Abstract
Prothrombin time (PT) is routinely used to monitor oral anticoagulant treatment in patients with the antiphospholipid antibody syndrome (APS). The fact that PT is a phospholipid (PL)-dependent coagulation test raises the possibility that lupus anticoagulant (LA) might interfere with this test, thus complicating the control of anticoagulant treatment. The effect of 6 affinity-purified preparations of anti- (a)β2-glycoprotein I (GPI) antibodies with LA activity on the PT was tested. Instead of prolonging PT as expected, the aβ2-GPI antibodies reduced the PT of both normal plasma and anticoagulated plasma by a mean of 2.4 seconds and 5.6 seconds, respectively. This effect was also observed using other 5 commercially available preparations of thromboplastin. The aβ2-GPI–mediated reduction in PT was dose-dependent and was lost upon removal of β2-GPI. The failure of aβ2-GPI antibodies to express LA activity in PT was found to depend on the fact that calcium ions were added together with PL at the beginning of the assay. In fact, modification of the standard diluted Russell viper venom time (dRVVT) test by adding calcium ions together with PL resulted in a loss of aβ2-GPI anticoagulant activity. The procoagulant effect was not as evident in an assay that used stimulated monocytes as a source of thromboplastin. These results show that aβ2-GPI antibodies exhibit an ‘in vitro’ procoagulant effect in PT and an anticoagulant effect in dRVVT only when the interaction with their antigen and PL occurs in the absence of calcium ions.
THE TERM ‘lupus anticoagulant’ (LA) defines a group of immunoglobulins able to prolong the clotting time of phospholipid (PL)-dependent coagulation tests.1 The interest in detecting LA is related to the fact that its presence is strongly associated with both arterial and venous thromboembolism.2,3 Ten years after demonstration of the antiphospholipid nature of these immunoglobulins in 1980,4it was shown that autoimmune antiphospholipid (aPL) antibodies require the plasma protein β2-glycoprotein I (β2-GPI) to bind anionic PL.5-7 It was subsequently recognized that β2-GPI is essential for the expression of the LA activity of some aPL antibodies,8,9 and that its adsorption removes LA activity from most LA-positive plasma samples.10 Affinity-purified aβ2-GPI antibodies from plasma of LA-positive patients behave as classical LA, with their activity disappearing at increasing PL concentrations.11 Moreover, some monoclonal antibodies to β2-GPI express LA activity,12,13 depending on the specific domain against which they are directed.12 The mechanism underlying LA activity of aβ2-GPI antibodies is related to their ability to enhance binding of β2-GPI to PL, thus impeding binding/activation of clotting factors.12
As suggested by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis,14 the laboratory diagnosis of LA requires the prolongation of at least one PL-dependent coagulation test, and the demonstration that the inhibitor is responsible for this effect (mixing test) by interfering with PL or PL-bound proteins (confirmatory test). Numerous tests measuring clotting parameters such as activated partial thromboplastin time, dilute Russell viper venom time (dRVVT), kaolin clotting time, and tissue thromboplastin inhibition (TTI) are currently used to detect LA. As the use of a single screening test will fail to identify approximately 30% to 40% of LA patients, a combination of two or more tests in the initial evaluation step is recommended. Among PL-dependent coagulation tests, prothrombin time (PT) is not included14because it is rather insensitive15 and sometimes is prolonged due to associated hypoprothrombinemia.16
On the other hand, PT is routinely used to monitor anticoagulation, the mainstream treatment of patients with antiphospholipid antibody syndrome (APS).17,18 As pointed out by Moll and Ortel,19 this monitoring approach could be compromised by the inhibitory effect of LA on PT. This possibility prompted us to test the effect of affinity-purified human aβ2-GPI antibodies with LA activity on PT.
MATERIALS AND METHODS
Blood sampling and screening.
Venous blood was collected in siliconized glass tubes containing one-tenth volume of 3.8% trisodium citrate. Plasma was obtained by centrifugation at 2,000g at room temperature. Anticardiolipin antibody enzyme-linked immunosorbent assay (ELISA), aβ2-GPI antibody ELISA, and dRVVT for the detection of LA activity were performed on patient plasma as described elsewhere.20
Purification of IgG autoantibodies to β2-GPI.
Filtered plasma was loaded onto a prepacked column of Superose (Pharmacia LKB Biotechnology, Uppsala, Sweden) that had been coupled with purified human β2-GPI11 (5 mg of protein per milliliter of Superose) according to the manufacturer’s instructions. After extensive washing with phosphate-buffered saline (PBS), aβ2-GPI antibodies were eluted from the column using 0.1 mol/L glycine 0.5 mol/L NaCl, pH 2.8,21 and immediately buffered with 1 mol/L Tris, pH 8.4. The purity of the resulting aβ2-GPI IgG preparations was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the protein concentration was measured by optical density at 280 nm as previously described.22Normal IgG from a healthy donor was purified from plasma by means of a protein A-Sepharose column (Pharmacia LKB Biotechnology). Purified aβ2-GPI antibodies and control IgG were tested after a 1:4 dilution in aβ2-GPI IgG ELISA as previously described.22
Preparation of β2-GPI–depleted plasma.
Pooled normal plasma was passed through a CL-octyl Sepharose column as previously reported.22 To estimate the quantity of residual β2-GPI in the flow through, 24-μL samples (diluted 1:2 in 20 mmol/L Tris, 150 mmol/L NaCl, pH 7.4 [TBS]) were applied to 4.0-mm wells of a 1% agarose gel containing 6% vol/vol polyclonal aβ2-GPI rabbit antiserum, and subjected to single radial immunodiffusion. Purified β2-GPI was serially diluted with TBS, pH 7.4, and analyzed in parallel to provide a calibration curve. Results of this analysis showed that β2-GPI was no longer detectable in the depleted plasma.
Coagulation studies.
Pooled plasma from 5 healthy subjects and pooled plasma from 5 patients on stable oral anticoagulant treatment were used as coagulation substrates. Clotting times were recorded using an automated fibrometer (Mechrolab Clot-timer Model 202A; Heller Laboratories, Santa Rosa, CA). LA activity of purified aβ2-GPI IgG preparations was evaluated by dRVVT as previously described.22 Standard PT was assayed by preincubating 100 μL of plasma at 37°C for 2 minutes, followed by the addition of 200 μL of calcium thromboplastin and measurement of clotting time. To introduce 100 μL of aβ2-GPI IgG into the test without altering the proportions between reagents, tissue calcium thromboplastin was reconstituted with one-half volume of the appropriate diluting agent. Distilled water was used for the thromboplastin preparations Innovin (Dade, Miami, FL), Thromborel S (Istituto Behring, L’Aquila, Italy), Thromboplastin IS (Baxter Diagnostic Division, Milan, Italy), and IL-HS (Instrumentation Laboratories, Milan, Italy). Recombiplastin (Ortho Clinical Diagnostics, Milan, Italy) was reconstituted with 30 mmol/L CaCl2. The final reaction mixture contained 100 μL of pooled plasma, 100 μL of either TBS or normal pooled or aβ2GPI IgG in Tris buffer, and 100 μL of concentrated calcium thromboplastin. In experiments performed to test the importance of the sequence of reagent addition, tissue thromboplastin was incubated with aβ2-GPI antibodies in the presence or absence of calcium ions for 2 minutes before addition of anticoagulated plasma. In some experiments, the reconstituted Recombiplastin was further diluted with 30 mmol/L CaCl2.
A modified dRVVT was performed by changing the sequence of added reagents. In the dRVVT used to measure the LA activity of purified antibodies, the addition sequence was (1) normal plasma; (2) TBS, normal IgG, or affinity-purified aβ2-GPI IgG; (3) viper venom; (4) PL, followed by incubation at 37°C for 30 seconds; and (5) CaCl2, followed by measurement of clotting time. In a modified test the sequence was (1), (2), and (3), followed by the addition of CaCl2 and PL and measurement of clotting time. A second modified dRVVT was performed by incubating (2), (3), (4), with or without (5) (calcium ions) for 30 seconds followed by initiation of the clotting reaction by adding (1) (normal pooled plasma). Some experiments were performed by using a range of CaCl2concentrations (3 mmol/L to 7 mmol/L).
Induction of tissue factor–like activity in monocytes.
Peripheral blood mononuclear cells (PBMCs) were obtained as described elsewhere.23 Briefly, blood was collected in heparin from a healthy staff volunteer. PBMCs were isolated by Ficoll-Hypaque gradient centrifugation (Pharmacia LKB Biotechnology Inc, Piscataway, NJ) and washed twice with RPMI. PBMCs were then resuspended in RPMI-15% fetal calf serum (FCS) and incubated in a tissue-culture flask (Falcon) for 1 hour at 37°C in a humidified, 5% CO2 atmosphere. Nonadherent cells were then discarded, and adherent cells were collected by scraping and incubated overnight in RPMI-10% FCS. This method resulted in a 3-fold enrichment in CD14+ mononuclear cells, as evaluated by cytofluorimetry after staining with an anti-CD14 antibody. The cells were resuspended at a concentration of 1 × 106 cells/mL, incubated for 6 hours with 10 μg/mL lipopolysaccharide (LPS) to stimulate monocytes, and then centrifuged and resuspended in TBS containing 30 mmol/L CaCl2. PT was measured by adding 50 μL of the stimulated monocyte/CaCl2 preparation to 50 μL of normal pooled plasma that had been preincubated with 50 μL of TBS or aβ2-GPI IgG for 2 minutes at 37°C.
Patients.
Six patients (2 males and 4 females, 40 to 58 years of age) with APS were studied; 4 patients had primary APS, and 2 patients had the syndrome associated with systemic lupus erythematosus. All patients tested positive for anticardiolipin IgG and LA activity. Two patients also had low titers of anticardiolipin IgM. Qualifying thrombotic events were venous thromboembolism in 3 patients and arterial thromboembolism in the remaining 3 cases (1 had acute myocardial infarction and 2 had acute arterial insufficiency in the lower limbs). All patients tested positive for aβ2-GPI IgG.
RESULTS
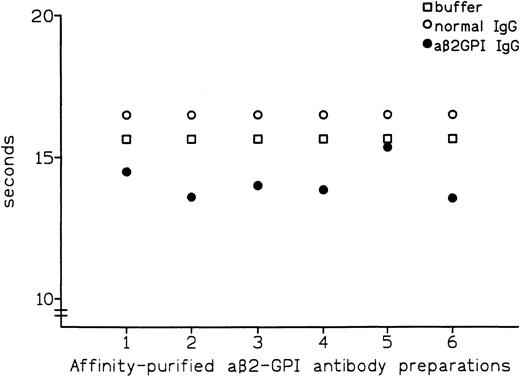
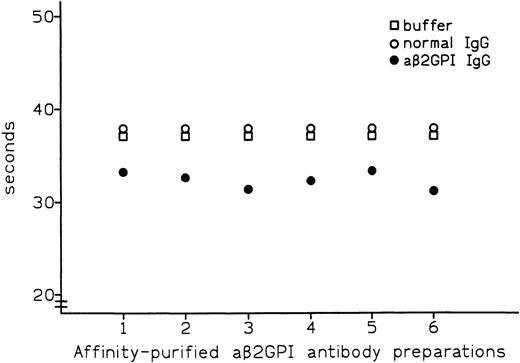
Affinity-purified preparations of aβ2-GPI IgG antibodies were obtained from plasma samples of 6 patients with APS. All 6 preparations showed LA activity in dRVVT, with prolongation of clotting time ranging from 3.7 to 8.3 seconds over control IgG. All 6 preparations also showed marked positivity in aβ2-GPI IgG ELISA, with the OD405 ranging from 2.587 U for patient no. 5 to 3.492 U for patient no. 1 (Table 1). These preparations were then tested by PT (see Materials and Methods) with the thromboplastin routinely used in our laboratory (Recombiplastin) and pooled normal plasma as a substrate. Comparison of results obtained with normal IgG showed that all of the aβ2-GPI antibody preparations slightly shortened the PT, with the reduction ranging from 1.1 seconds in patient no. 5 to 2.9 seconds in patient no. 2 (mean, 2.4 seconds) (Fig 1). As shown in Fig2, this procoagulant effect was also observed in assays performed using pooled plasma from 5 patients on stable long-term anticoagulant treatment (International Normalized Ratio [INR] of pooled plasma was 2.28). Compared with normal IgG, the aβ2-GPI antibody preparations shortened the PT of the anticoagulated pool by a mean of 5.6 seconds (from a minimum of 4.5 seconds in patient no. 5 to a maximum of 6.7 seconds in patient no. 6). Results were comparable when using different pools of normal or anticoagulated plasma (data not shown).
Anticoagulant and ELISA Activities of Affinity-Purified Anti–β2-GPI Antibody Preparations
| . | Normal IgG . | Affinity-Purified aβ2-GPI . | |||||
|---|---|---|---|---|---|---|---|
| No. 1 . | No. 2 . | No. 3 . | No. 4 . | No. 5 . | No. 6 . | ||
| Protein concentration (μg/mL) | 100 | 46 | 41 | 51 | 30 | 54 | 50 |
| dRVVT (seconds prolongation over buffer) | 0.0 | 6.7 | 3.7 | 4.2 | 3.9 | 8.3 | 4.9 |
| Anti–β2-GPI IgG ELISA (OD405) | 0.035 | 3.492 | 3.056 | 3.026 | 3.121 | 2.587 | 3.320 |
| . | Normal IgG . | Affinity-Purified aβ2-GPI . | |||||
|---|---|---|---|---|---|---|---|
| No. 1 . | No. 2 . | No. 3 . | No. 4 . | No. 5 . | No. 6 . | ||
| Protein concentration (μg/mL) | 100 | 46 | 41 | 51 | 30 | 54 | 50 |
| dRVVT (seconds prolongation over buffer) | 0.0 | 6.7 | 3.7 | 4.2 | 3.9 | 8.3 | 4.9 |
| Anti–β2-GPI IgG ELISA (OD405) | 0.035 | 3.492 | 3.056 | 3.026 | 3.121 | 2.587 | 3.320 |
Effect of affinity-purified aβ2-GPI antibody preparations on PT of normal plasma.
Effect of affinity-purified aβ2-GPI antibody preparations on PT of normal plasma.
Effect of affinity-purified aβ2-GPI antibody preparations on PT of plasma from patients on oral anticoagulant treatment.
Effect of affinity-purified aβ2-GPI antibody preparations on PT of plasma from patients on oral anticoagulant treatment.
We next performed additional PT assays to test the influence of the tissue thromboplastin preparations and the sequence of reagent addition on the observed reduction in clotting time. To limit experimental variability, these assays were performed using aβ2-GPI preparations (nos. 1A and 1B) obtained from one of the patients (no. 1).
Table 2 summarizes results of PT performed using 4 additional preparations of tissue thromboplastin (ie, Innovin, Thromborel S, Thromboplastin IS, and IL-HS) and either aβ2-GPI preparation no. 1A (52 μg/mL) or normal IgG (100 μg/mL). The aβ2-GPI IgG antibodies shortened the PT regardless of the thromboplastin preparation used, yielding a mean reduction of 2.1 and 10.1 seconds using normal and anticoagulated plasma, respectively. The reduction in PT was particularly evident in tests performed using anticoagulated plasma and either Thromborel S or Thromboplastin IS (9.8 and 19.3 seconds, respectively).
Effect of aβ2-GPI Antibodies From Patient No. 1 on PT of Normal Plasma (NP) and Plasma From Patients on Oral Anticoagulants (OA), Measured Using Different Thromboplastin Preparations (INR of pooled anticoagulated plasma = 2.7)
| Thromboplastin Preparation . | PT s (+buffer) . | PT s (+normal IgG) . | PT s (+IgG anti–β2-GPI) . | |||
|---|---|---|---|---|---|---|
| NP . | OA . | NP . | OA . | NP . | OA . | |
| Innovin | 12.7 | 36.9 | 12.9 | 37.3 | 11.5 (−1.4) | 31.7 (−5.6) |
| Thromborel S | 17.9 | 38.5 | 17.9 | 39.9 | 14.5 (−3.4) | 30.1 (−9.8) |
| Thromboplastin IS | 17.8 | 53.6 | 17.4 | 56.8 | 15.2 (−2.2) | 37.5 (−19.3) |
| IL-HS | 18.1 | 33.7 | 18.0 | 34.6 | 16.6 (−1.4) | 28.9 (−5.7) |
| Thromboplastin Preparation . | PT s (+buffer) . | PT s (+normal IgG) . | PT s (+IgG anti–β2-GPI) . | |||
|---|---|---|---|---|---|---|
| NP . | OA . | NP . | OA . | NP . | OA . | |
| Innovin | 12.7 | 36.9 | 12.9 | 37.3 | 11.5 (−1.4) | 31.7 (−5.6) |
| Thromborel S | 17.9 | 38.5 | 17.9 | 39.9 | 14.5 (−3.4) | 30.1 (−9.8) |
| Thromboplastin IS | 17.8 | 53.6 | 17.4 | 56.8 | 15.2 (−2.2) | 37.5 (−19.3) |
| IL-HS | 18.1 | 33.7 | 18.0 | 34.6 | 16.6 (−1.4) | 28.9 (−5.7) |
In the standard PT assay, plasma was preincubated at 37°C for 2 minutes before the addition of calcium-thromboplastin and measurement of clotting time. Results of modified assays in which aβ2-GPI preparation (no. 1B) and thromboplastin were incubated in the absence (−Ca) or presence (+Ca) of calcium ions for 2 minutes before the addition of anticoagulated plasma showed that the sequence of reagent addition did not influence the procoagulating effects of the antibodies with PT reduced by 9.2 seconds in standard assay, and by 7.6 and 10.1 seconds in the −Ca and +Ca assays, respectively.
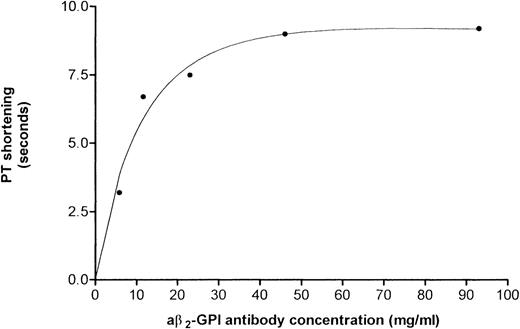
Results of PT assays performed using a pool of anticoagulated plasma (INR = 3.0) and increasing amounts of a aβ2-GPI preparation no. 1B showed that the reduction in PT was dose-dependent (Fig 3). This IgG preparation shortened PT by 3.2, 6.7, 7.5, 9 seconds when added at a concentration of 5.8, 11.6, 23.2, and 46.5 μg/mL, respectively. The undiluted preparation (93 μg/mL) shortened the PT by 9.2 seconds (ie, from 47.7 seconds using normal IgG to 38.5 seconds).
Reduction in PT induced by concentrations of aβ2-GPI antibody preparation no. 1B. A dose-response was obtained starting at an IgG concentration of 5.8 μg/mL. The reduction in PT was ascertained using pooled anticoagulated plasma (INR = 3.0).
Reduction in PT induced by concentrations of aβ2-GPI antibody preparation no. 1B. A dose-response was obtained starting at an IgG concentration of 5.8 μg/mL. The reduction in PT was ascertained using pooled anticoagulated plasma (INR = 3.0).
To ascertain the role of the target antigen, β2-GPI, in aβ2-GPI–mediated PT shortening, PT assays were performed using plasma that had been depleted of β2-GPI by affinity chromatography (see Materials and Methods). Results showed that β2-GPI–depleted plasma exhibited a substantial loss in the ability to shorten PT (ie, from a 9.2-second reduction to a 1.5-second reduction), indicating that the procoagulant effect of aβ2-GPI antibodies is dependent on the presence of their antigen. Moreover, in PT performed by incubating pooled anticoagulated plasma with PL and recombinant-tissue factor (r-TF) for 2 minutes before adding calcium ions, aβ2-GPI IgG did not exhibit procoagulant effect (ie, 45.8 seconds for aβ2-GPI preparation no. 1B v 47 seconds for normal IgG). Therefore, procoagulant activity of aβ2-GPI IgG is not detectable in PT when plasma is incubated with PL in the absence of calcium ions.
Previous results had shown that the use of diluted tissue thromboplastin in TTI yielded comparable PTs in the presence or absence of aβ2-GPI IgG with LA activity.24This prompted us to test whether diluting thromboplastin would affect the procoagulant activity of aβ2-GPI IgG. As shown in Fig4, dilution of Recombiplastin progressively abolished the aβ2-GPI–mediated reduction in PT of both normal and anticoagulated plasma. Therefore, the observed procoagulant effect also depend on the PL/r-TF concentration.
Effect of r-TF/PL (Recombiplastin) dilutions on reduction of PT of normal or anticoagulated plasma measured using anti-β2GPI antibody preparation no. 1A.
Effect of r-TF/PL (Recombiplastin) dilutions on reduction of PT of normal or anticoagulated plasma measured using anti-β2GPI antibody preparation no. 1A.
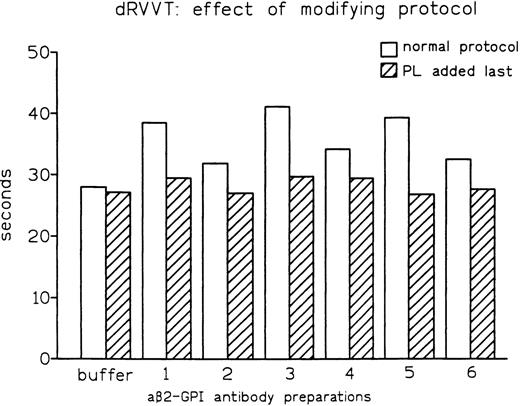
As both dRVVT and PT depend on PL, we reasoned that the distinct abilities of these tests to disclose LA might be influenced by the sequence in which the reagents are mixed. One major difference between the PT and dRVVT protocols is that calcium ions are added together with PL in the former, while they are added after PL in the latter. In fact, modification of the dRVVT protocol by concomitant addition of calcium ions and anionic PL (as in PT) resulted in normal clotting times (Fig5). The same effect was observed when dRVVT was performed by incubating all reagents for 30 seconds before adding normal pooled plasma. In fact, while affinity-purified aβ2-GPI antibody preparation no. 1B prolonged conventional dRVVT by 10.3 seconds, it showed no prolongation when normal pooled plasma was added last. The same behavior, ie, no prolongation, was observed when preparation no. 1B was used and calcium ions and normal pooled plasma were added after 30 seconds of incubation of the other reagents. Moreover, an increase in final concentration of calcium from 3 mmol/L to 7 mmol/L completely abolished the LA activity of aβ2-GPI antibodies (data not shown). These observations showed that detection of aβ2-GPI LA in dRVVT assay requires the incubation of plasma with PL in the absence of calcium ions.
Effect of modification of the assay protocol on LA activity expressed by affinity-purified aβ2-GPI antibody preparations.
Effect of modification of the assay protocol on LA activity expressed by affinity-purified aβ2-GPI antibody preparations.
Finally, we tested whether this procoagulant effect could be mediated by monocytes, cells known to express tissue factor activity on their surface when provided with specific stimuli. As shown in Table3, compared with nonactivated monocytes, activated monocytes shortened the clotting time of normal plasma from 220 to 42.6 seconds in the presence of Tris buffer and from 217 to 43.3 seconds in the presence of normal IgG; aβ2-GPI IgG preparations nos. 4 and 6 exhibited a modest procoagulant effect (ie, reduction in clotting time by 3 seconds and 2.4 seconds, respectively) in the presence of activated monocytes.
PT of Normal Plasma in the Presence of aβ2-GPI Antibody Preparations Measured Using Monocytes as a Source of Tissue Factor
| . | PT (s) (normal plasma) . |
|---|---|
| Buffer + nonactivated monocytes | 220 |
| Normal IgG + nonactivated monocytes | 217 |
| Buffer + activated monocytes | 42.6 |
| Normal IgG + activated monocytes | 43.2 |
| aβ2-GPI IgG no. 1 | 43.3 |
| aβ2-GPI IgG no. 2 | 42 |
| aβ2-GPI IgG no. 3 | 42.6 |
| aβ2-GPI IgG no. 4 | 40.2 |
| aβ2-GPI IgG no. 5 | 42.6 |
| aβ2-GPI IgG no. 6 | 40.8 |
| . | PT (s) (normal plasma) . |
|---|---|
| Buffer + nonactivated monocytes | 220 |
| Normal IgG + nonactivated monocytes | 217 |
| Buffer + activated monocytes | 42.6 |
| Normal IgG + activated monocytes | 43.2 |
| aβ2-GPI IgG no. 1 | 43.3 |
| aβ2-GPI IgG no. 2 | 42 |
| aβ2-GPI IgG no. 3 | 42.6 |
| aβ2-GPI IgG no. 4 | 40.2 |
| aβ2-GPI IgG no. 5 | 42.6 |
| aβ2-GPI IgG no. 6 | 40.8 |
DISCUSSION
LA is a strong risk factor for thrombosis, and its association with either arterial or venous thrombosis defines the APS syndrome.25 Most patients with this condition are treated with oral anticoagulants17,18,26 and undergo PT-INR monitoring, the internationally accepted method used to assess oral anticoagulant treatment. It has been reported that the prolongation of PT in patients with LA reflects both the effect of warfarin and the ‘in vitro’ anticoagulant effect of their autoantibodies; therefore, PT-INR values might not accurately reflect the true level of anticoagulation.19,27 The present study was undertaken to elucidate the effect of aβ2-GPI LA, a common LA in patients with APS syndrome,11 on PT. Results showed that, instead of prolonging PT, these LA accelerate coagulation of both normal plasma and plasma from patients on oral anticoagulant treatment. This explains the observation that (1) PT is seldom influenced in the presence of LA; (2) PT with Simplastin is not affected by monoclonal antibodies against β2-GPI28; and (3) the TTI is not influenced by aβ2-GPI LA.24
The mechanism by which aβ2-GPI antibodies shorten PT (with possible implications between this phenomenon and the occurrence of thromboembolic events) is difficult to explain. Our data show that aβ2-GPI–mediated PT shortening exhibits the following characteristics: (1) it is dose-dependent; (2) it requires the presence of the plasma antigen, β2-GPI; (3) it is not dependent on the type of thromboplastin; (4) it disappears upon dilution of TF and the template PL through which these antibodies recognize their antigen11 24; and (5) it is abolished when calcium ions are added last to initiate clotting. Therefore, aβ2-GPI, β2-GPI, and PL in the presence of calcium ions accelerate thrombin formation when the activator of coagulation is TF.
Some investigators suggest that aβ2-GPI antibodies determine a PL-dependent reduction in the activity of tissue factor pathway inhibitor (TFPI).29 Given that extrinsic factor Xa is more efficiently generated when β2-GPI, TFPI, and anionic PL are all present,29 the accumulated findings led to the hypothesis that antiβ2GPI-β2GPI complexes formed in the presence of anionic PL might inhibit TFPI by impeding the building of the quaternary complex (TFPI/FXa/FVIIa/TF). Our data focus attention on the tissue factor pathway of coagulation to explain the paradox that ‘in vitro’ anticoagulant antibodies have an ‘in vivo’ procoagulant effect.
The ‘in vitro’ anticoagulant effect of aβ2-GPI antibodies is in agreement with the observation that binding of aβ2-GPI/β2-GPI complexes to PL membranes severely impairs the absorption or hinders the lateral mobility and activation of clotting factors.12,30 It was recently shown that increasing ionic strength and calcium ions markedly inhibit the binding of β2-GPI to anionic PL in comparison with coagulation proteins30 and that β2-GPI/aβ2GPI complex formation on the PL surface (plannar membrane, PS/PC 20/80) is reduced in the presence of physiological concentrations of calcium ions.31 In the same way, the present study showed that the LA of aβ2-GPI IgG was no longer evident when the dRVVT was modified by adding PL in the presence of calcium ions or in any situation in which β2-GPI (ie, plasma), PL and aβ2-GPI antibodies were allowed to interact in the presence of calcium ions.
It was recently reported that aPL antibodies that induce APS are able to stimulate mononuclear cells to produce a procoagulant activity (PCA) that resembles tissue factor (TF).32 Interestingly, upregulation of the TF pathway with elevated circulating levels of TF and TFPI was demonstrated in patients with APS, thus implicating the TF pathway in the pathogenesis of aPL-related thrombosis.33Our observation that TF is able to induce a procoagulant effect in normal plasma in the presence of aβ2-GPI might be relevant in relation of the increased circulating levels of TF shown in these patients. On the contrary, TF produced by stimulated normal monocytes did not produce a substantial procoagulant effect, with only 2 of 6 aβ2-GPI IgG preparations producing a mild reduction in PT in comparison to normal IgG.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to V. Pengo, MD, Department of Clinical and Experimental Medicine, Thrombosis Center, University of Padova School of Medicine, ‘Ex Busonera’ Hospital, via Gattamelata 64, I-35128 Padova, Italy; e-mail: pengo@ux1.unipd.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal