Abstract
Chemokines play a central role for lymphocyte trafficking and homing. The mechanisms that direct the tissue localization of B cells from patients with chronic lymphocytic leukemia (B-CLL) are unknown. We found that CLL B cells express functional CXCR4 receptors for the chemokine stromal cell-derived factor-1 (SDF-1), as demonstrated by receptor endocytosis, calcium mobilization, and actin polymerization assays. Moreover, CLL B cells displayed chemotaxis to this chemokine that could be inhibited by monoclonal antibodies (MoAbs) against CXCR4, pertussis toxin, or Wortmannin, a phosphatidylinositol 3-kinase inhibitor. That this chemotaxis may be involved in the homing of CLL cells is argued by studies in which CLL B cells were cocultured with a murine marrow stromal cell line that secretes SDF-1. Within 2 hours, CLL B cells spontaneously migrated beneath such stromal cells in vitro (pseudoemperipolesis). This migration could be inhibited by pretreatment of CLL B cells with anti-CXCR4 MoAbs, SDF-1, or pertussis-toxin. Furthermore, we noted strong downmodulation of CXCR4 on CLL B cells that migrated into the stromal cell layer. These findings demonstrate that the chemokine receptor CXCR4 on CLL B cells plays a critical role for heterotypic adherence to marrow stromal cells and provide a new mechanism to account for the marrow infiltration by neoplastic B cells.
B-CHRONIC LYMPHOCYTIC leukemia (B-CLL) represents the most common type of adult leukemia in western societies and is characterized by the relentless accumulation of anergic, self-reactive, mature CD5+ B cells in the blood, secondary lymphoid tissues, and the marrow.1 The marrow invariably is infiltrated with leukemia cells. Furthermore, the extent of marrow infiltration correlates with clinical stage and prognosis.2,3 Some studies suggest that CLL B cells can respond to regulatory signals in the marrow microenvironment. In particular, close contact with marrow stromal cells can provide factors favorable for the accumulation and survival of CLL B cells.4 5 At this time, it is not known whether CLL cells originate or home to the marrow.
The trafficking and homing of normal lymphocytes between the blood and lymphoid tissues is a multistep process that requires the sequential engagement of adhesion molecules and the activation through chemokine receptors.6 7 These steps are thought to be critical for extravasation and homing to distinct lymphoid-tissue microenvironments that provide supportive growth and regulatory factors.
Stromal cell–derived factor-1 (SDF-1) is a CXC chemokine that is constitutively expressed at high levels by bone marrow stromal cells.8-10 It exists in 2 forms derived from alternative RNA splicing, SDF-1α or SDF-1β. The SDF-1 gene was highly conserved during evolution, and there exists only 1 amino acid difference between murine and human SDF-1, allowing for the action of this chemokine across species.8,11 SDF-1 signals through a G protein–coupled receptor termed CXCR4.12,13 In lymphocytes, SDF-1 triggers rapid integrin-dependent arrest under physiological flow conditions, indicating that SDF-1 can induce lymphocyte recruitment in vivo.14
There is substantial evidence from in vitro and in vivo experi- ments that SDF-1 plays an important role in B-cell development and trafficking. Mice lacking the gene encoding SDF-1 or CXCR4 have severely reduced B lymphopoiesis, but normal T lymphopoiesis.15-18 In vitro, SDF-1 is chemotactic for pro- and pre-B cells.19 These results suggest that SDF-1 may direct progenitor B cells into the appropriate bone marrow microenvironments, where regulatory factors are released.20This interpretation is supported by the recent finding that the marrow of CXCR4-deficient mice contains reduced numbers of pro-B and pre-B cells, whereas abnormally high numbers are found in the blood due to a premature release from the marrow.21
CLL B cells can be described as “incompetent” resting B cells. More than 99% are in the G0 phase of the cell cycle, they respond poorly to mitogenic signals, and are inefficient antigen-presenting cells. Therefore, mechanisms that control the trafficking of normal B cells may not be functional in CLL B cells, and it has not yet been established that CLL B cells can migrate.
Several studies, however, described the expression of adhesion molecules and soluble factors related to the trafficking of lymphocytes on CLL B cells or in the serum of CLL patients and related expression pattern to disease subsets and prognosis.22-24 Because of the importance of SDF-1 for B-lymphocyte development and trafficking, we examined for expression and function of the chemokine receptor CXCR4 on CLL B cells.
MATERIALS AND METHODS
Chemokine, antibodies, flow cytometry.
Synthetic, human SDF-1α (1-67) was provided by Dr I. Clark-Lewis (University of British Columbia, Vancouver, Canada) and purchased from Upstate Biotechnology (Lake Placid, NY). The following monoclonal antibodies (MoAbs) specific for human surface antigens were used: anti-CXCR4–phycoerythrin (PE) (12G5), anti-CD3–fluorescein isothiocyanate (FITC), anti-CD19–allophycocyanine (APC), anti-CD49d, anti-CD49e, anti-CD54, and the appropriate isotype controls from Pharmingen (San Diego, CA). The National Institutes of Health (NIH) AIDS research and reference reagent program (Rockville, MD) provided the nonconjugated CXCR4 MoAb 12G5 and the CCR3-specific MoAb 7B11. For flow cytometry, the cells were adjusted to a concentration of 5 × 106 cells/mL in RPMI 1640 with 0.5% bovine serum albumin (BSA). A total of 5 × 105 cells were stained with saturating antibody concentrations for 30 minutes at 4°C, washed 2 times, and then analyzed on a FACSCalibur (Becton Dickinson, Mountain View, CA). Flow cytometry data were analyzed by using the FlowJo 2.7.4 software (Tree Star, Inc, San Carlos, CA).
Cell purification, cell lines.
After informed consent, blood samples were obtained from patients fulfilling diagnostic and immunophenotypic criteria for common B-cell CLL at the University of California, San Diego (UCSD) Medical Center. Blood mononuclear cells (PBMC) were isolated via density gradient centrifugation over Ficoll Paque (Pharmacia, Uppsala, Sweden). Cells were used fresh or viably frozen in fetal calf serum (FCS) plus 10% dimethyl sulfoxide (DMSO) for storage in liquid nitrogen. Frozen cells were cultured overnight at 37°C in 5%CO2/air in RPMI-1640 supplemented with 10% FCS and penicillin-streptomycin-glutamine (GIBCO-BRL, Grand Island, NY). Fluorescence-activated cell sorting (FACS) analysis of the CLL cells showed an average of 93.1% ± 4.4% (mean ± standard deviation [SD]) CD19-positive cells, representing the CLL B cells. The T cells are the second most predominant population in the blood lymphocyte population of CLL patients. These cells constituted an average of 3.9% ± 2.1% (mean ± SD, n = 16) of the total lymphocytes of all patient samples examined. The viability of the CLL cells was always greater than 85%, as determined by staining with propidium iodine (PI). The human pro-B cell line, Reh, and the pre-B cell line, Nalm-6, were provided by Dr J. Scheele (Department of Biochemistry and Chemistry, UCSD). The murine stromal cell line M2-10B4 was purchased from the American Type Culture Collection (ATCC; Rockville, MD). Cell lines were cultured at 37°C in RPMI-1640 supplemented with 10% FCS and penicillin-streptomycin-glutamine (GIBCO-BRL).
CXCR4 reverse transcriptase-polymerase chain reaction (RT-PCR) analysis.
RNA was isolated from nonpurified (n = 12) or CD19-selected CLL PBMC (n = 3), using the Quiagen RNeasy kit (Quiagen, Santa Clarita, CA). RNA then was used for first strand cDNA synthesis with the SuperScript preamplification system (GIBCO-BRL, Rockville, MD), according to the manufacturer’s instructions. The following CXCR4-specific primers were used: 5′ primer: GGA GAA TTC TTA CCA TGG AGG GGA TCA; 3′ primer: GGA GAA TTC AGC TGG AGT GAA AAC TTG. The annealing temperature was 58°C and the reaction proceeded for 35 cycles. To normalize for the amount of RNA, we performed RT-PCR for human glyceraldehyde-3-phosphate dehydrogenase (GA3PD), as described.25
CXCR4 receptor endocytosis assay.
Receptor downmodulation of CXCR4 by SDF-1α was performed as described.26 27 Briefly, CLL cells, the pro-B cell line, Reh, or the pre-B cell line, Nalm-6, each were adjusted to 5 × 106/mL in RPMI-1640 with 0.5% BSA. The cells were cultured with SDF-1α at various concentrations for 1 hour at 37°C in 5%CO2 in air. Cells were washed with a 20-fold volume of ice-cold buffer without FCS, stained at 4°C with saturating concentrations of PE-conjugated anti-CXCR4 MoAb, and then analyzed by flow cytometry.
Ca2+ mobilization.
Ca2+ mobilization in response to SDF-1α was performed as described.28 Briefly, the cells were loaded with Indo-1AM (Molecular Probes, Eugene, OR), washed 2 times with Ca2+-free modified Gey’s buffer (MGB), suspended in MGB containing 1.5 mmol/L Ca2+, and then warmed to 37°C for 2 minutes in a stirred cuvette. The emission ratio at 400/480 nm was followed kinetically after addition of the chemokine on an SLM 8000 fluorometer (Spectronic Instruments, Inc, Rochester, NY). To induce maximal Ca2+ release, cells were subsequently stimulated with 2.5 μg/mL ionomycin (Sigma Chemicals Co, St Louis, MO).
Actin polymerization assay.
Actin polymerization was tested as described.8 29 Briefly, cells (1.25 × 106/mL) were suspended in RPMI-1640 medium with 0.5% BSA at 37°C and incubated with 100 ng/mL SDF-1α for varying amounts of time. To determine actin polymerization in CLL B cells, CLL cells were prelabeled with anti-CD19 MoAbs. At the indicated time points, 400 μL of the cell suspension were added to 100 μL of a solution containing 4 × 10−7 mol/L FITC-labeled phalloidin, 0.5 mg/mL 1-α-lysophosphatidylcholine (both from Sigma), and 18% formaldehyde in phosphate-buffered saline (PBS). The fixed cells were analyzed by flow cytometry on a FACSCalibur and all time points are plotted relative to the mean relative fluorescence of the sample before addition of the chemokine.
Chemotaxis assay.
The chemotaxis assay across bare polycarbonate was preformed as described.8 Briefly, CLL cells or B-cell lines were suspended in RPMI-1640 with 0.5% BSA. A total of 100 μL, containing 5 × 105 cells, was added to the top chamber of a 6.5-mm diameter Transwell culture inserts (Costar, Cambridge, MA) with a pore size of 5 μm. Filters then were transferred to wells containing medium with or without SDF-1α. The chambers were incubated for 2 hours at 37°C in 5% CO2. After this incubation, the cells in the lower chamber were suspended and divided into aliquots for counting with a FACSCalibur for 20 seconds at 60 μL/min in triplicates or for immunophenotyping.
A 1:20 dilution of input cells was counted under the same conditions. Antibody inhibition was performed by preincubating the cells with different concentrations of anti-CXCR4 MoAb 12G5 or anti-CCR3 MoAb 7B11 for 30 minutes at 4°C before use in the chemotaxis assay. For pertussis toxin treatment, cells were preincubated with 200 ng/mL pertussis toxin (List Biological Laboratories, Inc, Campbell, CA) at 37°C for 2 hours, washed twice, and subsequently applied to the top chamber of the chemotaxis assay. For inhibition of phosphatidylinositol 3-kinase (PI-3 kinase), B-CLL cells were incubated with different concentrations of Wortmannin (Calbiochem, San Diego, CA) at 37°C for 30 minutes and then examined for chemotaxis in response to 100 ng/mL SDF-1α, as described above.
SDF-1 expression by the murine M2-10B4 marrow stromal cell line.
For SDF-1 mRNA detection, RNA was extracted from the M2-10B4 stromal cell line and used for cDNA synthesis as described above. The sequences of the murine SDF-1β–specific primers we used were: 5′ primer: CCT AAG TCG ACA CGC CAT GGA CGC CAA; 3′ primer: CCT ATC TCG AGT CAC ACC TCT CAC ATC. The conditions of the PCR reaction were the same as described above. A sequenced plasmid containing the murine SDF-1β cDNA was used as a positive control. Conditioned medium from this cell line was used to assay for secretion of bioactive SDF-1. For this purpose, the culture medium was replaced with serum-free medium (X-VIVO 15, Bio Whittaker, Walkersville, MD) when cells had reached 70% confluency. After 3 days, the conditioned medium was removed and used for chemotaxis and receptor-endocytosis assays with the pro-B cell line, Reh, as described above. For blocking of the CXCR4 receptor, Reh cells were preincubated with 30 μg/mL anti-CXCR4 MoAb before being applied to the chemotaxis chambers.
In vitro migration assay of CLL cells beneath stromal cells (Pseudoemperipolesis).
To determine the role of SDF-1 in the interaction of CLL cells with stromal cells in vitro, we developed an assay that allows us to count and phenotype the cells that migrate into a stromal cell layer. The murine stromal cell line M2-10B4 was seeded the day before the assay onto collagen-coated 24-well plates at a concentration of 1.5 × 105 cell per well in RPMI-1640 supplemented with 10% FCS and penicillin-streptomycin-glutamine. CLL cells were suspended in RPMI-1640/10% FCS and added to the stromal cell layer. The plates were incubated at 37°C in 5%CO2. After incubation for 2 hours or at the indicated time points in the time course experiment, cells that had not migrated into the stromal cell layer were removed by vigorously washing the wells with RPMI medium 3 times. The complete removal of nonmigrated cells and the integrity of the stromal cell layer containing transmigrated cells was assessed by phase contrast microscopy and documented photographically. The stromal cell layer containing transmigrated cells was detached by incubation for 1 minute with trypsin/EDTA solution prewarmed to 37°C (ATV solution; GIBCO-BRL). Cells were then immediately suspended by adding 1 mL ice-cold RPMI/10% FCS, washed, and suspended in 0.5 mL cold medium for counting by flow cytometry or staining of aliquots for flow cytometry. A lymphocyte gate was set using the different relative size and granularity (forward scatter and side scatter) characteristics to exclude stromal cells from the counts. Duplicate samples were counted at high flow for 20 seconds to determine the relative number of migrated cells. Control samples, in which 1 × 107 CLL B cells were added to the wells immediately before the washing step, or samples that only contained stromal cells consistently had counts <200 events/20 seconds (background). To calculate the percentage of CLL cells that had migrated into the marrow stromal cells (MSC) layer, a 1/10 diluted sample of the input cell suspension was counted under the same conditions. For the analysis of the phenotype of the transmigrated CLL B cells, cells were costained with anti-human CD19 MoAb along with anti-CXCR4, anti-CD49d, anti-CD49e, anti-CD54, or anti-CD3 MoAbs. For the antibody inhibition studies, CLL cells were preincubated with 30 μg/mL anti-CXCR4 MoAb (12G5) for 30 minutes, washed twice, and applied to the assay. SDF-1 pretreatment was performed by preincubating CLL cells (1 × 107 cells/mL) with synthetic SDF-1α at a concentration of 200 ng/mL for 1 hour at 37°C in 5% CO2, before adding the SDF-1α containing CLL cell suspension to the assay. Pertussis toxin pretreatment was performed as described above.
Data analysis, statistics.
Results are shown as mean ± standard deviation (SD) or standard error about the mean (SEM) of at least 3 experiments each. For statistical comparison between groups, the Student pairedt-test or Bonferroni t-test were used. Analyses were performed using the Biostatistics software developed by Stanton A. Glantz (UC San Francisco). Flow cytometry data were analyzed using the FlowJo software.
RESULTS
Expression of CXCR4 mRNA and surface protein in B-CLL.
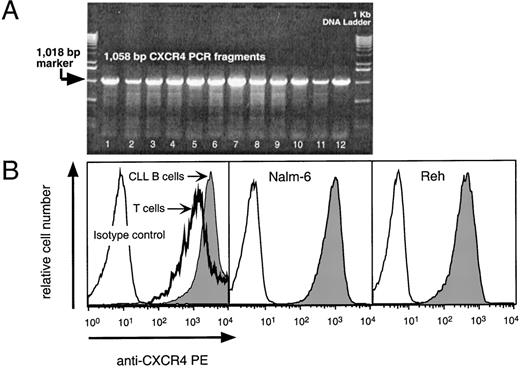
We detected CXCR4 mRNA in 12 of 12 CLL blood samples tested (Fig 1A). The amount of CXCR4 mRNA detected did not vary if the CLL B cells were purified to greater than 98% purity before RNA extraction. RT-PCR for the GA3PD gene indicated that the samples contained similar amounts of RNA (data not shown).
(A) RT-PCR analysis for CXCR4 mRNA in CLL samples from 12 different patients. The specific 1,058-bp PCR fragment is visible in 12 representative B-CLL samples (lanes 1 through 12). The arrow points to the 1,018-bp marker of the 1-Kb control DNA ladder shown in the lanes flanking the test samples. (B) Logarithmic fluorescence histograms depicting the expression of CXCR4 on mononuclear blood cells of a representative patient with CLL (left panel) and Nalm-6 (center) or Reh cells (right panel). The left panel depicts the logarithmic red fluorescence of electronically-gated CD3+ T cells (bold line) or the CD19+ CLL cells (shaded) stained with the anti-CXCR4–PE MoAb or with a PE-labeled isotype control antibody (thin lined histogram). The center and right histograms depict the Nalm-6 or Reh cells, respectively, stained with the anti-CXCR4–PE MoAb (shaded) or isotype control antibody (open histogram).
(A) RT-PCR analysis for CXCR4 mRNA in CLL samples from 12 different patients. The specific 1,058-bp PCR fragment is visible in 12 representative B-CLL samples (lanes 1 through 12). The arrow points to the 1,018-bp marker of the 1-Kb control DNA ladder shown in the lanes flanking the test samples. (B) Logarithmic fluorescence histograms depicting the expression of CXCR4 on mononuclear blood cells of a representative patient with CLL (left panel) and Nalm-6 (center) or Reh cells (right panel). The left panel depicts the logarithmic red fluorescence of electronically-gated CD3+ T cells (bold line) or the CD19+ CLL cells (shaded) stained with the anti-CXCR4–PE MoAb or with a PE-labeled isotype control antibody (thin lined histogram). The center and right histograms depict the Nalm-6 or Reh cells, respectively, stained with the anti-CXCR4–PE MoAb (shaded) or isotype control antibody (open histogram).
We stained CLL blood cells with anti-CD3–FITC, anti-CXCR4–PE, and anti-CD19–APC, and examined for B- and T-cell expression of CXCR4 by flow cytometry (Fig 1B). As expected, the pro-B cell line, Reh, and the pre-B cell line, Nalm-6, also expressed high levels of CXCR4 (Fig 1B).
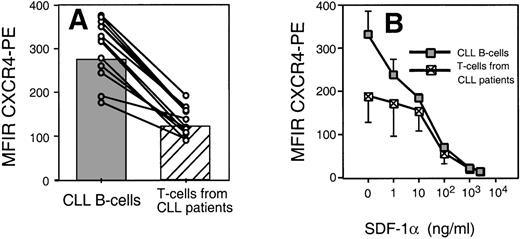
We found that CLL B cells from each of 12 patients expressed high levels of CXCR4. The T cells in the samples expressed lower levels of CXCR4 than CLL B cells (Fig 2A). The mean fluorescence intensity ratio of CLL cells from 12 different patients was 274 ± 68 (mean ± SD, n = 12), whereas it was 122 ± 34 for the T cells of the same patients (Fig 2A).
(A) Leukemic CLL B cells express higher levels of CXCR4 than the T cells from CLL B patients. The average MFIR of CLL B cells (n = 12; shaded) was significantly higher than the mean CXCR4-MFIR of T cells (n = 12; hatched). The dots represent CXCR4-MFIR values for CLL B and T cells from individual patients, with lines connecting the values from the same patient sample. (B) SDF-1 induces CXCR4 receptor downmodulation on CLL B cells and T cells. Using anti-CXCR4 along with anti-CD19 and anti-CD3 MoAbs, we determined the CXCR4 MFIR values for CLL B cells (shaded boxes) and T cells from the same CLL patients (hatched boxes) after preincubation with 1, 10, 100, 1,000, or 2,500 ng/mL SDF-1 or medium alone. The data represent the MFIR values (±SD) from 3 different patient samples.
(A) Leukemic CLL B cells express higher levels of CXCR4 than the T cells from CLL B patients. The average MFIR of CLL B cells (n = 12; shaded) was significantly higher than the mean CXCR4-MFIR of T cells (n = 12; hatched). The dots represent CXCR4-MFIR values for CLL B and T cells from individual patients, with lines connecting the values from the same patient sample. (B) SDF-1 induces CXCR4 receptor downmodulation on CLL B cells and T cells. Using anti-CXCR4 along with anti-CD19 and anti-CD3 MoAbs, we determined the CXCR4 MFIR values for CLL B cells (shaded boxes) and T cells from the same CLL patients (hatched boxes) after preincubation with 1, 10, 100, 1,000, or 2,500 ng/mL SDF-1 or medium alone. The data represent the MFIR values (±SD) from 3 different patient samples.
SDF-1α induces dose-dependent CXCR4 receptor endocytosis.
Receptors internalization by endocytosis is characteristic for chemokine receptors and may allow for continuous sampling of chemoattractants, permitting the cells to follow a chemotactic gradient. Figure 2B shows the mean fluorescence of B-CLL cells stained with anti-CXCR4 MoAbs after incubation with varying concentrations of SDF-1α or medium alone. We found that the amount of SDF-1α required to induce maximum downmodulation on CLL B cells was equal to 1,000 ng/mL, or 125 nmol/L. This is similar to the amount required for optimal downmodulation of CXCR4 on T cells of the same patient (Fig 2B) or Nalm-6 (data not shown). In addition, we observed a partial CXCR4 downmodulation on CLL B cells (Fig 2B) or Nalm-6 in response to SDF-1α at concentrations as low as 1 ng/mL. This was lower than the concentration of SDF-1α that was required to induce detectable CXCR4 downmodulation on T cells of the same CLL patients and might indicate high sensitivity of CXCR4 on CLL B cells to SDF-1.
SDF-1α induces calcium mobilization in CLL B cells.
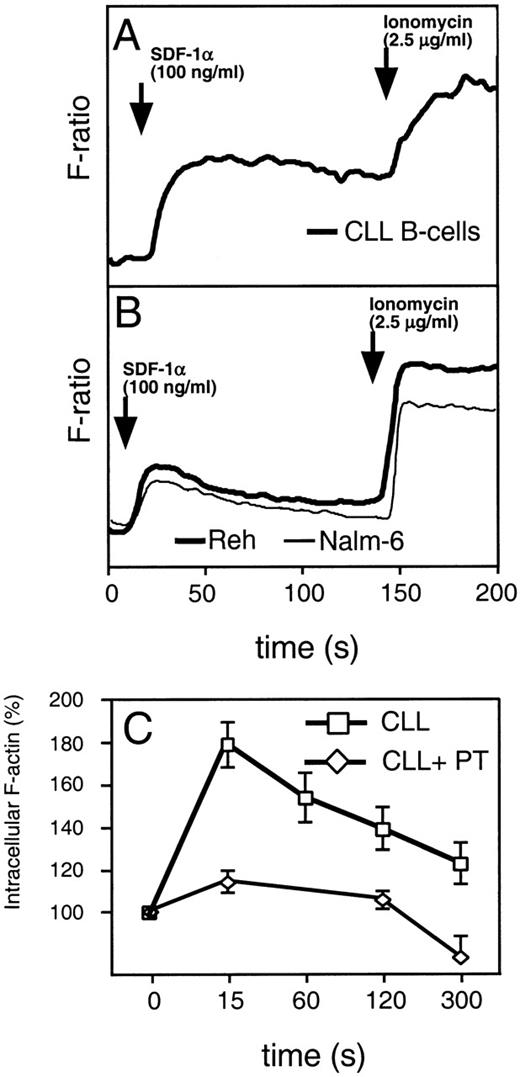
Binding of chemokines to their receptors causes a characteristic increase in cytosolic calcium. This is one of the earliest biochemical events that occur in response to chemokines. To examine intracellular calcium flux, we labeled CLL cells or the B-cell lines, Nalm-6 and Reh, with indo-1–AM before adding SDF-1α to 100 ng/mL. Evaluation of the fluorescence of stimulated cells showed that both CLL B cells and normal B cells mobilize Ca2+ in response to SDF-1α. Compared with the immature B-cell lines, Nalm-6 or Reh (Fig 3A and B), CLL cells (n = 6) had a prolonged elevation in intracellular free Ca2+ after stimulation with SDF-1α.
(A) SDF-1 induces mobilization of intracellular calcium in CLL B cells (bold line). (B) The immature B-cell lines, Reh (bold line) and Nalm-6 (thin line). Increases of intracellular Ca2+ were recorded on a fluorometer after addition of 100 ng/mL SDF-1 to cells loaded with Indo-1. Adding ionomycin induced maximum release of intracellular Ca2+. A representative experiment of at least 3 is shown. (C) SDF-1 induces actin polymerization in CLL B cells, which can be inhibited by pertussis toxin. Intracellular F-actin was measured using FITC-labeled phalloidin in CD19-prelabeled CLL B cells (boxes) after the addition of 100 ng/mL SDF-1 at time 0. Results are shown as percent of intracellular F-actin relative to the value before the addition of SDF-1 and are the mean and SD of 3 independent experiments. Pertussis toxin inhibits actin polymerization in CLL B cells (diamonds).
(A) SDF-1 induces mobilization of intracellular calcium in CLL B cells (bold line). (B) The immature B-cell lines, Reh (bold line) and Nalm-6 (thin line). Increases of intracellular Ca2+ were recorded on a fluorometer after addition of 100 ng/mL SDF-1 to cells loaded with Indo-1. Adding ionomycin induced maximum release of intracellular Ca2+. A representative experiment of at least 3 is shown. (C) SDF-1 induces actin polymerization in CLL B cells, which can be inhibited by pertussis toxin. Intracellular F-actin was measured using FITC-labeled phalloidin in CD19-prelabeled CLL B cells (boxes) after the addition of 100 ng/mL SDF-1 at time 0. Results are shown as percent of intracellular F-actin relative to the value before the addition of SDF-1 and are the mean and SD of 3 independent experiments. Pertussis toxin inhibits actin polymerization in CLL B cells (diamonds).
SDF-1α induces actin polymerization in CLL B cells.
Reorganization of the actin cytoskeleton is an early event in the migratory response to chemokines.20 To evaluate the ability of SDF-1α to induce changes in the actin cytoskeleton of CLL B cells, we examined for changes in filamentous actin (F-actin) of CLL cells in response to 100 ng/mL SDF-1α. We detected a significant, transient increase in F-actin within 15 seconds after exposure of the cells to the chemokine, followed by a subsequent depolymerization, as shown in Fig 3C. Actin polymerization after SDF-1α stimulation was inhibited by preincubation of CLL B cells with pertussis toxin (Fig3C), indicating that this response is mediated through pertussis toxin-sensitive Gi proteins.
Chemotaxis of CLL cells in response to SDF-1α.
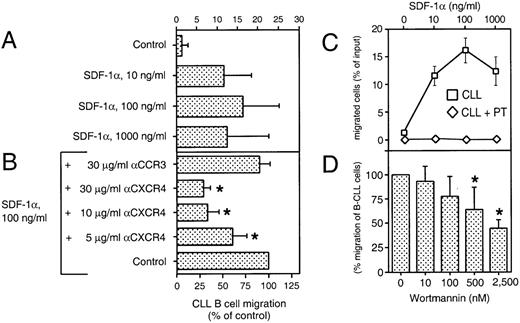
We performed a chemotaxis assay in which B-CLL cells were evaluated for their ability to migrate through 5-μm pores of bare polycarbonate filters. This assay allowed us to determine the absolute number, as well as the phenotype of the transmigrated cells. Figure 4A shows the chemotaxis response of CLL B cells to various concentrations of synthetic SDF-1α, as determined by immunophenotyping of input and transmigrated cells. The average proportion of CLL B cells that migrated to chambers with the optimal concentration of SDF-1α (100 ng/mL) was 16% ± 9% of input cells (mean ± SD, n = 16 patients). In contrast, the proportion of input cells that migrated to control chambers without SDF-1α was 1% ± 2% (Fig 4A). The response to SDF-1α had a biphasic curve that is characteristic for chemoattractant-induced chemotaxis (Fig 4C). The pro-B cell line, Reh, and the pre-B cell line, Nalm-6, also migrated to chambers containing SDF-1α, as described earlier.19 The maximum migration response also was noted to chambers containing 100 ng/mL SDF-1α.
SDF-1 induces chemotaxis in CLL B cells. (A) Blood lymphocytes from 16 CLL patients were assayed in the bare filter chemotaxis assay for migration to buffer (control) or different concentrations of SDF-1. Input and transmigrated cells were stained with anti-CD19 and anti-CD3 MoAbs to determine the percentages of the input CLL B cells that migrated into the chambers. The bars represent the mean values (±SD) for the migration of CLL B cells from 16 different patients. (B) For antibody inhibition, CLL PBMC were preincubated with different concentrations of MoAbs against the chemokine receptors CXCR4 (12G5) or a control MoAb directed against the chemokine receptor CCR3 (7B11) before addition to the chemotaxis assay. The controls were preincubated in buffer alone. Results indicate the relative migration compared with control samples migrating to 100 ng/mL SDF-1 (100%) and represent the mean values ± SD of 2 experiments with CLL B cells from 4 different patients. The stars indicate statistically different values, compared with the controls withP values < .05. (C) SDF-1 attracts CLL B cells by a pertussis toxin-sensitive mechanism. The migration of CLL B cells is completely blocked by pretreatment with 200 ng/mL pertussis-toxin (PT, diamonds). Data represent the mean values ± SEM of CLL B from 16 CLL B patients for the chemotaxis assays (boxes) and 4 different CLL samples for pertussis-toxin treatment (diamonds). (D) Migration of B-CLL cells is partially inhibited by Wortmannin, as selective inhibitor of PI-3 kinase. CLL cells were pretreated with 10, 100, 500, and 2,500 nmol/L concentrations of Wortmannin and subject to the chemotaxis assay in the presence of 100 ng/mL SDF-1. The bars represent the mean values (±SD) for migration of Wortmannin-treated B-CLL cells (n = 6), relative to the migration without the inhibitor. The stars indicate statistically different values, compared with the controls with P values < .05.
SDF-1 induces chemotaxis in CLL B cells. (A) Blood lymphocytes from 16 CLL patients were assayed in the bare filter chemotaxis assay for migration to buffer (control) or different concentrations of SDF-1. Input and transmigrated cells were stained with anti-CD19 and anti-CD3 MoAbs to determine the percentages of the input CLL B cells that migrated into the chambers. The bars represent the mean values (±SD) for the migration of CLL B cells from 16 different patients. (B) For antibody inhibition, CLL PBMC were preincubated with different concentrations of MoAbs against the chemokine receptors CXCR4 (12G5) or a control MoAb directed against the chemokine receptor CCR3 (7B11) before addition to the chemotaxis assay. The controls were preincubated in buffer alone. Results indicate the relative migration compared with control samples migrating to 100 ng/mL SDF-1 (100%) and represent the mean values ± SD of 2 experiments with CLL B cells from 4 different patients. The stars indicate statistically different values, compared with the controls withP values < .05. (C) SDF-1 attracts CLL B cells by a pertussis toxin-sensitive mechanism. The migration of CLL B cells is completely blocked by pretreatment with 200 ng/mL pertussis-toxin (PT, diamonds). Data represent the mean values ± SEM of CLL B from 16 CLL B patients for the chemotaxis assays (boxes) and 4 different CLL samples for pertussis-toxin treatment (diamonds). (D) Migration of B-CLL cells is partially inhibited by Wortmannin, as selective inhibitor of PI-3 kinase. CLL cells were pretreated with 10, 100, 500, and 2,500 nmol/L concentrations of Wortmannin and subject to the chemotaxis assay in the presence of 100 ng/mL SDF-1. The bars represent the mean values (±SD) for migration of Wortmannin-treated B-CLL cells (n = 6), relative to the migration without the inhibitor. The stars indicate statistically different values, compared with the controls with P values < .05.
The chemotaxis of CLL cells was significantly inhibited by preincubation of the input cells with the anti-CXCR4 MoAb 12G5, indicating that a direct interaction between SDF-1 and CXCR4 was necessary for chemotaxis (Fig 4B). In contrast, preincubation with a control MoAb against the chemokine receptor, CCR3, had no significant effect.
Chemotaxis of CLL B cells to SDF-1α was completely blocked by pertussis toxin (Fig 4C), indicating that this activity was dependent on signaling through a Gi protein(s). Furthermore, pretreatment with Wortmannin, a selective inhibitor of PI-3 kinase, partially inhibited SDF-1α–induced migration of CLL B cells (Fig4D). The phenotype of the migrated cells indicated that the proportion of CLL B cells and T cells did not differ between samples pretreated with Wortmannin or controls, indicating that Wortmannin inhibited the migration of both cell types.
M2-10B4 stromal cells express SDF-1 mRNA and secrete bioactive SDF-1.
Using SDF-1β–specific PCR primers, we amplified a PCR product of the expected size (296 bp) from M2-10B4 cDNA and a sequenced plasmid containing the murine SDF-1β cDNA served as a positive control, demonstrating SDF-1 expression by the stromal cell line (Fig 5A). Conditioned medium from this cell line induced chemotaxis of Reh cells in a dose-dependent fashion, and this migration was blocked by preincubation of the cells with anti-CXCR4 MoAbs (Fig 5B). Moreover, preincubation with M2-10B4–conditioned medium induced a dose-dependent downmodulation of CXCR4 receptors on Reh cells, compared with cells preincubated in medium alone (data not shown). These observations indicated that the M2-10B4 line expresses and secretes SDF-1.
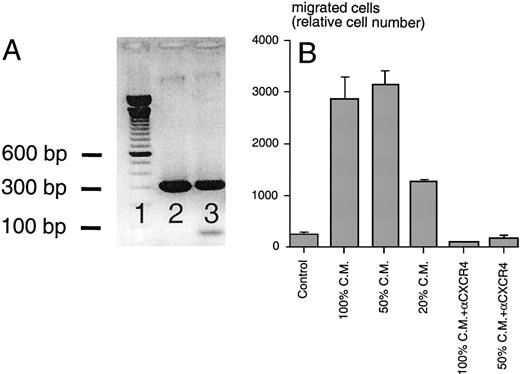
(A) RT-PCR analysis for murine SDF-1β mRNA. Using cDNA from M2-10B4 cells (lane 3) and plasmid DNA encoding murine SDF-1β as a positive control (lane 2), PCR fragments of the expected size of 296 bp were amplified in both test samples (100-bp marker in lane 1). (B) Chemotaxis of the Reh B-cell line in response to conditioned medium (CM) from M2-10B4 cells. Compared with medium (Control), M2-10B4 CM at different concentrations (100, 50, and 20 vol%) induced chemotaxis of Reh cells. This migration was inhibited by preincubation of Reh cells with 30 μg/mL CXCR4 MoAb. Error bars indicate the range of duplicate samples.
(A) RT-PCR analysis for murine SDF-1β mRNA. Using cDNA from M2-10B4 cells (lane 3) and plasmid DNA encoding murine SDF-1β as a positive control (lane 2), PCR fragments of the expected size of 296 bp were amplified in both test samples (100-bp marker in lane 1). (B) Chemotaxis of the Reh B-cell line in response to conditioned medium (CM) from M2-10B4 cells. Compared with medium (Control), M2-10B4 CM at different concentrations (100, 50, and 20 vol%) induced chemotaxis of Reh cells. This migration was inhibited by preincubation of Reh cells with 30 μg/mL CXCR4 MoAb. Error bars indicate the range of duplicate samples.
CLL B cells migrate beneath heterologous MSC (Pseudoemperipolesis).
Coculture of CLL cells with the murine-marrow-stroma cell-line, M2-10B4, results in spontaneous migration of CLL cells into the stromal cell layer. This in vitro phenomenon termed pseudoemperipolesis is characterized by the dark appearance of cells that have migrated into the same focal plane as the stromal cells, whereas the more superficial, nonmigrated cells remain refractile (Fig 6).30 Time-course experiments showed that pseudoemperipolesis of CLL cells mostly occurred within the first 2 hours of coculture (Fig 7B). Titration of the input CLL cell numbers showed that concentrations above 1 × 107 cells per 24 well plate did not significantly increase the number of migrated cells (Fig 7C). A 2-hour assay with 1 × 107 input cells was found to be the optimal condition for this assay and hence was used in subsequent inhibition studies. Under these conditions, an average of 7.4% ± 3.7% (mean ± SD) of input CLL cells from 6 different patients migrated into the stromal layer. For comparison, we assessed the migration of Nalm-6 cells and found that 6.9% ± 0.5% (mean ± SE of duplicate tests) of the input cells migrated into the stromal layer under the same experimental conditions.
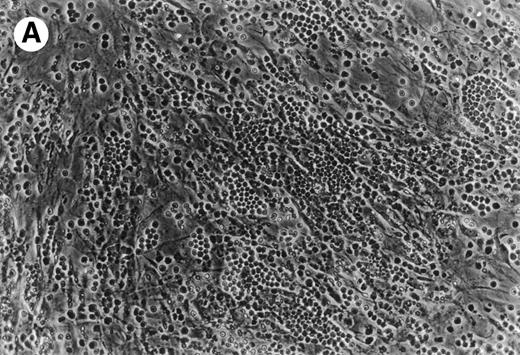
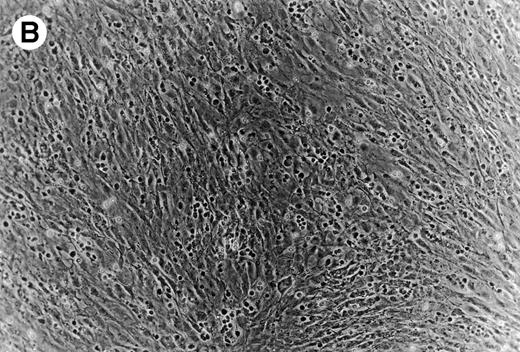
(A) Representative phase contrast photomicrograph of pseudoemperipolesis of CLL B cells after 2-hour culture on the heterologous murine stromal cell line, M2-10B4. Cells that had not migrated beneath the stromal cells washed off, and the stromal cell layer containing the migrated CLL cells was photographed (200x magnification). Pseudoemperipolesis is characterized by the dark appearance of lymphocytes that have migrated into the same focal plane as the stromal cells. (B) For comparison, this photomicrograph shows reduced pseudoemperipolesis after pretreatment of CLL cells with pertussis toxin.
(A) Representative phase contrast photomicrograph of pseudoemperipolesis of CLL B cells after 2-hour culture on the heterologous murine stromal cell line, M2-10B4. Cells that had not migrated beneath the stromal cells washed off, and the stromal cell layer containing the migrated CLL cells was photographed (200x magnification). Pseudoemperipolesis is characterized by the dark appearance of lymphocytes that have migrated into the same focal plane as the stromal cells. (B) For comparison, this photomicrograph shows reduced pseudoemperipolesis after pretreatment of CLL cells with pertussis toxin.
Measurement, time course, and titration of CLL B-cell pseudoemperipolesis. (A) To measure pseudoemperipolesis, CLL cells that had migrated into the stromal cell layer were harvested by treating the washed stromal cell layer with trypsin. The removed cells were analyzed via flow cytometry. We collected the data on the cells that had characteristic forward- and side-light scatter characteristics of lymphocytes, allowing us to exclude the marrow stromal cells from the analyses (for demonstrative purposes, the stromal cell population was centered for the acquisition of this sample). (B) The time course of pseudoemperipolesis of CLL B cells from 3 different patients. A continuous increase of CLL B cells, as determined by counting and anti-CD19 staining of cells that had migrated into the stromal cell layer, was detected over the first 2 hours. (C) Titration of pseudoemperipolesis of CLL cells using increasing numbers of input CLL cells. Lymphocytes from 3 different CLL patients that migrated into the stromal cell layer within 2 hours were counted for 20 seconds at high flow using a lymphocyte gate. Displayed are the mean (±SD) relative numbers of duplicate samples.
Measurement, time course, and titration of CLL B-cell pseudoemperipolesis. (A) To measure pseudoemperipolesis, CLL cells that had migrated into the stromal cell layer were harvested by treating the washed stromal cell layer with trypsin. The removed cells were analyzed via flow cytometry. We collected the data on the cells that had characteristic forward- and side-light scatter characteristics of lymphocytes, allowing us to exclude the marrow stromal cells from the analyses (for demonstrative purposes, the stromal cell population was centered for the acquisition of this sample). (B) The time course of pseudoemperipolesis of CLL B cells from 3 different patients. A continuous increase of CLL B cells, as determined by counting and anti-CD19 staining of cells that had migrated into the stromal cell layer, was detected over the first 2 hours. (C) Titration of pseudoemperipolesis of CLL cells using increasing numbers of input CLL cells. Lymphocytes from 3 different CLL patients that migrated into the stromal cell layer within 2 hours were counted for 20 seconds at high flow using a lymphocyte gate. Displayed are the mean (±SD) relative numbers of duplicate samples.
Pseudoemperipolesis of CLL B cells is associated with strong CXCR4 downmodulation and increased expression of CD49d.
CLL B cells that had migrated into the stromal cell layer and input CLL cells were analyzed for surface marker expression by costaining with FITC-labeled anti-CD19 MoAb and PE-conjugated MoAbs to the epitopes of interest. First, we examined for CD19 and CD3 expression on the migrated lymphocytes. Similar to the input CLL cells, the CD19-positive CLL B cells were the predominant population of transmigrated cells (82% ± 14.7% of the migrated lymphocytes, n = 6). Comparing the mean fluorescence intensity ratios (MFIR ± SD), we noted a significantly lower CXCR4 expression (CXCR4 MFIR, 50 ± 21 v423 ± 184, n = 4; P < .05) and higher CD49d expression (CD49d MFIR, 13 ± 2 v 7 ± 2; n = 4, P < .05) on the transmigrated CLL B cells than on the input CLL B cells (Fig 8A), whereas no difference was observed for the expression of CD49e and CD54 (CD49e MFIR, 6 ± 5v 6 ± 5; n = 4; CD54 MFIR, 24 ± 11 v 25 ± 9; n = 4).
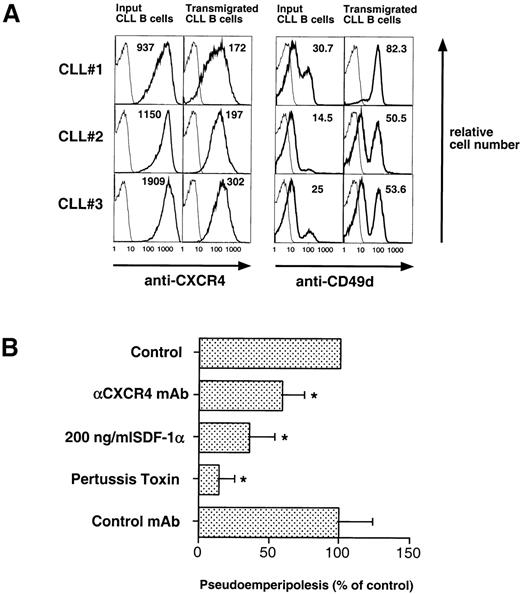
(A) CLL B cells that migrated into the stromal cell layer have lower CXCR4- and higher CD49d (VLA-4)-surface expression than input CLL B cells. CLL cells from 3 representative patients that migrated into the MSC layer were stained with anti-CD19 and anti-CXCR4 or anti-CD49d MoAbs. An aliquot of the input CLL cells before addition to the assay was stained for comparison. The bold lines represent the staining for the specific MoAb, while the thin lines show the isotype control staining of the respective sample. The numbers indicate the mean fluorescence intensity for CXCR4 or CD49d, respectively. (B) Pseudoemperipolesis of CLL cells was significantly inhibited by anti-CXCR4 MoAb, pretreatment with 200 ng/mL SDF-1, and pertussis toxin, while preincubation with an isotype-matched MoAb to an irrelevant antigen had no inhibitory effect. The bars represent the number of CLL B cells that had migrated into the stromal cell layer after 2 hours (1 × 107 input cells), relative to the nontreated controls (100%) and are the means (±SD) of 6 different CLL patients tested in 3 independent experiments. The stars indicate significant differences with P < .05, using Bonferroni’st-test.
(A) CLL B cells that migrated into the stromal cell layer have lower CXCR4- and higher CD49d (VLA-4)-surface expression than input CLL B cells. CLL cells from 3 representative patients that migrated into the MSC layer were stained with anti-CD19 and anti-CXCR4 or anti-CD49d MoAbs. An aliquot of the input CLL cells before addition to the assay was stained for comparison. The bold lines represent the staining for the specific MoAb, while the thin lines show the isotype control staining of the respective sample. The numbers indicate the mean fluorescence intensity for CXCR4 or CD49d, respectively. (B) Pseudoemperipolesis of CLL cells was significantly inhibited by anti-CXCR4 MoAb, pretreatment with 200 ng/mL SDF-1, and pertussis toxin, while preincubation with an isotype-matched MoAb to an irrelevant antigen had no inhibitory effect. The bars represent the number of CLL B cells that had migrated into the stromal cell layer after 2 hours (1 × 107 input cells), relative to the nontreated controls (100%) and are the means (±SD) of 6 different CLL patients tested in 3 independent experiments. The stars indicate significant differences with P < .05, using Bonferroni’st-test.
Role of SDF-1 in pseudoemperipolesis of CLL B cells.
To establish the role of SDF-1 in the migration of CLL cells into the stromal layer, we used inhibitors that specifically or nonspecifically interfered with the interaction of SDF-1 or CXCR4 on CLL cells. Pertussis toxin was the strongest inhibitor of CLL cell pseudoemperipolesis: only 14% ± 11% (mean ± SD; n = 6) cells compared with untreated control samples (100%) had migrated after 2 hours. Significant inhibition was also observed after SDF-1α pretreatment and addition to the coculture (35% ± 19%; n = 6), and anti-CXCR4 MoAb preincubation of CLL cells (58% ± 16%; n = 6; Fig 8B).
DISCUSSION
In this study, we investigated the expression and function of the chemokine receptor, CXCR4, on B cells from patients with CLL and characterized its role in heterotypic adherence to marrow stromal cells. First, we stimulated CLL B cells with synthetic SDF-1α and detected responses that are characteristic for the activation of leukocytes by chemoattractants (chemokine receptor endocytosis, calcium mobilization, actin polymerization). Moreover, CLL B cells migrated in response to SDF-1α, showing a biphasic dose response curve that is characteristic for chemokine-induced migration. The chemotaxis of CLL B cells toward SDF-1α was mediated by signaling through the CXCR4 receptor, as demonstrated by our ability to inhibit such migration with anti-CXCR4 MoAb 12G5. Preincubation with this MoAb significantly reduced chemotaxis of CLL B cells in response to SDF-1α (72% inhibition with 30 μg/mL anti-CXCR4 MoAb; Fig 4B). The failure to achieve 100% inhibition under these conditions has been noted earlier,31,32 and may reflect the competition of relatively high concentrations of chemokine (eg, 100 ng/mL) with anti-CXCR4 MoAbs during a 2-hour assay. Internalization and recycling of CXCR4 receptors after MoAb binding26 or partial dissociation of this MoAb at the physiologic temperatures of the chemotaxis assay may play a role in this context.
We also found that the migration of CLL B cells could be inhibited by pretreatment of the CLL cells with pertussis toxin or Wortmannin, indicating that SDF-1α signaling for migration of CLL B cells is linked to a CXCR4 coupled Gi α subunit of a heterotrimeric guanosine triphosphate (GTP)-binding protein and activation of PI3-kinase, respectively.
In hematopoietic cells, SDF-1α treatment also can activate p44/42 mitogen-activated protein kinase (Erk1 and Erk2) through CXCR4.33,34 This downstream signaling pathway may play a role in SDF-1α–induced transcriptional activation.34 In addition, there are reports that the specific p44/42 mitogen activated protein kinase (MAPK) inhibitor, PD98059, could inhibit chemotaxis of eosinophils induced by eotaxin35 or of neutrophils induced by N-formyl peptide, C5a, or interleukin-8,36 suggesting that the Erk-pathway also may be involved in signaling for chemotaxis. However, this remains controversial, as other reports indicate that PD98059 could not inhibit neutrophil-chemotaxis induced by f-Met-Leu-Phe (fMLP)37 or interleukin-838 or the platelet-derived growth factor-induced chemotaxis of fibroblasts.39 Consistent with such reports are our preliminary studies in which we did not observe significant inhibition of SDF-1α–induced chemotaxis with 50 μmol/L PD98059 for CLL cells from any 1 of 5 different donors (data not shown). As such, it appears that the p44/42 MAPK pathway may not be necessary for SDF-1–induced CLL cell migration.
Having established that CLL B cells express functional CXCR4 chemokine receptors, we went on to investigate the importance of this receptor for interactions with marrow stromal cells, which are a major source of the chemokine SDF-1 in vivo. We found that CLL B cells can spontaneously migrate beneath marrow stromal cells within 2 hours. This striking in vitro phenomenon termed pseudoemperipolesis was demonstrated by phase contrast microscopy by the dark appearance of lymphocytes that migrated into the same focal plane as the stromal cells. The term pseudoemperipolesis is used to describe symbiotic complexes of leukemia cells with their stromal cell component in vitro.30,40 In this context, stromal cells also are referred to as “nurse-like cells.” During this cell interaction, leukemia cells migrate beneath the adherent cells or are trapped by membrane projections, but do not become internalized.40 It is used to distinguish this type of cell interaction from “true” emperipolesis, the internalization of lymphocytes by sessile cells, first described in situ for thymocytes internalized by thymic stromal cells (thymic nurse cells).41
Earlier studies on the molecular mechanism of heterotypic adherence between marrow stromal cells and B cells demonstrated that this is a biphasic process, characterized by an early phase of adherence (≤15 minutes). This process partly depends on the interaction between very late antigen-4 (VLA-4; or CD49d) on B lymphocytes with vascular cell adhesion molecule-1 (VCAM-1; or CD106), expressed on stromal cells.40 42
The higher expression of CD49d (VLA-4) integrins on migrated CLL B cells compared with input CLL B cells can either be interpreted as an enrichment of CLL B cells expressing higher levels of CD49d, allowing those to enter the stromal cell layer, or could be explained as an induction of CD49d expression by signals delivered during the migration process. In either case, this observation suggests that CLL B cells interact with VCAM-1 or fibronectin on marrow stromal cells.
The late phase of heterotypic adherence (≥30 minutes), associated with pseudoemperipolesis, does not depend on CD49d/VLA-4 engagement.42 While the early phase of adherence may allow for the initial homing to the marrow, the late phase, where leukemia cells come into closer contact with stromal cells, may confine malignant B cells within the bone marrow, as suggested by Patrick et al.42
In this study, we demonstrated that the chemokine SDF-1 plays a critical role for pseudoemperipolesis, the late phase of heterotypic adherence of CLL B cells to marrow stromal cells according to the above-mentioned model. The almost complete inhibition of migration into the stromal cell layer by pertussis toxin demonstrates that signaling through a Gi protein coupled receptor is required for this migration. The pretreatment of CLL B cells with SDF-1α and the addition of this chemokine to the coculture significantly reduced the pseudoemperipolesis by about 65% compared with nontreated controls. This condition uses 2 antagonizing mechanisms that can interfere with SDF-1–induced migration of CLL B cells into the stromal layer. First, the pretreatment of the CLL cells with this chemokine induces a downmodulation of the CXCR4 receptor on CLL B cells to levels that are ≈10% of nontreated cells, as demonstrated in the receptor endocytosis assay (Fig 2B). Second, by adding these cells in SDF-1 containing medium to the coculture, the exogenous SDF-1 can interfere with a SDF-1 gradient established by SDF-1 secreting stromal cells that otherwise may allow for the directional migration into the stromal cell layer. Direct evidence for the importance on SDF-1 for this migration is provided by significantly blocking pseudoemperipolesis with an anti-CXCR4 MoAb, whereas a control antibody was without any effect (Fig8B). Finally, the strong downmodulation of CXCR4 on CLL B cells that had migrated into the stromal cell layer provides further evidence that SDF-1 contributes to this migration process. From these observations, we propose that heterotypic adhesion between CLL B cells and marrow stromal cells is a multistep process comparable to transendothelial migration, involving the sequential engagement of adhesion molecules and activation through chemokine receptors, in particular CD49d/VLA-4 and CXCR4, respectively.
Earlier studies by Lagneaux et al and others4,43demonstrated that CLL B cells could adhere to human marrow stromal cells. They noted that this adhesion was partly mediated through β1 and β2 integrins, and direct contact with stromal cells prevented spontaneous or steroid-induced apoptosis of CLL B cells in vitro.4 5 In contrast, only very few normal CD5+ B cells adhered to marrow stromal cells, and those were not protected from spontaneous apoptosis. It has therefore been proposed, that the marrow microenvironment, in particular marrow stromal cells, provide factors that allow for the accumulation of CLL B cells and make them more resistant to chemotherapy.
Because SDF-1 induces chemotaxis of CLL B cells and is critical for the spontaneous migration of leukemic CLL B cells beneath marrow stromal cells, we propose that this chemokine allows for the infiltration of the marrow by leukemic CLL cells. In addition, SDF-1 may not only direct, but also confine CLL B cells within the medullary cavity, similar to the recently demonstrated mechanism of progenitor B-cell retention in the marrow by SDF-1.21 Attraction through the CXCR4 chemokine receptor therefore provides a new mechanism to explain how neoplastic B cells can access limited supportive microenvironmental niches in the marrow, which usually are restricted to progenitor cells.
Further studies will have to define whether inhibiting the heterotypic adhesion of B-CLL cells to marrow stromal cells, for example by interfering with CXCR4-receptor signaling in CLL B cells, could modify the growth or survival of CLL B cells. Therefore, future studies on the role of SDF-1 in the interaction of CLL B cells with marrow stromal cells may lead to new therapeutic strategies for patients with this disease.
ACKNOWLEDGMENT
The authors are grateful to Drs L.Z. Rassenti and K. Kato for providing B-CLL cDNA samples and to T.A. Johnson for excellent technical assistance. We thank Dr I. Clark-Lewis for the generous gift of SDF-1α, Dr I.U. Schraufstatter for providing the p44/42 MAPK inhibitor PD98059, and Dr N.J. Zvaifler for suggestions and the critical evaluation of the manuscript.
Supported in part by Grant No. D-96-17136 (to J.A.B.) from the Deutsche Krebshilfe, Bonn, Germany, Grant No. SA 623/2-1 (to M.B.) from the Deutsche Forschungsgemeinschaft, Bonn, Germany, and Grant No. 5 R37CA49870-11 from the National Institutes of Health (to T.J.K.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Thomas J. Kipps, MD, PhD, Department of Medicine, Division of Hematology/Oncology, University of California San Diego, School of Medicine, 9500 Gilman Dr, La Jolla, CA 92093-0663; e-mail: tkipps@ucsd.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal