as IEGF/IC , where IEGF represents amplitudes of the EGF-induced K+ current and IC represents the K+ current measured from control ML-1 cells. (D) Single-channel recording of K+ channel in ML-1 cells. Inward current recorded as a downward deflection was obtained from cell-attached patches at a membrane potential of −60 mV in the symmetrical 140/140 mmol/L KCl condition. EGF (50 ng/mL) was directly applied to the patch chamber to activate K+ channels in the same patch. (E) Current trace demonstrates that application of 100 μmol/L 4-AP in the patch pipette prevented the EGF-induced increase of K+-channel activity. Channel activity (NPo) was plotted as a function of time in the lower portion of D and E. (F) Statistics of K+-channel activity stimulated by EGF and FBS in the absence and presence of 100 μmol/L 4-AP. Vertical bars represent mean K+-channel activity (horizontal bars represent standard error of the mean [SE]). *Significant difference (statistical tests: ANOVA and Tukey, P < .001). Data were collected from five independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.139.413k11_139_145/6/m_blod41311001ax.jpeg?Expires=1767876731&Signature=ybiN~aTtaQsAILXQc~dyUGV-Q4DM4c538MTfxpSrl-wwHpdQw6eC6yjHxvo3-CxwqH25iCb81uXHPNshcnAFxyT~B0lMmQyl0YpdSz6s8XJ6AeADx58t7V3qeKksyk25tKUcoW3zHyQWeuIV6rtl36aVfE4orC8ECRcQbYaALTzzIedVm8oUhu954dwmfdx2syCbDBTfIKdRa3iTytlcc2KOxdjv98V1GOHjpKzt8k7UifCDAxQgGfWVMpTquymdBd-5PfI0z-GIW4x7WylI1UbWN6lMusT9K1D6JWvnPPghQGj9~WMxLgKbECIqxemqUs49WjZFPKZ3fkbhbr83Yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Activation of a 4-AP–sensitive K+ channel by EGF stimulation. (A) Effect of EGF on the 4-AP–sensitive K+ current. The membrane potential was depolarized from a holding potential of −60 mV to +80 mV at 20-mV increments with a pulse protocol showing in the top panel. Whole-cell currents were recorded from (1) ML-1 cells in the absence (control) and presence of 2 mmol/L 4-AP (as indicated); and (2) ML-1 cells simulated with 10 ng/mL EGF in the absence (EGF) and presence of 4-AP (EGF + 4-AP). (B) Current-voltage relationship of the 4-AP–sensitive K+current activated by EGF in the absence and presence of 4-AP. (C) Time course of EGF-activated K+ current in the absence and presence of 4-AP. Currents were normalized as IEGF/IC , where IEGF represents amplitudes of the EGF-induced K+ current and IC represents the K+ current measured from control ML-1 cells. (D) Single-channel recording of K+ channel in ML-1 cells. Inward current recorded as a downward deflection was obtained from cell-attached patches at a membrane potential of −60 mV in the symmetrical 140/140 mmol/L KCl condition. EGF (50 ng/mL) was directly applied to the patch chamber to activate K+ channels in the same patch. (E) Current trace demonstrates that application of 100 μmol/L 4-AP in the patch pipette prevented the EGF-induced increase of K+-channel activity. Channel activity (NPo) was plotted as a function of time in the lower portion of D and E. (F) Statistics of K+-channel activity stimulated by EGF and FBS in the absence and presence of 100 μmol/L 4-AP. Vertical bars represent mean K+-channel activity (horizontal bars represent standard error of the mean [SE]). *Significant difference (statistical tests: ANOVA and Tukey, P < .001). Data were collected from five independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.139.413k11_139_145/6/m_blod41311001bx.jpeg?Expires=1767876731&Signature=RNNJo9NP7Er4WpNR7jB~VYEKhb5wkMKDJKw5GSOZqTRuR~MUI6OKvqd8QIQ4WPXcqHCilhcZQV2C~o7JBK2fWuKNANTK9-tabkcZReTO61hRkJdLDB3vDMFRVNOym8pXZ8gAGmx0iCO6MHPe4Q4O-KlZn64R9YcxZEtMUwYavnEnTempcrCAAtOo1DCrFQUBUtUrrr-Wsw9wCVXdLZ4baUnNj97aod8yvX7VrFrD5r1iDOlZR61VEcRu0OpUZnh~BA0pDrgBzxX8~HiEGS-R2PB~rMS0-2V4-~8cwLJdSvvunr-eO8MdTioXVS-S520HWwOD9NQ0Nok9KEOuKE2YWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Activation of a 4-AP–sensitive K+ channel by EGF stimulation. (A) Effect of EGF on the 4-AP–sensitive K+ current. The membrane potential was depolarized from a holding potential of −60 mV to +80 mV at 20-mV increments with a pulse protocol showing in the top panel. Whole-cell currents were recorded from (1) ML-1 cells in the absence (control) and presence of 2 mmol/L 4-AP (as indicated); and (2) ML-1 cells simulated with 10 ng/mL EGF in the absence (EGF) and presence of 4-AP (EGF + 4-AP). (B) Current-voltage relationship of the 4-AP–sensitive K+current activated by EGF in the absence and presence of 4-AP. (C) Time course of EGF-activated K+ current in the absence and presence of 4-AP. Currents were normalized as IEGF/IC , where IEGF represents amplitudes of the EGF-induced K+ current and IC represents the K+ current measured from control ML-1 cells. (D) Single-channel recording of K+ channel in ML-1 cells. Inward current recorded as a downward deflection was obtained from cell-attached patches at a membrane potential of −60 mV in the symmetrical 140/140 mmol/L KCl condition. EGF (50 ng/mL) was directly applied to the patch chamber to activate K+ channels in the same patch. (E) Current trace demonstrates that application of 100 μmol/L 4-AP in the patch pipette prevented the EGF-induced increase of K+-channel activity. Channel activity (NPo) was plotted as a function of time in the lower portion of D and E. (F) Statistics of K+-channel activity stimulated by EGF and FBS in the absence and presence of 100 μmol/L 4-AP. Vertical bars represent mean K+-channel activity (horizontal bars represent standard error of the mean [SE]). *Significant difference (statistical tests: ANOVA and Tukey, P < .001). Data were collected from five independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.139.413k11_139_145/6/m_blod41311001cx.jpeg?Expires=1767876731&Signature=yYDTM~L-bmivbvRKaoLe37rAi3uhX~aG3IQMLvrtRvOvK0BF7lgVvqPcGNg1Fa1rqGxZAWcAmoOkPooS6-lpEUZCyrQoBd4HJuiQoQzAL5rcUFoGkhwxnH7FX5EUN7HjNJKblpz7W7krT4yz2BOYCjDz~SHnHRkxc61JW81~1uvw0IHJ831xoMMh2TfHriqDImY6fVGnp0tma~-y-9OcOBAw~58GbIR5qiBDCU1DSrZLh1hRpZuq2hu7LQK7clAIUx6RVTNzTdGMBVFcB-NxJvzNye4ENEoSql-02D1mWNqhOcQhrQ7c5vDL8zoFbHZmZC8j40u7-lhl8plhAdVghg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Activation of a 4-AP–sensitive K+ channel by EGF stimulation. (A) Effect of EGF on the 4-AP–sensitive K+ current. The membrane potential was depolarized from a holding potential of −60 mV to +80 mV at 20-mV increments with a pulse protocol showing in the top panel. Whole-cell currents were recorded from (1) ML-1 cells in the absence (control) and presence of 2 mmol/L 4-AP (as indicated); and (2) ML-1 cells simulated with 10 ng/mL EGF in the absence (EGF) and presence of 4-AP (EGF + 4-AP). (B) Current-voltage relationship of the 4-AP–sensitive K+current activated by EGF in the absence and presence of 4-AP. (C) Time course of EGF-activated K+ current in the absence and presence of 4-AP. Currents were normalized as IEGF/IC , where IEGF represents amplitudes of the EGF-induced K+ current and IC represents the K+ current measured from control ML-1 cells. (D) Single-channel recording of K+ channel in ML-1 cells. Inward current recorded as a downward deflection was obtained from cell-attached patches at a membrane potential of −60 mV in the symmetrical 140/140 mmol/L KCl condition. EGF (50 ng/mL) was directly applied to the patch chamber to activate K+ channels in the same patch. (E) Current trace demonstrates that application of 100 μmol/L 4-AP in the patch pipette prevented the EGF-induced increase of K+-channel activity. Channel activity (NPo) was plotted as a function of time in the lower portion of D and E. (F) Statistics of K+-channel activity stimulated by EGF and FBS in the absence and presence of 100 μmol/L 4-AP. Vertical bars represent mean K+-channel activity (horizontal bars represent standard error of the mean [SE]). *Significant difference (statistical tests: ANOVA and Tukey, P < .001). Data were collected from five independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.139.413k11_139_145/6/m_blod41311001dx.jpeg?Expires=1767876731&Signature=RibhKOXp-FkLlLp~lzXJZdCHwT6PsG-e9ipFtwgivHiMxsXYDhFqjBtx8~~NlDiSYQiOcVfNIBn4x3hYfUqshzc-DKrz~c-7cA2NzRoWRSs6Fqp6YRbm0BF-~8smhsbn3xjchMgTfRxRlVJmmzZ4cehaiOdJd3auj6sEH~wOv3yCdl8U9JLTBES9vUx2Go3TC4D8htehqKOfEECNCY7IP1JPGX209eh2Ci-b2w~Wq8d0vk4r1DqmKei46zDQ2eARsXYaJ-Mwp8fYsOpexO5zCXuebQdVQH2qennZNO84owcJfk3QZ8XvWfPyCXaGitOxiiRsGVkYl1dCy4V8NC34Eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Activation of a 4-AP–sensitive K+ channel by EGF stimulation. (A) Effect of EGF on the 4-AP–sensitive K+ current. The membrane potential was depolarized from a holding potential of −60 mV to +80 mV at 20-mV increments with a pulse protocol showing in the top panel. Whole-cell currents were recorded from (1) ML-1 cells in the absence (control) and presence of 2 mmol/L 4-AP (as indicated); and (2) ML-1 cells simulated with 10 ng/mL EGF in the absence (EGF) and presence of 4-AP (EGF + 4-AP). (B) Current-voltage relationship of the 4-AP–sensitive K+current activated by EGF in the absence and presence of 4-AP. (C) Time course of EGF-activated K+ current in the absence and presence of 4-AP. Currents were normalized as IEGF/IC , where IEGF represents amplitudes of the EGF-induced K+ current and IC represents the K+ current measured from control ML-1 cells. (D) Single-channel recording of K+ channel in ML-1 cells. Inward current recorded as a downward deflection was obtained from cell-attached patches at a membrane potential of −60 mV in the symmetrical 140/140 mmol/L KCl condition. EGF (50 ng/mL) was directly applied to the patch chamber to activate K+ channels in the same patch. (E) Current trace demonstrates that application of 100 μmol/L 4-AP in the patch pipette prevented the EGF-induced increase of K+-channel activity. Channel activity (NPo) was plotted as a function of time in the lower portion of D and E. (F) Statistics of K+-channel activity stimulated by EGF and FBS in the absence and presence of 100 μmol/L 4-AP. Vertical bars represent mean K+-channel activity (horizontal bars represent standard error of the mean [SE]). *Significant difference (statistical tests: ANOVA and Tukey, P < .001). Data were collected from five independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.139.413k11_139_145/6/m_blod41311001ex.jpeg?Expires=1767876731&Signature=nLFS-afSYeKkgWQAAQsRUf8Hg0P9WnEBFJQ6THK7hAvqBBPtbibTyEDiD5Bv2gZWxgr89rgPuFCORTiKWuFpMJc1Nlq4fpJQ~Zt5CCSXCr~AeLP5pg5jZlTUVJsONKACM-jlF1gGfVIbQY8T87Yn8xqTq8bydQyH2UfZDZ-eenzOyIPMvFQ3zKxbdFvr4zfEsSig7eceJVCnJLsZ6S~4qGN6NR-QPHBRKV56effXJe3fAGB~jIvpze8UvRASFe5RuwlIcn0ii5mmpWR-2ubJ~p7tidBnKqp0ccWa7Eg~rQaqz12XB3m4NkJtauE54oN9zbNzsjA6vPNA3EFuNYQEbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Activation of a 4-AP–sensitive K+ channel by EGF stimulation. (A) Effect of EGF on the 4-AP–sensitive K+ current. The membrane potential was depolarized from a holding potential of −60 mV to +80 mV at 20-mV increments with a pulse protocol showing in the top panel. Whole-cell currents were recorded from (1) ML-1 cells in the absence (control) and presence of 2 mmol/L 4-AP (as indicated); and (2) ML-1 cells simulated with 10 ng/mL EGF in the absence (EGF) and presence of 4-AP (EGF + 4-AP). (B) Current-voltage relationship of the 4-AP–sensitive K+current activated by EGF in the absence and presence of 4-AP. (C) Time course of EGF-activated K+ current in the absence and presence of 4-AP. Currents were normalized as IEGF/IC , where IEGF represents amplitudes of the EGF-induced K+ current and IC represents the K+ current measured from control ML-1 cells. (D) Single-channel recording of K+ channel in ML-1 cells. Inward current recorded as a downward deflection was obtained from cell-attached patches at a membrane potential of −60 mV in the symmetrical 140/140 mmol/L KCl condition. EGF (50 ng/mL) was directly applied to the patch chamber to activate K+ channels in the same patch. (E) Current trace demonstrates that application of 100 μmol/L 4-AP in the patch pipette prevented the EGF-induced increase of K+-channel activity. Channel activity (NPo) was plotted as a function of time in the lower portion of D and E. (F) Statistics of K+-channel activity stimulated by EGF and FBS in the absence and presence of 100 μmol/L 4-AP. Vertical bars represent mean K+-channel activity (horizontal bars represent standard error of the mean [SE]). *Significant difference (statistical tests: ANOVA and Tukey, P < .001). Data were collected from five independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.139.413k11_139_145/6/m_blod41311001fx.jpeg?Expires=1767876731&Signature=V0itxwclsqe2bfPvAdMmx5z0FTLLAAjmmj9JBwYnX4R12nIC5GsfixN7QMK7Mtj6oBOwIl8Raucfc9RfmXIgOUiM-fHZXXAF-v3e6Rh-5i-RZxZzLkDw~6S-y3jT-JC9D18IvRKsv2Pc8hEr5adoTy7z-vPNKF-W~IfbvK8axtt6L2Oc4sI878Kg~aF46bjBbC2AX56R-PHmebONJzBWq4Nj9Io6tmUskC3kW7AbI0Zz4bbR5BpyQ42jGOrzDktUQU-~yBMtwHJTBCbqBs1z~Q12JvZBY6WBti1vWktUptqJyM0ZNsGiymUeVIhFSiZ0N9sfGBzajYIpqkvPY-V-YA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal