Retroviral vectors based on the Moloney murine leukemia virus (MoMuLV) are currently the most commonly used vehicles for stable gene transfer into mammalian hematopoietic cells. But, even with reasonable transduction efficiency, expression only occurs in a low percentage of transduced cells and decreases to undetectable levels over time. We have previously reported the modified MND LTR (myeloproliferative sarcoma virus enhancer,negative control region deleted, dl587rev primer-binding site substituted) to show increased expression frequency and decreased methylation in transduced murine embryonic stem cells and hematopoietic stem cells. We have now compared expression of the enhanced green fluorescent protein (eGFP) from a vector using the MoMuLV LTR (LeGFPSN) with that from the modified vector (MNDeGFPSN) in mature hematopoietic and lymphoid cells in the mouse bone marrow transplant (BMT) model. In primary BMT recipients, we observed a higher frequency of expression from the MND LTR (20% to 80%) in hematopoietic cells of all lineages in spleen, bone marrow, thymus, and blood compared with expression from the MoMuLV LTR (5% to 10%). Expression from the MND LTR reached 88% in thymic T lymphocytes and 54% in splenic B lymphocytes for up to 8 months after BMT. The mean fluorescence intensity of the individual cells, indicating the amount of protein synthesized, was 6- to 10-fold higher in cells expressing MNDeGFPSN compared with cells expressing LeGFPSN. Transduction efficiencies determined by DNA polymerase chain reaction of vector copy number were comparable for the 2 vectors. Therefore, the MND vector offers an improved vehicle for reliable gene expression in hematopoietic cells.
EFFECTIVE GENE TRANSFER and expression in pluripotent hematopoietic stem cells (HSC) could provide new therapies for inherited disorders of myeloid and lymphoid cells (such as hemoglobinopathies, storage disorders, and immune deficiencies), for infectious diseases (such as acquired immunodeficiency syndrome [AIDS]), and for acquired disorders (such as cardiovascular diseases).1,2 Efficient transduction, stable integration, and persistent expression of the relevant gene are prerequisites in gene therapy targeting HSC. The Moloney murine leukemia virus (MoMuLV), an endogenous murine retrovirus, is the most commonly used retroviral vector for gene transfer studies in vitro and in vivo. It has been widely used in clinical trials, for marking studies of repopulating stem cells or malignant cells, for replacement of the defective gene in patients with genetic disorders, and for insertion of anti-human immunodeficiency virus (HIV) genes into hematopoietic cells of HIV-infected patients.3
Cocultivation of murine bone marrow on vector-producing fibroblasts or transduction on recombinant fibronectin results in high transduction efficiency (70% to 100%) and stable integration into the genome of murine HSC and their progeny. However, expression of the desired gene driven by the MoMuLV LTR is often subject to silencing in vitro and in vivo.4-6 We have previously shown that a modified vector, MND (myeloproliferative sarcoma virus enhancer,negative control region deleted, dl587rev primer-binding site substituted), derived from the MoMuLV, shows a significantly higher frequency of expression than the standard MoMuLV vector in embryonic carcinoma (F9) and embryonic stem cells (CCE) in vitro7,8 and in murine HSC and their progeny in vivo.9 Although the analysis of secondary spleen foci (2° colony-forming units-spleen [CFU-S]) as performed by Robbins et al9 is a stringent assay for transduction of and expression in HSC and their progeny, expression in hematopoietic and lymphoid cells of primary transplant recipients is more relevant to clinical applications and therefore warrants detailed studies.
The enhanced green fluorescent protein (eGFP) gene has been shown previously to be useful as a reporter gene in hematopoietic cells in vitro and in vivo.10-13 Analysis of the expression of the eGFP reporter gene by fluorescence-activated cell sorting (FACS) allows assessment of expression in individual cells of each hematopoietic lineage from various tissues in vivo.
We therefore compared expression of eGFP in primary re- cipients of bone marrow, transduced with either the modified vector, MNDeGFPSN, or the MoMuLV-based vector, LeGFPSN. The time course and distribution of expression were monitored over 8 months to allow for hematopoietic cells derived from transduced, mature progenitors to be replaced by cells derived from transduced, primitive precursors and HSC. Secondary recipients were transplanted with marrow harvested from primary recipients 2 to 6 months after bone marrow transplantation (BMT) and 2°CFU-S were analyzed at 12 to 14 days after BMT. Semiquantitative polymerase chain reaction (PCR) was performed to assess vector copy number in cell populations. Our results show that, with equal vector copy numbers, the MND vector is expressed in a significantly higher percentage of hematopoietic and lymphoid cells than the MoMuLV vector.
MATERIALS AND METHODS
Retroviral vectors.
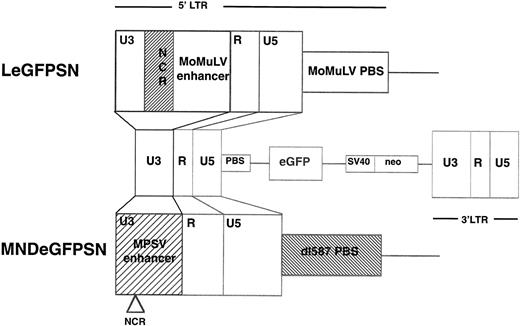
The LN vector, in which the MoMuLV LTR drives expression of the neomycin resistance gene, was constructed by A. Dusty Miller (Fred Hutchinson Cancer Center, Seattle, WA).14 The LeGFPSN vector was constructed by insertion of the BglII-Not I fragment containing the eGFP gene (Clontech Laboratories, Palo Alto, CA) into the Hpa I site in the polylinker of L-X-SN. The MNDeGFPSN vector was constructed as previously described by Robbins et al8(Fig 1).
Vector constructs. Vectors were constructed so that the LTR drives expression of the reporter gene for eGFP and the SV40 promoter drives expression of the gene for neomycin resistance. The 5′LTR of LeGFPSN is the MoMuLV LTR. The MND LTR is based on the MoMuLV LTR, but was modified by replacing the MoMuLV enhancer by the MPSV enhancer (M), deleting the negative control region (N) and replacing the primer binding site (PBS) of the MoMuLV by the PBS of the endogenous murine retrovirus dl587rev (D). Vectors are otherwise identical.
Vector constructs. Vectors were constructed so that the LTR drives expression of the reporter gene for eGFP and the SV40 promoter drives expression of the gene for neomycin resistance. The 5′LTR of LeGFPSN is the MoMuLV LTR. The MND LTR is based on the MoMuLV LTR, but was modified by replacing the MoMuLV enhancer by the MPSV enhancer (M), deleting the negative control region (N) and replacing the primer binding site (PBS) of the MoMuLV by the PBS of the endogenous murine retrovirus dl587rev (D). Vectors are otherwise identical.
Packaging of retroviral vectors.
The PA317 cell line, derived from NIH 3T3 fibroblasts, was obtained from the American Type Culture Collection (Rockville, MD).15 The GP+E-86 ecotropic packaging cells16 were a generous gift from Arthur Bank (Columbia University, New York, NY). LN was packaged in a high titer clone of PA317 packaging cells in A. Dusty Miller’s laboratory. The vectors LeGFPSN and MNDeGFPSN were packaged in the GP+E-86 packaging cells. High titer clones were generated for LN (1 × 106/mL), LeGFPSN (2 × 106/mL), and MNDeGFPSN (1 × 106/mL), as measured by G418 resistance on 3T3 fibroblasts.
BMT.
Bone marrow of C57BL/6 (Charles River Laboratories, Wilmington, MA) male donor mice was transplanted into lethally (10 Gy) irradiated female C57BL/6 recipients after transduction. Donor bone marrow cells were transduced by cocultivation on irradiated vector-producing fibroblasts in the presence of murine interleukin-3 (IL-3), human IL-6, and murine stem cell factor (SCF) as described by Robbins et al.8 Animals were injected intravenously with 3 to 5 × 106 nucleated cells transduced with either LN, LeGFPSN, or MNDeGFPSN retroviral vectors. Five separate BMTs were performed with each set, including all 3 vectors. Bone marrow of some primary recipients was used to reconstitute a second generation of lethally irradiated female recipients. Twelve to 14 days after the secondary transplants, 2°CFU-S were analyzed by PCR for the presence of the vector and by FACS for eGFP expression. All procedures involving animals were approved by the Animal Care Committee at Childrens Hospital Los Angeles (Los Angeles, CA).
Tissue processing.
Animals were killed at varying time points after BMT. Immediately after lethal CO2 inhalation, spleen, thymus, bone marrow, and blood were harvested on ice. Single-cell suspensions were prepared for FACS analysis and DNA preparation.
DNA analysis.
A semiquantitative PCR assay was established to measure the vector copy number in samples of murine tissue. We used a GP+E-86 clone, containing 5 copies of the MNDeGFPSN vector, to generate a standard curve. DNA of this clone was diluted with DNA of nontransduced GP+E-86 cells, such that the template input was maintained at 40 ng per reaction. The standard curve was based on samples diluted to represent 5, 3.75, 2.5, 1.25, 0.625, and 0 copies/cell. Semiquantitative PCR was performed with primers to eGFP (forward primer, 5′-TAC GGC AAG CTG ACC CTG AAG TTC-3′; reverse primer, 5′-CGT CCT TGA AGA AGA TGG TGC G-3′), yielding a product of 193 bp. Reactions were performed in a 50 μL reaction, with PCR buffer (10× buffer II; Perkin Elmer-Cetus, Norwalk, CT), 1.5 mmol/L MgCl2, primers at 1 μmol/L, and dNTP at 0.2 nmol/L using the Perkin Elmer Gene Amp PCR System 9600. The reaction was conducted for 20 cycles with denaturation at 94°C for 30 seconds, annealing at 58°C for 1 minute, and extension at 72°C for 1 minute. In parallel, an aliquot of each DNA sample was subjected to PCR with primers to β-actin to control for DNA content. β-Actin primers were previously described by Tanaka et al.17 PCR was conducted at the same conditions as used for eGFP amplification. The β-actin primers yield a PCR product of 226 bp. Reaction products were electrophoresed and transferred to nylon membranes. Southern blots were performed with a probe labeled with32P by random priming (Stratagene, La Jolla, CA) specific for eGFP or β-actin accordingly, generated with the above-mentioned primers. Kodak x-ray film (Eastman Kodak, Rochester, NY) was exposed to blots for 2 to 5 hours at room temperature, and the band intensity was quantitated by densitometry using a SciScan 5000 (US Biochemical, Cleveland, OH).
FACS analysis.
Nonspecific antibody binding was blocked with 10 μL/1 million cells of murine γ-globulin (IgG; 1 mg/mL; Sigma, St Louis, MO) and incubated for 30 minutes at 4°C. For analysis of lineage-specific eGFP expression, cells were stained with phycoerythrin (PE)-conjugated antibodies against murine CD4, CD8, B220, Mac-1, Gr-1, and Ter-119 (Pharmingen, San Diego, CA). Excess antibody was removed by washing twice, once with red cell lysis buffer (Ortho-mune TM; Ortho Diagnostic Systems Inc, Raritan, NJ) and once with Dulbecco’s phosphate-buffered saline (DPBS; Bio Whittaker, Walkersville, MD). Detection of eGFP expression and PE staining was accomplished on a FACScan cytometer equipped with a 488 nm argon laser for excitation and 530/30 nm (eGFP) and 575/28 nm (PE) bandpass filters for monitoring the fluorescent emissions.
Statistical analysis.
Statistical analysis was performed to compare frequency of expression, vector copy numbers, and mean fluorescence intensities between MNDeGFPSN and LeGFPSN. Two-sided tests were performed at α = .05 level of significance. The t-test was used when normality and equal variances were present. The Aspin-Welch Test was used when normality of the data was fulfilled, but equal variances were rejected. Either the Mann-Whitney U or the Wilcoxon Rank-Sum Test for Difference in Medians was applied when normality and equal variances were rejected, depending on the presence of ties.
RESULTS
Gene transfer/BMT.
Bone marrow from male C57BL/6 mice was collected, transduced with either LeGFPSN, MNDeGFPSN, or LN, and transplanted into irradiated female C57BL/6 recipient mice. At specific times after gene transfer/BMT, expression of eGFP was assessed by FACS analysis in cells of the myeloid, erythroid, and lymphoid lineages from the bone marrow, spleen, thymus, and blood of recipients. Tissues of recipients transplanted with bone marrow transduced by the LN vector, which does not contain eGFP, were used as negative controls. At each time-point, a set of mice transplanted with cells transduced by each of the 3 vectors was analyzed simultaneously. A total of 35 sets of mice were studied. Cells were stained with PE-conjugated antibodies against specific surface antigens on granulocytes (Gr-1), monocytes/macrophages (Mac-1), T lymphocytes (CD4 and CD8), B lymphocytes (B220), and the erythroid lineage (Ter-119).
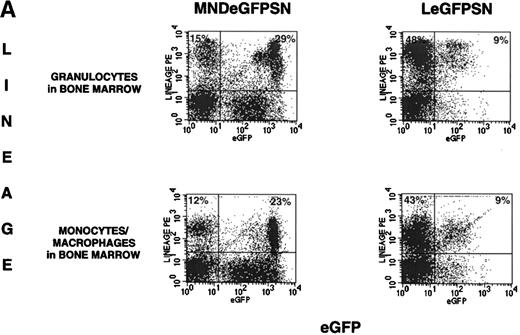
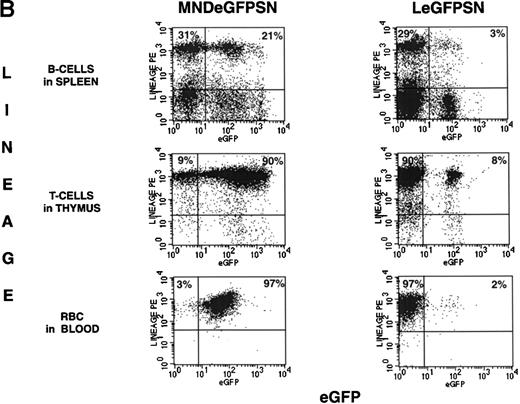
Representative FACS dot blots and histogram plots of a set of mice analyzed 4.5 months after BMT are shown in Fig 2A and B. These dot blots show a higher percentage of cells expressing eGFP with the MNDeGFPSN vector than with the LeGFPSN vector.
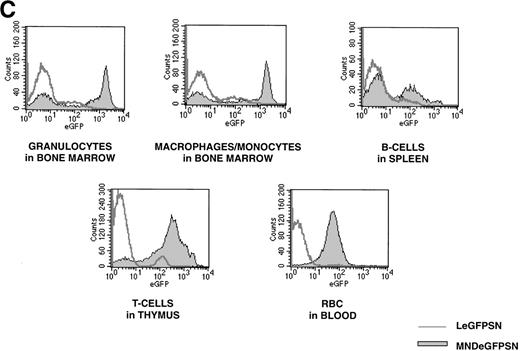
FACS analysis of eGFP expression. FACS dot blots and histogram blots from a representative pair of mice are shown. (A and B) Cells from bone marrow, spleen, thymus, and blood from recipients of LeGFPSN-transduced, MNDeGFPSN-transduced, and LN-transduced (data not shown) marrow were harvested and analyzed by FACS for eGFP expression (x axis) in granulocytes (Gr-1), monocytes/macrophages (MAC-1), B cells (B220), T cells (CD4, CD8), and RBC (Ter-119). Lineage-specific staining is shown on the y-axis. Quadrant statistics give the percentage of gated events. (C) Distribution of fluorescence intensity is shown in histogram plots for LeGFPSN (dark line) and MNDeGFPSN (solid area).
FACS analysis of eGFP expression. FACS dot blots and histogram blots from a representative pair of mice are shown. (A and B) Cells from bone marrow, spleen, thymus, and blood from recipients of LeGFPSN-transduced, MNDeGFPSN-transduced, and LN-transduced (data not shown) marrow were harvested and analyzed by FACS for eGFP expression (x axis) in granulocytes (Gr-1), monocytes/macrophages (MAC-1), B cells (B220), T cells (CD4, CD8), and RBC (Ter-119). Lineage-specific staining is shown on the y-axis. Quadrant statistics give the percentage of gated events. (C) Distribution of fluorescence intensity is shown in histogram plots for LeGFPSN (dark line) and MNDeGFPSN (solid area).
eGFP as a reporter also allows measurement of the level of expression as the fluorescence intensity of expressing cells. Mean fluorescence intensities were analyzed by gating only on the eGFP expressing cell populations to avoid skewing of the data by differences in the absolute numbers of expressing and nonexpressing cells.
In addition to the higher number of eGFP(+) cells, the level of expression was on average 6- to 10-fold higher in eGFP(+) cells of recipients of MNDeGFPSN-transduced marrow than in eGFP(+) cells of recipients of LeGFPSN-transduced marrow, as shown in representative histograms in Fig 2C and in Table 1.
Mean Fluorescence Intensity of eGFP-Expressing Cells
| Tissue/Vector . | Mean Fluorescence Intensity . | ||
|---|---|---|---|
| MNDeGFPSN . | LeGFPSN . | P Value . | |
| Bone marrow (n = 18) | 1,357.2 ± 184.2* | 152.4 ± 55.1* | .000013† |
| Spleen (n = 18) | 403.5 (147,1190)‡ | 72 (24,330)‡ | .000211-153 |
| Thymus (n = 17) | 1,109.3 ± 217* | 165.9 ± 31.4* | .00097† |
| Blood (n = 18) | 852.9 ± 106.3* | 102.5 ± 19.1* | .000001† |
| Tissue/Vector . | Mean Fluorescence Intensity . | ||
|---|---|---|---|
| MNDeGFPSN . | LeGFPSN . | P Value . | |
| Bone marrow (n = 18) | 1,357.2 ± 184.2* | 152.4 ± 55.1* | .000013† |
| Spleen (n = 18) | 403.5 (147,1190)‡ | 72 (24,330)‡ | .000211-153 |
| Thymus (n = 17) | 1,109.3 ± 217* | 165.9 ± 31.4* | .00097† |
| Blood (n = 18) | 852.9 ± 106.3* | 102.5 ± 19.1* | .000001† |
Mean ± SEM.
Student’s t-test.
Median (range).
Wilcoxon signed-rank test for difference in medians.
Copy number/cell by semiquantitative PCR.
The higher frequency and level of eGFP expression from MNDeGFPSN could be due to a higher gene transfer by MNDeGFPSN than by LeGFPSN, leading to differences in copy number/cell, rather than reflecting differences in LTR-driven expression. To assess the copy number/cell, semiquantitative PCR was performed on DNA samples derived from bone marrow, spleen, blood, and thymus or from cells of specific lineages sorted by FACS. A standard curve was generated based on a murine fibroblast vector-producer clone containing 5 copies/cell of an eGFP-containing vector. PCR with primers specific for murine β-actin served as an internal standard to control for DNA loading. DNA of tissues from recipients transplanted with the control vector LN were used as negative controls.
Results from all samples analyzed are shown in Table 2. The data on the percentages of eGFP-expressing cells in the table were derived only from those animals for which the vector copy number/cell was assessed. Differences in the percentages of expressing cells were significantly higher for MNDeGFPSN compared with LeGFPSN for this subset, whereas the copy numbers per cell were not statistically different between MNDeGFPSN and LeGFPSN for all tissues and cell lineages analyzed. Overall, the percentage of eGFP-expressing cells when normalized for a single vector copy/cell was 3.8 to 7.1 times higher for MNDeGFPSN than for LeGFPSN.
eGFP Expression and Vector Copy Number Per Cell
| . | Bone Marrow . | Spleen . | Thymus . | Blood . | Granulocytes in Bone Marrow . | B Cells in Spleen . | T Cells in Thymus . |
|---|---|---|---|---|---|---|---|
| LEGFPSN | |||||||
| % eGFP(+) cells* (n) | 5.5 ± 2.4 (14) | 4.7 ± 3.9 (15) | 2.7 ± 2.4 (13) | 3.6 ± 1.4 (8) | 7.2 ± 3.9 (7) | 4.1 ± 0.9 (8) | 8.6 ± 3.4 (7) |
| Vector Copy#/cell† (n) | 1.4 ± 0.6 (14) | 2 ± 0.7 (15) | 0.7 ± 0.4 (13) | 1.04 ± 0.9 (8) | 3.9 ± 2.3 (7) | 4.7 ± 1.5 (8) | 0.9 ± 0.4 (7) |
| MNDeGFPSN | |||||||
| % eGFP(+) cells* (n) | 26.3 ± 6.2 (17) | 30.6 ± 4.9 (17) | 29.8 ± 8.8 (15) | 31.6 ± 9.1 (9) | 28.9 ± 9.1 (8) | 35.2 ± 8.3 (6) | 31.9 ± 10.2 (12) |
| Vector Copy#/cell† (n) | 1.5 ± 0.5 (17) | 1.8 ± 0.7 (17) | 2 ± 0.7 (15) | 1.5 ± 0.8 (9) | 2.3 ± 0.9 (8) | 1.7 ± 0.8 (6) | 1.4 ± 0.6 (12) |
| . | Bone Marrow . | Spleen . | Thymus . | Blood . | Granulocytes in Bone Marrow . | B Cells in Spleen . | T Cells in Thymus . |
|---|---|---|---|---|---|---|---|
| LEGFPSN | |||||||
| % eGFP(+) cells* (n) | 5.5 ± 2.4 (14) | 4.7 ± 3.9 (15) | 2.7 ± 2.4 (13) | 3.6 ± 1.4 (8) | 7.2 ± 3.9 (7) | 4.1 ± 0.9 (8) | 8.6 ± 3.4 (7) |
| Vector Copy#/cell† (n) | 1.4 ± 0.6 (14) | 2 ± 0.7 (15) | 0.7 ± 0.4 (13) | 1.04 ± 0.9 (8) | 3.9 ± 2.3 (7) | 4.7 ± 1.5 (8) | 0.9 ± 0.4 (7) |
| MNDeGFPSN | |||||||
| % eGFP(+) cells* (n) | 26.3 ± 6.2 (17) | 30.6 ± 4.9 (17) | 29.8 ± 8.8 (15) | 31.6 ± 9.1 (9) | 28.9 ± 9.1 (8) | 35.2 ± 8.3 (6) | 31.9 ± 10.2 (12) |
| Vector Copy#/cell† (n) | 1.5 ± 0.5 (17) | 1.8 ± 0.7 (17) | 2 ± 0.7 (15) | 1.5 ± 0.8 (9) | 2.3 ± 0.9 (8) | 1.7 ± 0.8 (6) | 1.4 ± 0.6 (12) |
Abbreviation: (n), number of animals analyzed.
Percentage of eGFP(+) cells of tissues subjected to copy number/cell analysis.
Vector copy numbers between LeGFPSN and MNDeGFPSN are not statistically significantly different.
eGFP expression in tissues.
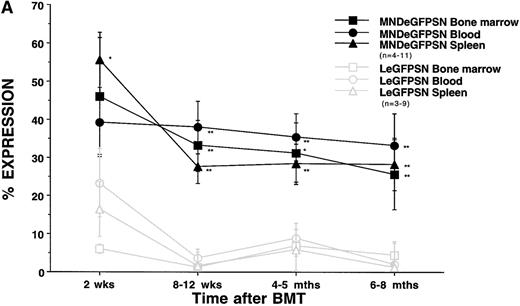
Mice were killed and tissues were analyzed by FACS for eGFP expression at 2 weeks, 8 weeks, 4 to 5 months, and 6 to 8 months after BMT. Two weeks after BMT, eGFP expression was detectable in 40% to 55% of cells from bone marrow, spleen, and blood from recipients of MNDeGFPSN-transduced marrow and in 5% to 25% of cells from recipients of LeGFPSN-transduced marrow (P = not significant; Fig 3A). Six to 8 months after transplant, expression levels were 25% to 35% of cells from recipients of MNDeGFPSN-transduced marrow, but were less than 5% in cells from recipients of LeGFPSN-transduced marrow (P < .05).
eGFP expression in tissues. eGFP expression was assessed in bone marrow, spleen, blood (A), and thymus (B) from recipients of LeGFPSN-transduced, MNDeGFPSN-transduced, and LN-transduced (data not shown) bone marrow 2 weeks, 8 to 12 weeks, 4 to 5 months, and 6 to 8 months after BMT. Values are given as the mean ± standard error of the mean. n is the number of animals analyzed for each time point and vector. * and ** mark differences in the percentage of expression between MNDeGFPSN and LeGFPSN that are statistically significant (*P < .05; **P < .005).
eGFP expression in tissues. eGFP expression was assessed in bone marrow, spleen, blood (A), and thymus (B) from recipients of LeGFPSN-transduced, MNDeGFPSN-transduced, and LN-transduced (data not shown) bone marrow 2 weeks, 8 to 12 weeks, 4 to 5 months, and 6 to 8 months after BMT. Values are given as the mean ± standard error of the mean. n is the number of animals analyzed for each time point and vector. * and ** mark differences in the percentage of expression between MNDeGFPSN and LeGFPSN that are statistically significant (*P < .05; **P < .005).
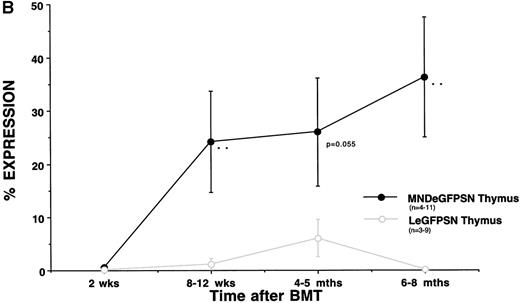
In contrast to the relatively stable percentage of cells showing eGFP expression in bone marrow, spleen, and blood from recipients of MNDeGPFSN-transduced marrow, there was an increase of expression over time in thymus (Fig 3B). Two weeks after BMT, eGFP expression occurred in 0.6% ± 0.4% of cells from the thymus of recipients of MNDeGFPSN-transduced marrow. Six to 8 months after transplant, frequency of expression reached 36.3% ± 11.3%. In recipients of LeGFPSN-transduced marrow, expression remained less than 10% of cells throughout the time course and was 0.2% ± 0.1% 6 to 8 months after BMT.
eGFP expression in cells of specific lineages.
Expression in cells of specific lineages was assessed for granulocytes, monocytes/macrophages, B lymphocytes, and T lymphocytes from bone marrow, spleen, and blood; for T lymphocytes from thymus; and for red blood cells (RBC) from blood. The percentage of expression by cells of a specific lineage was calculated by dividing the number of expressing, lineage-positive cells (RUQ in Fig 2A and B) by the total number of lineage-positive cells (LUQ + RUQ in Fig 2A and B). Cells from mice transplanted with LN-transduced marrow were analyzed in parallel as negative controls for eGFP expression.
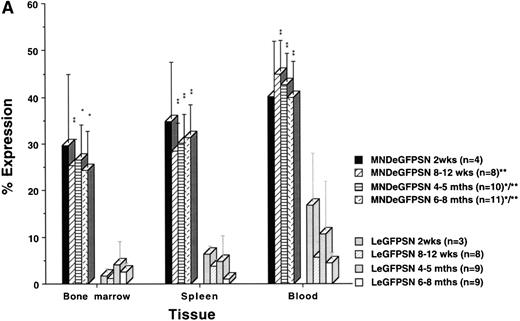
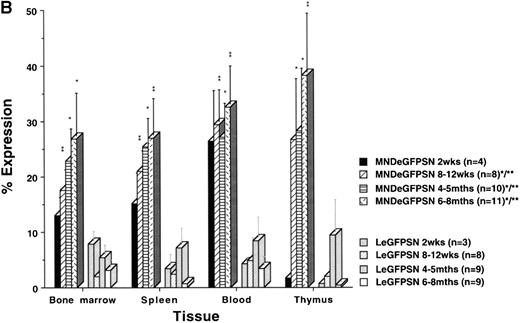
The percentage of eGFP expression in B lymphocytes from recipients of marrow transduced by MNDeGFPSN remained high (25% to 40%) from 2 weeks to 6 to 8 months after BMT, whereas it was less than 5% in recipients of marrow transduced by LeGFPSN by 6 to 8 months after BMT (Fig 4A). Overall, expression occurred in a 2- to 30-fold higher cell number under the control of the modified MND LTR than under the control of the MoMuLV LTR (P < .05). The time course and distribution of eGFP expression in monocytes, macrophages, and granulocytes and RBC were similar to the eGFP expression in B cells (data not shown).
eGFP expression in lineages. eGFP expression is shown for B lymphocytes (A) and T lymphocytes (B) in bone marrow, spleen, blood, and thymus from recipients of MNDeGFPSN-transduced and LeGFPSN-transduced (data for negative control LN not shown) bone marrow. Animals were killed and analyzed 2 weeks, 8 to 12 weeks, 4 to 5 months, and 6 to 8 months after BMT. Values for the percentage of cells showing expression were calculated by dividing the number of expressing lin+ cells by the total number of lin+ cells and are given as the mean ± standard error of the mean. n is the number of animals analyzed for each time point and vector. * and ** mark differences in the percentage of cells showing expression between MNDeGFPSN and LeGFPSN that are statistically significant (*P < .05 and **P < .005).
eGFP expression in lineages. eGFP expression is shown for B lymphocytes (A) and T lymphocytes (B) in bone marrow, spleen, blood, and thymus from recipients of MNDeGFPSN-transduced and LeGFPSN-transduced (data for negative control LN not shown) bone marrow. Animals were killed and analyzed 2 weeks, 8 to 12 weeks, 4 to 5 months, and 6 to 8 months after BMT. Values for the percentage of cells showing expression were calculated by dividing the number of expressing lin+ cells by the total number of lin+ cells and are given as the mean ± standard error of the mean. n is the number of animals analyzed for each time point and vector. * and ** mark differences in the percentage of cells showing expression between MNDeGFPSN and LeGFPSN that are statistically significant (*P < .05 and **P < .005).
As we saw for the analysis of the thymus (Fig 3B), the percentage of eGFP expressing T lymphocytes increased in bone marrow, spleen, blood, and thymus over time in recipients of MNDeGFPSN-transduced bone marrow (Fig 4B). In recipients of LeGFPSN-transduced marrow, expression levels increased only slightly up to 4 to 4.5 months after BMT, but decreased to less than 5% by 6 to 8 months after BMT. The increase in the percentage of eGFP(+) T lymphocytes from recipients of MNDeGFPSN-transduced marrow was most marked in thymus. Less than 5% of thymic T lymphocytes expressed eGFP 2 weeks after BMT with either vector. The percentage of eGFP expressing thymic T lymphocytes from recipients of MNDeGFPSN-transduced bone marrow increased to 24% ± 9.5% after 8 weeks, 26% ± 10.2% after 4 to 5 months, and 36.3% ± 11.3% after 6 to 8 months. At all time points, when under the control of the MoMuLV LTR, eGFP expression in thymic T lymphocytes reached 50% in only 1 of 29 mice and remained less than 5% in 24 of 29 mice. When under the control of the MND LTR, 8 of 33 mice showed expression in more than 50% of thymic T lymphocytes (5 of 33 in >85%) and only 12 of 33 mice showed levels less than 5% of thymic T lymphocytes (data not shown).
Secondary BMT.
To compare expression by the 2 vectors in cells derived from primitive HSC, we collected bone marrow from 5 randomly chosen pairs of mice transplanted with transduced bone marrow and transplanted cells into secondary recipients to generate 2°CFU-S. One primary pair of mice transduced with MNDeGFPSN and LeGFPSN was killed at 8 weeks and another at 4 months after transplant and 3 pairs were killed at 6 months after transplant. The bone marrow from each of the primary recipients was transplanted into 2 to 4 irradiated secondary recipients. Twelve to 14 days after the secondary transplant, 3 to 5 of the 2°CFU-S–derived colonies were harvested from the spleen of each secondary recipient and analyzed for the presence of the vector DNA by PCR for the eGFP gene and by FACS for eGFP expression. Recipients of LN-transduced marrow served as negative controls for PCR and FACS analysis.
DNA PCR analysis of the 2°CFU-S showed a transduction efficiency of 100% (54 of 54 foci) for MNDeGFPSN and 82% (46 of 56 foci) for LeGFPSN. Expression of eGFP was 100% (54 of 54 foci) in 2°CFU-S containing the MNDeGFPSN vector and 0% (0 of 46 foci) in 2°CFU-S containing the LeGFPSN vector (Table 3).
Vector Expression in 2°CFU-S
| Primary Recipient No.3-150 . | MNDeGFPSN . | LeGFPSN . | ||||
|---|---|---|---|---|---|---|
| Expression in 1° BM . | Vector3-151 (n) . | Expression3-152 . | Expression in 1° BM . | Vector3-151 (n) . | Expression3-152 . | |
| 1 | 60% | 13/13 | 13/13 | 0.4% | 13/15 | 0/13 |
| (8 wks) | (3) | (4) | ||||
| 2 | 78.5% | 6/6 | 6/6 | ND | ND | ND |
| (6 mos) | (2) | |||||
| 3 | 65.1% | 8/8 | 8/8 | 8.4% | 8/12 | 0/8 |
| (4 mos) | (3) | (3) | ||||
| 4 | 84.1% | 11/11 | 11/11 | 4.4% | 12/16 | 0/12 |
| (6 mos) | (4) | (4) | ||||
| 5 | 40.5% | 16/16 | 16/16 | 31% | 13/13 | 0/13 |
| (6 mos) | (4) | (4) | ||||
| 1-5 | 54/54 | 54/54 | 46/56 | 0/46 | ||
| 100% | 100% | 82% | 0% | |||
| Primary Recipient No.3-150 . | MNDeGFPSN . | LeGFPSN . | ||||
|---|---|---|---|---|---|---|
| Expression in 1° BM . | Vector3-151 (n) . | Expression3-152 . | Expression in 1° BM . | Vector3-151 (n) . | Expression3-152 . | |
| 1 | 60% | 13/13 | 13/13 | 0.4% | 13/15 | 0/13 |
| (8 wks) | (3) | (4) | ||||
| 2 | 78.5% | 6/6 | 6/6 | ND | ND | ND |
| (6 mos) | (2) | |||||
| 3 | 65.1% | 8/8 | 8/8 | 8.4% | 8/12 | 0/8 |
| (4 mos) | (3) | (3) | ||||
| 4 | 84.1% | 11/11 | 11/11 | 4.4% | 12/16 | 0/12 |
| (6 mos) | (4) | (4) | ||||
| 5 | 40.5% | 16/16 | 16/16 | 31% | 13/13 | 0/13 |
| (6 mos) | (4) | (4) | ||||
| 1-5 | 54/54 | 54/54 | 46/56 | 0/46 | ||
| 100% | 100% | 82% | 0% | |||
Abbreviation: (n), number of secondary transplant recipients; ND, not done.
Time after 1° transplant when 2° transplant was performed.
Number of CFU-S PCR (+) for vector DNA/number of CFU-S tested.
Number of CFU-S (+) for eGFP expression by FACS/number of CFU-S tested.
DISCUSSION
In this study, we compared the in vivo expression of the reporter gene eGFP from a modified vector, MNDeGFPSN, and from the MoMuLV vector, LeGFPSN, in murine hematopoietic and lymphoid cells. The data show a significantly higher expression in recipients of MNDeGFPSN-transduced marrow than in recipients of LeGFPSN-transduced marrow 8 weeks to 8 months after BMT. The frequency of expression on average was 6-fold higher in recipients of MNDeGFPSN-transduced marrow compared with recipients of LeGFPSN-transduced marrow. Mean fluorescence intensity, as a measure of the amount of synthesized protein per cell, was on average 6- to 10-fold higher in MNDeGFPSN-transduced eGFP(+) cells than in LeGFPSN-transduced eGFP(+) cells.
The higher frequency of eGFP expression and the higher mean fluorescence intensity for MNDeGFPSN-transduced cells could be related to a higher vector copy number per cell. A higher copy number could result in an increased probability of an expression-favoring insertion site of the vector and therefore to expression of the reporter gene. It could also lead to a greater intensity of fluorescence and a higher number of expressing cells through a summation effect of the reporter gene transcripts from all the vector copies in the cell. Therefore, we performed semiquantitative PCR analysis to assess average vector copy number/cell for the 2 vectors on tissues and sorted cell lineages. The results showed that the copy numbers were not statistically significantly different between the 2 vectors, ranging from 1 to 4 copies/cell on average, consistent with the equivalent titers of the vector-producing cell clones. Therefore, differences in expression were not due to a difference in copy number but were likely due to a higher probability of expression from the MND LTR than from the standard MoMuLV LTR. We have previously reported that embryonic carcinoma and embryonic stem cells expressed at a higher frequency from the MND LTR (>95%) than from the MoMuLV LTR (<5%).8
Long-term expression.
Two weeks after BMT, the frequency of expression was not significantly different between the 2 vectors. Both vectors initially expressed in a high percentage of hematopoietic cells and B lymphocytes in blood, spleen, and bone marrow. Over time, the percentage of cells expressing from the standard MoMuLV LTR decreased to a greater extent than from the modified MND LTR. The decrease in the number of expressing cells could be due to repression of expression (silencing) in mature hematopoietic cells that initially expressed the reporter gene. Alternatively, it could be due to the turnover of mature cells, resulting in later cell populations being derived from more primitive precursors, which either never expressed the reporter gene or in which it underwent silencing. These processes seem to be significantly less likely to occur in the modified vector, MNDeGPFSN, than in the MoMuLV-derived vector, LeGFPSN.
Distinct time courses of eGFP expression for different lineages.
In recipients of MNDeGFPSN-transduced marrow, the frequency of eGFP expression was relatively stable in cells of the myeloid lineage, B lymphocytes, and RBC. In contrast, in T lymphocytes there was a marked increase in eGFP expression over time. The increase in expression frequency was evident in all tissues, but was most prominent in thymus and least prominent in blood.
Most likely, in the lethally irradiated mice, T lymphocytes were initially derived from transduced donor primary T lymphocytes and mature lymphoid progenitors, resulting in high numbers of expressing T lymphocytes in peripheral blood 2 weeks after BMT. Over time, T lymphocytes were generated from transduced HSC and early lymphoid precursors by repopulation of the thymus and extrathymic T-cell development. Observed differences in expression over time in the myeloid and B-lymphoid lineages compared with T-lymphoid cells could result from a distinct pattern of regulation of peripheral hematopoietic and thymic engraftment.18
2°CFU-S.
Serial transplantation of transduced murine bone marrow is a stringent in vivo assay to test vectors targeting HSC. After reconstitution of a primary recipient for at least 2 months, cells that give rise to colonies forming in the spleens of secondary BMT recipients (2°CFU-S) are derived from cells that were HSC at the time of transduction.19 We have shown previously that the modifications contained in the MND LTR led to increased expression of a neomycin reporter gene in 2°CFU-S compared with the MoMuLV LTR.9 In this study, we observed eGFP expression in all of the MNDeGFPSN-transduced 2°CFU-S and none of the LeGFPSN-transduced 2°CFU-S, confirming that the higher expression frequency in primary recipients of MNDeGFPSN-transduced marrow extends to HSC.
Modification of vector sequences has previously been shown to improve transduction efficiency and expression in in vitro and in vivo systems targeting HSC and other primitive cells due to their wide clinical application and the difficulties to reach efficient transduction and expression in this entity. Riviere et al20 showed improvement of expression of the human ADA gene from MFG-derived vectors that included several modifications, such as replacement of the MoMuLV enhancer or LTR by the myeloproliferative sarcoma virus (MPSV) or Friend murine leukemia virus enhancer or LTR, respectively, or introduction of the B2 mutation in the viral tRNA primer binding site. Eckert et al21 showed improved expression of the multidrug resistance gene (mdr1) in 2 vectors based on the spleen focus-forming virus or the myeloproliferative sarcoma virus for the enhancer and the murine embryonic stem cell virus for the leader.
Pawliuk et al22 compared expression in a murine transplant model from 2 vectors, 1 containing the MPSV LTR and the other containing the PCC4 cell-passaged myeloproliferative sarcoma virus (PCMV) LTR and a substitution of the primer binding site to abrogate binding of the repressor binding protein (murine stem cell virus [MSCV]). In their BMT model, only FACS-sorted, vector-expressing bone marrow cells were transplanted. By this method, MSCV led to a high average percentage of expressing donor-derived cells in bone marrow (62%) and peripheral blood lymphocytes (28%) 6 months after BMT. In the thymus, expression was 15%, despite the presence of 87% donor-derived cells.22
In our study, whole bone marrow was transplanted without preselection for reporter gene expression. With the MND LTR, we saw similar values for expression in BM and peripheral blood, but an average expression frequency in the thymus of 38%.
In conclusion, these studies suggest that the modified vector, MND, provides an advantage to the MoMuLV LTR for the expression of genes in hematopoietic and lymphoid cells and especially in T lymphocytes. It remains to be shown whether the MND LTR provides an advantage in human cells, although preliminary studies suggest that it does.23Studies in primary human T lymphocytes in vitro as well as in immune deficient mice transplanted with CD34+ cells from human cord blood are in progress.
ACKNOWLEDGMENT
The authors thank Earl H. Leonard for help with the statistical analysis and Denise A. Carbonaro for reviewing the manuscript.
Supported by Grant No. CA59318 from the National Cancer Institute of the National Institutes of Health. D.B.K. is the recipient of a Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Donald B. Kohn, MD, Mailstop #62, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail:dkohn@chla.usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal