Abstract
Homing of hematopoietic stem cells to the bone marrow (BM) involves sequential interaction with adhesion molecules expressed on BM endothelium (BMEC) and chemokine stromal derived factor-1 (SDF-1). However, the mechanism whereby adhesion molecules regulate the SDF-1–induced transendothelial migration process is not known. E-selectin is an endothelial-specific selectin that is constitutively expressed by the BMEC in vivo. Hence, we hypothesized that E-selectin may mediate SDF-1–induced transendothelial migration of CD34+ cells. We show that CD34+ cells express both E-selectin ligand and fucosyltransferase-VII (FucT-VII). Soluble E-selectin–IgG chimera binds avidly to 75% ± 10% of CD34+ cells composed mostly of progenitors and cells with long-term culture-initiating cell (LTC-IC) potential. To assess the functional capacity of E-selectin to mediate CD34+ cell migration in a transendothelial migration system, CD34+ cells were placed on transwell plates coated with interleukin-1β–activated BMEC. In the absence of SDF-1, there was spontaneous migration of 7.0% ± 1.4% of CD34+ cells and 14.1% ± 2.2% of LTC-IC. SDF-1 induced migration of an additional 23.0% ± 4.4% of CD34+cells and 17.6% ± 3.6% of LTC-IC. Blocking MoAb to E-selectin inhibited SDF-1–induced migration of CD34+ cells by 42.0% ± 2.5% and LTC-IC by 90.9% ± 16.6%. To define the mechanism of constitutive expression of E-selectin by the BMEC in vivo, we have found that vascular endothelial growth factor (VEGF165) induces E-selectin expression by cultured endothelial cells. VEGF-stimulated endothelial cells support transendothelial migration of CD34+ cells that could be blocked by MoAb to E-selectin. These results suggest that trafficking of subsets of CD34+ cells with LTC-IC potential is determined in part by sequential interactions with E-selectin and SDF-1.
IN ADULTHOOD, HEMATOPOIESIS is restricted to the extravascular compartment of the bone marrow (BM) separated by a single layer of BM endothelial cells (BMEC). Thus, hematopoietic stem cells (HSC) arriving at the BM must first recognize or be recognized by the luminal surface of the BMEC.1-5 Molecules that mediate adhesion of HSC to BMEC are likely to play a pivotal role in the phenomenon of HSC homing.1-3,6-8 Similar to leukocyte trafficking,9 10 homing of HSC from the peripheral circulation to the BM is a multistep process and involves sequential interaction of CD34+ cells with adhesion molecules expressed on BMEC and specific chemokine(s) expressed within the BM. Chemokines orchestrate this process by providing directional cues for CD34+ cells to migrate into the BM microenvironment.
Based on recent studies, the chemokine stromal derived factor-1 (SDF-1) has been shown to play a key role in CD34+trafficking.11-14 Targeted gene knockout of either SDF-115 or its receptor CXCR416,17 resulted in a defect in BM hematopoiesis, whereas fetal liver hematopoietic activity remained intact. Recently, it was shown that CXCR4-dependent migration in response to SDF-1 is essential for the homing and engraftment of human stem cells into NOD/SCID mice.13However, transplantation of fetal-derived hematopoietic cells from CXCR4 knock out mice into lethaly irradiated syngenic mice resulted in a profound but not complete decrease in the engraftment of hematopoietic cells.18,19 These results suggest that homing and long-term maintenance of hematopoietic cells within the BM is only partially dependent on the expression of CXCR4 on the hematopoietic stem and progenitor cells.19
However, the mechanism whereby SDF-1 promotes transmigration of CD34+ cells and the role of adhesion molecules during the SDF-1–induced transendothelial migration are not well defined. Similar to leukocytes, transendothelial migration of CD34+ cells in response to SDF-1 most likely is initiated by tethering of CD34+ cells through interaction with selectins such as E- or P-selectins followed by firm adhesion mediated through intercellular adhesion molecule-1 (ICAM-1)/leukocyte function-associated antigen-1 (LFA-1), vascular adhesion molecule-1 (VCAM-1)/very late antigen 4 (VLA4) ligand pairs and engagement with junctional adhesion molecules such as platelet endothelial cell adhesion molecule (PECAM).9 10
Among these adhesion molecules, E-selectin is an endothelial-specific selectin that plays a critical role in tethering of leukocytes20 or hematopoietic progenitors21 to endothelial cells at high shear stress. Although E-selectin is mostly expressed by cytokine-activated endothelium, native BMEC in vivo constitutively express E-selectin,22 suggesting that E-selectin may play a role in the regulation of trafficking of CD34+ cells. Frenette et al7 have shown that recruitment of hematopoietic progenitor cells to the BM is reduced in P- and E-selectin knock-out P/E(−/−) mice. In this study, lethally irradiated recipient P/E(−/−) mice, transplanted with minimal numbers (5 × 104) of wild-type BM cells, engrafted poorly compared with the wild-type recipients. Based on these results, we hypothesized that combinatorial interaction of HSC with selectins and SDF-1 may promote selective homing of HSC to the BM.
E-selectin interacts with leukocyte counter-receptors E-selectin ligand-1 (ESL-1) and P-selectin glycoprotein ligand-1 (PSGL-1).23-25 ESL-126 and PSGL-127,28 are active as E-selectin counterreceptors only when modified posttransitionally by fucosylated oligosaccharides represented by Sialyl Lewis x (sCD15, sLex) or its structural variants. Synthesis of functional selectin ligands requires an ordered series of glycosylation reactions, including addition of a fucose residue in an α1,3-linkage, a reaction catalyzed by fucosyltransferase type VII (FucT-VII), which is expressed at significant levels in leukocytes.24,25,29-32 The targeted gene knockout of FucT-VII results in impaired leukocyte extravasation and faulty lymphocyte homing. Whether there is a dysregulation of HSC homing in FucT-VII null mice24 is not known. In addition, the exact nature of expression of various fully glycosylated functional E-selectin ligands on CD34+ cells is not well defined. The majority of freshly isolated CD34+ cells do not express CD15. Therefore, whether CD34+ cells with long-term culture-initiating cell (LTC-IC) potential express functional E-selectin ligands and their role in chemokine-induced transendothelial migration is not known and is the subject of the present study.
In this report, we have evaluated the role of E-selectin in SDF-1–induced transendothelial migration of CD34+ cells. We show that subsets of CD34+ cells with LTC-IC potential bind to the soluble E-selectin–IgG chimera. SDF-1–induced transendothelial migration of subsets of CD34+ cells with LTC-IC potential was partially dependent on the interaction with E-selectin. In addition, vascular endothelial growth factor (VEGF) induces the upregulation of E-selectin and promotes migration of CD34+ cells through endothelial cells. These data suggest that constitutive expression of E-selectin by BMEC may facilitate SDF-1–mediated homing of CD34+ cells to the BM.
MATERIALS AND METHODS
Purification of CD34+ cells.
Cord blood was obtained after informed consent was received through approval of the Institutional Review Board. Mononuclear cells were isolated from cord blood (CB) by Ficoll (Nycomed Pharmacia A.S., Oslo, Norway) density gradient centrifugation. CD34+ cells were isolated the same day by the standard Minimacs magnetic isolation technique (Miltenyi Biotec, Auburn, CA). Isolated CD34+cells were passaged up to 3 times through Minimacs column to achieve a very highly enriched population of CB-derived CD34+ cells (92% ± 3% purity).
Isolation of E-selectin ligand-positive CD34+ cells with E-selectin–IgG2a chimera.
Freshly isolated CD34+ cells were incubated with (1 μg/mL) soluble E-selectin–(mouse)IgG2a chimera at 4°C for 30 minutes in Hanks' balanced salt solution (HBSS) supplemented with 2 mmol/L calcium and magnesium. After 3 washes, the cells were treated with sheep antimouse Fc-coated immunomagnetic beads (Dynal A.S., Oslo, Norway) at 4°C for 30 minutes. CD34+ cells bound to beads were obtained by magnetic separation.
RNA isolation and reverse transcription (RT) and polymerase chain reaction (PCR).
Poly A RNA isolated from CD34+ cells, obtained from umbilical CB, granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood (PB), fetal liver (FL), and BM, HL60 myeloid cell line, and BMEC were used in RT-PCR reactions. Oligonucleotide primers spanning the insertion/deletion site of human ESL-1 and FucT-VII were synthesized. The forward primer for ESL-1 was 5′-CTA CCT GTC CTT GAG CTC-3′ and the reverse primer was 5′-TCC AGT CTA TGT GCT GAA. RT-PCR of m-RNA encoding ESL-1 resulted in a PCR product 450 bp long. The forward primer for FucT-VII was 5′-CAC CTC CGA GGC ATC TTC AAC TG-3′ and the reverse primer was 5′-CGT TGG TAT CGG CTC TCA TTC ATG-3′. The expected RT-PCR mRNA product encoding FucT-VII was 497 bp long. After 35 cycles, the samples were analyzed by gel electrophoresis.
Flow cytometry.
All fluorescein isothiocyanate (FITC)-and phycoerytherin (PE)-conjugated monoclonal antibodies (MoAbs) used for flow cytometry experiments, including CD34-PE (HPCA2; Becton Dickinson, Mountain View, CA), E-selectin–FITC (Biosource, Cama- rillo, CA), CD15-FITC (Becton Dickinson), and IgG isotype control FITC/PE (Immunotech, Miami, FL) were obtained from commercially available sources. To quantify the number of cells that have the capacity to bind to E-selectin, freshly isolated CD34+ cells were incubated with human soluble E-selectin–mouse IgG2a chimeric molecule (Texas Biotechnology, Houston, TX) followed by goat antimouse FITC-conjugated secondary antibody against mouse IgG2a in HBSS containing 2 mmol/L calcium and magnesium with and without 2 mmol/L EDTA. Subsequently, the cells were washed fixed with 1% formalin and analyzed by a Coulter Elite flow cytometer (Coulter, Miami, FL).
Cell lines.
Murine stromal cell line MS-5 (kindly Provided by K. Mori) were grown in α-minimum essential medium (α-MEM; GIBCO-BRL Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT) and passaged weekly. The cells support the proliferation of human HSC in long-term culture. The myeloid cell line HL60 (American Type Culture Collection, Rockville, MD) was cultured in RPMI 1640 medium (GIBCO) supplemented with 10% FBS.
Preparation of BMEC and human umbilical vein endothelial cells (HUVEC) monolayers.
Primary HUVEC and BMEC were isolated by a standard method as described previously4 and placed in medium 199 (M199; GIBCO-BRL) containing 20% FBS, 1 ng/mL VEGF, 5 ng/mL basic fibroblast growth factor (b-FGF; FGF-2), and heparin (5 U/mL). For transmigration assay, 5-mm pore transwells were plated with BMEC. After 3 days, the monolayers reached full confluency. Before transmigration assay, the integrity of the monolayer was confirmed by placing 100 μL medium M199 and 20% FBS containing 14C albumin (American Radiolabelled Chemicals, St Louis, MO) on the cells for 6 hours and measuring the amount of radioactivity accumulating in the lower chamber.
Adhesion studies.
Freshly isolated CB CD34+ cells (105cells/well) were incubated with interleukin-1β (IL-1β; 10 U/mL) –stimulated BMEC monolayers in HBSS supplemented with 2 mmol/L calcium and magnesium in the presence of either 2 mmol/L EDTA or blocking MoAbs to ICAM-1 (10 μg/mL; R&D Systems, Minneapolis, MN), E-selectin (10 μg/mL; R&D Systems), or VCAM-1 (10 μg/mL; Immunotech). After removal of the nonadherent cells, the adherent population was quantified by flow cytometry and phase contrast microscopy. After an incubation period of 1 hour at 37°C, the nonadherent population was removed and the number of attached CD34+ cells bound to endothelium was quantified by flow cytometry.
Transmigration assay.
Freshly isolated CB CD34+ cells were washed once with HBSS solution and resuspended with X-vivo-20 at a concentration of 106/mL. HUVEC monolayers were prestimulated with 10 U/mL of IL-1β and 40 ng/mL of VEGF for 12 to 16 hours. Subsequently, after washing the monolayer of IL-1 and VEGF, aliquots of CB CD34+ cell suspension (100 μL) were applied on 5-mm pore transwells covered with confluent monolayers of IL-1β and VEGF activated BMEC or HUVEC in a 24-well plate (Costar Corp, Cambridge, MA). Immediately, 600 μL of serum-free media containing SDF-1 (200 ng/mL) was placed in the lower chamber. After incubation for 24 hours at 37°C in a CO2 incubator, the migrated and the nonmigrated cells were counted on a hemacytometer. Transmigration through both IL-1β VEGF-activated BMEC or HUVEC was inhibited by preincubation with 10 μg/mL of anti–E-selectin (CD62E) in the upper chamber for 15 minutes.
LTC-IC assay.
MS-5 cells were seeded on T-12.5 flasks in α-MEM with 10% FBS. When cells reached confluence, medium was replaced with LTC medium consisting of α-MEM, 12.5% FCS, 5 × 10−4mol/L 2-β-mercaptoethanol (Fisher Scientific, Pittsburgh, PA), and 10−6 mol/L hydrocortisone (Sigma, St Louis, MO). The migrated population was plated at a density of 104 cells on the stromal feeder layers for LTC. These were then incubated for the first 3 to 4 days at 37°C and subsequently at 33°C in 5% CO2. Cultures were weekly demipopulated and replenished with fresh LTC medium. The cell suspension as well as the adherent population from week-5 LTC were plated at a density of 1 × 104 cells in triplicate in 35-mm tissue culture dishes (Corning, Acton, MA) containing 1 mL Iscove's modified Dulbecco's medium (IMDM; GIBCO), 0.36% agarose (FMC Bioproducts, Rockland, MD), and 20% FBS together with human kit-ligand (KL; 20 ng/mL; Immunex, Seattle, WA) and human granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 U/mL; Immunex). After 14 days of incubation at 37°C and 5% CO2, colony-forming unit–granulocyte-macrophage (CFU-GM) was scored.
Colony-forming cell (agarose/CFC) assays.
The cells from the migrated populations were plated at a cell concentration of 1 × 103/mL per 35-mm dish (Corning) in triplicate, containing 1 mL IMDM, 0.36% agarose, and 20% FBS together with human KL (20 ng/mL), GM-CSF (100 U/mL), erythropoietin (Epo; 6 U/mL; Amgen, Thousand Oaks, CA), IL-6 (20 ng/mL), and IL-3 (50 ng/mL). After 14 days of incubation at 37°C and 5% CO2, colonies were scored for burst-forming unit-erythroid (BFU-E), CFU-GM, and CFU-Mix.
Stimulation of HUVEC monolayer with VEGF.
HUVEC or BMEC monolayers were placed in serum-free medium X-Vivo-20 (BioWhittaker, Walkersville, MD) for 18 hours with 40 ng/mL of VEGF and stained with FITC-labeled MoAb to E-selectin (Biosource) to confirm upregulation of E-selectin by fluorescence-activated cell sorting (FACS) analysis.
Statistical analysis.
Data are expressed as the mean ± SEM of 3 to 5 independent experiments. To detect differences between migrating and nonmigrating cells, the t-test for the paired samples was applied.P < .05 was considered statistically significant.
RESULTS
CD34+ cells express ESL and FucT-VII by RT-PCR.
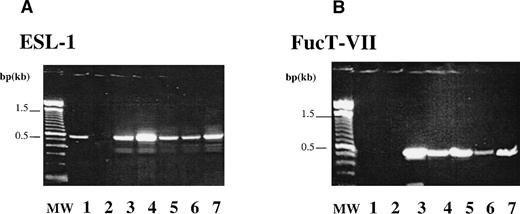
Creation of functional E-selectin ligands requires addition of a fucose residue in a α1,3 linkage to α2,3-Sialyllactosamine precursor, a reaction catalyzed by FucT-VII.24,25 29 The expression of ESL and FucT-VII on CD34+ cells was analyzed by RT-PCR. CD34+ cells isolated from CB, PB, BM, and FL express the expected product of both ESL (Fig 1A) and FucT-VII (Fig 1B). Expression of FucT-VII leads credence to the possibility that ESL or its variants expressed on CD34+cells is most likely a fucosylated, functional ligand capable of binding to E-selectin expressed on native BMEC or activated endothelial cells.
Expression of ESL-1 and FucT-VII by CD34+cells. RT-PCR analysis was performed to examine the capacity of CD34+ cells to express ESL-1 and FucT-VII. (A) The expected product of 450 bp for ESL-1 could be detected in BMEC (lane 1, EC), HL60 (lane 3, positive control), PB CD34 cells (lane 4, PB), BM CD34 cells (lane 5, BM), FL CD34 cells (lane 6, FL), and CB CD34 cells (lane 7, CB). RT (lane 2) is the PCR reaction without addition of RT. (B) The expected product of 497 bp for FucT-VII could be detected in HL60 (lane 3, positive control) and in PB- (lane 4), BM- (lane 5), FL- (lane 6), CB-derived CD34(+) cells (lane 7). FucT-VII expression is only limited to hematopoietic cells, and BMEC (EC, lane 1) did not express FucT-VII. RT control (lane 2) is the PCR reaction without addition of RT.
Expression of ESL-1 and FucT-VII by CD34+cells. RT-PCR analysis was performed to examine the capacity of CD34+ cells to express ESL-1 and FucT-VII. (A) The expected product of 450 bp for ESL-1 could be detected in BMEC (lane 1, EC), HL60 (lane 3, positive control), PB CD34 cells (lane 4, PB), BM CD34 cells (lane 5, BM), FL CD34 cells (lane 6, FL), and CB CD34 cells (lane 7, CB). RT (lane 2) is the PCR reaction without addition of RT. (B) The expected product of 497 bp for FucT-VII could be detected in HL60 (lane 3, positive control) and in PB- (lane 4), BM- (lane 5), FL- (lane 6), CB-derived CD34(+) cells (lane 7). FucT-VII expression is only limited to hematopoietic cells, and BMEC (EC, lane 1) did not express FucT-VII. RT control (lane 2) is the PCR reaction without addition of RT.
Chimeric E-selectin binds to CD34+ cells with LTC-IC potential.
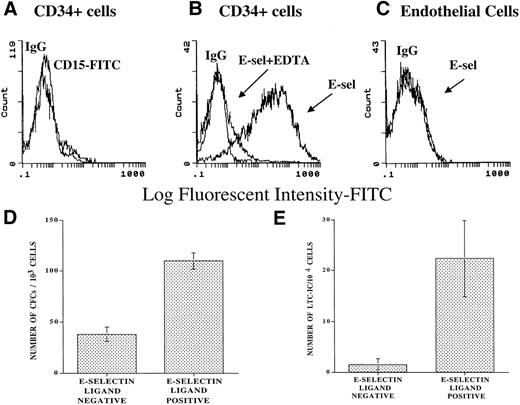
To explore the capacity of soluble E-selectin to bind to CD34+ cells, a chimeric E-selectin–IgG2a molecule was generated. Using flow cytometry, we demonstrated that the E-selectin–IgG2a chimera binds to 75% ± 10% of CB-derived CD34+ cells (CD34+ESL+ cells) in a calcium-dependent manner, as demonstrated by complete abrogation of binding in the presence of EDTA (Fig 2B). Soluble E-selectin fails to bind to endothelial cells that lack FucT-VII and therefore does not generate a functional ESL (Fig 2C). Almost all freshly isolated CD34+ cells used in these experiments did not express CD15 (Lewis X), suggesting that soluble E-selectin chimera is interacting with a variant type of fucosylated ESL on the surface of CD34+ cells (Fig 2A). Agarose and LTC-IC assays showed that, when compared with the CD34+ESL(−) cells, CD34+ESL+cells composed of a population of cells that showed a 2.1- ± 0.6-fold higher progenitor (Fig 2D) and 14.9- ± 10.7-fold higher LTC-IC content (Fig 2E). In fact, CD34+ cells that failed to bind to E-selectin were depleted of cells with LTC-IC potential (Fig2E).
Chimeric E-selectin–IgG2a binds to subsets of CD34+ cells. Soluble E-selectin–IgG2a chimera was used to quantify the number of ESL-expressing CD34+immediately after purification from CB. Using FACS analysis, 75% ± 5% of CD34+ cells bind to E-selectin–IgG2a chimeric molecule (B) (N = 3). The binding of E-selectin/IgG2a was dependent on divalent cations and was inhibited in the presence of 2 mmol/L EDTA (B). Endothelial cells that do not express FucT-VII showed no binding to the soluble E-selectin–IgG chimera (C). Freshly isolated CD34+ cells do not express CD15 (A). Agarose and LTC-IC assays performed on CD34+ cells bound to soluble E-selectin chimera showed a 2.09 ± 0.58-fold higher progenitor (D) and 14.9- ± 10.7-fold higher LTC-IC content than the population that did not bind to soluble E-selectin chimera (E). The CD34+cells that failed to bind to E-selectin were virtually devoid of CD34+ cells with LTC-IC potential (E) (N = 3, P< .05).
Chimeric E-selectin–IgG2a binds to subsets of CD34+ cells. Soluble E-selectin–IgG2a chimera was used to quantify the number of ESL-expressing CD34+immediately after purification from CB. Using FACS analysis, 75% ± 5% of CD34+ cells bind to E-selectin–IgG2a chimeric molecule (B) (N = 3). The binding of E-selectin/IgG2a was dependent on divalent cations and was inhibited in the presence of 2 mmol/L EDTA (B). Endothelial cells that do not express FucT-VII showed no binding to the soluble E-selectin–IgG chimera (C). Freshly isolated CD34+ cells do not express CD15 (A). Agarose and LTC-IC assays performed on CD34+ cells bound to soluble E-selectin chimera showed a 2.09 ± 0.58-fold higher progenitor (D) and 14.9- ± 10.7-fold higher LTC-IC content than the population that did not bind to soluble E-selectin chimera (E). The CD34+cells that failed to bind to E-selectin were virtually devoid of CD34+ cells with LTC-IC potential (E) (N = 3, P< .05).
Under static conditions, adhesion of CD34+ cells to BMEC monolayers is independent of E-selectin.
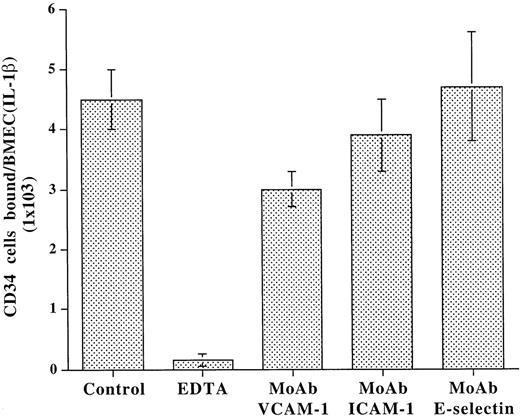
Adhesion studies were performed under static conditions to assess the role of E-selectin in the modulation of CD34+ progenitor cell adhesion to BMEC. Given that primary cultured BMEC lose their capacity to express E-selectin in vitro, BMEC monolayers were treated with IL-1β to induce E-selectin expression. Freshly isolated CD34+ cells from CB were incubated with IL-1β–activated BMEC monolayers in the presence of divalent cations and blocking MoAb to various adhesion molecules. As shown in Fig 3, after incubation for 1 hour at 37°C, under static conditions, only MoAb to VCAM-1 partially blocked the adhesion of CD34+ cells to BMEC monolayers. The blocking MoAb to E-selectin did not inhibit adhesion even at high concentrations (30 μg/mL). The binding of CD34+ cells to the BMEC was completely inhibited by EDTA, suggesting that the binding of CD34+ cells to adhesion molecules on BMEC is dependent on divalent cations. These data suggest that, under static conditions, E-selectin plays a minimal role in supporting the adhesion of CD34+ cells to BMEC.
Under static conditions, adhesion of CD34+cells to BMEC monolayers is independent of E-selectin. CD34+ cells derived from CB were plated on IL-1β–activated BMEC monolayers in the presence or absence of blocking MoAb to either VCAM-1 (10 μg/mL), ICAM-1 (10 μg/mL), E-selectin (10 μg/mL), or EDTA (2 mmol/L). After incubation for 1 hour, EDTA resulted in a complete inhibition of adhesion. However, only MoAb to VCAM-1 partially blocked the adhesion of CD34+cells by 44.0% ± 5.8%, whereas MoAb to E-selectin even at high concentrations of 30 μg/mL had no effect on adhesion of CD34+ cells to endothelium. MoAb to ICAM-1 also did not have a significant effect on adhesion of CD34+ cells to BMEC (N = 4, P < .01).
Under static conditions, adhesion of CD34+cells to BMEC monolayers is independent of E-selectin. CD34+ cells derived from CB were plated on IL-1β–activated BMEC monolayers in the presence or absence of blocking MoAb to either VCAM-1 (10 μg/mL), ICAM-1 (10 μg/mL), E-selectin (10 μg/mL), or EDTA (2 mmol/L). After incubation for 1 hour, EDTA resulted in a complete inhibition of adhesion. However, only MoAb to VCAM-1 partially blocked the adhesion of CD34+cells by 44.0% ± 5.8%, whereas MoAb to E-selectin even at high concentrations of 30 μg/mL had no effect on adhesion of CD34+ cells to endothelium. MoAb to ICAM-1 also did not have a significant effect on adhesion of CD34+ cells to BMEC (N = 4, P < .01).
SDF-1–induced transendothelial migration of CD34+cells is dependent on E-selectin.
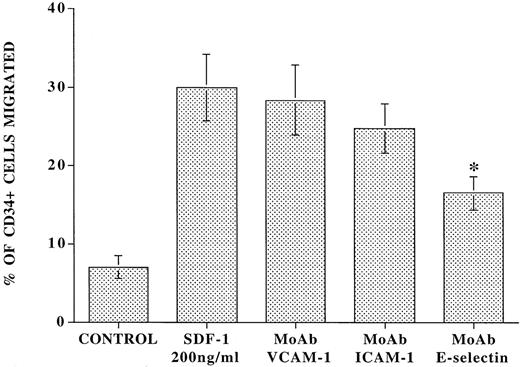
To assess the role of E-selectin in a more physiological chemokine-driven transendothelial migration model, CD34+cells isolated from CB were added to the upper chamber of 5-μm transwell plates that were coated with confluent monolayers of resting or IL-1β–activated BMEC in the presence or absence of a blocking MoAb to E-selectin (Fig 4). Immediately, 200 ng/mL of SDF-1 was placed in the lower chamber, and after an incubation period of 24 hours, the number of migrated cells was examined. In the absence of SDF-1, there was spontaneous migration of 7.0% ± 1.4% of total CD34+ cells added. However, SDF-1 induced migration of an additional 23.0% ± 4.4% of total CD34+ cells through IL-1β–activated endothelial cells. In the presence of blocking MoAb to E-selectin, there was a 42.0% ± 2.5% inhibition of the SDF-1–induced migration of CD34+ cells (Fig 4). In contrast, MoAbs to either VCAM-1 or ICAM-1 did not significantly block SDF-1–induced migration of CD34+ cells. Similar results were obtained with IL-1β–activated HUVEC monolayers (data not shown).
Blocking MoAb to E-selectin inhibits SDF-1–induced transmigration of CD34+ cells through IL-1β–activated endothelial cells. Freshly isolated CD34+ cells (105 cells) from CB were placed in the upper chamber of 5-μm transwell plates coated with confluent monolayers of IL-1β–stimulated intact BMEC monolayers in the presence or absence of 10 μg/mL blocking MoAb to E-selectin. Immediately, 200 ng/mL of SDF-1 was placed in the lower chamber and the number of migrated cells was evaluated by flow cytometry. After a period of 24 hours, in the absence of SDF-1, there was migration of only 7.0% ± 1.4% of the added CD34+ cells to the upper chamber. In the presence of 200 ng/mL SDF-1, there was migration of an additional 23.0% ± 4.4% of the added CD34+ cells. In the presence of blocking MoAb to E-selectin (10 μg/mL), there was a 42.0% ± 2.5% inhibition of the SDF-1–induced migration of CD34+ cells (N = 4, P < .003). Blocking MoAb to VCAM-1 and ICAM-1 did not significantly influence the SDF-1–induced migration process.
Blocking MoAb to E-selectin inhibits SDF-1–induced transmigration of CD34+ cells through IL-1β–activated endothelial cells. Freshly isolated CD34+ cells (105 cells) from CB were placed in the upper chamber of 5-μm transwell plates coated with confluent monolayers of IL-1β–stimulated intact BMEC monolayers in the presence or absence of 10 μg/mL blocking MoAb to E-selectin. Immediately, 200 ng/mL of SDF-1 was placed in the lower chamber and the number of migrated cells was evaluated by flow cytometry. After a period of 24 hours, in the absence of SDF-1, there was migration of only 7.0% ± 1.4% of the added CD34+ cells to the upper chamber. In the presence of 200 ng/mL SDF-1, there was migration of an additional 23.0% ± 4.4% of the added CD34+ cells. In the presence of blocking MoAb to E-selectin (10 μg/mL), there was a 42.0% ± 2.5% inhibition of the SDF-1–induced migration of CD34+ cells (N = 4, P < .003). Blocking MoAb to VCAM-1 and ICAM-1 did not significantly influence the SDF-1–induced migration process.
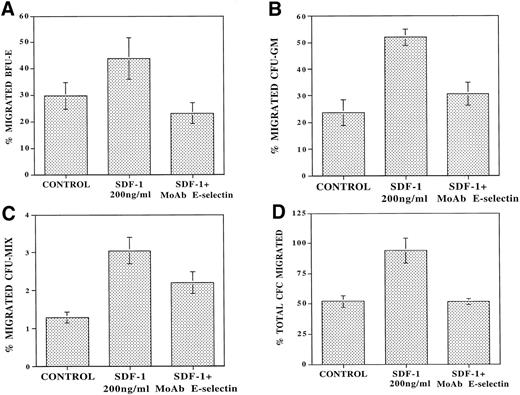
To determine the role of E-selectin in regulating the migration of cells with progenitor potential, the migrating CD34+ cells from each condition from the above experiments were also assayed in an agarose assay to quantitate the number of CFC. In the absence of SDF-1, there was spontaneous migration of 53% ± 1.5% of progenitors (Fig 5D). SDF-1 induced migration of an additional 41.8% ± 11.0% of CFC applied to the upper wells. Pretreatment of the endothelial monolayer with MoAb to E-selectin (10 μg/mL) resulted in virtually complete inhibition (91.6% ± 13.0%) of SDF-1–induced transendothelial migration of CFCs (Fig 5D). These results suggest that engagement of E-selectin with its ligand on CD34+ cells plays a role in the SDF-1–induced chemokinesis of CD34+ cells.
MoAb to E-selectin blocks transendothelial migration of primary CFU. CB-derived CD34+ cells (105cells/plates) were plated in the upper chamber of 5-μm 24-well costar plates coated with confluent monolayers of IL-1β–activated BMEC. Serum-free X-vivo medium was added to the control wells. After 24 hours of incubation at 37°C, the number of migrating CFU were examined by agarose assay. In the absence of SDF-1, there was spontaneous migration of 29.6% ± 4.9% of added BFU-E (A), 22.3% ± 5% of CFU-GM (B), and 1.4% ± 0.3% of CFU-Mix (C). In the presence of SDF-1, there was an additional migration of 14.2% ± 9.3% of BFU-E (A), 19.1% ± 8.6% of CFU-GM (B), and 1.75% ± 0.37% of CFU-Mix (C) (N = 4,P < .01). However, in the presence of MoAb to E-selectin (10 μg/mL), there was inhibition of the SDF-1–induced migration of 61.8% ± 14.5% of CFU-GM (B) and 48.6% ± 15.0% of CFU-MIX (C) (N = 4). In addition, MoAb to E-selectin inhibited the SDF-1–induced BFU-E migration even below spontaneous levels to 23.2% ± 5.0% (A) (N = 4, P < .05). When taking the total CFU (D) into account, there was spontaneous migration of 53% ± 7% of the added CFC in the control samples. SDF-1 induced additional migration of 41.8% ± 11.0% of the added CFU, and MoAb to E-selectin completely blocked this SDF-1–induced migration by 91.6% ± 13.0% (N = 4, P < .05).
MoAb to E-selectin blocks transendothelial migration of primary CFU. CB-derived CD34+ cells (105cells/plates) were plated in the upper chamber of 5-μm 24-well costar plates coated with confluent monolayers of IL-1β–activated BMEC. Serum-free X-vivo medium was added to the control wells. After 24 hours of incubation at 37°C, the number of migrating CFU were examined by agarose assay. In the absence of SDF-1, there was spontaneous migration of 29.6% ± 4.9% of added BFU-E (A), 22.3% ± 5% of CFU-GM (B), and 1.4% ± 0.3% of CFU-Mix (C). In the presence of SDF-1, there was an additional migration of 14.2% ± 9.3% of BFU-E (A), 19.1% ± 8.6% of CFU-GM (B), and 1.75% ± 0.37% of CFU-Mix (C) (N = 4,P < .01). However, in the presence of MoAb to E-selectin (10 μg/mL), there was inhibition of the SDF-1–induced migration of 61.8% ± 14.5% of CFU-GM (B) and 48.6% ± 15.0% of CFU-MIX (C) (N = 4). In addition, MoAb to E-selectin inhibited the SDF-1–induced BFU-E migration even below spontaneous levels to 23.2% ± 5.0% (A) (N = 4, P < .05). When taking the total CFU (D) into account, there was spontaneous migration of 53% ± 7% of the added CFC in the control samples. SDF-1 induced additional migration of 41.8% ± 11.0% of the added CFU, and MoAb to E-selectin completely blocked this SDF-1–induced migration by 91.6% ± 13.0% (N = 4, P < .05).
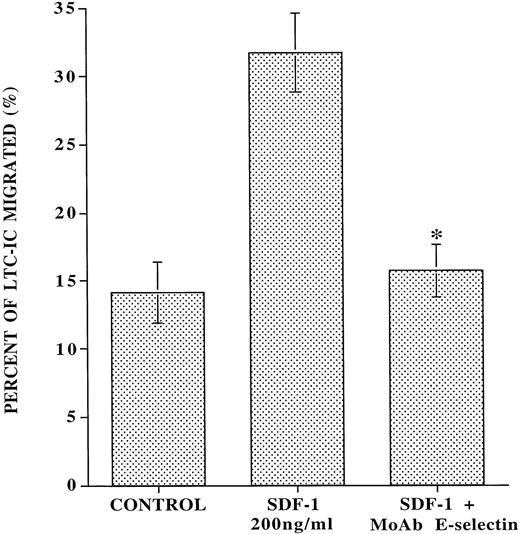
MoAb to E-selectin inhibits the SDF-1–induced transendothelial migration of CD34+ cells with LTC-IC potential.
SDF-1 has been shown to induce the migration of CD34+CD38-cells, which phenotypically can be considered as HSC.14 However, whether SDF-1 also support the migration of LTC-IC has not been evaluated. We demonstrate that, in the absence of any added SDF-1, IL-1β–activated endothelial monolayers induce spontaneous migration of 14% ± 1.5% of added LTC-IC (Fig 6). However, SDF-1 induced migration of an additional 17.6% ± 3.6% of the added LTC-IC through IL-1β–activated endothelial monolayer. Blocking MoAb to E-selectin almost completely inhibited (90.9% ± 16.6%) the SDF-1–induced transendothelial migration of LTC-IC. These data suggest that the forced SDF-1–induced migration of CD34+ cells with LTC-IC potential is dependent on interaction with E-selectin. However, LTC-IC populations migrating independently of SDF-1 do not require interaction with E-selectin.
MoAb to E-selectin inhibits the SDF-1–induced transmigration of LTC-IC through IL-1β–activated endothelial monolayer. Ten thousand CB-derived CD34+ cells were placed on the upper chamber of transwell plates that were covered with confluent monolayers of BMEC in the presence or absence of SDF-1 in the lower chamber and blocking MoAb to E-selectin in the upper chamber. After 24 hours, migrating cells from each condition were placed on MS-5 feeder layers to quantitate the number of migrated cobblestone area-forming cells (LTC-IC), which at week 5 were quantified by agarose assay. In the absence of SDF-1, there was a spontaneous migration of 14.0% ± 1.5% LTC-IC that was independent of SDF-1 and E-selectin. However, with the addition of SDF-1, an additional 17.6% ± 3.6% of the added LTC-IC migrated through IL-1β–activated endothelial cells. Blocking MoAb to E-selectin (10 μg/mL) almost completely inhibited (90.9% ± 16.6%) all SDF-1–induced migration of LTC-IC (N = 5,P < .007).
MoAb to E-selectin inhibits the SDF-1–induced transmigration of LTC-IC through IL-1β–activated endothelial monolayer. Ten thousand CB-derived CD34+ cells were placed on the upper chamber of transwell plates that were covered with confluent monolayers of BMEC in the presence or absence of SDF-1 in the lower chamber and blocking MoAb to E-selectin in the upper chamber. After 24 hours, migrating cells from each condition were placed on MS-5 feeder layers to quantitate the number of migrated cobblestone area-forming cells (LTC-IC), which at week 5 were quantified by agarose assay. In the absence of SDF-1, there was a spontaneous migration of 14.0% ± 1.5% LTC-IC that was independent of SDF-1 and E-selectin. However, with the addition of SDF-1, an additional 17.6% ± 3.6% of the added LTC-IC migrated through IL-1β–activated endothelial cells. Blocking MoAb to E-selectin (10 μg/mL) almost completely inhibited (90.9% ± 16.6%) all SDF-1–induced migration of LTC-IC (N = 5,P < .007).
VEGF stimulation of HUVEC results in the induction of E-selectin expression and enhanced migration of CD34+ cells.
BMEC constitutively express E-selectin in vivo. However, cultivation of BMEC results in the downregulation of E-selectin expression. In fact, adhesion molecule repertoire of in vitro-cultured unstimulated BMEC monolayers simulates that of resting HUVECs.33 This phenomenon is due to the finding that isolation and subsequent propagation of BMEC monolayers results in the downregulation of E-selectin, suggesting that factors released by the hematopoietic cells within the BM microenvironment may confer organ specificity to BMEC by inducing upregulation of E-selectin. Most experiments described here have been performed with IL-1β–activated BMEC or HUVEC monolayers to maintain the expression of E-selectin.
Among the factors that could potentially modulate endothelial cell function is VEGF. We and others have shown that hematopoietic cells, including megakaryocytes, secrete significant amount of VEGF165.34 35 Given that high levels of VEGF are produced by hematopoietic cells, we explored the possibility that VEGF may modulate adhesion molecule repertoire on endothelial cells.
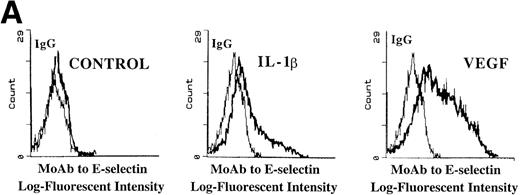
Incubation of either BMEC or HUVEC monolayers under serum-free conditions, in the presence of recombinant endotoxin-free VEGF165 (40 ng/mL), resulted in upregulation of E-selectin (Fig 7A) and, to a smaller degree, VCAM-1 and ICAM-1. This suggests that sustained E-selectin expression by BMEC may be dependent on VEGF released by hematopoietic cells.
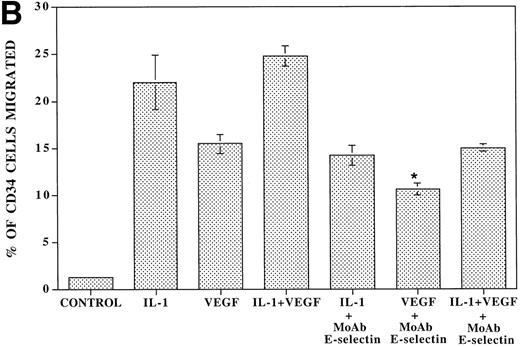
VEGF stimulation of endothelial cells induces expression of E-selectin and enhances SDF-1–induced CD34+ cell transendothelial cell migration. (A) Confluent monolayers of either BMEC or HUVEC in serum-free conditions were incubated with recombinant endotoxin-free VEGF (40 ng/mL). After an incubation period of 16 hours, the level of E-selectin expression was analyzed by FACS analysis. VEGF upregulated E-selectin by BMEC monolayers (N = 5). Similar results were obtained for HUVEC monolayers (data not shown). IL-1β–stimulated endothelium served as the positive control. (B) Blocking MoAb to E-selectin inhibited SDF-1–induced transmigration of CD34+ cells through VEGF-activated endothelial cells. To determine if the E-selectin expression induced by VEGF supported the SDF-1–induced migration, CB-derived CD34+ cells were plated on VEGF-primed BMEC monolayers. In the absence of any stimulation to the endothelial monolayers, SDF-1 induced migration of only 1.5% ± 0.2% of the added CD34+ cells. SDF-1 induced migration of 15.5% ± 1.1% of CD34+ cells through VEGF-activated endothelial monolayers. Blocking MoAb to E-selectin (10 μg/mL) inhibited this migration of CD34+cells by 31.6% ± 1.6% (N = 3, P < .05). In addition, SDF-1 induced migration of 22% ± 2.8% of CD34+ cells through IL-1β–activated endothelial cells (N = 3, P < .05), which was inhibited by MoAb to E-selectin by 36.0% ± 2.8%. When the endothelial monolayers were treated with both VEGF and IL-1β, SDF-1 induced the migration of 24.8% ± 1.1% of CD34+ cells through the endothelial monolayer; MoAb to E-selectin inhibited this migration by 40.4% ± 0.4%.
VEGF stimulation of endothelial cells induces expression of E-selectin and enhances SDF-1–induced CD34+ cell transendothelial cell migration. (A) Confluent monolayers of either BMEC or HUVEC in serum-free conditions were incubated with recombinant endotoxin-free VEGF (40 ng/mL). After an incubation period of 16 hours, the level of E-selectin expression was analyzed by FACS analysis. VEGF upregulated E-selectin by BMEC monolayers (N = 5). Similar results were obtained for HUVEC monolayers (data not shown). IL-1β–stimulated endothelium served as the positive control. (B) Blocking MoAb to E-selectin inhibited SDF-1–induced transmigration of CD34+ cells through VEGF-activated endothelial cells. To determine if the E-selectin expression induced by VEGF supported the SDF-1–induced migration, CB-derived CD34+ cells were plated on VEGF-primed BMEC monolayers. In the absence of any stimulation to the endothelial monolayers, SDF-1 induced migration of only 1.5% ± 0.2% of the added CD34+ cells. SDF-1 induced migration of 15.5% ± 1.1% of CD34+ cells through VEGF-activated endothelial monolayers. Blocking MoAb to E-selectin (10 μg/mL) inhibited this migration of CD34+cells by 31.6% ± 1.6% (N = 3, P < .05). In addition, SDF-1 induced migration of 22% ± 2.8% of CD34+ cells through IL-1β–activated endothelial cells (N = 3, P < .05), which was inhibited by MoAb to E-selectin by 36.0% ± 2.8%. When the endothelial monolayers were treated with both VEGF and IL-1β, SDF-1 induced the migration of 24.8% ± 1.1% of CD34+ cells through the endothelial monolayer; MoAb to E-selectin inhibited this migration by 40.4% ± 0.4%.
To evaluate the capacity of VEGF-stimulated HUVEC monolayers to support the migration of CD34+ cells, freshly isolated CB CD34+ cells were placed on the upper chamber of transwell plates that were coated with endothelial monolayers stimulated with either IL-1β or VEGF165 or a combination of IL-1β or VEGF165 for 16 hours. Subsequently, the number of migrating CD34+ cells in response to SDF-1 was quantified with phase contrast microscopy. In the absence of any stimulation to the endothelial monolayer, SDF-1 induced migration of only 1.5% ± 0.2% of the added CD34+ cells. Both VEGF165-activated and IL-1β–activated endothelial cells supported migration of 15.5% ± 1.1% and 22% ± 2.8% of the added CD34+ cells. MoAb to E-selectin inhibited the SDF-1–induced migration by 31.6% ± 1.6% and 36.0% ± 2.8% through the VEGF165-activated and IL-1β–activated endothelial monolayers, respectively. These results suggest that VEGF165 facilitates SDF-1–induced migration of CD34+ cells by induction of E-selectin expression.
DISCUSSION
Emerging data suggest that selective homing of transplanted HSC to the BM is mediated in part through interaction of CD34+ cells with adhesion molecules1-6 expressed on BMEC as well as chemokines expressed within the bone marrrow microenvironment.12,13 BMEC exhibit unique features that may convey organ specificity to the BM microenvironment.35Although most microvascular beds require activation with inflammatory cytokines to express the endothelial-specific adhesion molecule E-selectin, BMEC constitutively express E-selectin.22E-selectin has been shown to facilitate tethering of leukocytes20 or hematopoietic progenitors21 on activated endothelial cells at high flow rates. Although the flow rate within the BM microenvironment is relatively low, rapid forced transendothelial migration of hematopoietic cells in response to chemokines may also necessitate tethering with selectins, including E-selectin.
Most studies related to the biology of E-selectin ligands have been hampered by the lack of availability of specific antibodies that could identify surface expression of fully glycosylated functional E-selectin ligands on the hematopoietic cells. Therefore, we have used soluble human E-selectin mouse IgG chimera to detect potential E-selectin ligands that may bind to CD34+ cells. We demonstrate that soluble E-selectin–IgG chimera binds to 75% ± 5% of CD34+ cells in a calcium-dependent manner, suggesting that functional ESL is expressed on a subset of CD34+ cells that, when isolated, contain almost all of the cells with LTC-IC potential (Fig 2). Furthermore, MoAb to E-selectin effectively blocked the SDF-1–induced transendothelial migration of CD34+cells with LTC-IC potential. Taken together, these data lend credence to the possibility that CD34+ cells with LTC-IC potential express some form of functional ESL.
As shown in Figs 5 and 6, there is spontaneous SDF-1–independent migration of significant numbers of both primary CFU and LTC-IC. These data suggest that subsets of LTC-IC or progenitors may respond to another as yet unrecognized adhesion molecule(s) or chemokine(s) produced by the IL-1β–activated endothelial cells that mediate SDF-1–independent transendothelial migration of CD34+cells. These data are in agreement with other previously published reports demonstrating spontaneous migration of CD34+ cells through a monolayer of BMEC-1 cell line.36 37
In the experiments reported here, E-selectin expression was induced on BMEC and HUVEC by IL-1β, which also stimulates the expression of ICAM-1 and VCAM-1. However, blocking MoAb to VCAM-1 or ICAM-1 had a minor effect on SDF-1–induced transendothelial migration of CD34+ cells. VCAM-1, the inducible ligand for VLA4, has been shown to be constitutively expressed in the BM stromal as well as sinusoidal BMEC.39 Papayannopoulou et al1,6,8have shown that homing of BM-derived HSC to the BM of the irradiated mice was blocked by pretreatment of HSC with MoAb to VLA4. In addition, MoAb to VLA4 or VCAM-1 induce significant mobilization of HSC, suggesting that the VCAM-1/VLA4 ligand pair may modulate homing as well as mobilization of HSC.1,40 We show that, under static conditions, binding of human CB-derived CD34+ cells to BMEC is dependent on VCAM-1/VLA4, but transendothelial migration of LTC-IC in response to SDF-1 was not significantly inhibited by blocking MoAb to VCAM-1. Therefore, it is possible that constitutive expression of E-selectin may play a critical role in the initial phases of SDF-1–induced transendothelial migration, whereas constitutive expression of VCAM-1 by BM reticular and sinusoidal cells may support long-term lodgment of HSC, once they have completed the transmigration process. It is also conceivable that engagement of VCAM-1/VLA4 may be more critical for SDF-1–independent transendothelial migration of HSC. Pretreatment of CD34+ cells with cytokines such IL-3 has been shown to enhance transendothelial migration of CD34+cells.37 Imai et al38 have shown that SDF-1 induces migration of the murine IL-3–dependent HSC line through VCAM-1–expressing murine endothelial cell lines. However, these SV40-transformed cell lines do not express E-selectin, even with IL-1β stimulation, and therefore the role of E-selectin could not have been evaluated.38
Frenette et al7 have shown that recruitment of transplanted hematopoietic progenitor cells to the BM of recipient P- and E-selectin knock out mice P/E(−/−) was significantly reduced. In addition, Zannettino et al41 have shown that primitive hematopoietic cells bind to P-selectin. However, based on these studies, the relative contribution of either P- or E-selectin in supporting SDF-1–induced transendothelial migration of HSC has not been studied. Resting or IL-1β–activated BMEC do not express P-selectin.33 However, given that tumor necrosis factor-α (TNF-α) or thrombin could upregulate P-selectin on BMEC,33 P-selectin may play a role in HSC trafficking during inflammatory processes.
KL not only enhances SDF-1–induced chemokinesis of CD34+progenitor cells,12 but also plays a role in VCAM-1/VLA4–mediated mobilization of HSC.1 SDF-1 and KL have also been shown to share common signaling pathways.42Therefore, because endothelial cells express both soluble and membrane bound KL,43 it is possible that KL may also augment expression of functional ESL expression on CD34+ cells.
Isolation and cultivation of BMEC in vitro results in downregulation of E-selectin expression, suggesting that factors released in the BM microenvironment may confer organ specificity to BMEC and induce upregulation of E-selectin. Among the factors modulating endothelial cell function is VEGF. VEGF is not only the principal specific mitogenic and survival factor for endothelial cells, but it may also regulate the adhesion molecule repertoire on endothelial cells. We have previously shown that one of the secreted isoforms of VEGF (VEGF165) is expressed in large quantities by different hematopoietic cells, including megakaryocytes34 and CD34+ cells (manuscript in preparation). Hence, we explored the possibility that VEGF may influence E-selectin expression by BMEC. Incubation of either unstimulated monolayers of HUVEC or BMEC with endotoxin-free recombinant VEGF165resulted in upregulation of E-selectin expression. In contrast to unstimulated endothelial monolayers, there was substantial increase in the number of SDF-1–induced CD34+ cell migration through VEGF-stimulated endothelial monolayers. VEGF-induced migration was partially mediated through interaction of CD34+ cells with E-selectin.
These results suggest that reciprocal interaction between endothelial cells and CD34+ cells is critical for homing of HSC and maintenance of native BMEC phenotype. Whether combined interaction of E-selectin, SDF-1, and KL with HSC conveys signals that modify adhesion molecule expression and homing properties of HSC is the subject of ongoing experiments.
S.R. is supported by the American Heart Association Grant-In-Aid, NHLBI Grants No. RO1 HL58707 and RO1 HL61849, the Dorothy Rodbell Foundation for Sarcoma Research, and the Rich Foundation. M.A.S.M. is supported by NHLBI Grant No. RO1 HL61401. R.L.S. is supported by NHLBI Grant No. PO1 HL46403.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Shahin Rafii, MD, Weill Medical College of Cornell University, Hematology-Oncology Division, 1300 York Ave, Room C-606, New York, NY 10021; e-mail: srafii@mail.med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal