Interaction of von Willebrand factor (vWF) with the platelet is essential to hemostasis when vascular injury occurs. This interaction elevates the intracellular free calcium concentration ([Ca2+]i) and promotes platelet activation. The present study investigated the temperature dependence of vWF-induced [Ca2+]i signaling in human platelets. The influence of temperature can provide invaluable insight into the underlying mechanism. Platelet [Ca2+]i was monitored with Fura-PE3. Ristocetin-mediated binding of vWF induced a transient platelet [Ca2+]i increase at 37°C, but no response at lower temperatures (20°C to 25°C). This temperature dependence could not be attributed to a reduction in vWF binding, as ristocetin-mediated platelet aggregation and agglutination were essentially unaffected by temperature. Most other platelet agonists (U-46619, -thrombin, and adenosine 5′-diphosphate [ADP]) induced a [Ca2+]isignal whose amplitude did not diminish at lower temperatures. The [Ca2+]i signal in response to arachidonic acid, however, showed similar temperature dependence to that seen with vWF. Assessment of thromboxane A2 production showed a strong temperature dependence for metabolism of arachidonic acid by the cyclo-oxygenase pathway. vWF induced thromboxane A2production in the platelet. Aspirin treatment abolished the vWF-induced [Ca2+]i signal. These observations suggest that release of arachidonic acid and its conversion to thromboxane A2 play a central role in vWF-mediated [Ca2+]i signaling in the platelet at physiological temperatures.
VON WILLEBRAND FACTOR (vWF) is a multimeric, plasma glycoprotein that plays a major role in hemostasis and thrombosis.1,2 It can interact with a receptor, the glycoprotein Ib-IX-V complex (GP Ib-IX-V), on the platelet membrane and thereby promote platelet adhesion. Such interaction is not spontaneous, but may be triggered through surface immobilization of vWF3or by an exogenous modulator, such as the antibiotic ristocetin.4 Binding of vWF to exposed subendothelial elements may play a similar role in eliciting its interaction with GP Ib-IX-V in vivo.5 Recent data indicate that exposure to high shear stress, such as encountered in a stenosed artery, also promotes binding of vWF to GP Ib-IX-V.6
Considerable evidence has accrued to suggest that interaction of vWF with GP Ib-IX-V causes platelet activation.1,3,7-9 The signaling mechanism that leads to such activation is now under investigation. Several investigators have reported that binding of vWF to GP Ib-IX-V evokes an increase in intracellular free calcium concentration ([Ca2+]i) in the platelet.10-17 By analogy with other platelet agonists,18 this calcium signal is presumed to play an essential role in platelet activation.
Studies of the vWF-induced [Ca2+]i signal have used several approaches to elicit the interaction of vWF with platelet GP Ib-IX-V. Some investigators have adopted a static system, in which either an exogenous modulator (ristocetin or botrocetin) mediates the binding of native human vWF11,15,17 or spontaneous binding results from the use of a mutant human vWF14 or of porcine vWF16; others have chosen a dynamic approach, where shear stress acts as the trigger for vWF binding.10,12,13 There have been several significant discrepancies among the results obtained with these diverse approaches. In particular, the intracellular signaling mechanism that is responsible for the [Ca2+]i increase appears to differ depending on the means for triggering vWF interaction. Observations with the static approaches have suggested that activation of the phospholipase A2 pathway and subsequent production of thromboxane A2 may play a major role in vWF-induced [Ca2+]i signaling.11,19 In contrast, studies using shear stress to trigger vWF binding have implicated calcium influx through channels in the platelet plasma membrane as the primary signaling mechanism, with negligible contribution from thromboxane A2.1,12 13
Such a divergence in the signaling mechanism for vWF would present an attractive target for selective therapeutic intervention to prevent thrombosis in a region of stenosis without impairing hemostasis in the event of a vascular injury. It is premature, however, to conclude at this time that the signaling mechanism differs in this way. Before reaching such a conclusion, it is necessary to rule out any more mundane, technical explanation for the divergent findings. For example, the investigations of vWF-mediated platelet [Ca2+]i signaling under shear stress appear, for technical reasons, to have been conducted at room temperature,10,12,13 whereas those under static conditions were generally performed at 37°C.11,14,16,17 19 This difference in temperature might conceivably explain the apparent difference in the signaling mechanism.
To address this concern, this study examines the influence of temperature on the [Ca2+]i signal that arises when ristocetin triggers interaction of vWF with platelet GP Ib-IX-V. The pattern of temperature dependence is compared with that observed for other platelet agonists. The influence of temperature on a biological process may also provide insight into the nature of the mechanism that underlies it, as a diffusion-limited process is usually less affected by temperature than an enzyme-mediated process.20 Not only do our findings stress the importance of temperature as a factor that has a profound influence on vWF-induced signaling in the platelet, they also highlight a major difference in the signaling mechanism between vWF and most other platelet agonists.
MATERIALS AND METHODS
Evaluation of kd for binding of calcium to Fura-PE3.
The dissociation constants for binding of calcium to Fura-PE3 at 20°C and 37°C were assessed using a calcium calibration buffer kit (Molecular Probes, Eugene, OR) in accordance with the manufacturer’s instructions. In brief, for measurements at each temperature, two solutions containing 2 μmol/L Fura-PE3 (K+ salt; Texas Fluorescence Laboratory, Austin, TX) were prepared, one with 10 mmol/L EGTA and the other with 10 mmol/L CaEGTA. Both solutions also contained 100 mmol/L KCl, 1 mmol/L free Mg2+, and 10 mmol/L 3-(N-morpholino) propanesulfonic acid (MOPS), and they were adjusted to pH 7.0 at the desired temperature. A series of 11 solutions of known free Ca2+ concentration was prepared by appropriate mixing. Fluorescence excitation spectra were collected for each solution at the appropriate temperature with an emission wavelength of 510 nm. The kd value was calculated from a Hill plot of the fluorescence intensity with excitation at 340 nm.17
Isolation of human platelets and loading with Fura-PE3.
Venous blood was drawn from healthy adult volunteers who had not taken aspirin or a nonsteroidal antiinflammatory drug for at least 10 days before phlebotomy. Female donors were not taking an oral contraceptive. The protocol was approved by the local Institutional Review Board, and informed consent was obtained from each donor. Platelets were isolated using the method described previously.17 In brief, blood was collected into 1/6 volume of acid-citrate-dextrose anticoagulant (65 mmol/L citric acid, 85 mmol/L sodium citrate, 110 mmol/L D-glucose). Platelet-rich plasma was treated with 5 mmol/L creatine phosphate and 25 U/mL creatine phosphokinase (Sigma Chemicals, St Louis, MO), and the platelets were isolated by centrifugation at 1,000g for 15 minutes at 23°C. The platelets were resuspended in calcium-free, magnesium-free, Tyrode’s solution (137 mmol/L NaCl, 2.7 mmol/L KCl, 12 mmol/L NaHCO3, 0.4 mmol/L NaH2PO4, 5.5 mmol/L D-glucose), supplemented with 10 mmol/L HEPES, pH 7.35, and 0.35% (wt/vol) bovine serum albumin (BSA). This solution was also supplemented with 5 mmol/L creatine phosphate, 25 U/mL creatine phosphokinase, and 1 mmol/L EGTA during indicator loading.
Platelets were loaded with fluorescent calcium indicator by a 1-hour incubation at 23°C in the dark with 5 μmol/L Fura-PE3 acetoxymethyl ester (AM) (Texas Fluorescence Laboratory) in the presence of 0.004% (wt/vol) Pluronic F-127; before addition to the platelets, the Fura-PE3/AM stock (3.3 mmol/L in dimethylsulfoxide) was mixed with Tyrode’s solution containing 4% BSA to facilitate its dispersal. A separate portion of the platelet suspension was retained as an autofluorescence control. After loading, the platelets were washed twice by centrifugation and resuspension.17 21 The final resuspension (in buffered Tyrode’s solution with 0.35% BSA and 1 mmol/L MgCl2) was adjusted to a platelet count of 50,000 cells/μL.
Measurement of platelet [Ca2+]i signals.
Platelet [Ca2+]i was assayed on a Photon Technology International (South Brunswick, NJ) RF-M2004 spectrofluorometer. All studies were conducted in a cylindrical glass cuvette (8-mm diameter) designed for aggregometry (Payton Scientific, Buffalo, NY). Reflections from its curved surface were eliminated by using a vertical polarizer in the excitation beam and a horizontal one in the emission beam. A specially designed stirrer was used; this comprised a Teflon cylinder with a bar magnet embedded at its base and an ultraviolet (UV)-grade methacrylate stirring vane attached at the top.17 This stirrer ensures efficient stirring of a platelet suspension without significant interference with the optical signal, and its use has been validated extensively in studies with both classical platelet agonists and multimeric human vWF.17
Platelets (50,000 cells/μL) that had been loaded with fluorescent calcium indicator were stirred continuously at the selected temperature. They were equilibrated briefly (1 to 2 minutes) with extracellular calcium (1 mmol/L CaCl2), unless otherwise indicated. Stimulation was usually effected by sequential addition of 1 mg/mL ristocetin (Bio/Data Corp, Hatboro, PA) and 5 μg/mL purified human vWF. Human vWF was either obtained from a commercial source (Calbiochem, San Diego, CA) or purified from plasma by cryoethanol precipitation, differential polyethylene glycol fractionation, and gel filtration on Sepharose CL-4B.22 23 Similar results were obtained with these two vWF preparations; each was essentially pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and included the full range of multimers present in plasma vWF. In other studies, platelets were stimulated by addition of 5 μmol/L adenosine 5′-diphosphate (ADP), 0.02 U/mL human α-thrombin, 0.1 μmol/L U-46619, or 20 μmol/L arachidonic acid (all from Sigma Chemicals). Fluorescence intensity was monitored at 510 nm emission with excitation alternating between 340 and 380 nm wavelengths. Autofluorescence was determined in platelets that had not been loaded with Fura-PE3 and was subtracted from each fluorescence measurement (before ratio calculation).
Calibration data were obtained in each experiment by the procedure of Merritt et al24 (on a separate aliquot of platelets). The maximal fluorescence ratio (Rmax) was assessed by adding 60 μmol/L digitonin (Calbiochem) in the presence of 1 mmol/L CaCl2, and the minimal fluorescence ratio (Rmin) determined by adding excess (20 mmol/L) EGTA, buffered to pH 8.5. Experimental measurements of the fluorescence ratio (R) were converted to [Ca2+]i values by the equation25:
where Ff and Fb are the fluorescence intensities with 380 nm excitation for free and calcium-bound indicator, respectively. Calculations at 20°C and 37°C are based on measured kd values of 251 and 146 nmol/L, respectively, for binding of calcium to Fura-PE3 (see Results); calculations at intermediate temperatures used interpolated kd estimates of 220 nmol/L at 25°C and 189 nmol/L at 30°C.
Platelet aggregometry.
Platelet aggregation was assessed simultaneously with the [Ca2+]i measurements in some studies. The Photon Technology RF-M2004 spectrofluorometer was used in an unorthodox configuration for these studies (Fig 1); one emission monochromator (380 nm wavelength) was repositioned to monitor transmitted light, and thereby assess aggregation, while platelet [Ca2+]i was evaluated from Fura-PE3 fluorescence emission measurements (510 nm) perpendicular to the excitation beam. The lower part of the transmitted light beam was blocked with an opaque panel in a specially designed cuvette holder so that the novel stirrer (which protrudes into the excitation beam) did not interfere with the aggregation measurements. The excitation wavelength alternated between 340 and 380 nm for the [Ca2+]i measurements; transmitted light was monitored during the 380-nm excitation phase only. Aggregation is expressed as the change in light transmittance relative to the transmittance in the absence of platelets. This experimental approach yields aggregation data that are indistinguishable from data obtained with a traditional aggregometer.
Optical configuration and stirring arrangement for simultaneous assessment of platelet aggregation and [Ca2+]i signaling. Measurements are conducted in a cylindrical glass aggregometer cuvette (8-mm diameter) in a reconfigured dual-emission spectrofluorometer. The platelet suspension is stirred with a novel stirrer, comprising an opaque Teflon cylinder with a bar magnet at its base and a UV-grade methacrylate stirring vane at its top. This stirrer protrudes into the excitation beam of the spectrofluorometer to ensure that platelet aggregates cannot settle below its detection zone.17 Platelet aggregation is monitored through measurement of transmitted light intensity, with the lower part of the transmitted light beam blocked so that the stirrer does not interfere with these measurements. Platelet [Ca2+]i is monitored through fluorescence measurements perpendicular to the excitation beam. Reflections from the curved surfaces of the cuvette are eliminated by use of a vertical polarizer in the excitation beam and a horizontal one in the emission beam. This arrangement provides efficient stirring of the platelet suspension and ensures that the fluorescence signal is representative of the entire population of platelets regardless of the extent of aggregation.
Optical configuration and stirring arrangement for simultaneous assessment of platelet aggregation and [Ca2+]i signaling. Measurements are conducted in a cylindrical glass aggregometer cuvette (8-mm diameter) in a reconfigured dual-emission spectrofluorometer. The platelet suspension is stirred with a novel stirrer, comprising an opaque Teflon cylinder with a bar magnet at its base and a UV-grade methacrylate stirring vane at its top. This stirrer protrudes into the excitation beam of the spectrofluorometer to ensure that platelet aggregates cannot settle below its detection zone.17 Platelet aggregation is monitored through measurement of transmitted light intensity, with the lower part of the transmitted light beam blocked so that the stirrer does not interfere with these measurements. Platelet [Ca2+]i is monitored through fluorescence measurements perpendicular to the excitation beam. Reflections from the curved surfaces of the cuvette are eliminated by use of a vertical polarizer in the excitation beam and a horizontal one in the emission beam. This arrangement provides efficient stirring of the platelet suspension and ensures that the fluorescence signal is representative of the entire population of platelets regardless of the extent of aggregation.
Thromboxane A2 production.
Production of thromboxane A2 was assessed in the samples used to monitor the platelet [Ca2+]i signal. Platelets were incubated with 1 mg/mL ristocetin and 5 μg/mL human vWF for 10 minutes, a time sufficient for the [Ca2+]i transient to be complete at 37°C. Incubations of the same duration were performed at the lower temperatures, and equivalent control incubations were conducted at each temperature with 1 mg/mL ristocetin in the absence of vWF. Analogous studies were conducted with 20 μmol/L arachidonic acid, except that the incubation time was 3 minutes. Each reaction was stopped by adding 1 mmol/L aspirin and 10 mmol/L EDTA (in buffered Tyrode’s solution); the supernatant fraction was collected after centrifugation for 1 minute at 16,000g. Thromboxane A2 production during the incubation was assessed by competitive enzyme immunoassay of thromboxane B2 (its stable breakdown product) in the supernatant fraction, using a commercial assay kit (Cayman Chemicals, Ann Arbor, MI). All thromboxane B2 samples from studies on platelets from a particular donor were included in a single assay; the within-assay coefficient of variation for these assays averaged 4%.
Agglutination of paraformaldehyde-fixed platelets.
Human platelets were fixed with 0.6% (wt/vol) paraformaldehyde by the method of Brinkhous and Read26 and washed extensively. Ristocetin-mediated agglutination of the fixed platelets by vWF was assessed by monitoring light transmittance at 380 nm (as for the aggregation measurements on live platelets).
Statistical analysis.
Most data on platelet [Ca2+]i and thromboxane A2 production in this study were not normally distributed. Such data are thus presented as a median value with the interquartile range, and nonparametric tests were used to determine statistical significance (specific tests indicated in Results). Data on kd values for binding of calcium to Fura-PE3 were consistent with a normal distribution and are presented as a mean value ± standard error of mean (SEM).
RESULTS
Temperature dependence of the calcium-binding characteristics of Fura-PE3.
The calcium-binding characteristics of Fura-PE3 were evaluated from excitation spectra measured in the presence of a series of free calcium concentrations. Three studies at 20°C yielded an average kd value of 251 ± 9 nmol/L (mean ± SEM) for Fura-PE3 at pH 7.0. A significantly lower kd value (146 ± 9 nmol/L,17 based on four studies; P < .001, Student’s t-test) was found at 37°C and pH 7.0.
Temperature dependence of the vWF-induced platelet [Ca2+]i signal.
Ristocetin-mediated binding of multimeric human vWF in the presence of extracellular calcium induced a gradual and transient platelet [Ca2+]i increase at 37°C (Fig 2C). This [Ca2+]i signal arose after an appreciable lag phase. At 20°C or 25°C, in contrast, vWF did not induce a perceptible increase in [Ca2+]i (Fig 2A and Table 1). Studies at 30°C showed either a small increase in platelet [Ca2+]i (Fig 2B) or no discernible increase (four of the eight studies). Analogous studies performed in the absence of extracellular calcium (with 1 mmol/L EGTA) showed the same pattern of temperature dependence (data not shown).
Temperature dependence of the [Ca2+]i signal induced by ristocetin-mediated binding of vWF. Human platelets were loaded with 5 μmol/L Fura-PE3/AM. Measurements were performed in an aggregometer cuvette, with the platelet suspension (50,000 cells/μL) stirred by the novel stirrer. The platelets were stimulated at 20°C (A), 30°C (B), or 37°C (C) by adding 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF (solid traces) in the presence of extracellular calcium (1 mmol/L CaCl2). Data obtained in parallel control studies with 1 mg/mL ristocetin alone (dotted traces) are also displayed. The fluorescence intensity at 510-nm emission wavelength was measured with excitation alternating between 340 and 380 nm. Platelet [Ca2+]i was calculated from the fluorescence ratio (340:380 nm), using the kd value for Fura-PE3 at the corresponding temperature. Observations at 25°C (not shown) were indistinguishable from those at 20°C. The results illustrated are from a typical one of eight such studies.
Temperature dependence of the [Ca2+]i signal induced by ristocetin-mediated binding of vWF. Human platelets were loaded with 5 μmol/L Fura-PE3/AM. Measurements were performed in an aggregometer cuvette, with the platelet suspension (50,000 cells/μL) stirred by the novel stirrer. The platelets were stimulated at 20°C (A), 30°C (B), or 37°C (C) by adding 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF (solid traces) in the presence of extracellular calcium (1 mmol/L CaCl2). Data obtained in parallel control studies with 1 mg/mL ristocetin alone (dotted traces) are also displayed. The fluorescence intensity at 510-nm emission wavelength was measured with excitation alternating between 340 and 380 nm. Platelet [Ca2+]i was calculated from the fluorescence ratio (340:380 nm), using the kd value for Fura-PE3 at the corresponding temperature. Observations at 25°C (not shown) were indistinguishable from those at 20°C. The results illustrated are from a typical one of eight such studies.
Influence of Temperature on the Amplitude of Platelet [Ca2+]i Transients
| Agonist . | Agonist-Induced Peak Increment in Platelet [Ca2+]i (nmol/L) . | |||
|---|---|---|---|---|
| 20°C . | 25°C . | 30°C . | 37°C . | |
| vWF* (5 μg/mL) | 1† (1 to 5) | 1† (−2 to 1) | 9† (−1 to 21) | 61 (40 to 83) |
| U-46619 (0.1 μmol/L) | 178 (164 to 192) | 167 (160 to 193) | 157 (134 to 169) | 143 (129 to 179) |
| α-Thrombin (0.02 U/mL) | 165†,‡,1-153 (139 to 207) | 103†,‡ (64 to 167) | 75† (44 to 112) | 52 (33 to 62) |
| ADP (5 μmol/L) | 141† (126 to 159) | 155† (142 to 179) | 138† (107 to 145) | 101 (97 to 131) |
| Arachidonic acid (20 μmol/L) | 0†,‡ (0 to 1) | 0†,‡ (0 to 1) | 4† (3 to 7) | 49 (24 to 51) |
| Resting platelet [Ca2+]i | 7†,‡,1-153 (6 to 9) | 9†,‡ (7 to 10) | 11† (10 to 13) | 13 (11 to 15) |
| Agonist . | Agonist-Induced Peak Increment in Platelet [Ca2+]i (nmol/L) . | |||
|---|---|---|---|---|
| 20°C . | 25°C . | 30°C . | 37°C . | |
| vWF* (5 μg/mL) | 1† (1 to 5) | 1† (−2 to 1) | 9† (−1 to 21) | 61 (40 to 83) |
| U-46619 (0.1 μmol/L) | 178 (164 to 192) | 167 (160 to 193) | 157 (134 to 169) | 143 (129 to 179) |
| α-Thrombin (0.02 U/mL) | 165†,‡,1-153 (139 to 207) | 103†,‡ (64 to 167) | 75† (44 to 112) | 52 (33 to 62) |
| ADP (5 μmol/L) | 141† (126 to 159) | 155† (142 to 179) | 138† (107 to 145) | 101 (97 to 131) |
| Arachidonic acid (20 μmol/L) | 0†,‡ (0 to 1) | 0†,‡ (0 to 1) | 4† (3 to 7) | 49 (24 to 51) |
| Resting platelet [Ca2+]i | 7†,‡,1-153 (6 to 9) | 9†,‡ (7 to 10) | 11† (10 to 13) | 13 (11 to 15) |
Platelet [Ca2+]i was monitored with Fura-PE3 using an aggregometer cuvette and the novel stirrer. Parallel studies were performed at four temperatures (20°C to 37°C); all measurements were conducted in the presence of extracellular calcium (1 mmol/L CaCl2). The various agonists were used at concentrations that gave [Ca2+]i transients of similar amplitude at 37°C. Peak increments in platelet [Ca2+]i were calculated from the difference between an agonist-stimulated sample and the corresponding control; in the absence of a perceptible [Ca2+]itransient (vWF and arachidonic acid stimulation at the lower temperatures), this difference was determined at the time of the peak for the transient at 37°C. Data are presented as the median value from eight studies with vWF, seven studies with arachidonic acid, and six studies with each of the other agonists; the interquartile range is shown in parentheses. Data on resting platelet [Ca2+]i (based on average values before addition of each agonist) are also included.
Stimulation was with 5 μg/mL multimeric human vWF in the presence of 1 mg/mL ristocetin.
Statistical analysis used Friedman’s test with a nonparametric Student-Newman-Keuls posthoc test; significant differences (P < .05) from measurements at the higher temperatures are designated:
compared with 37°C;
compared with 30°C; and
compared with 25°C.
Stirring of the platelet suspension was required for vWF to induce a [Ca2+]i increase at 37°C, suggesting that platelet aggregation might be necessary. The role of vWF binding to the glycoprotein IIb-IIIa complex (GP IIb-IIIa) in the signaling mechanism was evaluated using a monoclonal antibody against GP IIb-IIIa (10E5; kindly provided by Dr Barry Coller, Mount Sinai Medical Center, New York, NY).27 An antibody concentration of 10 μg/mL proved sufficient to prevent binding of vWF to GP IIb-IIIa; it abolished the aggregation by vWF of platelets that had been activated by U-46619 (Fig 3A). In contrast, preincubation with this concentration of antibody did not significantly affect the platelet [Ca2+]i signal evoked by ristocetin-mediated binding of vWF at 37°C (Fig 3B and C). Median values (from four studies) for the peak [Ca2+]i increase were 98 nmol/L in untreated platelets, 86 nmol/L in platelets pretreated with an isotype-matched control Ig (IgG2a), and 89 nmol/L after treatment with 10E5 antibody (P > .2, Friedman’s test). Blockade of GP IIb-IIIa with the tetrapeptide Arg-Gly-Asp-Ser (1 mmol/L) also had negligible effect on the vWF-induced [Ca2+]i signal.17These data imply that binding of vWF to GP Ib-IX-V triggers the platelet [Ca2+]i increase.
Effect of GP IIb-IIIa blockade on the vWF-induced [Ca2+]i signal. (A) The ability of the 10E5 antibody to block GP IIb-IIIa was evaluated by aggregometry. Washed human platelets (50,000 cells/μL, suspended in Tyrode’s solution with 1 mmol/L CaCl2) were preincubated (5 minutes at 37°C) with isotype-matched control IgG (solid trace) or with 10E5 IgG (dotted trace). Each IgG was essentially azide-free and was used at a final concentration of 10 μg/mL. Platelets were stimulated with 0.1 μmol/L U-46619 and aggregation was assessed at 37°C in the presence of 5 μg/mL multimeric human vWF. (B and C) The effect of the 10E5 antibody on the platelet [Ca2+]isignal was evaluated. Human platelets (50,000 cells/μL, loaded with Fura-PE3) were preincubated with 10 μg/mL control IgG (B) or 10E5 IgG (C). The platelets were then stimulated at 37°C by 1 mg/mL ristocetin and 5 μg/mL vWF (solid traces) in the presence of 1 mmol/L CaCl2. Data obtained in parallel control studies with 1 mg/mL ristocetin alone (dotted traces) are also displayed. Fluorescence measurements were performed in a cylindrical aggregometer cuvette, with the platelet suspension stirred by the novel stirrer. Platelet [Ca2+]i was calculated from the fluorescence excitation ratio (340:380 nm). Results of a typical one of four such studies are illustrated. Aggregation and platelet [Ca2+]i were also assessed in the absence of either IgG (not shown); results were indistinguishable from those in the presence of the control IgG.
Effect of GP IIb-IIIa blockade on the vWF-induced [Ca2+]i signal. (A) The ability of the 10E5 antibody to block GP IIb-IIIa was evaluated by aggregometry. Washed human platelets (50,000 cells/μL, suspended in Tyrode’s solution with 1 mmol/L CaCl2) were preincubated (5 minutes at 37°C) with isotype-matched control IgG (solid trace) or with 10E5 IgG (dotted trace). Each IgG was essentially azide-free and was used at a final concentration of 10 μg/mL. Platelets were stimulated with 0.1 μmol/L U-46619 and aggregation was assessed at 37°C in the presence of 5 μg/mL multimeric human vWF. (B and C) The effect of the 10E5 antibody on the platelet [Ca2+]isignal was evaluated. Human platelets (50,000 cells/μL, loaded with Fura-PE3) were preincubated with 10 μg/mL control IgG (B) or 10E5 IgG (C). The platelets were then stimulated at 37°C by 1 mg/mL ristocetin and 5 μg/mL vWF (solid traces) in the presence of 1 mmol/L CaCl2. Data obtained in parallel control studies with 1 mg/mL ristocetin alone (dotted traces) are also displayed. Fluorescence measurements were performed in a cylindrical aggregometer cuvette, with the platelet suspension stirred by the novel stirrer. Platelet [Ca2+]i was calculated from the fluorescence excitation ratio (340:380 nm). Results of a typical one of four such studies are illustrated. Aggregation and platelet [Ca2+]i were also assessed in the absence of either IgG (not shown); results were indistinguishable from those in the presence of the control IgG.
The absence of a vWF-induced platelet [Ca2+]isignal at the lower temperatures could reflect a strong temperature dependence for either binding of vWF to the GP Ib-IX-V complex or a subsequent step in the signal transduction process triggered by vWF. These two possibilities were discriminated primarily by evaluating the temperature dependence of ristocetin-mediated platelet aggregation by vWF. (“Aggregation” is used here in an inclusive sense to encompass both physical agglutination through binding of vWF to different platelets and true aggregation involving platelet activation. Both processes occur when live platelets are incubated with vWF in the presence of ristocetin, and the measurement of light transmittance cannot distinguish between them.) Aggregation was assessed simultaneously with the platelet [Ca2+]imeasurements by using a novel configuration of the spectrofluorometer (Fig 1). The aggregation of live platelets by vWF was essentially unaffected by temperature over the range 20°C to 37°C (Fig 4A and B). Temperature also had negligible effect when aggregation was assessed in the presence of Arg-Gly-Asp-Ser (data not shown). Ristocetin-mediated agglutination of fixed platelets by vWF declined marginally when the temperature was lowered from 37°C to 20°C (Fig 4C and D), but there was no significant reduction at 30°C (data not shown). These data together imply that the marked temperature dependence of the vWF-induced platelet [Ca2+]i signal cannot be attributed to differences in vWF binding to GP Ib-IX-V.
Temperature dependence of platelet aggregation and agglutination by ristocetin-mediated binding of vWF. (A and B) Aggregation of live platelets was monitored simultaneously with platelet [Ca2+]i. The platelet suspension (50,000 cells/μL) was incubated at 20°C (A) or 37°C (B) in the presence of 1 mmol/L CaCl2. Aggregation was deduced from the transmitted light intensity (during the 380 nm fluorescence excitation phase). Aggregation patterns at 25°C and 30°C (not shown) were indistinguishable from those at 20°C and 37°C. The results illustrated were acquired in parallel with the [Ca2+]i measurements shown in Fig 2; these aggregation data are typical of four such studies. (C and D) Agglutination of paraformaldehyde-fixed platelets (50,000 cells/μL) was evaluated at 20°C (C) and 37°C (D) in an analogous manner. Agglutination patterns at 30°C (not shown) were comparable to those at 37°C. These data are typical of three such studies. Panels A through D present the responses on incubation either with 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF (solid traces) or with 1 mg/mL ristocetin alone (dotted traces).
Temperature dependence of platelet aggregation and agglutination by ristocetin-mediated binding of vWF. (A and B) Aggregation of live platelets was monitored simultaneously with platelet [Ca2+]i. The platelet suspension (50,000 cells/μL) was incubated at 20°C (A) or 37°C (B) in the presence of 1 mmol/L CaCl2. Aggregation was deduced from the transmitted light intensity (during the 380 nm fluorescence excitation phase). Aggregation patterns at 25°C and 30°C (not shown) were indistinguishable from those at 20°C and 37°C. The results illustrated were acquired in parallel with the [Ca2+]i measurements shown in Fig 2; these aggregation data are typical of four such studies. (C and D) Agglutination of paraformaldehyde-fixed platelets (50,000 cells/μL) was evaluated at 20°C (C) and 37°C (D) in an analogous manner. Agglutination patterns at 30°C (not shown) were comparable to those at 37°C. These data are typical of three such studies. Panels A through D present the responses on incubation either with 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF (solid traces) or with 1 mg/mL ristocetin alone (dotted traces).
Temperature dependence of platelet [Ca2+]isignals for other agonists.
The influence of temperature on the [Ca2+]isignal was also evaluated for other platelet agonists. For the stable thromboxane A2 analog U-46619, the amplitude of the agonist-induced [Ca2+]i transient was not significantly affected by temperature (Fig5 and Table 1; P > .2, Friedman’s test). For α-thrombin and ADP, the amplitude of the [Ca2+]i signal tended to diminish as the temperature was increased from 20°C to 37°C (Table 1); this diminution was marked for α-thrombin, but modest for ADP. The time course of the [Ca2+]i transient for each of these three agonists tended to be prolonged at the lower temperatures (Fig 5). Studies with different concentrations of U-46619 (0.05 to 1 μmol/L) and α-thrombin (0.01 to 0.1 U/mL) yielded similar observations (data not shown). Resting platelet [Ca2+]i rose gradually with increasing temperature (Table 1). Analogous findings were made in studies conducted with these agonists in the absence of extracellular calcium (data not shown).
Temperature dependence of the [Ca2+]i signals induced by U-46619, -thrombin, and ADP. The [Ca2+]itransient in a stirred suspension of human platelets (50,000 cells/μL) was monitored with Fura-PE3. The platelets were stimulated at 20°C (dotted traces) or 37°C (solid traces) by 0.1 μmol/L U-46619 (A), 0.02 U/mL human -thrombin (B), or 5 μmol/L ADP (C) in the presence of extracellular calcium (1 mmol/L CaCl2). Platelet [Ca2+]i was calculated from the fluorescence excitation ratio (340:380 nm) using the kd value for Fura-PE3 at the corresponding temperature. Observations at 25°C and 30°C (not shown) were intermediate between those at 20°C and 37°C. The results shown are from a typical one of six such studies.
Temperature dependence of the [Ca2+]i signals induced by U-46619, -thrombin, and ADP. The [Ca2+]itransient in a stirred suspension of human platelets (50,000 cells/μL) was monitored with Fura-PE3. The platelets were stimulated at 20°C (dotted traces) or 37°C (solid traces) by 0.1 μmol/L U-46619 (A), 0.02 U/mL human -thrombin (B), or 5 μmol/L ADP (C) in the presence of extracellular calcium (1 mmol/L CaCl2). Platelet [Ca2+]i was calculated from the fluorescence excitation ratio (340:380 nm) using the kd value for Fura-PE3 at the corresponding temperature. Observations at 25°C and 30°C (not shown) were intermediate between those at 20°C and 37°C. The results shown are from a typical one of six such studies.
The platelet [Ca2+]i signal induced by arachidonic acid showed a markedly different pattern of temperature dependence than these direct platelet agonists. A robust [Ca2+]i increase was observed at 37°C in the presence of extracellular calcium (Fig6C), a very modest increase generally occurred at 30°C (Fig 6B), and no [Ca2+]i signal was seen at 20°C and 25°C (Fig 6A). The same pattern was observed in the absence of extracellular calcium (data not shown). This pattern of temperature dependence was closely analogous to that observed with vWF (Table1).
Temperature dependence of the [Ca2+]i signal induced by exogenous arachidonic acid. The [Ca2+]i transient in a stirred suspension of human platelets (50,000 cells/μL) was monitored with Fura-PE3. The platelets were stimulated at 20°C (A), 30°C (B), or 37°C (C) by 20 μmol/L arachidonic acid (solid traces) in the presence of extracellular calcium (1 mmol/L CaCl2). Data obtained in parallel control studies with 0.2% (wt/vol) dimethylsulfoxide (vehicle for arachidonic acid; dotted traces) are also displayed. Observations at 25°C (not shown) were indistinguishable from those at 20°C. The results illustrated are from a typical one of seven such studies.
Temperature dependence of the [Ca2+]i signal induced by exogenous arachidonic acid. The [Ca2+]i transient in a stirred suspension of human platelets (50,000 cells/μL) was monitored with Fura-PE3. The platelets were stimulated at 20°C (A), 30°C (B), or 37°C (C) by 20 μmol/L arachidonic acid (solid traces) in the presence of extracellular calcium (1 mmol/L CaCl2). Data obtained in parallel control studies with 0.2% (wt/vol) dimethylsulfoxide (vehicle for arachidonic acid; dotted traces) are also displayed. Observations at 25°C (not shown) were indistinguishable from those at 20°C. The results illustrated are from a typical one of seven such studies.
Temperature dependence of arachidonic acid metabolism by the cyclo-oxygenase pathway.
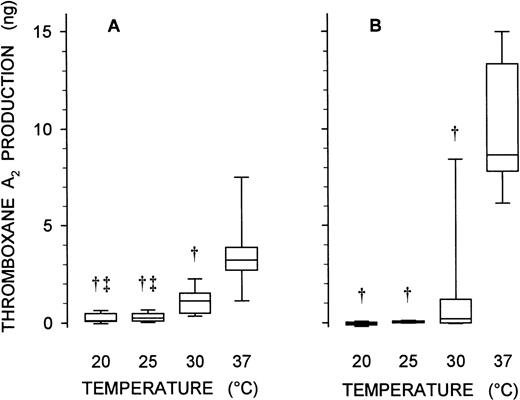
The strong temperature dependence of the platelet [Ca2+]i signal induced by arachidonic acid, but lack of such dependence when U-46619 was the agonist, suggested that metabolism of arachidonic acid by the cyclo-oxygenase pathway might be severely curtailed at the lower temperatures. This possibility was assessed directly by assay of thromboxane A2 production in platelets incubated with exogenous arachidonic acid at different temperatures. Metabolism of arachidonic acid to thromboxane A2 was substantially abrogated when the incubation temperature was reduced from 37°C to 30°C (Fig 7A; P < .01, Friedman’s test with a nonparametric Student-Newman-Keuls posthoc test) and was virtually abolished at the lower temperatures (20°C and 25°C).
Temperature dependence of thromboxane A2production induced by exogenous arachidonic acid and vWF. A stirred suspension of human platelets (500 μL at 50,000 cells/μL) was stimulated with either 20 μmol/L arachidonic acid (A) or a combination of 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF (B) at 20°C, 25°C, 30°C, or 37°C (as indicated). Platelet thromboxane A2 production was assessed by enzyme immunoassay of its stable breakdown product, thromboxane B2. Control samples were incubated with dimethylsulfoxide (vehicle for arachidonic acid) or 1 mg/mL ristocetin alone; thromboxane A2 production in the control (typically, 0.4 ng in both cases) was subtracted from that in the corresponding experimental sample. Data are presented as a Tukey box plot: the central line in the box shows the median value for the agonist-induced increment in thromboxane A2 production from seven studies, the lower and upper limits of the box designate the quartiles, and the error bars extending below and above the box represent the 10th and 90th percentiles, respectively. Statistical analysis was based on Friedman’s test with a nonparametric Student-Newman-Keuls posthoc test; significant differences (P < .01) from measurements at the higher temperatures are designated: †compared with 37°C, and ‡compared with 30°C.
Temperature dependence of thromboxane A2production induced by exogenous arachidonic acid and vWF. A stirred suspension of human platelets (500 μL at 50,000 cells/μL) was stimulated with either 20 μmol/L arachidonic acid (A) or a combination of 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF (B) at 20°C, 25°C, 30°C, or 37°C (as indicated). Platelet thromboxane A2 production was assessed by enzyme immunoassay of its stable breakdown product, thromboxane B2. Control samples were incubated with dimethylsulfoxide (vehicle for arachidonic acid) or 1 mg/mL ristocetin alone; thromboxane A2 production in the control (typically, 0.4 ng in both cases) was subtracted from that in the corresponding experimental sample. Data are presented as a Tukey box plot: the central line in the box shows the median value for the agonist-induced increment in thromboxane A2 production from seven studies, the lower and upper limits of the box designate the quartiles, and the error bars extending below and above the box represent the 10th and 90th percentiles, respectively. Statistical analysis was based on Friedman’s test with a nonparametric Student-Newman-Keuls posthoc test; significant differences (P < .01) from measurements at the higher temperatures are designated: †compared with 37°C, and ‡compared with 30°C.
vWF induces thromboxane A2 production in the platelet.
The similar patterns of temperature dependence for platelet [Ca2+]i signaling with vWF and arachidonic acid suggested that these signals might arise through similar mechanisms. Assay of thromboxane A2 production in response to the interaction of vWF with the platelet provided an independent test of this hypothesis. Ristocetin-mediated binding of vWF promoted a substantial increase in platelet thromboxane A2 production at 37°C (Fig 7B). vWF-induced thromboxane A2 production was substantially diminished at 30°C (P < .01) and abolished at 20°C and 25°C (Fig 7B). This pattern of temperature dependence closely matches the patterns seen for thromboxane A2 production with arachidonic acid and for platelet [Ca2+]i signaling with both vWF and arachidonic acid.
Effect of aspirin on the vWF-induced platelet [Ca2+]i signal.
If thromboxane A2 plays an essential role in the mechanism by which vWF elevates platelet [Ca2+]i, treatment with a cyclo-oxygenase inhibitor should abolish the [Ca2+]i signal. Studies were conducted in aspirin-treated platelets to evaluate this possibility. Pretreatment with 0.2 mmol/L aspirin consistently abrogated the platelet [Ca2+]i increase induced by ristocetin-mediated binding of vWF at 37°C (Fig 8A and B; P < .005, Wilcoxon signed-rank test). Such treatment also abolished vWF-induced thromboxane A2 production (Fig 8C; P < .005). Platelet [Ca2+]i signaling and thromboxane A2 production in response to 20 μmol/L arachidonic acid were also eliminated (data not shown), confirming the effectiveness of cyclo-oxygenase inhibition.
Effect of cyclo-oxygenase inhibition on the vWF-induced [Ca2+]i signal and thromboxane A2 production. Human platelets (50,000 cells/μL, loaded with Fura-PE3) were preincubated (5 minutes at 37°C) with 0.1% dimethylsulfoxide (vehicle) or 0.2 mmol/L aspirin. The platelets were then stimulated at 37°C by 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF in the presence of 1 mmol/L CaCl2. Parallel control studies were undertaken with 1 mg/mL ristocetin alone. (A) The results from a typical one of nine such studies are shown. The solid trace denotes the vWF-induced [Ca2+]isignal in vehicle-treated platelets, and the dotted trace that after aspirin treatment. (B) The amplitude of the vWF-induced [Ca2+]i increment, relative to ristocetin alone, in the whole set of studies is presented as a Tukey box plot. (C) Thromboxane A2 production was assessed in parallel. The vWF-induced increment in thromboxane A2 production, relative to ristocetin alone (typically, 0.3 ng), is displayed as an analogous Tukey box plot. The hatched bars in panels B and C summarize data for platelets preincubated with vehicle and the open bars data for aspirin-treated platelets.
Effect of cyclo-oxygenase inhibition on the vWF-induced [Ca2+]i signal and thromboxane A2 production. Human platelets (50,000 cells/μL, loaded with Fura-PE3) were preincubated (5 minutes at 37°C) with 0.1% dimethylsulfoxide (vehicle) or 0.2 mmol/L aspirin. The platelets were then stimulated at 37°C by 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF in the presence of 1 mmol/L CaCl2. Parallel control studies were undertaken with 1 mg/mL ristocetin alone. (A) The results from a typical one of nine such studies are shown. The solid trace denotes the vWF-induced [Ca2+]isignal in vehicle-treated platelets, and the dotted trace that after aspirin treatment. (B) The amplitude of the vWF-induced [Ca2+]i increment, relative to ristocetin alone, in the whole set of studies is presented as a Tukey box plot. (C) Thromboxane A2 production was assessed in parallel. The vWF-induced increment in thromboxane A2 production, relative to ristocetin alone (typically, 0.3 ng), is displayed as an analogous Tukey box plot. The hatched bars in panels B and C summarize data for platelets preincubated with vehicle and the open bars data for aspirin-treated platelets.
DISCUSSION
Temperature has only a modest influence on the amplitude and kinetics of [Ca2+]i signals in response to most agonists in many types of cell.20,28,29 The limited nature of this influence has served as a rationale for performing some [Ca2+]i signaling studies at ambient temperature when the demands of a particular experimental approach make it technically difficult to perform the study at a physiological temperature. For this reason, confocal microscopic evaluation of [Ca2+]i signals in individual cells is often performed at ambient temperature.30-32 This rationale also explains the choice of an ambient temperature for studies of platelet [Ca2+]i signaling under shear stress.10,12 13
The limited influence of temperature on [Ca2+]i signals induced by the classical platelet agonists U-46619, α-thrombin, and ADP in the present study (Fig 5 and Table 1) is consistent with the patterns reported previously for α-thrombin and ADP in the platelet33,34 and for many agonists in various cells.20,28,29 The platelet [Ca2+]i transients with these classical agonists tended to be somewhat prolonged at the lower temperatures. This pattern parallels previous reports of a slower removal of excess calcium from the cytosol at lower temperatures.35,36U-46619 triggers a platelet [Ca2+]i increase primarily through stimulation of phospholipase C and inositol 1,4,5-trisphosphate-mediated calcium release from the intracellular stores,37 whereas influx of calcium through receptor-operated channels in the plasma membrane is thought to play the main role in ADP-induced [Ca2+]isignaling.38 39 The amplitude of an agonist-induced [Ca2+]i transient reflects a balance between calcium entry into the cytosol and its extrusion. That the [Ca2+]i signal with U-46619 is temperature invariant might be related to the enzyme-mediated nature of both calcium release from the intracellular stores and its extrusion from the cytosol. A lesser effect of temperature on calcium influx with ADP (a diffusion-limited process) than on extrusion might also explain the modest increase in amplitude of its [Ca2+]isignal at lower temperatures. The pattern of inverse temperature dependence with α-thrombin, however, cannot be rationalized so readily.
In striking contrast to our findings with U-46619, ADP, and α-thrombin, the platelet [Ca2+]i signal induced by ristocetin-mediated binding of vWF was inhibited substantially by a reduction in temperature to 30°C and abrogated completely by a further reduction to 20°C or 25°C (Fig 2 and Table 1). This marked difference in temperature dependence strongly suggests a substantive difference in the signaling mechanism for vWF compared with the other agonists. The protracted time course of the [Ca2+]i signal with vWF (at 37°C) also supports such a difference. In particular, the pronounced lag phase between addition of vWF and the platelet [Ca2+]i increase argues in favor of an indirect signaling mechanism for vWF. Furthermore, the similarity in temperature dependence of the platelet [Ca2+]i signals with vWF (Fig 2) and arachidonic acid (Fig 6) suggests that release of arachidonic acid may be a proximal event in [Ca2+]i signaling with vWF. The finding that vWF promotes thromboxane A2production in the platelet (Fig 7B) lends further support to this possibility. Moreover, the abolition of the [Ca2+]i signal by the cyclo-oxygenase inhibitor aspirin (Fig 8A and B) provides direct evidence that thromboxane A2 plays an essential role in the signaling mechanism for vWF. Metabolism of arachidonic acid to thromboxane A2 by the cyclo-oxygenase pathway may exhibit such a strong temperature dependence (Fig 7A) because it involves three catalytic steps, cyclization and peroxidation by cyclo-oxygenase (prostaglandin H synthase) and isomerization by thromboxane A synthase. The temperature dependence of these steps, in combination, can explain the marked temperature dependence for platelet [Ca2+]isignaling with vWF.
Prior studies of vWF-induced [Ca2+]isignaling in the platelet have yielded inconsistent findings. One of the earliest studies by Kroll et al,11 using ristocetin to trigger vWF binding, led its authors to postulate that activation of the phospholipase A2 pathway and subsequent production of thromboxane A2 play a major role in vWF-induced [Ca2+]i signaling. There have been no further detailed studies of the signaling mechanism using this experimental approach. However, one subsequent study, using a mutant type 2B vWF that binds spontaneously to platelet GP Ib-IX-V, yielded data that broadly support this mechanism.19 In contrast, other investigators have suggested that the proximal event in vWF signaling leading to a [Ca2+]i increase is an influx of calcium through channels in the plasma membrane1,10,12,13,16,40; these investigators contend that thromboxane A2 does not contribute significantly to the process. Support for the latter mechanism is derived largely from dynamic studies where shear stress acted as the trigger for vWF binding to platelet GP Ib-IX-V.10,12,13 One study with a static system, measuring the response to spontaneous binding of porcine vWF, has also lent support to this mechanism.16 The putative ability of vWF to signal by two distinct mechanisms when its interaction with platelet GP Ib-IX-V is instigated by different means has not yet been explained. It does, nonetheless, present an attractive therapeutic target for prevention of pathological thrombosis without impairment of normal hemostasis.
It is not uncommon for a signal transduction pathway to bifurcate downstream of a receptor.41 42 Such bifurcation could enable platelet GP Ib-IX-V, when occupied by vWF, to signal through both phospholipase A2 and a plasma membrane calcium channel. This would provide a partial explanation for the putative ability of vWF to stimulate a different primary signaling pathway when its interaction with GP Ib-IX-V is initiated by different means. This hypothesis, however, does not explain why one signaling pathway should predominate (in general) under static conditions, but a different pathway under shear stress.
There is not yet sufficient evidence to conclude that the signaling mechanism for vWF really differs in this manner. Instead, a more mundane explanation for the divergent findings among prior studies should, perhaps, be sought. Could the discrepancies simply reflect technical differences between the various experimental approaches? The present study does provide a simple technical explanation for the apparent lack of involvement of the phospholipase A2pathway in signaling for vWF under shear stress. Prior investigations into platelet [Ca2+]i signaling under shear stress appear to have been conducted at room temperature.10,12,13 Our study shows that arachidonic acid effectively cannot be metabolized to thromboxane A2 (a necessary step in the phospholipase A2 pathway) in the platelet at this temperature (Figs 6 and 7). Although the isolated cyclo-oxygenase enzyme has demonstrable activity at 25°C,43 this need not pertain in the cellular milieu where other competing reactions and regulatory mechanisms exist.44 Clearly, further studies of vWF-induced [Ca2+]i signaling under shear stress must be undertaken at 37°C to establish unequivocally whether the phospholipase A2 pathway plays a significant role under these circumstances. It is worth noting, in this context, that the classical studies of vWF-mediated platelet adhesion and aggregation on exposed vascular subendothelium in a Baumgartner chamber showed a strong temperature dependence for platelet activation under shear stress.45 Our earlier studies, moreover, have raised doubts about the validity of most [Ca2+]imeasurements in aggregating platelets.17,46 47 As these doubts apply particularly to studies where a [Ca2+]i signal is observed only in the presence of extracellular calcium (Kermode et al, manuscript submitted), the apparent ability of vWF to induce calcium influx into the platelet (under any circumstances) should be questioned. It is possible that the prior studies of vWF-induced [Ca2+]i signaling under shear stress failed to uncover the real signaling pathway because they were performed at ambient temperature, but, instead, reported an artifactual [Ca2+]i increase due to platelet aggregation.
In conclusion, platelet [Ca2+]i signaling induced by ristocetin-mediated interaction of vWF with GP Ib-IX-V is markedly influenced by temperature. A similar pattern of temperature dependence is seen for [Ca2+]i signaling with arachidonic acid, but not with other platelet agonists. Treatment of platelets with the cyclo-oxygenase inhibitor aspirin abolishes the vWF-induced [Ca2+]i signal. These findings are consistent with the concept that stimulation of phospholipase A2 is a necessary and proximal event in platelet [Ca2+]i signaling with vWF.
ACKNOWLEDGMENT
We are greatly indebted to Joe Ed Smith for his expertise in constructing the cuvette holder and novel stirrer (Fig 1). The generous gift of the 10E5 antibody by Dr Barry Coller is most appreciated. Thanks are due to Dr Rodney Baker for his critical review of the manuscript. We are also grateful to the volunteer donors who provided blood samples for these studies.
Supported by Grant-in-Aid MS-G-970048 from the American Heart Association, Mississippi Affiliate, Inc, by National Heart Foundation Starter Grants No. NHF-97302 and NHF-98301 from the American Health Assistance Foundation, and by a Biomedical Research Support Grant from the University of Mississippi Medical Center (all to J.C.K.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John C. Kermode, PhD, Department of Pharmacology and Toxicology, University of Mississippi Medical Center, 2500 N State St, Jackson, MS 39216-4505.

![Fig. 1. Optical configuration and stirring arrangement for simultaneous assessment of platelet aggregation and [Ca2+]i signaling. Measurements are conducted in a cylindrical glass aggregometer cuvette (8-mm diameter) in a reconfigured dual-emission spectrofluorometer. The platelet suspension is stirred with a novel stirrer, comprising an opaque Teflon cylinder with a bar magnet at its base and a UV-grade methacrylate stirring vane at its top. This stirrer protrudes into the excitation beam of the spectrofluorometer to ensure that platelet aggregates cannot settle below its detection zone.17 Platelet aggregation is monitored through measurement of transmitted light intensity, with the lower part of the transmitted light beam blocked so that the stirrer does not interfere with these measurements. Platelet [Ca2+]i is monitored through fluorescence measurements perpendicular to the excitation beam. Reflections from the curved surfaces of the cuvette are eliminated by use of a vertical polarizer in the excitation beam and a horizontal one in the emission beam. This arrangement provides efficient stirring of the platelet suspension and ensures that the fluorescence signal is representative of the entire population of platelets regardless of the extent of aggregation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.199.413k14_199_207/6/m_blod41314001x.jpeg?Expires=1765891167&Signature=Ot5nW1YL7dInxYNaqgTrVJxqqL5LQAgH-Cz9hRIneiD3aBNzN8UzYbpu7xJIsI4Vble9Jg7TM0ahqTU~Kar9SUXsLev76sPGKzwt0BI1rk0FXXFUM6WVYPG0CIDdy44KV-s4cR3xVlvD-VpaOeLR0hPT185cy8t7dKIjTwRp6yIyw6isOoHSaSyHwe9m9zGHGctupmO9mFM9GNZvTfNsnx1~XzRwoE9gwaLVjB-W~-msWXIKtLzILXFt7FfTFBHmrFU38C3GiLWB-65VZiqbDgjIBQ~9fQtpdkBtv31f6n9580kcfHSscRffBQiz3EIP9GsqP8LXmVlhiUiH4iMMmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Temperature dependence of the [Ca2+]i signal induced by ristocetin-mediated binding of vWF. Human platelets were loaded with 5 μmol/L Fura-PE3/AM. Measurements were performed in an aggregometer cuvette, with the platelet suspension (50,000 cells/μL) stirred by the novel stirrer. The platelets were stimulated at 20°C (A), 30°C (B), or 37°C (C) by adding 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF (solid traces) in the presence of extracellular calcium (1 mmol/L CaCl2). Data obtained in parallel control studies with 1 mg/mL ristocetin alone (dotted traces) are also displayed. The fluorescence intensity at 510-nm emission wavelength was measured with excitation alternating between 340 and 380 nm. Platelet [Ca2+]i was calculated from the fluorescence ratio (340:380 nm), using the kd value for Fura-PE3 at the corresponding temperature. Observations at 25°C (not shown) were indistinguishable from those at 20°C. The results illustrated are from a typical one of eight such studies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.199.413k14_199_207/6/m_blod41314002x.jpeg?Expires=1765891167&Signature=33namEqcliUXiAf9F9nJtAULb~-dWyBs2stM6H1nTiDMz2esnFBOdzxYKutjS4q0gfskLf4qiq5Tyo~J2BFiYQdGV1u3GqEb1cM27Hw~R0bd16V-yFg29S4jtU6OL5OJKOPVkCPnEsptsUaU3ET2GdVjtSD66BeyQjWCJ55qmhb~rBsJu7AX7odbpMavYJoWOiylQc-17p5ywbdCzB4Rqad0HREwFEfErAuInvoqbgtiGYExj5SuJFjZdWHnRpfdp8aqIy1--jButvfYR2HN~RsUS7GSb6RbsGWySNrzl8LRfY1lKkwoUPcZ05XM01b3PGecoUUpL2llu4mNMaC1Pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of GP IIb-IIIa blockade on the vWF-induced [Ca2+]i signal. (A) The ability of the 10E5 antibody to block GP IIb-IIIa was evaluated by aggregometry. Washed human platelets (50,000 cells/μL, suspended in Tyrode’s solution with 1 mmol/L CaCl2) were preincubated (5 minutes at 37°C) with isotype-matched control IgG (solid trace) or with 10E5 IgG (dotted trace). Each IgG was essentially azide-free and was used at a final concentration of 10 μg/mL. Platelets were stimulated with 0.1 μmol/L U-46619 and aggregation was assessed at 37°C in the presence of 5 μg/mL multimeric human vWF. (B and C) The effect of the 10E5 antibody on the platelet [Ca2+]isignal was evaluated. Human platelets (50,000 cells/μL, loaded with Fura-PE3) were preincubated with 10 μg/mL control IgG (B) or 10E5 IgG (C). The platelets were then stimulated at 37°C by 1 mg/mL ristocetin and 5 μg/mL vWF (solid traces) in the presence of 1 mmol/L CaCl2. Data obtained in parallel control studies with 1 mg/mL ristocetin alone (dotted traces) are also displayed. Fluorescence measurements were performed in a cylindrical aggregometer cuvette, with the platelet suspension stirred by the novel stirrer. Platelet [Ca2+]i was calculated from the fluorescence excitation ratio (340:380 nm). Results of a typical one of four such studies are illustrated. Aggregation and platelet [Ca2+]i were also assessed in the absence of either IgG (not shown); results were indistinguishable from those in the presence of the control IgG.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.199.413k14_199_207/6/m_blod41314003x.jpeg?Expires=1765891167&Signature=X51T9xRS~CXvuKLPO3vNLuAXkUPULTMSu60VAAfCUL8wJHl2ImsJw1CVlmHke1PLRvXJu9FGJYOd27M2lzLn5Q0eaZClkcihFXIomdie9GrvNlQQXwuTqppYqznVA0837MZO1ByipnoeVmnwA3GtJLTbj-W23-cWH~b28rYuAdV-~KKLY8OuZr7uZ-HqPulPAvIn4Fv6wdgqTp~Etf8naOum9fS3ZMlWB-QHFCng~XTQjtIs2WrxGfCg1whImooaT2OaV-qfkQuFJBVpuaRm8vHQ4Ru4RnSAtXfOaItFrSwcDsbE69vS0y6lSP4yhEKi~Ei71Al9sY1lgOuGYrY44w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Temperature dependence of platelet aggregation and agglutination by ristocetin-mediated binding of vWF. (A and B) Aggregation of live platelets was monitored simultaneously with platelet [Ca2+]i. The platelet suspension (50,000 cells/μL) was incubated at 20°C (A) or 37°C (B) in the presence of 1 mmol/L CaCl2. Aggregation was deduced from the transmitted light intensity (during the 380 nm fluorescence excitation phase). Aggregation patterns at 25°C and 30°C (not shown) were indistinguishable from those at 20°C and 37°C. The results illustrated were acquired in parallel with the [Ca2+]i measurements shown in Fig 2; these aggregation data are typical of four such studies. (C and D) Agglutination of paraformaldehyde-fixed platelets (50,000 cells/μL) was evaluated at 20°C (C) and 37°C (D) in an analogous manner. Agglutination patterns at 30°C (not shown) were comparable to those at 37°C. These data are typical of three such studies. Panels A through D present the responses on incubation either with 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF (solid traces) or with 1 mg/mL ristocetin alone (dotted traces).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.199.413k14_199_207/6/m_blod41314004x.jpeg?Expires=1765891167&Signature=XewrgCbZLLWdabsuh21xdaY-lNAVVcsr38nZzyINPaNB4YMThnJUAPm2I64T2ESz21iUk85Xe1~K8mFNtReTAWhYzTAQhNSg0kQAqo16et944THq5AerJ1hrap-TXfNWmvvIoC-yc5XoNvacGwf87Ppfd9I9~~n12A-nuChDLWsGSUqxZ0OwiNtppjV9L~ViTFXzPH2zQXU4ExtLXasSWc8bF4JWO4w4iOO8S897-CybpLP614fmvsRBA6awEkzp-u8N1zXv9-qEZvE7dpYLFwypbRdKTuIBYXPT9WY9Qr7qgW8JgyZsQ1j9bVXOJ4XPhL7yvhvbT2Tvy3Gz~vKLXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Temperature dependence of the [Ca2+]i signals induced by U-46619, -thrombin, and ADP. The [Ca2+]itransient in a stirred suspension of human platelets (50,000 cells/μL) was monitored with Fura-PE3. The platelets were stimulated at 20°C (dotted traces) or 37°C (solid traces) by 0.1 μmol/L U-46619 (A), 0.02 U/mL human -thrombin (B), or 5 μmol/L ADP (C) in the presence of extracellular calcium (1 mmol/L CaCl2). Platelet [Ca2+]i was calculated from the fluorescence excitation ratio (340:380 nm) using the kd value for Fura-PE3 at the corresponding temperature. Observations at 25°C and 30°C (not shown) were intermediate between those at 20°C and 37°C. The results shown are from a typical one of six such studies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.199.413k14_199_207/6/m_blod41314005x.jpeg?Expires=1765891167&Signature=Rj4vIQ1c8K15t2PwNK~Ib5uUApLpug-x7hWOVVza~5teD9rlfzw01JaDojXQbV90HD-4l-6ZjoN~9lNOknTPN~TTaBu~95vqgt0~gF14OjZ9LhW~YKiFXFVyqtUvl2lwJjOxIq7p6AA1DP0yvWdUACAP4nwww-X4aNiHSlc10Sjqjt75dq1PfkNL0ejXUfIOuhbQooYoWDMO5ZBFOjbMW1NvB4PlA9Dv31aZ3GM12VMj-pTaEXlPixeibkRGq0dtKzEIQ6DC7V2Sd6j5T1UZFDvwZdxeNdeno0~-zo7Vp0laTVHxxWoh3x~mBJR~uTlhGwHHPIJK~09WbidAeS-rFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Temperature dependence of the [Ca2+]i signal induced by exogenous arachidonic acid. The [Ca2+]i transient in a stirred suspension of human platelets (50,000 cells/μL) was monitored with Fura-PE3. The platelets were stimulated at 20°C (A), 30°C (B), or 37°C (C) by 20 μmol/L arachidonic acid (solid traces) in the presence of extracellular calcium (1 mmol/L CaCl2). Data obtained in parallel control studies with 0.2% (wt/vol) dimethylsulfoxide (vehicle for arachidonic acid; dotted traces) are also displayed. Observations at 25°C (not shown) were indistinguishable from those at 20°C. The results illustrated are from a typical one of seven such studies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.199.413k14_199_207/6/m_blod41314006x.jpeg?Expires=1765891167&Signature=Bxzuxy6yO~FqV2ky5Mgrw3ZZ5E3XZyouDuj3~ADcVfvp7jcOjcShvtFdYZ-HSIVzZxymFQBaH3gha1a7g3cQn2VALUABGIoDOT7w~NJuH~wxCBTCGTEmeEWz5yiMqrcpQCxc3y7InEi5PqKvxraMiyPGZR~4gXpX7ain0ozR62xeK3ml5Zfhc~49DHqDT~S~ltspvISFeD2Fem2p3UF7B9sD8WDaiwR3J6xCY-ZAqIM3HD~OZX42wzQBfPhQKapNwhISwu82yIBKMQZtEz5yPz-puR~DkOjVG3t~BmlzbSM2t8fn7St-99NvQ6gDuti2eS8QebuCfwAvxM2-pGFHDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effect of cyclo-oxygenase inhibition on the vWF-induced [Ca2+]i signal and thromboxane A2 production. Human platelets (50,000 cells/μL, loaded with Fura-PE3) were preincubated (5 minutes at 37°C) with 0.1% dimethylsulfoxide (vehicle) or 0.2 mmol/L aspirin. The platelets were then stimulated at 37°C by 1 mg/mL ristocetin and 5 μg/mL multimeric human vWF in the presence of 1 mmol/L CaCl2. Parallel control studies were undertaken with 1 mg/mL ristocetin alone. (A) The results from a typical one of nine such studies are shown. The solid trace denotes the vWF-induced [Ca2+]isignal in vehicle-treated platelets, and the dotted trace that after aspirin treatment. (B) The amplitude of the vWF-induced [Ca2+]i increment, relative to ristocetin alone, in the whole set of studies is presented as a Tukey box plot. (C) Thromboxane A2 production was assessed in parallel. The vWF-induced increment in thromboxane A2 production, relative to ristocetin alone (typically, 0.3 ng), is displayed as an analogous Tukey box plot. The hatched bars in panels B and C summarize data for platelets preincubated with vehicle and the open bars data for aspirin-treated platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.199.413k14_199_207/6/m_blod41314008x.jpeg?Expires=1765891167&Signature=KS6U9WnfRxFrc3KbTm-LD15y3vp4PjMVq3BCiWXCQrUd4WSR6ibw0Ks8n5FiFRdzXbgsZ6-5cuhmOVcB9zIF~tARWsn-UDgLfp5LM7s-DdJIcEMFDZm3VfnWxNdZPE7i-YD0B~jq61gOa0rn8igNcD3~zc0D9O~JIY7njvAoBwC225CbIAS-M3EDvIifmW~pe4DMwm1LHHhFTti-~BcePOah029lsB9piNYfIX2io2WHhhfIi8CIHR1EerHEcePNuMZZXtgo~fcsMHyBtUM33jXpII4QVrdnTI15Cw5LoYhQ2CQOXoisk1pdYrI3VCG9cZEgSu646VNdlRUjeLDeWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal