Abstract
This study evaluated the contributing roles of flow cytometric immunophenotyping of blood and bone marrow and immunohistochemical paraffin section staining of bone marrow biopsies in the staging of B-cell malignant lymphoma. Flow immunophenotyping was performed on a marrow specimen in 175 cases; a corresponding blood specimen was also immunophenotyped in 135 of these cases. Morphologic marrow involvement by lymphoma was found in 59 cases; flow immunophenotyping identified 54 cases with a monoclonal B-cell process: morphology-positive/flow-positive (n = 49), morphology-positive/flow-negative (n = 10), morphology-negative/flow-positive (n = 5), and morphology-negative/flow-negative (n = 111). The 10 morphology-positive/flow-negative cases included 5 follicular and 5 large-cell lymphomas with minimal marrow involvement. All 5 morphology-negative/flow-positive cases were from patients with large-cell lymphomas and bulky clinical disease. Because the blood contained the same B-cell clone in 2 of 2 morphology-negative/flow-positive cases studied, blood contamination of marrow may account for these findings. Blood flow cytometric immunophenotyping studies were positive in 32 cases; 30 had marrow involvement by morphology and were from patients with follicular, mantle cell, lymphoplasmacytic, small lymphocytic, or marginal zone lymphomas. From our results, we conclude that (1) bone marrow flow cytometric immunophenotyping is not a cost-effective replacement for good morphologic evaluation in lymphoma staging and that (2) a positive peripheral blood flow cytometric immunophenotyping study when performed in low-grade lymphomas correlates with marrow involvement.
MALIGNANT LYMPHOMA is the most common hematologic malignancy encountered in the Western world.1,2Over the last decade there has been a continual growth in the number of ancillary tools that can be used in the laboratory to evaluate malignant lymphoma.3-10 This has proved critical in providing the consistent and accurate information that is needed for clinical decision making in these patients. The laboratory evaluation of patients with malignant lymphoma remains centered on 4 primary aspects: (1) recognition and diagnosis of disease; (2) appropriate classification11; (3) providing information regarding disease stage; and (4) providing prognostic indications that predict the risk of death from disease.12 13
A complete and accurate staging of the patient with malignant lymphoma is essential in determining the extent of disease, which may affect both the prognosis and the potential therapeutic options. Staging of patients with malignant lymphoma has become an increasingly noninvasive, radiologic-based process.1,2,14 However, obtaining a bone marrow (BM) biopsy has remained an important part of evaluating these patients. BM involvement by lymphoma has traditionally been detected by morphologic assessment of biopsy specimens. Compared with unilateral BM biopsies, the evaluation of bilateral trephine specimens apparently increases the rate of detection of lymphoma by 10% to 20%,15-17 although studies have not precisely controlled for the size of the specimen examined.
Other ancillary laboratory studies are available that can be incorporated to aid in the assessment of lymphoma. These include flow cytometric immunophenotyping with monoclonal antibodies (MoAbs) directed against lymphoid-associated antigens, immunohistochemical staining on paraffin-embedded BM biopsy material, and molecular studies of Ig genes/T-cell receptor genes or lymphoma-associated gene translocations.3-10 The precise role of these latter 3 techniques over and above morphologic assessment has not been fully established. Despite this lack of scientific evidence, there appears to be an increasing belief that immunophenotyping studies are crucial in the examination of a BM specimen for possible lymphoma. Most previous studies have looked at immunophenotyping in specific disease entities18-22 and not at the larger issue of how these modalities should be used in the global approach to the patient with malignant lymphoma. With this as background, it was the intention of this study to evaluate the contributing roles of flow cytometric immunophenotyping of blood and BM and immunohistochemical paraffin section staining of BM biopsies in the staging of B-cell malignant lymphoma and to determine how best to use these laboratory resources.
MATERIALS AND METHODS
Adult patients undergoing a BM aspiration and biopsy who had a preceding or concurrent lymph node or other tissue biopsy that was diagnosed as malignant lymphoma were identified for this study. Because κ and λ surface Ig analysis offers an objective method for determining B-cell clonality, only those lymphoma cases with a B-cell immunophenotype were selected; T-cell lymphoma and Hodgkin’s disease patients were excluded. Marrow-based lymphoproliferative disorders, such as chronic lymphocytic leukemia (CLL), Waldenstrom’s macroglobulinemia, and hairy cell leukemia, were excluded, because marrow involvement is an integral part of those diseases. One hundred seventy-five consecutive Mayo Clinic bilateral BM specimens from patients with a diagnosis of B-cell malignant lymphoma and meeting the restrictions given above were identified and were collected over a 7-month period. The study protocol was reviewed and approved by the Institutional Review Board with consent obtained before the beginning of this study.
Wright-Giemsa–stained slides of peripheral blood (PB) and BM aspirate smears were reviewed for atypical lymphoid/lymphoma cells, independent of immunophenotypic studies. Bilateral BM trephine biopsies were obtained in all cases, fixed in B5 fixative, decalcified, and paraffin-embedded. Five biopsy levels were cut for each specimen with the first, third, and fifth levels stained with hematoxylin and eosin (H&E) for morphologic assessment of malignant lymphoma.
Flow cytometric immunophenotyping of an EDTA, anticoagulated BM aspirate specimen was performed in each case. A PB specimen obtained within 2 days of the BM specimen was also immunophenotyped by flow cytometry in 135 of the 175 cases. A standard, whole-blood/marrow assay with erythrocyte cell lysis was used for preparing all of the BM aspirate and PB specimens.23-25 Because the lymphomas associated with these cases were classified on the basis of the lymph node/tissue biopsy, the BM and PB immunophenotypic analyses were solely centered on whether there was involvement by malignant lymphoma, ie, whether there was a monoclonal B-cell population present. As such, all cases had, at minimum, immunophenotyping performed with MoAbs directed against CD19 and κ and λ Ig. These antibodies were combined as CD19-phycoerythrin (PE)/κ-fluorescein isothiocyanate (FITC) and CD19-PE/λ-FITC (BioSource International, Camarillo, CA). The anti-κ and anti-λ MoAb solutions were titrated for protein concentration and κ/λ signals.
Determination of monoclonality was based on a combination of flow cytometric histogram evaluation and correlation with the percentage of cellular positivity.21,26 A normal range of κ-to-λ ratios had been previously determined in the laboratory to be 0.3:1 to 3.0:1.26 Other MoAbs evaluated in the majority of cases included CD3, CD5, CD10, CD11c, CD16/56, CD20, CD22, CD23, and CD45. Two-color or 3-color flow cytometric analysis was used depending on the case findings, previous immunophenotyping studies, sample availability, etc. Both forward/side light scatter and CD45/side light scatter were used in each case as the primary gating methodologies. Further gating was performed as necessary on either lymphoid subpopulations based on cell size or as backgating on CD19+ B-cell staining events.25
Immunohistochemical staining of the paraffin-embedded BM biopsies was performed in all cases and used standard techniques that have been previously published.27 Antibodies used included polyclonal anti-CD3 and monoclonal anti-CD20 (Dako Corp, Carpinteria, CA).
RESULTS
Clinical.
The 175 patients included in this study consisted of 105 men and 70 women. The ages of these patients ranged from 19 to 94 years (median, 64 years of age); 1 patient was less than 20 years old and 11 patients were greater than 80 years of age. One-hundred seven of the 175 patients were evaluated as part of the initial disease presentation requiring a diagnostic biopsy procedure. The remaining 68 patients were being evaluated for a variety of reasons, including restaging of disease while in complete or partial remission, restaging for relapsed disease, pre-BM transplantation study, pre-protocol study, or suspected transformation to a more aggressive disease. The 175 patients were staged as follows: stage I = 31, stage II = 38, stage III = 14, and stage IV = 82; the remaining 10 patients could not be accurately staged for various reasons. The international prognostic index (IPI)12 13 showed the following: IPI of 0 = 25, IPI of 1 = 63, IPI of 2 = 48, IPI of 3 = 26, IPI of 4 = 11, and IPI of 5 = 2.
Lymph node/tissue/marrow biopsy.
Table 1 shows the various lymph node/tissue diagnoses from the cases included in this study. Large-cell lymphoma and follicular lymphomas accounted for the majority of diagnoses. Morphologic assessment alone of the 175 BM biopsies demonstrated that 59 cases had involvement by malignant lymphoma. As expected, the small B-cell lymphoid neoplasms (follicular lymphoma, mantle cell lymphoma, small lymphocytic lymphoma, lymphoplasmacytic lymphoma, and marginal zone lymphoma) had the highest percentage of cases with marrow infiltration, whereas marrow involvement was less common in the large-cell lymphomas and the low-grade lymphomas of MALT type.
Lymph Node, BM, and Flow Cytometric Findings
| Lymphoma Diagnosis . | n . | Morphology +* . | BM Flow +† . |
|---|---|---|---|
| Large cell | 74 | 5 | 5 |
| Follicular | 58 | 30 | 25 |
| Extranodal marginal zone‡ | 12 | 0 | 0 |
| Mantle cell | 10 | 8 | 8 |
| Small lymphocytic | 9 | 9 | 9 |
| Lymphoplasmacytic | 9 | 6 | 6 |
| Splenic marginal zone | 1 | 1 | 1 |
| Burkitt’s | 1 | 0 | 0 |
| Not classifiable | 1 | 0 | 0 |
| Total | 175 | 59 | 54 |
| Lymphoma Diagnosis . | n . | Morphology +* . | BM Flow +† . |
|---|---|---|---|
| Large cell | 74 | 5 | 5 |
| Follicular | 58 | 30 | 25 |
| Extranodal marginal zone‡ | 12 | 0 | 0 |
| Mantle cell | 10 | 8 | 8 |
| Small lymphocytic | 9 | 9 | 9 |
| Lymphoplasmacytic | 9 | 6 | 6 |
| Splenic marginal zone | 1 | 1 | 1 |
| Burkitt’s | 1 | 0 | 0 |
| Not classifiable | 1 | 0 | 0 |
| Total | 175 | 59 | 54 |
BM biopsy morphology positive for lymphoma.
BM flow cytometric study positive for lymphoma.
Low-grade lymphoma of MALT type.
Immunophenotyping: BM aspirate.
Flow cytometric immunophenotyping of the BM aspirate identified 54 cases with a monoclonal B-cell process (Table 1): 29 with κ Ig and 25 with λ Ig light chain restriction. The percentage of monoclonal B cells detected by immunophenotyping ranged from 0.09% to 58.6% (median, 3.2%) of the overall marrow cell population (not just the lymphoid gate). Thirty-seven cases had small monoclonal populations that accounted for less than 5% of the overall BM aspirate cell specimen; 14 demonstrated less than 1% monoclonal B cells.
The 121 BM cases with a negative flow cytometric study typically showed a mixture of polyclonal B cells and a small population of CD19+/Ig− cells that represent normal CD19+, precursor B cells. The median κ-to-λ ratio in these 121 cases was 1.29:1 (range, 0.27:1 to 2.94:1), confirming the normal range previously determined in the laboratory.
The concurrence rate of morphologic assessment and flow cytometric immunophenotyping in evaluating for the presence of lymphoma was 91.4% (160 of 175 BM cases). Forty-nine cases showed evidence of malignant lymphoma by both morphologic and flow cytometric immunophenotypic modalities, whereas 111 cases were negative for malignant lymphoma by both methods (Table 2).
Flow Cytometric Results Versus BM Morphology
| . | Flow Results . | |
|---|---|---|
| Monoclonal . | Polyclonal . | |
| Morphology positive | 49 | 10 |
| Morphology negative | 5 | 111 |
| . | Flow Results . | |
|---|---|---|
| Monoclonal . | Polyclonal . | |
| Morphology positive | 49 | 10 |
| Morphology negative | 5 | 111 |
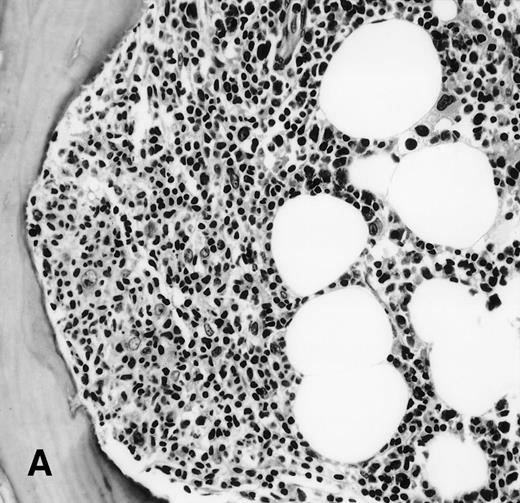
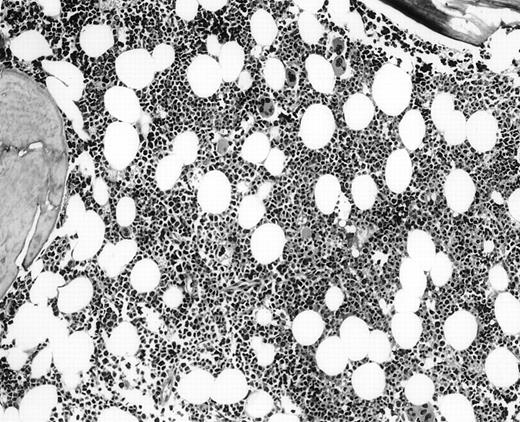
It is the remaining 15 cases that deserve additional description. Ten cases demonstrated morphologic features of lymphoma on the BM biopsy specimen and were confirmed by immunohistochemical staining, but had a polyclonal B-cell immunophenotypic pattern. Subgating based on cell size and backgating with CD19 or other antibodies failed to identify a monoclonal or abnormal B-cell population. κ/λ ratios ranged from 0.20 to 1.8:1 in these 10 cases. Five of the 10 cases were from patients with follicular lymphoma, predominantly small cleaved type, and 5 were from patients with large-cell lymphoma (Fig 1). One of the latter 5 patients with large-cell lymphoma had a discordant BM morphology with primarily a small, cleaved cell infiltrate seen in the BM sections. In addition to morphologic features indicative of lymphoma, all 10 cases demonstrated a prominent CD20+, lymphomatous infiltrate by immunohistochemistry on paraffin sections of the marrow biopsies. The extent of involvement by lymphoma in these cases was quite variable, with 4 showing small, focal, paratrabecular patterns of infiltration; 4 showing less than 20% involvement in a random, nodular pattern; and 2 cases with single, albeit relatively large, foci. It seems reasonable to conclude that the discordance seen in these 10 cases may be ascribed to either variation in sampling between the aspirate and biopsy, the minimal marrow disease seen in some biopsies and not represented in the aspirate specimen, or failure to aspirate the diagnostic cells due to reticulin fibrosis that can be associated with a lymphomatous infiltrate.16 28
(A) BM biopsy showing a paratrabecular lymphoid infiltrate of atypical lymphoid cells, diagnostic of involvement by malignant lymphoma and confirmed by CD20+immunohistochemical staining. A lymph node biopsy showed a follicular lymphoma. Flow cytometric studies demonstrated polyclonal B cells with no monoclonal B-cell population identified (H&E; original magnification × 400). (B) BM biopsy showing a nodular lymphoid infiltrate of atypical, large lymphoid cells, diagnostic of involvement by malignant lymphoma and confirmed by CD20+ immunohistochemical staining. A lymph node biopsy showed a large-cell lymphoma. Flow cytometric studies of the marrow aspirate demonstrated polyclonal B cells with no monoclonal B-cell population identified (H&E; original magnification ×400).
(A) BM biopsy showing a paratrabecular lymphoid infiltrate of atypical lymphoid cells, diagnostic of involvement by malignant lymphoma and confirmed by CD20+immunohistochemical staining. A lymph node biopsy showed a follicular lymphoma. Flow cytometric studies demonstrated polyclonal B cells with no monoclonal B-cell population identified (H&E; original magnification × 400). (B) BM biopsy showing a nodular lymphoid infiltrate of atypical, large lymphoid cells, diagnostic of involvement by malignant lymphoma and confirmed by CD20+ immunohistochemical staining. A lymph node biopsy showed a large-cell lymphoma. Flow cytometric studies of the marrow aspirate demonstrated polyclonal B cells with no monoclonal B-cell population identified (H&E; original magnification ×400).
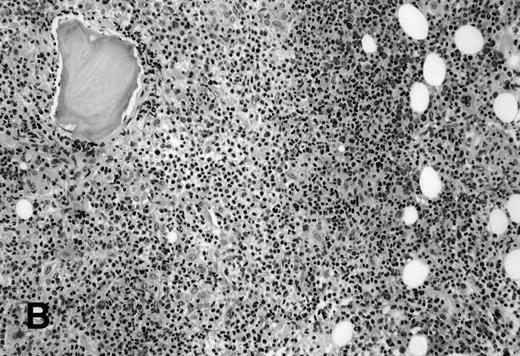
The 5 BM morphology-negative, flow cytometric-positive cases were all from patients with large-cell lymphomas (Table 3). Four patients, independent of the flow results, had localized, but bulky clinical disease: 1 with stage I, 2 with stage II, 1 with stage III, and 1 with widespread stage IV disease. All were treated with chemotherapy, independent of knowing the positive flow cytometric immunophenotyping results. No morphologic or immunohistochemical evidence of lymphoma was seen in these 5 BM biopsy specimens (Fig2). Substantial bilateral biopsy material was available, ranging from 2 to 6.5 cm of trephine biopsy. Interestingly, the PB contained the same B-cell clone in 2 of 2 cases in which concomitant blood immunophenotyping was also performed. These 5 cases showed small monoclonal populations by flow cytometric analysis that ranged from 0.09% to 3% of the overall cell population analyzed, indicating that all cases had an extremely small population of clonal cells. Unfortunately, insufficient follow-up time has accrued that would allow clinical outcome analysis in this interesting subset of patients.
BM Morphology-Negative/Flow Cytometric-Positive Cases (n = 5)
| Diagnosis . | Presentation . | Stage . | IPI . |
|---|---|---|---|
| 1. Large cell | Inguinal mass; proximity to femoral artery-? pseudo-aneurysm | IA | 1 |
| 2. Large cell | Right neck mass; 8 × 10 cm | IIAE | 1 |
| 3. Large cell | Pelvic mass; perforation of inguinal wall with associated abscess | IIE | 2 |
| 4. Large cell | Pelvic mass; sigmoid perforation | IIIA | 2 |
| 5. Large cell | Epidural disease | IV | 4 |
| Diagnosis . | Presentation . | Stage . | IPI . |
|---|---|---|---|
| 1. Large cell | Inguinal mass; proximity to femoral artery-? pseudo-aneurysm | IA | 1 |
| 2. Large cell | Right neck mass; 8 × 10 cm | IIAE | 1 |
| 3. Large cell | Pelvic mass; perforation of inguinal wall with associated abscess | IIE | 2 |
| 4. Large cell | Pelvic mass; sigmoid perforation | IIIA | 2 |
| 5. Large cell | Epidural disease | IV | 4 |
BM biopsy showing a normocellular marrow with no morphologic features of lymphoma, confirmed by negative immunohistochemical staining. A lymph node biopsy showed a large-cell lymphoma. Flow cytometric studies of the marrow aspirate demonstrated a small population of monoclonal B cells (H&E; original magnification × 400).
BM biopsy showing a normocellular marrow with no morphologic features of lymphoma, confirmed by negative immunohistochemical staining. A lymph node biopsy showed a large-cell lymphoma. Flow cytometric studies of the marrow aspirate demonstrated a small population of monoclonal B cells (H&E; original magnification × 400).
Immunophenotyping: PB.
PB immunophenotyping studies showed a monoclonal B-cell population in 32 of the 135 cases analyzed (Table 4). Thirty of these 32 flow-positive cases had concurrent BM involvement as assessed by morphologic and immunophenotypic findings. The 2 remaining flow-positive cases were the morphology-negative, large-cell lymphomas previously discussed. Table 4 shows the lymph node diagnoses in these 32 patients with positive blood studies. Not surprisingly, the blood-positive cases were from patients with follicular lymphoma, mantle cell lymphoma, small lymphocytic lymphomas, lymphoplasmacytic lymphoma, or splenic marginal zone lymphoma, all known to have a relatively high incidence of PB involvement.3,6,16,20,22,29 30 Although a positive PB immunophenotyping study was usually associated with BM involvement by lymphoma, a polyclonal blood study did not exclude the possibility of lymphoma involving the marrow. Table 4 indicates that follicular lymphoma, mantle cell lymphoma, lymphoplasmacytic lymphoma, and even small lymphocytic lymphoma do not always demonstrate a monoclonal B-cell population in the PB. The percentage of monoclonal B cells detected in this study by immunophenotyping of the PB ranged from 0.01% to 49.2% (median, 2.1%) of the overall leukocyte population (not the percentage of the lymphoid gate alone). Eighteen cases had small monoclonal populations that accounted for less than 5% of the overall blood cell specimen; 15 demonstrated less than 1% monoclonal B cells.
PB: Flow Cytometric Analysis
| Lymphoma Diagnosis . | n . | BM Morphology +4-150 . | PB Flow +4-151 . |
|---|---|---|---|
| Large cell | 62 | 3 | 2 |
| Follicular | 42 | 21 | 15 |
| Extranodal marginal zone‡ | 8 | 0 | 0 |
| Mantle cell | 9 | 7 | 7 |
| Small lymphocytic | 5 | 5 | 4 |
| Lymphoplasmacytic | 6 | 4 | 3 |
| Splenic marginal zone | 1 | 1 | 1 |
| Burkitt’s | 1 | 0 | 0 |
| Not classifiable | 1 | 0 | 0 |
| Total | 135 | 41 | 32 |
| Lymphoma Diagnosis . | n . | BM Morphology +4-150 . | PB Flow +4-151 . |
|---|---|---|---|
| Large cell | 62 | 3 | 2 |
| Follicular | 42 | 21 | 15 |
| Extranodal marginal zone‡ | 8 | 0 | 0 |
| Mantle cell | 9 | 7 | 7 |
| Small lymphocytic | 5 | 5 | 4 |
| Lymphoplasmacytic | 6 | 4 | 3 |
| Splenic marginal zone | 1 | 1 | 1 |
| Burkitt’s | 1 | 0 | 0 |
| Not classifiable | 1 | 0 | 0 |
| Total | 135 | 41 | 32 |
The 2 large-cell lymphomas with positive blood flow studies showed no morphologic features of lymphoma in the corresponding BM specimens.
BM biopsy morphology positive for lymphoma.
BM flow cytometric study positive for lymphoma.
Low-grade lymphoma of MALT type.
Immunophenotyping: histogram evaluation.
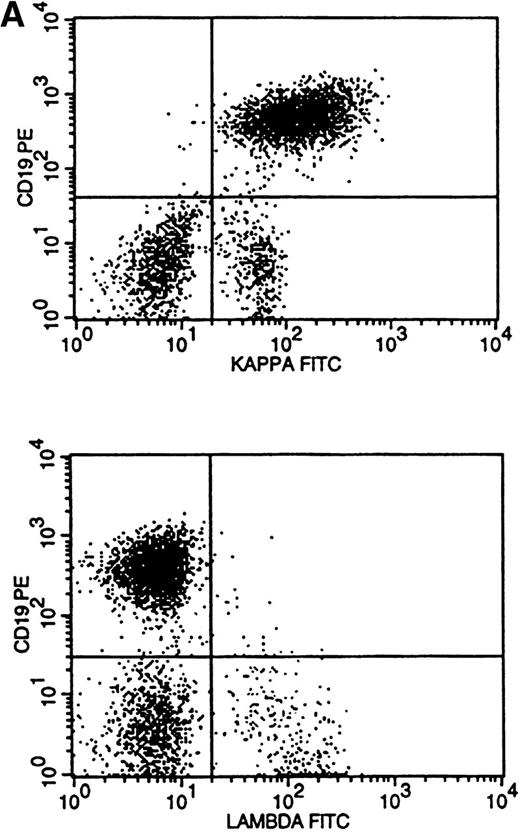
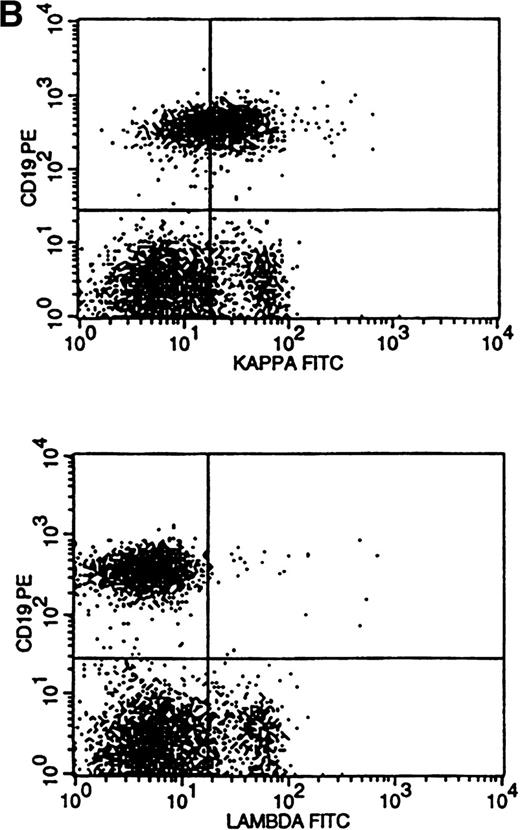
Flow cytometric histogram evaluation was critical in determining κ or λ monoclonality. Four patterns of κ/λ expression were seen in these histograms. Bright surface Ig (sIg) expression was the most obvious pattern to recognize and was found in 28 cases (Fig 3A). This was reflected in the κ-to-λ ratio seen in these specimens (for κ, median of 24:1 and range of 9 to 189:1; for λ, median of 0.1:1 and range of 0.01 to 0.2:1).
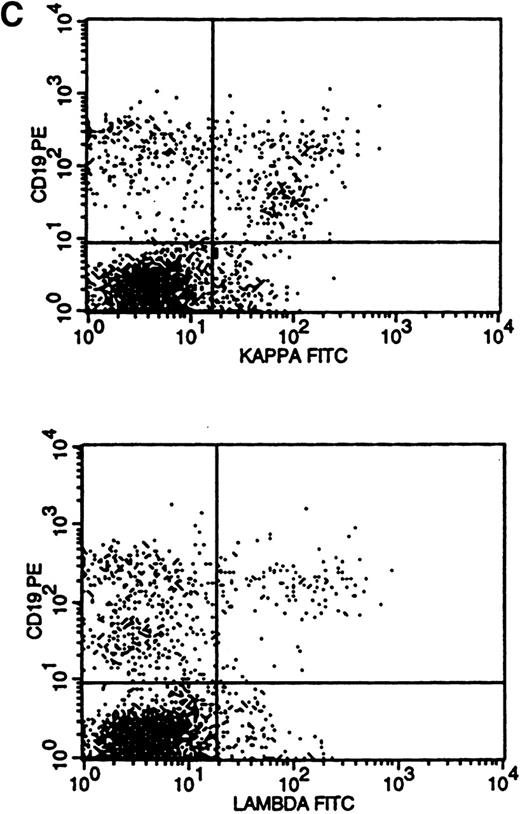
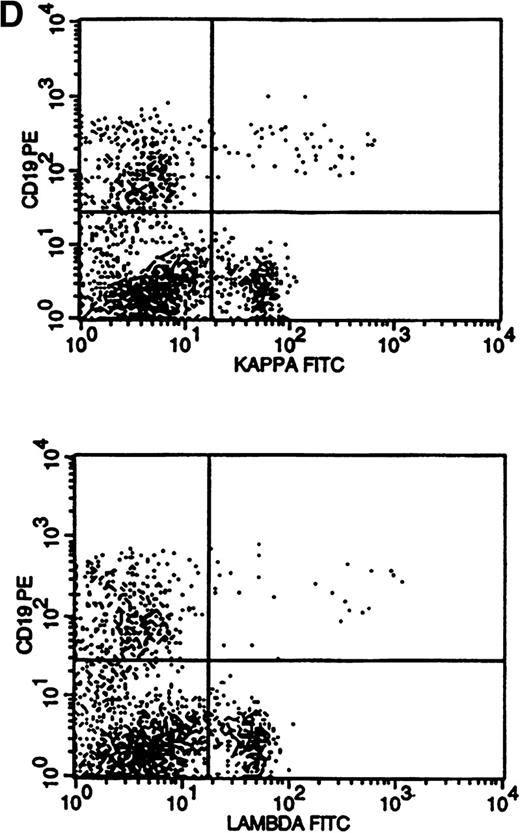
Flow cytometric histograms showing distribution of CD19/κ and CD19/λ staining. (A) Histograms showing bright monoclonal κ surface Ig expression. (B) Histograms showing dim monoclonal κ surface Ig expression. (C) Histograms showing a mixed monoclonal κ surface Ig expression and polyclonal B cells with a normal κ-to-λ ratio. (D) Histograms showing a B-cell population with dim CD19 and no surface Ig expression.
Flow cytometric histograms showing distribution of CD19/κ and CD19/λ staining. (A) Histograms showing bright monoclonal κ surface Ig expression. (B) Histograms showing dim monoclonal κ surface Ig expression. (C) Histograms showing a mixed monoclonal κ surface Ig expression and polyclonal B cells with a normal κ-to-λ ratio. (D) Histograms showing a B-cell population with dim CD19 and no surface Ig expression.
Dim sIg expression, as typically seen in the small lymphocytic lymphomas and chronic lymphocytic leukemia,4,6 21 reflects a diminished number of Ig molecules on the cell surface. This pattern is easily recognized when comparing the histograms of κ and λ staining using the same fluorochrome (FITC in this study) conjugated to the anti-κ and anti-λ antisera (Fig 3B). The distribution pattern of surface Ig light chains can be analyzed statistically, but direct histogram observation/analysis is easier and more reliable than using ratios or statistics. As such, the κ/λ ratios were less dramatic in these 11 cases, with 3 having ratios that were within the normal range (for κ, median of 4.9:1 and range of 2.9 to 11.0:1; for λ, median of 0.2:1 and range of 0.04 to 0.8:1).
The most difficult histogram interpretations were in those 14 cases that had a mixture of polyclonal and monoclonal B cells (Fig 3C). κ-to-λ ratios were of little value in these cases, and monoclonal populations could only be recognized by κ and λ histogram pattern comparisons (κ/λ ratios: for κ, median of 2.9:1 and range of 1.3 to 4.4:1; for λ, median of 0.7:1 and range of 0.5 to 1.0:1).
A single case of surface Ig-negative lymphoma was identified (Fig 3D), demonstrating a distinctly dim CD19 population that differed from the usual CD19+ precursor cells. Surface Ig negativity was subsequently confirmed in the diagnostic lymph node specimen of this case by frozen section immunophenotyping.
Immunohistochemistry.
Immunohistochemical staining of the paraffin-embedded trephine sections was performed in all cases with antibodies to CD3 (polyclonal) and CD20. The immunohistochemical stains showed close correlation with the morphologic findings with concurrence in 97.7% of cases (171 of 175; Table 5). Three cases with morphologic features of malignant lymphoma did not show the lymphomatous infiltrate on the recut, biopsy sections, thus obviating the ability to assess interpretation. One case showed 2 tiny, distinctly paratrabecular foci of CD20+ B cells, which are suggestive, but not definitive for involvement by that patient’s known follicular lymphoma. Lymphoid aggregates were identified in 48 cases, but were not interpreted as representing malignant lymphoma by the assessment of the morphologic features of these aggregates. All of these latter 48 cases showed either a mixture of CD20+, small B cells and CD3+ T cells or an exclusive T-cell population.
DISCUSSION
The role of evaluating BM trephine biopsy sections is well-established in the staging of malignant lymphoma. Staging for disease as determined by marrow involvement has a clear implication for survival of patients with malignant lymphoma and current therapeutic protocols may be directed according to stage of disease.1,2,14 The international prognostic index for patients with malignant lymphoma includes BM involvement as 1 of its prognostic factors.12 13 Determination of marrow involvement, therefore, remains a crucial component of the workup of the patient with malignant lymphoma.
The findings from this study lead to several observations and conclusions. Morphologic evaluation of bilateral BM trephine biopsies remains the gold standard in the staging process of malignant lymphoma. An accepted corollary is that sufficient biopsy tissue needs to be obtained and stained with a high-quality H&E stain when relying on morphologic findings in this manner. Because morphology alone identified all relevant cases with marrow involvement, we conclude that routine BM flow cytometric immunophenotyping and immunohistochemical staining for malignant lymphoma is not a cost-effective replacement for good morphologic evaluation in lymphoma staging. These laboratory tools do have a role in the laboratory, but should be reserved for those BM cases with equivocal morphologic findings or marginally adequate biopsy specimens. Too often, these ancillary studies are interpreted in isolation from the clinical and morphologic perspectives. There needs to be judicious use of these technologies and development of skills and trust in traditional morphologic assessment.
The exact role of flow cytometric analysis and immunohistochemistry over and above morphologic assessment has never been fully established. A study published by Naughton et al31 concluded that flow cytometric immunophenotyping had a limited role in the staging and follow-up of patients with malignant lymphoma. Only 3 of 273 marrow specimens showed a combination of flow immunophenotypic positivity and negative BM morphology. Interestingly, 2 of these 3 were from patients with large-cell lymphomas, similar to the results found in our study. Dunphy18 compared BM morphology and flow cytometric immunophenotyping in 188 specimens that also included many BM-based disorders, such as CLL and hairy cell leukemia. More than 80% of the cases in that study showed concordance between morphology and immunophenotypic studies, whereas less than 5% demonstrated immunophenotypic positivity in morphologically negative or borderline BM cases. Fineberg et al19 also evaluated the yield of immunophenotyping in BMs for malignant lymphoma. However, all cases in the report of Fineberg et al had morphologic evidence of BM involvement by either lymphoma or CLL, and it was not a broad-based study of malignant lymphoma. Most previous studies using immunophenotypic laboratory methods have looked at specific disease entities, such as Waldenstrom’s macroglobulinemia, mantle cell lymphoma, etc,20,22 and not at the larger issue of how these modalities should be used in the global approach to the patient with malignant lymphoma. Coad et al10 looked at the role of molecular gene rearrangement studies by polymerase chain reaction in BM aspirates and biopsies from patients with malignant lymphoma; no comparison with immunophenotyping was performed. This study showed that 43% of marrows that had morphologic evidence of lymphoma failed to show clonality by molecular methods, again raising awareness of the limitations of interpreting laboratory tests independent of the morphologic findings.10
The 5 morphologically negative, flow cytometric immunophenotypic-positive cases associated with large-cell lymphoma raise interesting questions without obvious answers. All 5 of these cases were from patients with large-cell lymphoma, with 4 having localized, but bulky clinical disease. Biologically, are these findings the result of distant, PB seeding by the bulky malignant lymphoma with blood contamination of the marrow aspirate? Thus, if this phenomena did occur, these would not represent true stage IV disease and should be staged independently of the flow cytometric results. Alternatively, if these cases actually have definite marrow involvement arising from perhaps closely adjacent BM sites that were not represented in the biopsy specimens obtained, they would represent true stage IV marrow involvement. It is hard to fathom the latter possibility, because these 5 specimens were exceedingly generous in quantity and showed absolutely no morphologic or immunohistochemical evidence of involvement, both reliable means of detecting disease. Therapeutically, these 5 patients with large-cell lymphomas were treated with systemic chemotherapy, regardless of the flow findings. However, prognostically, if a positive flow immunophenotyping study is to be equated with stage IV disease, then the possible change in the international prognostic index if more than 1 extranodal site is involved would imply an increased risk of death from disease.12 13 However, insufficient follow-up time is available in these patients from our study to determine if, indeed, there is a long-term clinical significance to this finding. Care must be taken in potentially changing our historic definition of stage IV disease without appropriate clinical follow-up and study. The real answer has to come from following a larger cohort of large-cell lymphoma patients who have similar findings and determining their eventual clinical outcome.
The PB flow cytometric immunophenotyping component of this study raises some interesting and perhaps controversial perspectives. Lymphomas known to have a relatively high rate of PB involvement include the follicular lymphomas, mantle cell lymphoma, small lymphocytic lymphoma, lymphoplasmacytic lymphoma, and marginal zone lymphoma.3,6,16,20,22,29 30 The majority of patients in this series with these diagnoses demonstrated clonal B-cell populations in the PB and correspondingly had lymphoma involvement in the BM by both morphology and immunophenotypic analysis. Whether a PB flow cytometric immunophenotyping study could be used as a lymphoma screening test remains to be determined. A negative study would obviously not exclude marrow involvement. Such PB immunophenotyping studies also do not appear to be routinely warranted in patients with other types of lymphoma having very infrequent evidence of circulating lymphoma cells. Regardless, it must be recognized that BM specimens can still provide important information regarding the extent of involvement by lymphoma and documentation of normal or abnormal hematopoietic activity.
The determination of κ/λ monoclonality is an inadequately described and understood process. Relying exclusively on κ-to-λ ratios is misleading and certainly could lead to false interpretations.21,26,32 In this series, 16 of the 54 positive BM flow cases would have been excluded if based solely on κ-to-λ ratios. Based on our experience, determination of κ/λ monoclonality by flow cytometry must be primarily based on examination of the respective CD19/κ and CD19/λ histograms. It is perhaps a more costly approach to analyze 2 tubes containing these antibodies as opposed to a single tube. However, the ability to directly compare κ and λ conjugated with the same fluorochrome is essential when trying to determine whether a subtle shift in Ig expression represents monoclonal expression.21,26 32 Such subtle shifts are difficult to recognize if the 2 Ig light chains are conjugated to different fluorochromes or titered to different protein concentrations. Although flow cytometric analysis gives the perception of precise objectivity due to the number of cells evaluated and the exact numbers that are generated, indeed, the flow cytometric analytic process is a culmination of a variety of subjective steps, including the setting of gates, quadrants, and other parameters. This subjectiveness must be recognized by both the clinician and laboratorian. Experience in interpreting flow cytometric studies is a crucial component in obtaining accurate and sensitive results, especially in the search for relatively small populations of malignant cells.
Immunohistochemical staining of paraffin section material has become an increasingly useful tool in the evaluation of malignant lymphoma.8,9,33,34 Changes in laboratory techniques and available antibodies have led to more comprehensive immunoperoxidase panels that can be performed in paraffin-embedded biopsies. Antibodies directed against CD10, CD20, CD23, CD43, κ, λ, bcl-2, and cyclin d1 are now available that work in paraffin sections and allow for the majority of cases of B-cell malignant lymphoma to be immunophenotyped effectively by this more pathologist-friendly medium.35However, the findings from our study should not encourage the routine use of these stains in the staging of malignant lymphoma and support the conclusion that immunohistochemical staining of marrow biopsies does not add much diagnostic value to the lymphoma staging process. Lymphoid aggregates in BM biopsies that are of uncertain significance usually remain uncertain after immunohistochemical stains.
Several immunologic and molecular techniques are now available that can be used in the clinical laboratory for aiding in the assessment of BM staging for malignant lymphoma. The findings from this study suggest that BM flow cytometric immunophenotyping and immunohistochemistry do not significantly help in the diagnostic work-up of the patient with B-cell malignant lymphoma. In our opinion, immunophenotyping of blood or BM may be warranted in those cases that cannot be adequately phenotyped in the diagnostic lymph node/tissue specimen, if that is crucial for lymphoma classification. There may also be other situations in which BM immunophenotyping may be beneficial, such as when evaluating marginal quality BM biopsies with equivocal morphologic features. It is imperative that we gain a thorough understanding of these assays, of how these laboratory tests can contribute diagnostically and clinically to our patients, and, just as importantly, of their diagnostic and clinical limitations. As health care finances become more limited and focused, judicial use of our laboratory resources becomes essential.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Curtis A. Hanson, MD, Mayo Clinic, 200 First St, SW, Rochester, MN 55905.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal