Rearrangements involving the MLL gene at chromosome 11q23 are associated with leukemia and are present in up to 70% of infant leukemias. Loss of heterozygosity (LOH) has been shown for anonymous polymorphic markers at 11q23 in adult leukemias. To study LOH at theMLL locus, we have identified two new polymorphic microsatellite markers: a GAA repeat (mllGAAn) in intron 6 of theMLL gene and a GA (mllGAn) repeat in the 5′ flanking region of the gene, approximately 2 kb upstream of the translation initiation codon. The heterozygosity index of mllGAAn is 0.54, which renders it useful for analyzing LOH. We screened two groups of leukemia patients to study LOH at the mllGAAn marker. Group A (n = 18) was selected on the basis of presentation before 18 months. Cytogenetic and reverse transcription-polymerase chain reaction analysis showed that 9 of these 18 children had translocations involving MLL. No LOH was observed. Group B (n = 36) were randomly selected children who had presented with leukemia between 1993 and 1994. Cytogenetic analysis of this group showed a variety of different chromosomal abnormalities. LOH was shown in 9 of 20 individuals (45%) who were informative. Microsatellite instability (MSI) was demonstrated in 1 of 18 individuals in group A and 5 of 36 individuals (13.9%) in group B. MSI and LOH were observed simultaneously in three individuals. Loss of an allele was confirmed in one individual by fluorescence in situ hybridization. Individuals with MSI or LOH at mllGAAn were selected for analysis at anonymous polymorphic markers D11S1364 and D11S1356, which flank the MLL gene. No LOH or MSI was observed at these markers in those individuals who were informative. These results show that LOH at the MLL gene locus is a common event during leukemogenesis. Furthermore, the presence of MSI at this locus suggests that the region is a hotspot for genetic instability.
NONRANDOM REARRANGEMENTS in the mixed lineage leukemia (MLL) gene (also known as ALL-1,HRX, and HTRX1) at chromosome 11q23 are frequently observed in leukemia.1 It is estimated that over 70% of leukemias in infants below 1 year involve rearrangements of MLL,and such rearrangements define a subgroup of patients who respond badly to treatment and have a poor prognosis.2-4 The rearrangements are normally translocations, and over 20 different translocation partners have been identified to date. The most common of these partners is AF-4 (ALL-1fusion gene on chromosome 4), but AF-9 and ENL are also common partners.5-7 Duplications of exons 2-6 or 2-8 of the MLL gene, resulting in “self fusion,” and interstitial deletions of exon 8 have also been reported.8 9
MLL is a large gene: it spans 100 kb, has at least 35 exons, and the mRNA is 14.5 kb long.10-12 The MLL protein comprises 3969 amino acids, has an estimated size of 431 kD, and appears to comprise a number of distinct functional domains. Near the amino-terminus are three closely spaced A·T hooks that have been shown to bind to cruciform DNA and to SAR DNA.12,13Sequences in this region have also been shown to bind the leukemia-associated SET protein, which appears to mediate complex formation between MLL and protein phosphatase 2A.14Further downstream are two regions that can repress or activate transcription of reporter genes, respectively. The former has homology to DNA methyltransferases.13,15 There are also several regions of homology to the trithorax gene product, which is involved in positive regulation of homeotic gene transcription inDrosophila. One of these, located downstream of the methyltransferase homology region, is predicted to form three plant homology domain (PHD)-type zinc-finger motifs, which are presumed to interact with DNA in a sequence-specific manner. Another, located at the carboxyl terminus of MLL, is named the SET domain, because it is found in the Drosophila Suppressor of variegation and Enhancer of zeste gene products as well as in the protein encoded by trithorax. This domain has recently been shown to bind sbp (SETbinding protein), which is predicted to be an antiphosphatase.16 Taken together, these data suggest thatMLL normally makes contact with both proteins and DNA, and functions as part of a large multimeric complex of proteins that regulate transcription of specific target genes, probably by modifying chromatin structure. In support of this idea, mice that are hemizygous for a null mutation of the MLL gene show abnormal Hoxgene expression patterns.17
As yet, it is unclear how MLL gene rearrangements contribute to leukemogenesis. The translocation breakpoints cluster between exons 5 and 8 (exon numbering of Tkachuk et al, 1992).12 Such rearrangements result in synthesis of proteins that have lost the SET domain, transcription activation domain, and some of the zinc-finger domain of MLL, but which retain most of the transcription repression domain and the A·T hook region.13,18 The most common fusion partners, AF-4, AF-9 and ENL, contribute their own transcription regulation domains to the fusion proteins, but other partners have disparate functions. It is possible that the fusion proteins represent gain-of-function mutations that contribute to leukemogenesis. On the other hand, the loss of some MLLfunctions in fusion proteins, and the lack of consistency in the functions contributed by the fusion partners, have led to the suggestion that MLL may be a tumor suppressor gene. Corral et al19 recreated the MLL/AF-9 fusion gene in transgenic mice by “knock-in” of the human AF-9 gene to the mouse MLL gene, and found a high incidence of acute myeloid leukemias in these animals. Similarly, Lavau et al20 found that retrovirally encoded MLL/ENL fusion protein could immortalize and transform myeloid cells when used to infect mouse bone marrow cells enriched in hematopoietic stem cells. These findings show that MLL gene rearrangements are important primary events in leukemogenesis, but do not distinguish between the hypotheses that the fusion proteins are gain-of-function mutants, or dominant-negative mutants that disrupt function of the normal MLLprotein.1 Deletions at 11q23 have been reported in leukemia but are not a common nonrandom event.21 If MLL is a tumor suppressor gene, then loss of heterozygosity (LOH) at this locus would be expected to be a common event in the development of leukemias and, possibly, other types of cancer. In a genome-wide study of microsatellite markers in adults with leukemia, LOH at 11q23 was reported in 14% of patients.22 The investigators proposed that there was a tumor suppressor gene in that region and suggestedMLL as a candidate, but the markers used flanked MLL. Ideally, to determine whether there is LOH at the MLL locus, a marker within that locus should be used.
To determine whether LOH at the MLL locus is a common event in childhood leukemia, we identified a polymorphic GAA repeat in the breakpoint cluster region of the MLL gene. We have shown 45% LOH and 13.9% MSI in a group of randomly selected children with leukemia. We also analyzed this marker in a group of patients selected by presentation before 18 months. MSI was detected in 1 of 18 patients. Despite a high incidence of gross MLL rearrangements in this latter group, no LOH was detected.
MATERIALS AND METHODS
Patients.
Patients attended the Childhood Leukaemia Clinic at Great Ormond Street Hospital NHS Trust (London, UK) unless otherwise stated. DNA was prepared from bone marrow at presentation and at remission. We studied two groups of patients. Members of group A were selected on the basis of presentation before 18 months. Such patients have a high incidence of MLL rearrangements and we wished to determine whether they also exhibited a high incidence of more subtle abnormalities at theMLL locus, irrespective of whether they had an 11q23 translocation. Group B patients were selected by sequential admissions in 1993 and 1994 to the Childhood Leukaemia Clinic at Great Ormond Street Hospital NHS Trust. They were therefore not selected on the basis of any phenotypic or genotypic criteria. In group A, sample A16 was generously provided by E. Grace (Department of Cytogenetics, Royal Hospital for Sick Children, Edinburgh Sick Children NHS Trust, Edinburgh, UK) and sample A17 was generously provided by M. McKinley (Oxford Medical Genetics Laboratories, The Churchill Hospital, Oxford Radcliffe Hospital NHS Trust, Oxford, UK). Details of the karyotypes of these samples were generously provided by Dr Christine Harrison (Leukaemia Research Fund UKCCG ALL Karyotype Database, Royal Free Hospital Medical School, London, UK). DNA was prepared by the salt precipitation method,23 unless otherwise stated.
DNA samples were obtained with permission from 50 normal volunteer Caucasian adults by extraction from mouthwash samples, as described previously,24 or from peripheral blood. DNA samples were also prepared with permission from the peripheral blood of normal volunteer Caucasians from three families.
Isolation of PAC clones.
Pools of the de Jong RPCI1 human PAC library,25 obtained from HGMP (Cambridge, UK), were screened by polymerase chain reaction (PCR) using primers corresponding to sequences in exon 8 of theMLL gene (sense primer: 5′-gagctccttatagatgaagagg-3′; antisense primer 5′-tcctatccgatcctgagcagta-3′). PCR cycling conditions were as follows: 95°C, 1 minute; 55°C, 1 minute; 72°C, 1 minute, 25 cycles. DNA was prepared from positive PACs using maxiprep columns (Qiagen, Crawley, UK). PAC DNA was digested with multiple restriction enzymes according to the manufacturer’s conditions (GIBCO-BRL, Paisley, UK), electrophoresed on 0.8% (wt/vol) agarose gels, and transferred to Hybond-N+ nylon membranes (Amersham, Little Chalfont, UK) by Southern blotting. Oligonucleotides corresponding to sequences in exon 1 (5′-gatggcgcacagctgtcggtgg-3′), exon 3 (5′-gcagactagtgctccggcagagcc-3′), and exon 11 (5′-acacccagtttattctccaacacag-3′) of the MLL gene were labeled with γ32P-ATP (Amersham) using T4 polynucleotide kinase (GIBCO-BRL), as recommended by the manufacturer, and hybridized to Southern-blotted PAC DNA under stringent conditions, as described previously.26 A 5.5-kbHindIII-NotI fragment of PAC dJ217a21, containing part of MLL exon 1 and upstream sequences, and a 3.7-kb TaqI fragment from PAC dJ59j2, spanning from intron 5 to intron 8, were subcloned into pBluescript SK+ (Stratagene, Amsterdam, The Netherlands) and sequenced using the Sequenase kit (Amersham) as recommended by the manufacturer.
Microsatellite analysis.
The forward primer was labeled with γ32P-ATP, and PCR was performed using Qiagen buffer and Taq polymerase as recommended by the manufacturers. A two-step PCR cycling protocol was used: 95°C, 30 seconds; 65°C, 30 seconds; 30 cycles. Primers used were: mllGAAn, forward 5′-tgaggtgggaggattgcttgag-3′, reverse 5′-agtacctgggacactacgcaactg-3′; mllGAn, forward 5′-ctctgcagaccgttatgc-3′, reverse 5′-ggattccctcaagcatcc-3′; D11S1356, forward 5′-gttgctcatctgttgctca-3′, reverse 5′-acctgccctgacttgc-3′; D11S1364 forward 5′-ggatccactccagcctgggcaa-3′, reverse 5′-gatagatggatcatggatacagg-3′.27 Labeled PCR products were separated on 6% (wt/vol) polyacrylamide denaturing gels and exposed to Kodak XAR5 film (Eastman Kodak, Rochester, NY) for 0.5 to 17 hours at −70°C. The reactions were repeated three times independently.
Cytogenetics.
Bone marrow samples obtained at presentation were processed by standard methods and the GTG-banded karyotypes were described according to ISCN.28 Whole chromosome paints (Cambio, UK) and locus-specific probes for BCR/ABL (Appligene Oncor, Durham, UK) and TEL/AML-1 (Vysis, Richmond, UK) were processed according to the manufacturers’ instructions. Fixed cells from bone marrow aspirates were available for fluorescent in situ hybridization (FISH) analysis of the MLL locus from patients B22, B32, and B34, who demonstrated loss at mllGAAn. A digoxygenin-labeled, locus-specific probe for the MLL locus (Appligene Oncor) was prepared according to the manufacturer’s instructions and FISH was performed as described previously.29 Cells were viewed using a CCD camera (Photometrics, Tuscon, AZ) and Smartcapture software (Digital Scientific, Cambridge, UK).
RESULTS
Isolation of MLL PAC clones.
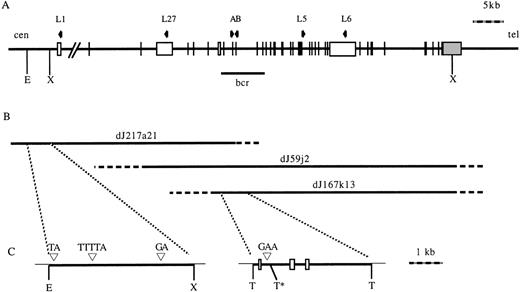
To identify polymorphic microsatellite markers, a human genomic PAC library was screened by PCR to obtain clones spanning the MLLgene locus. The PCR primers corresponded to sequences in exon 8 of theMLL gene. Three positive PACs were identified: dJ217a21, dJ167k13, and dJ59j2. To characterize these PACs, and to check for integrity, they were digested with a number of restriction enzymes, Southern blotted, and hybridized to labeled oligonucleotides complimentary to sequences in exons 1, 3, and 11 of the MLLgene. As shown in Fig 1, dJ217a21 extends from approximately 6 kb upstream of MLL exon 1 to downstream of exon 8, dJ59j2 extends from upstream of exon 3 to downstream of exon 37, and dJ167k13 extends from upstream of exon 7 to downstream of exon 37.
(A) Diagram of the MLL gene. Vertical lines and open boxes represent exons. Shaded box represents 3′ UTR. Arrows represent oligonucleotides used to screen the PAC library (A and B) or used to map the PAC (L1, L27, L5, and L6). (B) Three PACs isolated which span the gene. Solid lines indicate mapped regions of the PACs, dashed lines indicate unmapped regions of the PACs. (C) Subcloned regions of PACs containing repetitive elements. Open boxes represent exons. (▿), Positions of small repetitive elements. E, EcoRI; X, Xho; T, Taq I. *No restriction digestion at this site due to overlapping Dam methylation. cen, centromere; tel, telomere; bcr, breakpoint cluster region.
(A) Diagram of the MLL gene. Vertical lines and open boxes represent exons. Shaded box represents 3′ UTR. Arrows represent oligonucleotides used to screen the PAC library (A and B) or used to map the PAC (L1, L27, L5, and L6). (B) Three PACs isolated which span the gene. Solid lines indicate mapped regions of the PACs, dashed lines indicate unmapped regions of the PACs. (C) Subcloned regions of PACs containing repetitive elements. Open boxes represent exons. (▿), Positions of small repetitive elements. E, EcoRI; X, Xho; T, Taq I. *No restriction digestion at this site due to overlapping Dam methylation. cen, centromere; tel, telomere; bcr, breakpoint cluster region.
Identification of polymorphisms.
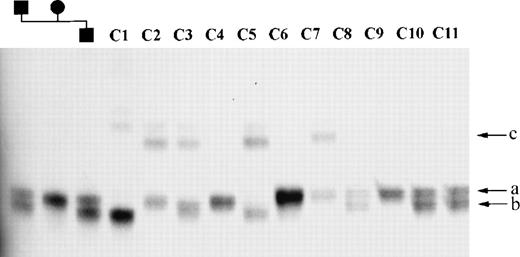
A 5.7-kb HindIII-NotI fragment containing part ofMLL exon 1 and upstream (centromeric) sequences, and a 3.7-kbTaqI fragment spanning from intron 5 to intron 8, were subcloned into pBluescript SK+ and sequenced. As shown in Fig 1, four repetitive elements were identified: a CA repeat, a GA repeat, and a TTTTA repeat in the HindIII-NotI fragment, and a GAA repeat in the TaqI fragment. These repetitive elements were amplified from 50 normal individuals and members of two families to identify which, if any, were polymorphic. The CA and TTTTA repeats were not polymorphic. The GA repeat upstream of exon 1 (mllGAn) had three alleles, two of which were rare (<5%) and one of which was predominant in the population analyzed. The heterozygosity index of this polymorphism was 0.06. The GAA repeat located in intron 6 (mllGAAn) had three common alleles, designated a, b and c; the frequency of these alleles is 0.5, 0.43, and 0.07 respectively (Fig 2). The heterozygosity index of this polymorphic marker was 0.54 and the alleles were in Hardy-Weinberg equilibrium in the normal population. The nucleotide sequence of the region of intron 6 of the MLL gene containing the GAA repeat has been lodged with the EMBL Nucleotide Sequence Database (accession no. AJ131191).
mllGAAn microsatellite alleles in normal controls. Part of MLL intron 6 was amplified from normal DNA samples and separated on a denaturing PAGE gel (see Materials and Methods). Samples 1 through 3: Mother, father, and son, respectively, from a normal family showing inheritance of alleles. Samples 4 through 14: normal control samples. (a, b, and c) Designation of mllGAAn alleles. Faint bands above the main bands in lanes C1 through C7 are nondenatured PCR products.
mllGAAn microsatellite alleles in normal controls. Part of MLL intron 6 was amplified from normal DNA samples and separated on a denaturing PAGE gel (see Materials and Methods). Samples 1 through 3: Mother, father, and son, respectively, from a normal family showing inheritance of alleles. Samples 4 through 14: normal control samples. (a, b, and c) Designation of mllGAAn alleles. Faint bands above the main bands in lanes C1 through C7 are nondenatured PCR products.
LOH and microsatellite instability (MSI) analysis.
We used the mllGAAn marker to investigate whether LOH at theMLL locus was a common event in two groups of patients with leukemia. The first group (group A) were selected on the basis of presentation before 18 months of age. Half the members of group A had a rearrangement of the MLL gene previously identified by cytogenetic analysis (Table 1). The second group (group B) were patients selected randomly, by sequential admission, from children presenting with acute leukemia from 1993 to 1994. Cytogenetic analysis showed a number of different chromosomal abnormalities in this group but no translocations at 11q23 (Table 2). DNA prepared from bone marrow taken at presentation and after remission was amplified by PCR, and the presentation and remission samples for each individual were compared to ascertain whether the leukemic samples exhibited LOH or MSI. One sample in group A was heterozygous at presentation but homozygous at remission, indicating there was MSI. No LOH was detected in this group.
Phenotype, Genotype, and Karyotype of Patient Group A (infants)
| Patient No. . | Age (mo) . | Diagnosis . | mllGAAn Genotype . | Karyotype . | |
|---|---|---|---|---|---|
| P . | R . | ||||
| A1 | 4 | AML M7 | bc | bc | 46,XY[8]/46,Y,t(X;6)(p11.2;q23)[22] |
| A2 | 7 | Null ALL | ab | ab | 46,XY,t(11;19)(q23;p13)[10] |
| A3 | 4 | Null ALL | ab | ab | 46,XX[6]/46,XX,t(4;11)(q21;q23)[2] |
| A4 | 14 | ALL | ab | ab | 46,XX[1]/47,XX,+der(8)[3] / 49,XX,+der(8)+der(8) +der(8)[3]/50,XX, +der(8)+der(8)+der(8)+der(8)[2] |
| A5 | 3 | AML M5 | ab | ab | 46,XX [13]/46,XX,t(10;11)p11;q23)[6] |
| A6 | 8 | Pre-B ALL | ab | ab | 46,XX[5]/46,XX,t(9;11)(p21;q23)[15] |
| A7 | 15 | Pre-B ALL | ab | ab | 46,XY,der(1)t(1;19)(q23;p13)[2]/ 48,XY,idem+2+4[1] |
| A8 | 2 | Null ALL | bb | bb | 46,XY [2]/46,XY,t(4;11)(q21;q23)[8] |
| A9 | 7 | Null ALL | aa | aa | 46,XX[1]/46,XX,t(4;11)(q21;q23)[3] |
| A10 | 11 | Null ALL | ab | ab | 46,XY[10]/48,XY,t(11;19;?)(q23;p13;?),+21[5] |
| A11 | 10 | Null ALL | ab | ab | Not done |
| A12 | 12 | T-ALL | ab | ab | 48,XX,+19,+21[3]/49XX,+8,+19,+21[8] |
| A13 | 17 | ALL | ab | ab | 46,XX[1]/46,XX,t(1;19)(q23;p13)[9] |
| A14 | 7 | ALL | ab | ab | 46,XY[1]/46,XY,−5,del(17)(p11),−20,+mar,+mar[8] |
| A15 | 15 | ALL | ab | aa | 46,XY[2]/53,XY,+4,+9,+12,+13,+18,+21,+22[2] |
| A16 | U | U | ab | ab | 46,XY,t(11;17)(q23;p13) |
| A17 | U | U | aa | aa | 46,XY,t(4;11)(q?25;q23) |
| A18 | 8 | AML M2 | ab | ab | 46,XY[4]/46,XY,del(8)(q13q22)[10] |
| Patient No. . | Age (mo) . | Diagnosis . | mllGAAn Genotype . | Karyotype . | |
|---|---|---|---|---|---|
| P . | R . | ||||
| A1 | 4 | AML M7 | bc | bc | 46,XY[8]/46,Y,t(X;6)(p11.2;q23)[22] |
| A2 | 7 | Null ALL | ab | ab | 46,XY,t(11;19)(q23;p13)[10] |
| A3 | 4 | Null ALL | ab | ab | 46,XX[6]/46,XX,t(4;11)(q21;q23)[2] |
| A4 | 14 | ALL | ab | ab | 46,XX[1]/47,XX,+der(8)[3] / 49,XX,+der(8)+der(8) +der(8)[3]/50,XX, +der(8)+der(8)+der(8)+der(8)[2] |
| A5 | 3 | AML M5 | ab | ab | 46,XX [13]/46,XX,t(10;11)p11;q23)[6] |
| A6 | 8 | Pre-B ALL | ab | ab | 46,XX[5]/46,XX,t(9;11)(p21;q23)[15] |
| A7 | 15 | Pre-B ALL | ab | ab | 46,XY,der(1)t(1;19)(q23;p13)[2]/ 48,XY,idem+2+4[1] |
| A8 | 2 | Null ALL | bb | bb | 46,XY [2]/46,XY,t(4;11)(q21;q23)[8] |
| A9 | 7 | Null ALL | aa | aa | 46,XX[1]/46,XX,t(4;11)(q21;q23)[3] |
| A10 | 11 | Null ALL | ab | ab | 46,XY[10]/48,XY,t(11;19;?)(q23;p13;?),+21[5] |
| A11 | 10 | Null ALL | ab | ab | Not done |
| A12 | 12 | T-ALL | ab | ab | 48,XX,+19,+21[3]/49XX,+8,+19,+21[8] |
| A13 | 17 | ALL | ab | ab | 46,XX[1]/46,XX,t(1;19)(q23;p13)[9] |
| A14 | 7 | ALL | ab | ab | 46,XY[1]/46,XY,−5,del(17)(p11),−20,+mar,+mar[8] |
| A15 | 15 | ALL | ab | aa | 46,XY[2]/53,XY,+4,+9,+12,+13,+18,+21,+22[2] |
| A16 | U | U | ab | ab | 46,XY,t(11;17)(q23;p13) |
| A17 | U | U | aa | aa | 46,XY,t(4;11)(q?25;q23) |
| A18 | 8 | AML M2 | ab | ab | 46,XY[4]/46,XY,del(8)(q13q22)[10] |
Abbreviations: P, presentation sample; R, remission sample; U, unknown.
Phenotype, Genotype, and Karyotype of Patient Group B (children)
| Patient No. . | Age (yr) . | Diagnosis . | mllGAAn Genotype . | LOH/MSI . | Karyotype . | |
|---|---|---|---|---|---|---|
| P . | R . | |||||
| B1 | 9 | T-ALL | aa | aa | NI | 46,XY[1]/46,XY,add(7)(q32 or q36), del(9)(p13)[4] |
| B2 | 8 | ALL | ab | ab | Normal | 46,XY[7]/46,XY,t(8;14)(q24;q23)[3] |
| B3 | 2 | Pre B-ALL | ab | aa | MSI | 46,XX[16]/46,XX,t(1;19)(q23;p13)[2] |
| B4 | 3 | ALL | bb | bb | NI | 46,XX[7]/55,XX,+add(1)(p2)+5,+6,+9, +10,+13,+17,+21,+21[6] |
| B5 | 5 | ALL | aa | aa | NI | 46,XY[2]/54,XY,+6,+8,+del(10) (q22q26),+14,+17,+18,+21,+21 [12] |
| B6 | 4 | ALL | ab | ab | Normal | 46,XX[4]/46,XX,t(8;14)(q24;q11)[9] |
| B7 | 2 | ALL | ac | ac | Normal | 46,XX[4]/46,XX,t(9;16)(p13;q24)[14] |
| B8 | 2 | ALL | bb | bb | NI | 46,XY[9] ?clone >50 by DNA index |
| B9 | 3 | ALL | a(b) | ab | LOH | 46,XX,del(11)(q13?q25),add(19)(p13) [2]/47, idem +21 [1]/47,XX, add(19)(p13),+21[2] |
| B10 | 8 | AML M3 | aa | aa | NI | 46,XY,t(15;17)(q22;q21)[50] |
| B11 | 5 | ALL | ab | ab | Normal | 46,XY |
| B12 | 6 | ALL | a(b) | ab | LOH | 46,XX[7]/55,+mars,inc[1] |
| B13 | 8 | AML M7 | ab | bb | MSI | 46,XY[11]/92-95,+3,+13,inc[8] |
| B14 | 3 | ALL | aa | aa | NI | 51,XX,t(12;21)(p13;q22),+17,+18,+19,+21,+22[9] |
| B15 | 4 | AML | b | ab | LOH | 47,XY,del(7)(q22q32),+21 |
| B16 | 5 | Pre B-ALL | ab | ab | Normal | 46,XY,[4]/47,XY,der(7)t(3;7)(q25;q36),t(12;21)(p13;q22),+21[5] |
| B17 | 6 | T-ALL | aa | aa | NI | 46,XY[1]/46,XY,del(5)(q13q31)[9] |
| B18 | 4 | ALL | ab | ab | Normal | 50,XY,+11,+15,+21,+21[9] |
| B19 | 11 | ALL | aa | aa | NI | 46,XY[7]/45,XY,−8,del(12)(p13), t(12;21)(p13;q22),add(14)(q32)[3] |
| B20 | 3 | ALL | aa | aa | NI | 46,XX[2]/54,XX,+X,+X,+4,+7,+8,+14, +17,−20,+21,+21[3] |
| B21 | 1.75 | ALL | aa | aa | NI | 46,XY[20] |
| B22 | 10 | AML | b(c) | ac | LOH/MSI | 46,XY[3]/46,XY,dup(1)(q?)[10] |
| B23 | 3 | AML M | aa | aa | NI | 47,XX,+21c[2]/48,XX,+8,+21c[13] |
| B24 | 5 | ALL | ab | ab | Normal | 46,XY[7]/45,XY,del(6)(q1?4q23),−13[1] |
| B25 | 2 | ALL | aa | aa | NI | No karyotype, TEL/AML-1 translocation detected by RT-PCR |
| B26 | 2 | Pre B-ALL | ab | ab | Normal | 46,XX[1]/46,XX,t(1;19)(q23;p13)[5] |
| B27 | 4 | AML M2 | a | ab | LOH | 47,XXYc[6]/47,XXYc,t(6;12)(q21;q24)[8]/47,XXYc,t(X;6)(p11.2;p23)[3] |
| B28 | 2 | ALL | aa | aa | NI | 46,XY[4]/58,XY,+X,t(2;12)(p11;p13),+4,+6,+8,+10,+11,+14,+17,+18, +21,+21, +mar[8] |
| B29 | 4 | ALL | a(b) | ab | LOH | 66,XXY,−1,−3,−4,−5,del (6)(q15q23), −7,+8,+10,+12,−15,+17, −20[cp8] |
| B30 | 3 | ALL | ab | ab | Normal | 46,XX[1]/54,XX,dup(1)(q11q24), +4,+5,+8,+12,+13,+18,+21,+21[17] |
| B31 | 7 | T-ALL | aa | aa | NI | 46,XY[3]/46,XY,del(2)(q?21), del(9)(q?13),add12(q12), der(14) t(2;14)(q21;q32)[11] |
| B32 | 3 | Pre B-ALL | a | bb | LOH/MSI | 47,XX,+21c[2]/48,XX,+X,+21c[9] |
| B33 | 4 | ALL | bb | bb | NI | 46,XX,t(4;8)(q27;q24),add(11)(p15), del(15)(q2) |
| B34 | 4 | ALL | a | ab | LOH | 46,XX[16]/45,XX,t(3;12)(q25;q22), del(12)(p13),t(12;21)(p13;q22), −14[3] |
| B35 | 4 | ALL | b(c) | ac | LOH/MSI | 46,XX[3]/54,XX,+8,+13,+16,+17,+18, +20,+21,+21[1] |
| B36 | 8 | AML M5 | bb | bb | NI | 46,XY[2]/46,XY,t(3;5)(q21;q31)[3] |
| Patient No. . | Age (yr) . | Diagnosis . | mllGAAn Genotype . | LOH/MSI . | Karyotype . | |
|---|---|---|---|---|---|---|
| P . | R . | |||||
| B1 | 9 | T-ALL | aa | aa | NI | 46,XY[1]/46,XY,add(7)(q32 or q36), del(9)(p13)[4] |
| B2 | 8 | ALL | ab | ab | Normal | 46,XY[7]/46,XY,t(8;14)(q24;q23)[3] |
| B3 | 2 | Pre B-ALL | ab | aa | MSI | 46,XX[16]/46,XX,t(1;19)(q23;p13)[2] |
| B4 | 3 | ALL | bb | bb | NI | 46,XX[7]/55,XX,+add(1)(p2)+5,+6,+9, +10,+13,+17,+21,+21[6] |
| B5 | 5 | ALL | aa | aa | NI | 46,XY[2]/54,XY,+6,+8,+del(10) (q22q26),+14,+17,+18,+21,+21 [12] |
| B6 | 4 | ALL | ab | ab | Normal | 46,XX[4]/46,XX,t(8;14)(q24;q11)[9] |
| B7 | 2 | ALL | ac | ac | Normal | 46,XX[4]/46,XX,t(9;16)(p13;q24)[14] |
| B8 | 2 | ALL | bb | bb | NI | 46,XY[9] ?clone >50 by DNA index |
| B9 | 3 | ALL | a(b) | ab | LOH | 46,XX,del(11)(q13?q25),add(19)(p13) [2]/47, idem +21 [1]/47,XX, add(19)(p13),+21[2] |
| B10 | 8 | AML M3 | aa | aa | NI | 46,XY,t(15;17)(q22;q21)[50] |
| B11 | 5 | ALL | ab | ab | Normal | 46,XY |
| B12 | 6 | ALL | a(b) | ab | LOH | 46,XX[7]/55,+mars,inc[1] |
| B13 | 8 | AML M7 | ab | bb | MSI | 46,XY[11]/92-95,+3,+13,inc[8] |
| B14 | 3 | ALL | aa | aa | NI | 51,XX,t(12;21)(p13;q22),+17,+18,+19,+21,+22[9] |
| B15 | 4 | AML | b | ab | LOH | 47,XY,del(7)(q22q32),+21 |
| B16 | 5 | Pre B-ALL | ab | ab | Normal | 46,XY,[4]/47,XY,der(7)t(3;7)(q25;q36),t(12;21)(p13;q22),+21[5] |
| B17 | 6 | T-ALL | aa | aa | NI | 46,XY[1]/46,XY,del(5)(q13q31)[9] |
| B18 | 4 | ALL | ab | ab | Normal | 50,XY,+11,+15,+21,+21[9] |
| B19 | 11 | ALL | aa | aa | NI | 46,XY[7]/45,XY,−8,del(12)(p13), t(12;21)(p13;q22),add(14)(q32)[3] |
| B20 | 3 | ALL | aa | aa | NI | 46,XX[2]/54,XX,+X,+X,+4,+7,+8,+14, +17,−20,+21,+21[3] |
| B21 | 1.75 | ALL | aa | aa | NI | 46,XY[20] |
| B22 | 10 | AML | b(c) | ac | LOH/MSI | 46,XY[3]/46,XY,dup(1)(q?)[10] |
| B23 | 3 | AML M | aa | aa | NI | 47,XX,+21c[2]/48,XX,+8,+21c[13] |
| B24 | 5 | ALL | ab | ab | Normal | 46,XY[7]/45,XY,del(6)(q1?4q23),−13[1] |
| B25 | 2 | ALL | aa | aa | NI | No karyotype, TEL/AML-1 translocation detected by RT-PCR |
| B26 | 2 | Pre B-ALL | ab | ab | Normal | 46,XX[1]/46,XX,t(1;19)(q23;p13)[5] |
| B27 | 4 | AML M2 | a | ab | LOH | 47,XXYc[6]/47,XXYc,t(6;12)(q21;q24)[8]/47,XXYc,t(X;6)(p11.2;p23)[3] |
| B28 | 2 | ALL | aa | aa | NI | 46,XY[4]/58,XY,+X,t(2;12)(p11;p13),+4,+6,+8,+10,+11,+14,+17,+18, +21,+21, +mar[8] |
| B29 | 4 | ALL | a(b) | ab | LOH | 66,XXY,−1,−3,−4,−5,del (6)(q15q23), −7,+8,+10,+12,−15,+17, −20[cp8] |
| B30 | 3 | ALL | ab | ab | Normal | 46,XX[1]/54,XX,dup(1)(q11q24), +4,+5,+8,+12,+13,+18,+21,+21[17] |
| B31 | 7 | T-ALL | aa | aa | NI | 46,XY[3]/46,XY,del(2)(q?21), del(9)(q?13),add12(q12), der(14) t(2;14)(q21;q32)[11] |
| B32 | 3 | Pre B-ALL | a | bb | LOH/MSI | 47,XX,+21c[2]/48,XX,+X,+21c[9] |
| B33 | 4 | ALL | bb | bb | NI | 46,XX,t(4;8)(q27;q24),add(11)(p15), del(15)(q2) |
| B34 | 4 | ALL | a | ab | LOH | 46,XX[16]/45,XX,t(3;12)(q25;q22), del(12)(p13),t(12;21)(p13;q22), −14[3] |
| B35 | 4 | ALL | b(c) | ac | LOH/MSI | 46,XX[3]/54,XX,+8,+13,+16,+17,+18, +20,+21,+21[1] |
| B36 | 8 | AML M5 | bb | bb | NI | 46,XY[2]/46,XY,t(3;5)(q21;q31)[3] |
Abbreviations: P, presentation sample; R, remission sample; NI, not informative.
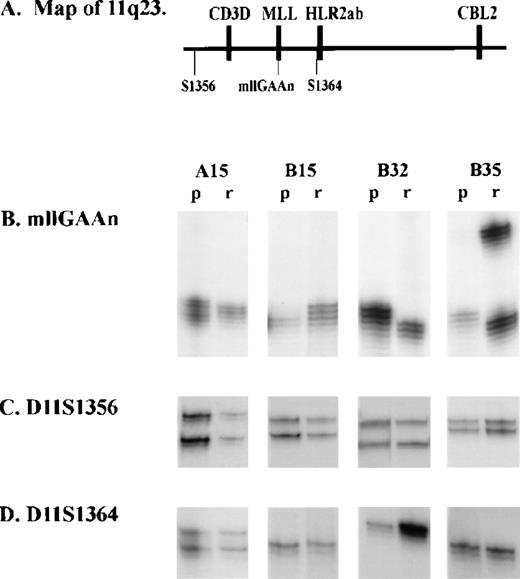
In group B, 17 of 36 remission samples were heterozygous. Of these 17, 8 (47%) showed either complete or partial loss of one allele in the corresponding presentation sample (Fig 3A). Partial loss reflects the fact that presentation samples contain some normal cells, at an estimated frequency of 0% to 20% of total cells present. In 2 of these 8 samples, the remaining allele was not the same size as either allele in the remission sample, indicating that both LOH and MSI had occurred. One additional patient (B32) was homozygous at remission but exhibited a single different allele at presentation, again indicating that both LOH and MSI had occurred. An additional 2 patients were homozygous at remission but heterozygous at presentation, indicating that MSI but not LOH had occurred. In total, therefore, 9 of 20 informative samples (45%) had LOH and 5 of 36 samples (13.9%) had MSI. Because some presentation samples may contain high levels of normal cells, the frequency of LOH is likely to be an underestimate. Similarly, because some remission samples may contain leukemic cells, the frequency of MSI could be an underestimate.
Analysis of microsatellite polymorphic markers at 11q23 in children with acute leukemias. Microsatellite markers were amplified and separated on a denaturing polyacrylamide gel electrophoresis (see Materials and Methods). p, presentation sample; r, remission sample. (A) Diagram showing location of MLL gene, microsatellite markers (mllGAAn, D11S1356, and D11S1364) and nearby genes (CD3D and CBL2) at 11q23. (B) mllGAAn marker. (C) D11S1356 marker. (D) D11S1364 marker.
Analysis of microsatellite polymorphic markers at 11q23 in children with acute leukemias. Microsatellite markers were amplified and separated on a denaturing polyacrylamide gel electrophoresis (see Materials and Methods). p, presentation sample; r, remission sample. (A) Diagram showing location of MLL gene, microsatellite markers (mllGAAn, D11S1356, and D11S1364) and nearby genes (CD3D and CBL2) at 11q23. (B) mllGAAn marker. (C) D11S1356 marker. (D) D11S1364 marker.
Analysis of other polymorphic microsatellite markers.
To determine the extent of LOH at other markers, we analyzed the flanking markers D11S1364 and D11S1356 in all samples in which the mllGAAn haplotypes were different at presentation and remission (Fig 3B and C, and Table 3). No LOH or MSI was identified at these markers in those samples which were informative, indicating that LOH is confined to a region between them. As part of a different study, some patients were analyzed for microsatellite markers at the TEL locus on chromosome 12. Samples A15, B9, B12, B15, B27, B32, and B35 were analyzed for D12S89; and samples A15, B9, B12, B22, B32, B34, and B35 were analyzed for D12S98. No LOH or MSI was detected (data not shown).
Genotype of Patients With LOH or MSI in the MLLLocus at Microsatellite Markers Flanking MLL
| Sample . | D11S1364 . | D11S1356 . | ||
|---|---|---|---|---|
| P . | R . | P . | R . | |
| A15 | het | het | het | het |
| B3 | het | het | ND | ND |
| B9 | hom | hom | hom | hom |
| B12 | hom | hom | hom | hom |
| B13 | het | het | het | het |
| B15 | hom | hom | het | het |
| B22 | hom | hom | hom | hom |
| B27 | het | het | hom | hom |
| B29 | hom | hom | het | het |
| B32 | hom | hom | het | het |
| B34 | hom | hom | het | het |
| B35 | hom | hom | het | het |
| Sample . | D11S1364 . | D11S1356 . | ||
|---|---|---|---|---|
| P . | R . | P . | R . | |
| A15 | het | het | het | het |
| B3 | het | het | ND | ND |
| B9 | hom | hom | hom | hom |
| B12 | hom | hom | hom | hom |
| B13 | het | het | het | het |
| B15 | hom | hom | het | het |
| B22 | hom | hom | hom | hom |
| B27 | het | het | hom | hom |
| B29 | hom | hom | het | het |
| B32 | hom | hom | het | het |
| B34 | hom | hom | het | het |
| B35 | hom | hom | het | het |
Abbreviations: P, presentation sample; R, remission sample; het, heterozygous; hom, homozygous; ND, not done.
Cytogenetic analysis.
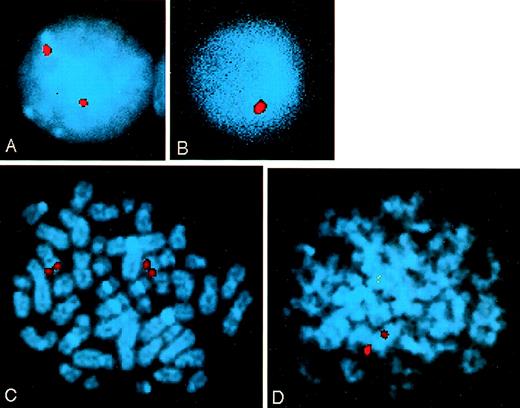
Conventional cytogenetics and FISH analysis was performed to determine whether the LOH observed correlated with cytogenetic abnormalities. G-banding of chromosomes from patients in group B showed no translocations involving MLL. However, sample B9, in which partial LOH of the mllGAAn polymorphism was observed, had an interstitial deletion of 11q, which is likely to involve the 11q23 region (Table 3). FISH analysis was performed on three samples (B22, B32, and B34,) in which LOH at mllGAAn had been observed. Between 89 and 104 interphase cells and 16 and 24 metaphase cells from each patient were analyzed for loss of MLL signal. Patient B22 had loss of signal in 28 of 101 (27.7%) interphase cells (Fig 4B) and 2 of 16 (12.5%) metaphase cells (Fig 4D). The percentage of cells with loss of an MLLallele may be an underrepresentation because there is often loss of leukemic cells during cell culture. No significant loss of signal was observed in samples B32 and B34.
Analysis of the MLL locus by FISH in patient B22. An MLL locus specific probe was hybridized to cells from bone marrow aspirates taken at presentation. Chromosomes were stained with DAPI. (A) Normal interphase cell with two MLL alleles. (B) Interphase cell with one MLL allele deleted. (C) Normal metaphase cell with two MLL alleles. (D) Metaphase cell with one MLL allele deleted.
Analysis of the MLL locus by FISH in patient B22. An MLL locus specific probe was hybridized to cells from bone marrow aspirates taken at presentation. Chromosomes were stained with DAPI. (A) Normal interphase cell with two MLL alleles. (B) Interphase cell with one MLL allele deleted. (C) Normal metaphase cell with two MLL alleles. (D) Metaphase cell with one MLL allele deleted.
Comparison of genotype and phenotype.
Four informative children in group B had acute myeloid leukemia, and of these 3 (75%) had LOH. In contrast, out of 20 informative children in group B with acute lymphoblastic leukemia, 6 (30%) had LOH. The level of MSI was not different in children with AML and ALL.
DISCUSSION
We have shown in this report that 45% of patients in a randomly selected group of children with leukemia exhibit LOH at a polymorphic marker within the MLL gene. The rate of LOH was highest in patients with AML (75%), and although the sample size was small (n = 4), this may indicate that LOH at MLL is a common event in the development of AML. LOH is common in some solid tumors but rarer in leukemia. LOH in childhood leukemia has been described at theTEL gene at chromosome 12p21, particularly when anAML/TEL fusion gene is present on the other allele30 and also at chromosome 9p21 where the p16 tumor suppressor gene is located.31,32 LOH at chromosome 6p has also been described in childhood ALL.33
These results indicate that loss of an allele at the MLL locus is a common event during the development of childhood leukemia. It is interesting that no LOH was observed in the infants with knownMLL translocations. A high frequency of LOH at a particular locus in tumor cells is often an indication of the presence of a tumor suppressor gene. The results presented here are consistent with the notion that MLL is a tumor suppressor gene, but further studies are required to prove this. No LOH was observed at the flanking markers D11S1364, which are estimated to be 3.5 and 11.5 centirays, respectively, from the MLL locus.34 Therefore, the LOH appears to be highly localized. Small interstitial deletions of exon 8 of the MLL gene have been reported in childhood AML and ALL,9 but these deletions are not thought to extend to intron 6 where this microsatellite is located. We were able to demonstrate in two patients that the LOH was due to a submicroscopic deletion by FISH. Unfortunately, due to lack of material, we were not able to confirm the presence of deletions in the other patients with LOH. In these samples it remains a possibility that the LOH observed at this single intronic microsatellite does not extend to the coding regions of the gene.
MSI at the mllGAAn polymorphism was observed in 13.9% of children in our study. This level of MSI is higher than has been described for most other loci in acute leukemias. In solid tumors, MSI is associated with errors in mismatch repair genes but it is not common in hematological malignancies.35 We have not ruled out that there is a mismatch repair defect in the patients described here, but this is unlikely because MSI was not detected for the four other microsatellite markers studied at 11q23 and the TEL locus (Table 3). Takeuchi et al36 investigated MSI in childhood ALL and found MSI in 10% of patients, located at “hotspots” on chromosomes 12p13 (intron 1 of the TEL gene), 9p21, and 6q22. They proposed that MSI is more common in regions that are also hotspots for rearrangements and deletions in hematological malignancies. The polymorphism described here is located in the breakpoint cluster region of the MLLgene, which is also regarded as a hotspot for rearrangement in hematological malignancies. However, rearrangements at the MLLlocus are commonly seen in secondary leukemias resulting from treatment with topoisomerase II (topo II) inhibitors.37 This observation, combined with the fact that the breakpoint cluster region of MLL contains topo II recognition sequences and SARs, has led to the theory that MLLtranslocations are related to abnormal activity of topo II.38-40 Therefore, it is unlikely that translocations, deletions, and LOH at the MLL locus are caused by the same mechanism which causes MSI, but there may be a shared factors such as chromatin organization or the alignment of DNA during mitotic recombination which makes the region a hotspot for instability.
The results of this study indicate that LOH at the MLL locus is a common event in the development of childhood leukemia. The MSI observed is indicative of genetic instability in this region.
ACKNOWLEDGMENT
We are grateful to E. Grace and M. McKinley for providing samples, and to Christine Harrison for providing cytogenetic data on those samples.
Supported by the Leukaemia Research Fund. I.G. was the recipient of a Royal Society/NATO Postdoctoral Fellowship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Julie C. Webb, PhD, Leukaemia Research Fund, Paul O’Gorman Centre for Childhood Leukaemia, Molecular Haematology Unit, Institute for Child Health, 30 Guilford St, London WC1N IEH, UK.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal