Abstract
Recently, primitive human bone marrow (BM) progenitors supporting hematopoiesis in extended (>60 days) long-term BM cultures were identified. Such extended long-term culture-initiating cells (ELTC-IC) are of the CD34+CD38− phenotype, are quiescent, and are difficult to recruit into proliferation, implicating ELTC-IC as the most primitive human progenitor cells detectable in vitro. However, it remains to be established whether ELTC-IC can proliferate and potentially expand in response to early acting cytokines. Here, CD34+CD38− BM ELTC-IC (12-week) were efficiently recruited into proliferation and expanded in vitro in response to early acting cytokines, but conditions for expansion of ELTC-IC activity were distinct from those of traditional (5-week) LTC-IC and murine long-term repopulating cells. Whereas c-kit ligand (KL), interleukin-3 (IL-3), and IL-6 promoted proliferation and maintenance or expansion of murine long-term reconstituting activity and human LTC-IC, they dramatically depleted ELTC-IC activity. In contrast, KL, flt3 ligand (FL), and megakaryocyte growth and development factor (MGDF) (and KL + FL + IL-3) expanded murine long-term reconstituting activity as well as human LTC-IC and ELTC-IC. Expansion of LTC-IC was most optimal after 7 days of culture, whereas optimal expansion of ELTC-IC activity required 12 days, most likely reflecting the delayed recruitment of quiescent CD34+CD38− progenitors. The need for high concentrations of KL, FL, and MGDF (250 ng/mL each) and serum-free conditions was more critical for expansion of ELTC-IC than of LTC-IC. The distinct requirements for expansion of ELTC-IC activity when compared with traditional LTC-IC suggest that the ELTC-IC could prove more reliable as a predictor for true human stem cell activity after in vitro stem cell manipulation.
THE HALLMARK OF TRUE hematopoietic stem cells is their ability to long-term reconstitute large numbers of all blood cell lineages.1 Considerable efforts have been devoted towards promoting in vitro proliferation and potential expansion of human stem cells for applications in stem cell transplantation and gene therapy.2-4 A requisite for successful stem cell expansion and gene transfer would be to efficiently promote proliferation of true stem cells without a concomitant loss of long-term reconstituting ability. However, so far results from clinical gene marking protocols have been disappointing, in particular in adults, suggesting little or no ability to mimic the self-renewal process in vitro.2,5 6
A major obstacle to development of successful clinical stem cell expansion and gene marking has been lack of easy, accessible, and optimal human stem cell assays. Although ultimately clinical gene marking protocols will be needed to unequivocally demonstrate successful ex vivo expansion and retroviral marking, there is an obvious need for further development and evaluation of the ability of surrogate human stem cell assays to predict long-term reconstituting potential.7-10 Over the last 2 decades, in vitro stromal cell-based surrogate human stem cell assays have been developed,7,11-14 taking advantage of the ability of an infrequent and primitive hematopoietic cell population to produce myeloid progenitor cells for prolonged time (long-term culture-initiating cells [LTC-IC]).15,16 This assay was originally developed for stem cells in mice,17 in which LTC-IC and long-term in vivo reconstituting stem cells (LTRC) were demonstrated to be present at similar frequencies and to have similar phenotypes.18-21 Although closely overlapping, more recent studies have suggested that the requirements for maintenance and expansion of murine LTC-IC and LTRC might differ.22,23 More importantly, whereas high efficiency retroviral-mediated transduction of human 5- to 8-week LTC-IC has been obtained through short-term stimulation of human adult bone marrow (BM) cells with c-kit ligand (KL), interleukin-3 (IL-3), and IL-6,24-27 this has primarily translated into retroviral marking of short-term repopulating cells in transplanted patients.2,5,6,28,29 Thus, the traditional LTC-IC assay might predominantly reflect the presence of primitive hematopoietic cells with short-term rather than long-term in vivo reconstituting activity, thus limiting its potential utility in predicting gene transfer and expansion of true stem cells. Interestingly, Hao et al27,30 recently identified a small population of primitive human BM cells capable of producing myeloid progenitors through more extended long-term cultures (beyond 60 days). The finding that such extended LTC-IC (ELTC-IC) appear to be quiescent, more exclusively of CD34+CD38−phenotype, and difficult to recruit into proliferation27,30suggested that ELTC-IC might be a better predictor of long-term repopulating stem cells than standard LTC-IC. Most importantly, conditions that promoted efficient retroviral marking of standard LTC-IC failed to mark ELTC-IC,27,31 as they had previously failed to mark long- term repopulating stem cells in clinical gene marking protocols2,5,6,28,29 and NOD/SCID repopulating cells (SRC).32
The studies of Shah et al33 suggested that the cloning efficiency of BM CD34+CD38− cells supplemented with KL + IL-3 + IL-6 or KL + IL-3 + IL-6 + flt3 ligand (FL) was rather low (0.5% and 11.7%, respectively). It is noteworthy that most of these clones, in particular those thought to reflect ELTC-IC, could only be detected after as much as 35 days of incubation or more.33 Collectively, these findings raised a question as to whether ELTC-IC, although likely to be attractive targets for ex vivo expansion and retroviral gene transduction, might prove difficult to recruit into proliferation. However, the cloning efficiency was only investigated in long-term stroma-supported cultures, and only CD34+CD38− cells producing a minimum of 100 cells were defined as clones.33 This might be of importance, because others have demonstrated efficient recruitment and expansion of BM CD34+CD38− LTC-IC in the absence of stroma and that such expanded LTC-IC are predominantly produced after a limited number of cell divisions.34 35Thus, the present studies were designed to investigate to what degree ELTC-IC derived from CD34+CD38− BM cells could be induced to proliferate, in the absence of stromal cells, without detrimental effects on their ability to long-term reconstitute in vitro.
MATERIALS AND METHODS
Hematopoietic growth factors.
Recombinant human megakaryocyte growth and development factor (rhMGDF), recombinant human granulocyte colony-stimulating factor (rhG-CSF), recombinant human KL (rhKL), recombinant rat KL (rrKL; stem cell factor), rhIL-3, and recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) were generously provided by Amgen Corp (Thousand Oaks, CA). Recombinant human erythropoietin (rhEpo) was kindly supplied by Boehringer Mannheim Corp (Mannheim, Germany) and rmIL-3 was from PeproTech Inc (Rocky Hill, NJ). rhIL-6 was a generous gift from Genetics Institute (Cambridge, MA). rhFL was kindly supplied by Immunex (Seattle, WA). Unless otherwise indicated, all growth factors were used at the following predetermined optimal concentrations: rhMGDF, rhKL, rhFL, and rhIL-6: all at 250 ng/mL; rhIL-3 and rrKL: 50 ng/mL; rhEpo: 5 U/mL; rhG-CSF: 25 ng/mL; and rmIL-3 and rmGM-CSF: 20 ng/mL.
Enrichment and purification of CD34+CD38− and CD34+CD38+ human BM cells.
After informed consent was given by healthy adults, and with the approval of the Ethics Committee at the Medical Faculty at the University Hospital of Lund, BM cells were obtained from the posterior iliac crest and collected in syringes containing preservative-free heparin (Pharmacia, Stockholm, Sweden). Mononuclear cells (MNC) were isolated as previously described.36 Positive selection of CD34+ cells was performed using a magnetically activated cell sorting (MACS) CD34 progenitor cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of CD34+ cells was between 56% and 91% (average 77%), as determined by flow cytometric analysis.
CD34+CD38− and CD34+CD38+ cells were obtained by incubating enriched CD34+ cells with a phycoerythrin (PE)-conjugated mouse antihuman CD38 monoclonal antibody (MoAb; 2.5 μg/mL) and a fluorescein isothiocyanate (FITC)-conjugated mouse antihuman CD34 MoAb (5 μg/mL; or isotype-matched irrelevant control antibodies; all from Becton Dickinson [BD], San Jose, CA) for 15 minutes at 6°C to 12°C. Subsequently, the 75% CD34+ cells expressing the highest levels of CD38 (CD34+CD38+) and those lacking detectable CD38 expression (CD34+CD38−) were sorted on a FACSVantage (BD). As previously described,30 a conservative approach was taken to only sort the 3% lowest CD34+CD38− cells, in an effort to obtain a highly enriched population of primitive progenitors.
Enrichment and purification of Lin−Sca1+c-kit+murine BM cells.
Lineage-depleted (Lin−) BM cells were isolated from normal 6- to 10-week-old female C57BL/6 mice (Ly5.2) according to previously described protocols.37-39 Lin−cells were incubated for 30 minutes on ice with a PE-conjugated goat antirat antibody (Southern Biotechnology, Birmingham, AL). Subsequently, cells were washed and stained with a Sca1-FITC-conjugated antibody and a c-kit-Allophycocyanin (APC)-conjugated antibody (or isotype-matched control antibodies; all from PharMingen, San Diego, CA). Lin−Sca1+c-kit+ cells (purity of 96% to 99%) were sorted on a FACSVantage.
Limiting dilution assays.
CD34+CD38− (180 cells per group) and Lin−Sca1+c-kit+cells (120 cells per group) were seeded in Terasaki plates (Nunc, Kamstrup, Denmark) at a density of 1 cell per well in 20 μL X-vivo 15 (BioWhittaker, Walkersville, MD) supplemented with 1% detoxified bovine serum albumin (BSA; StemCell Technologies, Vancouver, British Columbia, Canada) containing 100 U/mL penicillin (BioWhittaker), 100 U/mL streptomycin (BioWhittaker), 2 mmol/L L-glutamine (BioWhittaker), 10−4 mol/L 2-mercaptoethanol (Sigma, St Louis, MO; serum-free [SF] medium), and various cytokines. In some experiments, Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 20% fetal calf serum (FCS; both from BioWhittaker) was used as well. Wells were scored for cell growth after 10 to 12 days of incubation at 37°C in a humidified atmosphere with 5% CO2 in air. Because the statistical probability (based on Poisson probability distribution) of a well not receiving any cell is 37% by this method, the maximum expected clones were 76 for Lin−Sca1+c-kit+ cells and 113 for CD34+CD38− cells. In some experiments, cells were deposited by a single-cell depositor coupled to a FACSVantage and subsequently carefully visualized by microscopy to only include wells containing a single cell. The cytokine response pattern was similar with these 2 methods.
High-resolution cell division tracking of candidate murine and human stem cells.
Staining and flow procedures for high-resolution cell division tracking of CD34+CD38− and Lin−Sca1+c-kit+ cells using 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) were performed based on previously described procedures.40 Briefly, CFSE was added to cells at 5 × 106 cells/mL in Dulbecco's phosphate-buffered saline (DPBS; BioWhittaker) to give a final concentration of 1 μmol/L. Murine Lin− or human CD34+ cells were incubated at 37°C for 10 minutes, and the staining reaction was stopped by adding a 10-fold excess of DPBS with 10% to 20% FCS. Subsequently, cells were washed 2 to 3 times in DPBS with 1% FCS. To allow any unbound dye to diffuse out of the cells, CFSE-labeled cells were incubated overnight (12 to 15 hours) at 37°C in SF-medium supplemented with either KL + FL + MGDF (250 ng/mL each; human cells) or KL (50 ng/mL; murine cells). These cytokines induced proliferation of only a minor fraction of cells within the 12- to 15-hour incubation period (Bryder and Jacobsen, unpublished observations, May 1998). Murine cells were subsequently stained with rat antimouse Sca1-PE and rat antimouse c-kit-APC or appropriate isotype control antibodies (all PharMingen). Cells were sorted on a FACSVantage based on dual expression of Sca1 and c-kit and a 40 to 50 channel wide sorting gate set around the mean fluorescence channel for CFSE. After several days of culture, cells were analyzed with identical instrument settings and sorted based on number of cell divisions. Lin−Sca1+c-kit+ CFSE-stained cells were run on the FACSVantage before culture as a control for undivided cells. However, because some CFSE diffusion appeared to occur also after the 12- to 15-hour preincubation, sort gates were also set based on preliminary studies in which cells were analyzed every 12 hours throughout the 7 days of culture. This allowed a careful and accurate tracking of cell divisions and demonstrated, in agreement with others,40 that a maximum number of 7 cell divisions could reproducibly be detected with high resolution (Bryder and Jacobsen, unpublished observations, May 1998).
Human cells preincubated in CFSE for 12 to 15 hours were stained with mouse antihuman CD34-PE and mouse antihuman CD38-APC, or appropriate isotype control antibodies (all BD), and sort regions were then set to allow sorting of CD34+CD38− cells with a defined gate of 40 to 50 channels based on CFSE-fluorescence.
Long-term and extended long-term culture-initiating cell (LTC-IC and ELTC-IC) assay.
Long-term cultures were established and maintained as previously described.41 Briefly, stroma layers were initiated with human BM MNC by seeding 20 to 30 × 106 cells in a 80-cm2 culture flask in 15 mL myeloid long-term culture medium (Myelocult; StemCell) supplemented with 10−6mol/L hydrocortisone 21-hemisuccinate (Sigma). Half of the medium was changed weekly, and at confluency (usually after 4 to 6 weeks), cells were irradiated with an absorbed dose of 15 Gy with 6 MV x-rays from a medical linear accelerator (Philips SL25; Philips Medical Systems, Crawley, West Sussex, UK). After irradiation, cells were trypzinated (Trypsin Versene; BioWhittaker) and transferred to 24-well microtiter plates (Nunc; each flask sufficient for 35 wells). Freshly isolated and in vitro cultured cells were added in triplicates (1 well for each replicate of cultured cells was transferred to 1 well of stroma after counting) to irradiated allogeneic BM stroma layer, always using the same stroma for fresh and expanded cells. Cocultures were maintained by half medium changes weekly.
For ELTC-IC experiments, nonadherent and adherent cells were transferred to new irradiated stroma every 4 weeks throughout the 12 weeks of stromal cocultivation. We chose 12 weeks (84 days) as the endpoint of our ELTC-IC assay, because it has been demonstrated that stromal cocultivation for a period of 60 to 100 days detects a distinct and more primitive population of hematopoietic cells than the standard 5-week LTC-IC.27 30
After 5 weeks (LTC-IC) and 12 weeks (ELTC-IC), nonadherent and adherent cells were transferred to methylcellulose cultures containing predetermined optimal concentrations of MGDF, G-CSF, KL, FL (all at 25 ng/mL), Epo (5 U/mL), and IL-3 (10 ng/mL). To ensure formation of a reliable number of colonies from the long-term cultures, the content of each stroma coculture well was transferred to methylcellulose cultures at both a low and a high cell concentration. Colony-forming cells (CFC; read out of LTC-IC and ELTC-IC) were scored after an additional 10 to 12 days in culture.
Competitive repopulating assay for murine BM stem cells.
C57BL/6 recipient mice (Ly5.2) were lethally irradiated by a single exposure to 9.5 Gy of gamma irradiation from a 137Cs source (Instrument AB Scanditronix; Husbyborg, Uppsala, Sweden). Irradiated recipients were transplanted intravenously (0.5 mL/mouse) by tail injection with 750 CFSE-stained Lin−Sca1+c-kit+ cells (from B6SJL mice, Ly5.1) cultured for 12 to 15 hours in KL (control) or 750 expansion equivalents (EE) of CFSE-stained Lin−Sca1+c-kit+ cells. Donor cells were cotransplanted with 200,000 unfractionated congenic (Ly5.2) BM cells as a competitor and survival population. All mice (4 mice/group) were kept in individually ventilated cages throughout the experiment and given sterile food and autoclaved acidified water. Mice were bled from the retroorbital sinus and analyzed for donor reconstitution on a FACSCalibur (BD), after staining with antibodies against Ly5.1, Ly5.2, and lineage-specific antigens (all from PharMingen).
Statistics.
The statistical significance of differences between groups were determined using the paired Student's t-test. For LTC-IC and ELTC-IC data, the Student's t-test was performed with log transformation of the data.
RESULTS
KL + IL-3 + IL-6 efficiently promote proliferation of murine Lin−Sca1+c-kit+LTRC.
A main rationale for using KL + IL-3 + IL-6 (K36) to promote stem cell cycling in clinical gene marking protocols was their ability to efficiently promote retroviral-mediated gene transfer to LTRC from mice primed with 5-fluorouracil (5-FU) in vivo.42,43However, previous and recent studies have suggested that cytokine combinations containing K36 are inefficient at promoting gene transfer to unprimed murine BM LTRC.44 45 Thus, it remains to be established whether K36 can efficiently promote cycling and proliferation of unprimed murine BM stem cells without compromising their long-term reconstituting ability.
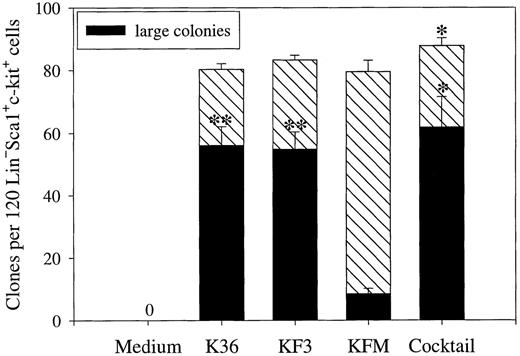
K36 recruited most if not all clonable candidate (Lin−Sca1+c-kit+) BM stem cells into proliferation under SF-conditions (Fig 1). K36 were equally efficient at promoting recruitment of Lin−Sca1+c-kit+progenitors into proliferation as KL + FL + IL-3 (KF3) and KL + FL + MGDF (KFM) (Fig 1), 2 cytokine combinations that recently have been suggested to be particularly promising at promoting growth of candidate human BM stem cells.35,36 46 In fact, stimulating Lin−Sca1+c-kit+ cells with a cocktail of 7 cytokines resulted in recruitment of only a few additional progenitors, indicating that K36, KF3, and KFM optimally induce in vitro growth of candidate murine stem cells. Whereas K36, KF3, and the cocktail resulted in formation of predominantly large colonies, most of the colonies formed in response to KFM were small (Fig 1).
Recruitment of candidate murine stem cells into proliferation. Lin−Sca1+c-kit+ BM cells were plated at 1 cell per well in SF-medium (X-vivo 15 with 0.5% detoxified BSA) supplemented with indicated cytokines at 50 ng/mL each, except for IL-3 (20 ng/mL). The cocktail contained the following 7 cytokines: GM-CSF, KL, IL-3, IL-6, MGDF, FL, and G-CSF. The total number of clones (containing 2 or more cells) and large colonies (clones covering more than 50% of well) were scored after 10 days of incubation. 0 = no clones. Results are presented as the means (±SEM) of 4 separate experiments. Paired Student's t-test was performed comparing KFM with the other cytokine combinations presented. *P < .05; **P < .005.
Recruitment of candidate murine stem cells into proliferation. Lin−Sca1+c-kit+ BM cells were plated at 1 cell per well in SF-medium (X-vivo 15 with 0.5% detoxified BSA) supplemented with indicated cytokines at 50 ng/mL each, except for IL-3 (20 ng/mL). The cocktail contained the following 7 cytokines: GM-CSF, KL, IL-3, IL-6, MGDF, FL, and G-CSF. The total number of clones (containing 2 or more cells) and large colonies (clones covering more than 50% of well) were scored after 10 days of incubation. 0 = no clones. Results are presented as the means (±SEM) of 4 separate experiments. Paired Student's t-test was performed comparing KFM with the other cytokine combinations presented. *P < .05; **P < .005.
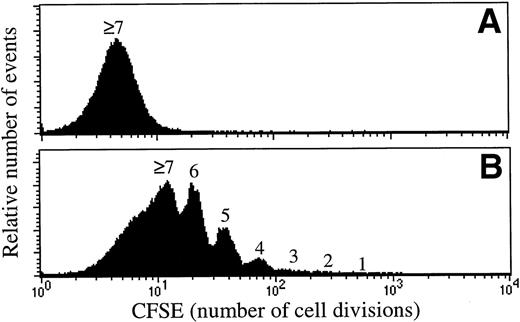
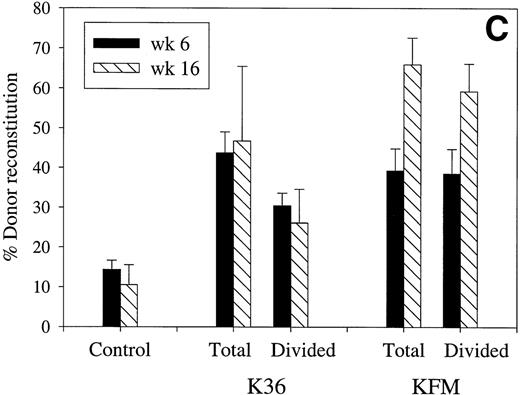
Lin−Sca1+c-kit+ cells cultured for 5 to 7 days in the presence of both K36 or KFM demonstrated a preserved ability to short- and long-term in vivo reconstitute (Bryder and Jacobsen, unpublished observations, January 1999). However, to unequivocally demonstrate that the reconstituting activities of the in vitro cultured stem cell populations were derived from cells that had undergone proliferation, high-resolution cell division tracking was performed to allow distinction and purification of cells that had undergone cell divisions. These studies demonstrated that almost all Lin−Sca1+c-kit+ cells had undergone multiple cell divisions after 7 days of culture in response to K36 and KFM (Fig 2A and B) and that most if not all of the reconstituting ability was derived from cells that had proliferated (Fig 2C). Furthermore, the long-term repopulating activities of the expanded cells were enhanced when compared with that of unexpanded CFSE-sorted cells (Fig 2C) and were comparable with that of freshly isolated Lin−Sca1+c-kit+ cells (Bryder and Jacobsen, unpublished observations, April 1999).
Most in vivo reconstituting murine stem cells proliferate in response to KL + IL-3 + IL-6 as well as KL + FL + MGDF. CFSE-stained Lin−Sca1+c-kit+(Ly5.1) BM cells were cultured in SF-medium supplemented with either K36 (A) or KFM (B) (all at 50 ng/mL, except for IL-3 at 20 ng/mL). (A) and (B) show the proliferation history (number of cell divisions) after 7 days of incubation. Cultured CFSE-stained cells were resorted to include either all cells (total) or only cells that had proliferated (a conservative approach was taken to only include cells that had undergone ≥2 divisions). Both cell populations, as well as the unexpanded CFSE-stained cells (control), were transplanted into lethally irradiated mice (C) together with unfractionated BM cells (Ly5.2). Analysis of the percentage of total donor reconstitution in peripheral blood was performed 6 and 16 weeks posttransplantation. Control and expanded cell populations showed comparable repopulation of all (myeloid, B, and T) lineages. All data are the means (±SEM) of 4 mice, except for KFM (total) 16 weeks posttransplantation, where n = 3. Data are from 1 of 2 experiments with similar results.
Most in vivo reconstituting murine stem cells proliferate in response to KL + IL-3 + IL-6 as well as KL + FL + MGDF. CFSE-stained Lin−Sca1+c-kit+(Ly5.1) BM cells were cultured in SF-medium supplemented with either K36 (A) or KFM (B) (all at 50 ng/mL, except for IL-3 at 20 ng/mL). (A) and (B) show the proliferation history (number of cell divisions) after 7 days of incubation. Cultured CFSE-stained cells were resorted to include either all cells (total) or only cells that had proliferated (a conservative approach was taken to only include cells that had undergone ≥2 divisions). Both cell populations, as well as the unexpanded CFSE-stained cells (control), were transplanted into lethally irradiated mice (C) together with unfractionated BM cells (Ly5.2). Analysis of the percentage of total donor reconstitution in peripheral blood was performed 6 and 16 weeks posttransplantation. Control and expanded cell populations showed comparable repopulation of all (myeloid, B, and T) lineages. All data are the means (±SEM) of 4 mice, except for KFM (total) 16 weeks posttransplantation, where n = 3. Data are from 1 of 2 experiments with similar results.
KL + IL-3 + IL-6 only recruit a minor subpopulation of candidate human BM CD34+CD38− stem cells into proliferation.
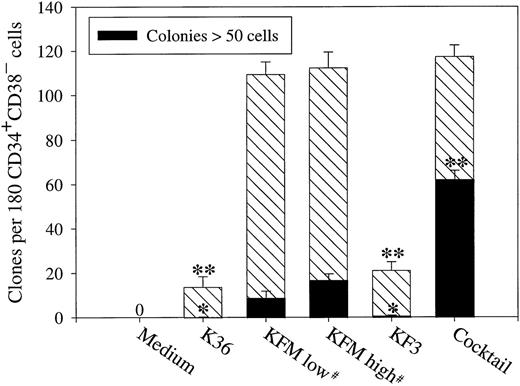
K36 have frequently, but with limited success, been used to promote retroviral-mediated gene transfer into human stem cells in clinical gene marking protocols.2,5,6,28,29 Surprisingly, in a SF-medium K36 only recruited approximately 10% of CD34+CD38− cells into proliferation (Fig 3). Expansion of stem cells has been suggested to depend on high concentrations of cytokines47; however, the growth response to K36 was not altered by enhancing the concentration of each of the 3 cytokines 5-fold (250 ng/mL each; Ramsfjell and Jacobsen, unpublished observations, August 1998).
Recruitment of candidate human stem cells into proliferation. CD34+CD38− BM cells were plated at 1 cell per well in SF-medium and supplemented with indicated cytokines at 250 ng/mL, except for IL-3, which was used at 50 ng/mL. #KFM were compared at high (250 ng/mL each) and low (50 ng/mL each) concentrations. The cocktail consisted of the following 6 cytokines: KL, IL-3, IL-6, MGDF, FL, and G-CSF. The total number of clones and colonies (>50 cells) were scored after 10 to 12 days of incubation. 0 = no clones. Results are presented as the means (±SEM) of 4 separate experiments. Paired Student's t-test was performed comparing KFM high with the other cytokine conditions presented. *P < .05; **P < .005.
Recruitment of candidate human stem cells into proliferation. CD34+CD38− BM cells were plated at 1 cell per well in SF-medium and supplemented with indicated cytokines at 250 ng/mL, except for IL-3, which was used at 50 ng/mL. #KFM were compared at high (250 ng/mL each) and low (50 ng/mL each) concentrations. The cocktail consisted of the following 6 cytokines: KL, IL-3, IL-6, MGDF, FL, and G-CSF. The total number of clones and colonies (>50 cells) were scored after 10 to 12 days of incubation. 0 = no clones. Results are presented as the means (±SEM) of 4 separate experiments. Paired Student's t-test was performed comparing KFM high with the other cytokine conditions presented. *P < .05; **P < .005.
As previously demonstrated, KFM efficiently recruited CD34+CD38− BM cells into proliferation,36 resulting in 62% of the wells containing proliferative clones (Fig 3). The addition of 3 additional cytokines (IL-3, IL-6, and G-CSF) did not further enhance the recruitment seen in response to KFM (P = .56; Fig 3), and the cloning frequency and size of clones in KFM-stimulated cultures were similar at low and high cytokine concentrations (Fig 3).
Because KF3 have been demonstrated to efficiently expand CD34+CD38− BM LTC-IC,35 it was surprising that this cytokine combination only promoted growth of approximately 15% of CD34+CD38− cells, similar to K36 (Fig 3). Again, a 5-fold increase in the concentration of KL, FL, and IL-3 did not affect the number or size of clones formed (Ramsfjell and Jacobsen, unpublished observations, August 1998). Because KFM were much more efficient than K36 (P < .005) and KF3 (P < .005) at promoting growth of CD34+CD38− cells under SF-conditions, we also compared the effects of these 3 cytokine combinations in FCS-containing medium (IMDM + 20% FCS). Interestingly, although the cloning frequency seen in response to K36 and KF3 was considerably higher in FCS-containing medium than in SF-medium, resulting in the growth of 31% and 52% of CD34+CD38−cells, respectively, KFM remained most efficient at promoting recruitment of CD34+CD38− cells (68% of the cells; means of 2 experiments).
KL + IL-3 + IL-6 efficiently promote expansion of CD34+CD38− LTC-CFC but deplete the more primitive ELTC-CFC.
Next, we investigated whether the different levels of recruitment of CD34+CD38− cells into cycling by the cytokine combinations investigated might reflect activation and potential expansion of functionally distinct subpopulations within the CD34+CD38− cell compartment. Of particular relevance, we evaluated and compared the usefulness of the standard (5-week) and extended (12-week) LTC-IC for predicting expansion of candidate human stem cells. Standard LTC-IC cultures initiated with 10 times as many CD34+CD38+ as CD34+CD38− cells resulted in a similar level of CFC production, in that 73 (experiment no. 1) to 148 (experiment no. 2) LTC-CFC were produced from 1,500 CD34+CD38+ cells and 27 (experiment no. 1) to 113 (experiment no. 2) LTC-CFC from 150 CD34+CD38− cells (means of triplicates from 2 experiments), demonstrating that LTC-IC were highly enriched in the CD34+CD38− (lowest 3%) cell population, but were also present in the less primitive CD34+CD38+ population (highest 75%). In contrast, and in agreement with others,30 we found ELTC-IC exclusively in the CD34+CD38− population. Specifically, 1,000 CD34+CD38− cells generated 14 to 150 12-week LTC-CFC (ELTC-CFC), whereas no CFC was detected from 10,000 CD34+CD38+ cells in ELTC-IC cultures (n = 2).
The abilities of K36, KF3, and KFM (all cytokines at 250 ng/mL; except for IL-3 at 50 ng/mL) to expand CD34+CD38−-derived 5-week LTC-IC were investigated (for experimental set up, see Fig 4). A number of previous studies have shown that the average number of CFC produced per LTC-IC remains constant when comparing fresh and cultured LTC-IC,34,47 48thus supporting the validity of using the number of LTC-CFC generated under various conditions as an indication for differences in LTC-IC activity. K36 increased the number of CFC produced after 5 weeks of long-term culture (5-week LTC-CFC) from CD34+CD38− cells many-fold when cultured for 7 as well as 12 days (Table 1). KF3 expanded 5-week LTC-CFC more than K36 (P < .05). However, KFM were most efficient (both at day 7 and 12; P < .05 for KFMv KF3 and KFM v K36) at expanding 5-week LTC-CFC (up to 244-fold). Noteworthy, for all 3 cytokine combinations (except for KF3 in experiment no. 2), the expansion of 5-week LTC-CFC was higher at day 7 than after 12 days of ex vivo culture (Table 1), although only K36 reached statistical significance (P < .05).
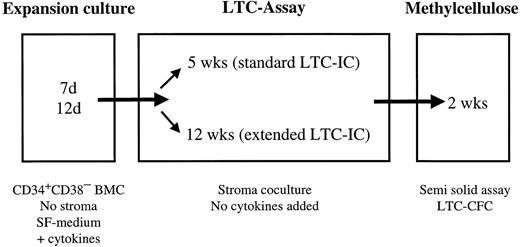
Experimental design of ex vivo expansion of LTC-IC and ELTC-IC. CD34+CD38− BM cells (BMC; maximum 1,000 cells/mL) were cultured in SF medium supplemented with cytokines. After 7 or 12 days of expansion culture, cells were counted and transferred to either 5- or 12-week LTC. At the end of the 5 or 12 weeks of stroma coculture (no cytokines added), LTC-IC–derived CFC (LTC-CFC) were detected in a CFC assay.
Experimental design of ex vivo expansion of LTC-IC and ELTC-IC. CD34+CD38− BM cells (BMC; maximum 1,000 cells/mL) were cultured in SF medium supplemented with cytokines. After 7 or 12 days of expansion culture, cells were counted and transferred to either 5- or 12-week LTC. At the end of the 5 or 12 weeks of stroma coculture (no cytokines added), LTC-IC–derived CFC (LTC-CFC) were detected in a CFC assay.
KL + IL-3 + IL-6 as Well as KL + FL + MGDF Expand 5-Week LTC-CFC
| Experiment No. . | Cytokines . | 5-wk LTC-CFC, % of Control . | |
|---|---|---|---|
| d7 . | d12 . | ||
| 1 | K36 | 360 (55) | 176 (62) |
| KF3 | 448 (324) | 248 (234) | |
| KFM | 3,522 (396) | 2,070 (1,647) | |
| 2 | K36 | 952 (829) | 344 (242) |
| KF3 | 3,254 (1,470) | 5,026 (1,957) | |
| KFM | 8,862 (1,604) | 8,069 (458) | |
| 3 | K36 | 5,889 (2,143) | 816 (408) |
| KF3 | 21,778 (5,551) | 6,186 (652) | |
| KFM | 24,384 (11,729) | 18,333 (7,638) | |
| Experiment No. . | Cytokines . | 5-wk LTC-CFC, % of Control . | |
|---|---|---|---|
| d7 . | d12 . | ||
| 1 | K36 | 360 (55) | 176 (62) |
| KF3 | 448 (324) | 248 (234) | |
| KFM | 3,522 (396) | 2,070 (1,647) | |
| 2 | K36 | 952 (829) | 344 (242) |
| KF3 | 3,254 (1,470) | 5,026 (1,957) | |
| KFM | 8,862 (1,604) | 8,069 (458) | |
| 3 | K36 | 5,889 (2,143) | 816 (408) |
| KF3 | 21,778 (5,551) | 6,186 (652) | |
| KFM | 24,384 (11,729) | 18,333 (7,638) | |
One hundred fifty human CD34+CD38− cells were cultured in SF-medium in the presence of the indicated cytokines. As a control for the content of 5-week LTC-CFC in freshly isolated CD34+CD38− cells, the same number of cells was transferred directly to pre-established irradiated stroma. After 5 weeks of stroma cocultivation, the number of CFC produced was evaluated in methylcellulose cultures. Results are presented as the mean (SD) of triplicate wells per group and time point. Controls (fresh, nonexpanded CD34+CD38− cells) were set to represent 100% and reflected 253 ± 6 colonies in experiment no. 1, 19 ± 13 colonies in experiment no. 2, and 5 ± 2 colonies in experiment no. 3.
Experiments in which CD34+CD38− cells were stained with CFSE before culture in SF-medium supplemented with KFM demonstrated that most if not all cells had undergone cell division(s) in response to KFM after 7 days of incubation, with the majority of the cells having divided 3 to 5 times. In agreement with this, cells sorted into CFSE-high (undivided) and CFSE-low (divided) cells demonstrated that the majority of BM CD34+CD38− LTC-IC had undergone proliferation after 7 days of incubation in KFM (Kornfält and Jacobsen, unpublished observations, November 1998).
Whereas the LTC-IC assay has been used extensively to evaluate ex vivo expansion of primitive human progenitors, the ELTC-IC assay has not yet been used for this purpose. Interestingly, whereas K36 expanded CD34+CD38−-derived 5-week LTC-CFC many-fold (Table 1), the number of 12-week LTC-CFC was consistently and dramatically reduced after 7 as well as 12 days of culture (Table 2). In contrast, both KF3 and KFM expanded 12-week LTC-CFC, although less efficiently than 5-week LTC-CFC. Noteworthy, and in contrast to 5-week LTC-CFC, 12-week LTC-CFC were more efficiently expanded after 12 than 7 days of ex vivo expansion in the presence of KFM (P < .05). Furthermore, after 12 days of culture, KFM-stimulated cultures contained consistently more 12-week LTC-CFC (3- to 30-fold expansion) than KF3-stimulated cultures (Table 2; P < .05).
Prolonged Exposure to KL + IL-3 + IL-6, But Not KL + FL + MGDF, Deplete ELTC-CFC
| Experiment No. . | Cytokines . | 12-wk LTC-CFC, % of Control . | |
|---|---|---|---|
| d7 . | d12 . | ||
| 1 | K36 | 9 (16) | 3 (5) |
| KF3 | 171 (28) | 291 (267) | |
| KFM | 529 (193) | 1,568 (268) | |
| 2 | K36 | 16 (15) | 33 (19) |
| KF3 | 202 (147) | 68 (84) | |
| KFM | 44 (38) | 269 (88) | |
| 3 | K36 | 17 (30) | 53 (91) |
| KF3 | 156 (136) | 158 (80) | |
| KFM | 64 (50) | 777 (874) | |
| 4 | K36 | 0 | 0 |
| KF3 | 1,217 (507) | 2,136 (1,176) | |
| KFM | 1,589 (404) | 3,002 (1,331) | |
| Experiment No. . | Cytokines . | 12-wk LTC-CFC, % of Control . | |
|---|---|---|---|
| d7 . | d12 . | ||
| 1 | K36 | 9 (16) | 3 (5) |
| KF3 | 171 (28) | 291 (267) | |
| KFM | 529 (193) | 1,568 (268) | |
| 2 | K36 | 16 (15) | 33 (19) |
| KF3 | 202 (147) | 68 (84) | |
| KFM | 44 (38) | 269 (88) | |
| 3 | K36 | 17 (30) | 53 (91) |
| KF3 | 156 (136) | 158 (80) | |
| KFM | 64 (50) | 777 (874) | |
| 4 | K36 | 0 | 0 |
| KF3 | 1,217 (507) | 2,136 (1,176) | |
| KFM | 1,589 (404) | 3,002 (1,331) | |
One thousand to 1,500 human CD34+CD38−cells were cultured in SF-medium in the presence of the indicated cytokines. As a control for the content of ELTC-IC in freshly isolated CD34+CD38− cells, the same number of cells was transferred directly to pre-established irradiated stroma. After 12 weeks of stroma cocultivation, the number of CFC produced was evaluated in methylcellulose cultures. Results are presented as the mean (SD) of triplicate wells per group and time point. Controls (fresh, nonexpanded CD34+CD38− cells) were set to represent 100% and reflected 55 ± 7 colonies in experiment no. 1, 40 ± 15 colonies in experiment no. 2, 11 ± 10 colonies in experiment no. 3, and 9 ± 5 colonies in experiment no. 4.
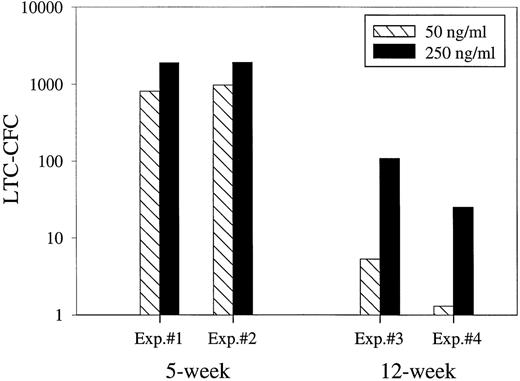
In agreement with Zandstra et al,47 we found that lowering the concentrations of KFM to 50 ng/mL each resulted in production of 54% less 5-week LTC-CFC (Fig 5). Interestingly, the difference in expansion was more evident with 12-week LTC-CFC, which were expanded as much as 20-fold more with the high than low cytokine concentrations (Fig 5).
Efficient expansion of ELTC-CFC requires very high concentrations of early acting cytokines. Fifty (LTC-IC assay) and 1,000 (ELTC-IC assay) human CD34+CD38−cells were cultured in SF-medium in the presence of KFM at low (50 ng/mL) and high (250 ng/mL) concentrations. After 12 days of stroma-free culture, expanded cells were transferred to irradiated stroma cocultures. After either 5 or 12 weeks, the number of CFC produced was evaluated in methylcellulose. Results are the means of triplicate wells per group from 4 separate experiments.
Efficient expansion of ELTC-CFC requires very high concentrations of early acting cytokines. Fifty (LTC-IC assay) and 1,000 (ELTC-IC assay) human CD34+CD38−cells were cultured in SF-medium in the presence of KFM at low (50 ng/mL) and high (250 ng/mL) concentrations. After 12 days of stroma-free culture, expanded cells were transferred to irradiated stroma cocultures. After either 5 or 12 weeks, the number of CFC produced was evaluated in methylcellulose. Results are the means of triplicate wells per group from 4 separate experiments.
Serum-containing medium negatively affects prolonged ex vivo expansion of ELTC-CFC.
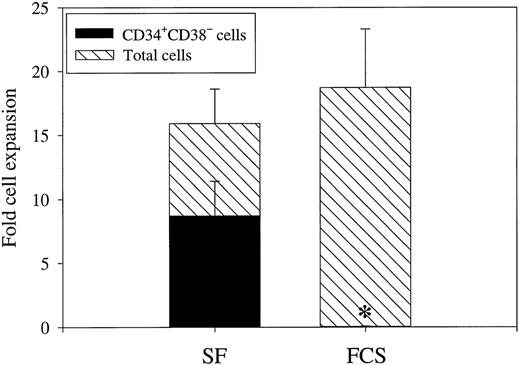
KFM were equally efficient at promoting recruitment of CD34+CD38− cells into proliferation in FCS-containing medium as in SF-medium (114 ± 9 and 117 ± 5 clones derived from 180 CD34+CD38− cells, respectively; n = 4). Despite this, expansion of ELTC-CFC in SF-medium was 17-fold higher than in FCS-containing medium (Table 3;P < .05). Again, the effect was more pronounced on ELTC-CFC than LTC-CFC, because both SF- and FCS-containing medium supported expansion of LTC-CFC (experiment no. 1: 81-fold [SF] and 71-fold [FCS]; and experiment no. 2: 183-fold [SF] and 24-fold [FCS]). Although its predictive value has been questioned, the potential usefulness of a phenotypic quantification of candidate stem cells is obvious, because functional surrogate human stem cell assays are tedious and require multiple weeks before a final read out. In good correlation with the 12-week LTC-CFC data, as much as 52% of KFM-derived cells maintained a CD34+CD38− phenotype after 12 days of SF-culture, whereas virtually no (0.2%) CD34+CD38− cells could be recovered from FCS-supported cultures (Fig 6; P< .05). Further phenotypic analysis showed that more than 80% of day 12 KFM-derived CD34+CD38− cells also remained negative for lineage markers (CD2, CD4, CD8, CD19, CD14, CD15, CD16, CD56, and glycophorin A; n = 2).
Effect of FCS-Containing Medium on Expansion of ELTC-CFC
| Experiment No. . | Fold Cell Expansion . | 12-wk LTC-CFC, % of Control . | ||
|---|---|---|---|---|
| SF . | 20% FCS . | SF . | 20% FCS . | |
| 1 | 14 | 17 | 269 | 7 |
| 2 | 7 | 8 | 137 | 29 |
| 3 | 42 | 44 | 3,002 | 394 |
| 4 | 19 | 13 | 2,811 | 211 |
| Experiment No. . | Fold Cell Expansion . | 12-wk LTC-CFC, % of Control . | ||
|---|---|---|---|---|
| SF . | 20% FCS . | SF . | 20% FCS . | |
| 1 | 14 | 17 | 269 | 7 |
| 2 | 7 | 8 | 137 | 29 |
| 3 | 42 | 44 | 3,002 | 394 |
| 4 | 19 | 13 | 2,811 | 211 |
One thousand CD34+CD38− cells were cultured in FCS-containing or in SF-medium supplemented with KFM at 250 ng/mL each for 12 days. As a control, the same number of freshly isolated CD34+CD38− cells was transferred directly to pre-established irradiated stroma. After 12 weeks of stroma cocultivation, the number of CFC produced was established. Results are the means of triplicate wells per group.
Phenotypic characterization of CD34+CD38− cells expanded under serum-free and serum-containing conditions. CD34+CD38−BM cells were cultured in 1.5 mL SF-medium or FCS-containing medium supplemented with KFM. After 12 days of incubation, cell numbers were counted and cells were analyzed for CD34 and CD38 expression by flow cytometry. Results are presented as the means (±SEM) of 3 separate experiments. Paired Student's t-test was performed comparing the 2 different growth conditions. *P < .05.
Phenotypic characterization of CD34+CD38− cells expanded under serum-free and serum-containing conditions. CD34+CD38−BM cells were cultured in 1.5 mL SF-medium or FCS-containing medium supplemented with KFM. After 12 days of incubation, cell numbers were counted and cells were analyzed for CD34 and CD38 expression by flow cytometry. Results are presented as the means (±SEM) of 3 separate experiments. Paired Student's t-test was performed comparing the 2 different growth conditions. *P < .05.
DISCUSSION
The potential utility of ex vivo expanded stem cells for clinical transplantation and gene therapy purposes is obvious.2-4 A successful approach to ex vivo expand HSC would also provide a means of addressing the molecular regulation of stem cell self-renewal and cell cycle status of reconstituting stem cells. For transplantation purposes, ex vivo expanded grafts must contain a minimum number of stem cells to promote efficient long-term reconstitution. Although it has been postulated that ex vivo expanded committed progenitor cells could facilitate short-term engraftment,3,4,49-51 studies in animal models suggest that LTRC might also be the best short-term repopulating cells.52 53 Thus, a primary objective of all ex vivo expansion protocols designed to improve engraftment or promote retroviral gene transfer must be to preserve and/or expand the number of LTRC.
Because of the absence of optimal preclinical human stem cell assays, studies of murine long-term reconstituting stem cells continue to play an important role for preclinical development of stem cell and gene therapy. Murine BM LTRC primed with 5-FU in vivo have been demonstrated to be excellent targets for retroviral-mediated gene transfer if prestimulated with K36, suggesting that they can self-renew in response to these cytokines in vitro.42,43 Thus, the ability of this cytokine combination to promote gene transfer into murine BM stem cells in vitro, combined with its ability to transduce human LTC-IC,24-27 provided a rationale for using K36 to promote gene transfer into human stem cells. However, clinical studies have failed to demonstrate efficient transduction of long-term reconstituting human BM stem cells using this approach.2,5,6,28,29 Furthermore, retroviral gene targeting of unprimed murine BM stem cells (more closely resembling human BM target cells in previous gene marking protocols) has also been inefficient.44 45 One obvious reason could be that K36 do not efficiently promote self-renewal divisions of unprimed BM stem cells. However, in the present studies, we demonstrate that the LTRC activity is retained in the fraction of cells that has undergone 2 or more cell divisions, unequivocally demonstrating that K36 (and KFM) promote proliferation and potential expansion of murine LTRC. Although a quantification of stem cell numbers was not performed, K36 and KFM expanded stem cells showed enhanced long-term reconstituting activity when compared with unexpanded CFSE-stained Lin−Sca1+c-kit+ cells. Thus, K36 and KFM efficiently promote proliferation of murine BM stem cells without compromising their long-term reconstituting ability.
Similarly to what we observed with candidate (Lin−Sca1+c-kit+) murine stem cells, KFM efficiently induced recruitment of candidate (CD34+CD38−) human BM stem cells into proliferation. Actually, KFM were as efficient at stimulating recruitment of CD34+CD38− cells as a cocktail containing 3 additional cytokines. In contrast, a much smaller fraction of CD34+CD38− BM cells proliferated in response to K36. Surprisingly, also KF3, shown to potently expand BM LTC-IC,35 were rather inefficient (when compared with KFM) at promoting recruitment of CD34+CD38− BM cells in the present studies. We cannot rule out that our specific SF-conditions might selectively support KFM- and not K36- or KF3-stimulated proliferation of CD34+CD38− cells. However, using the same SF-conditions, K36 and KF3 induced proliferation and cellular expansion of total CD34+ cells more efficiently than KFM (Ramsfjell and Jacobsen, unpublished observations, August 1998).
In the absence of an optimal human stem cell assay, the LTC-IC assay has played an instrumental role in the identification and characterization of candidate stem cells.9,14-16 However, in support of such standard (5- to 8-week) LTC-IC not necessarily representing the most primitive hematopoietic progenitor/stem cells, recent studies of freshly isolated BM and cord blood cells suggested that an extension of the long-term culture beyond 60 days selects for detection of a distinct and more infrequent progenitor, with unique phenotypic and cell cycle characteristics thought to be associated with true hematopoietic stem cells.27,30 Furthermore, previous studies had implicated a low cloning efficiency, delayed onset of proliferation, and in particular resistance to retroviral marking of ELTC-IC (unlike LTC-IC).27 30 However, in the present studies, we provide new data supporting that ELTC-IC can be recruited into proliferation and expanded in response to early acting cytokines.
Because our ELTC-IC assay, in contrast to previous studies,30 was performed without addition of cytokines, we cannot conclude that we are evaluating the same cells. However, in agreement with the studies of Hao et al,30 we found ELTC-CFC to be derived from CD34+CD38−cells and never from the sorted CD34+CD38+population. However, our findings do not rule out that CD34+CD38low cells might also contain ELTC-IC, because our CD34+CD38+ population only contained the 75% highest CD38+ cells.
Through a direct comparison of the conditions required for expansion of LTC-CFC and ELTC-CFC, we observed a number of distinct differences, all supporting the more primitive and quiescent nature of ELTC-IC. After 7 as well as 12 days of incubation, K36 expanded 5-week LTC-CFC derived from CD34+CD38− BM cells many-fold, while dramatically depleting ELTC-CFC. This finding provides one plausible explanation as for why Hao et al27 and Case et al31 were unable to retrovirally transduce ELTC-IC using the same cytokine combination, while obtaining efficient gene marking of LTC-IC. These data might (at least in part) also explain the inefficient stem cell gene marking observed in previous clinical gene therapy/marking protocols.2,6,28 29 It is also noteworthy that the inability of K36 to promote proliferation of ELTC-IC could not be predicted from our parallel studies on murine stem cells. Thus, the present studies implicate an important qualitative difference in the in vitro cytokine responsiveness of candidate murine and human BM stem cells.
Because limiting dilution was not performed, we cannot conclude to what degree the observed increase in ELTC-CFC activity results from an increased number of ELTC-IC with sustained or reduced level of CFC production. The ability of KFM and KF3 to efficiently expand not only LTC-CFC but also ELTC-CFC agrees with previous studies suggesting that these 2 cytokine combinations might be particularly useful at promoting expansion of candidate adult human stem cells.35,36,46 Our studies also further implicate MGDF as an essential and potent cytokine for optimal recruitment of BM CD34+CD38−cells into proliferation, because KFM were both required and sufficient for optimal recruitment. Although KFM were somewhat more efficient than KF3 at expanding ELTC-CFC after 12 days of culture, the ELTC-CFC activity derived per clonable CD34+CD38−cell was comparable. Furthermore, because others have demonstrated a higher cloning efficiency of CD34+CD38−BM cells in response to KF3,47 the abilities of these 2 cytokine combinations to expand candidate BM stem cells are likely to be comparable.
Optimal expansion of LTC-CFC was observed after 7 rather than 12 days of culture, whereas expansion of ELTC-CFC was most pronounced after more prolonged (12-day) culture. This observation is likely to be explained by a quiescent/primitive fraction of CD34+CD38− cells being recruited into the first cell division after prolonged incubation.34,36 Thus, previous retroviral-mediated gene marking protocols, including those seeking to transduce BM ELTC-IC,27 31 might have proved more efficient if the period of prestimulation had been significantly extended.
Previous studies have convincingly demonstrated that very high concentrations of early acting cytokines (250 to 300 ng/mL) might be required to preferentially promote stem cell self-renewal, rather than cell divisions associated with commitment and loss of stem cell function.47 Our studies support that this effect is strictly associated with preservation of stem cell function and not proliferation, because the level of recruitment and expansion of CD34+CD38− BM cells were not affected by increasing the cytokine concentrations. Of particular interest is our finding that increasing the concentration of KFM from 50 ng/mL to 250 ng/mL preferentially expanded ELTC-CFC.
Hogge et al54 have demonstrated that the number of LTC-IC detectable in normal BM, the average number of CFC produced per LTC-IC, as well as the maintenance of LTC-IC are all increased when using engineered murine fibroblast feeders as compared with normal BM feeders used in the present studies. Thus, obviously normal BM feeders only detect a fraction of the LTC-IC (and ELTC-IC) supported by engineered murine fibroblast cell lines. Whether the LTC-IC detected in these different assays are also qualitatively different remains to be established.
Another intriguing finding in the present studies was the observation that culturing of CD34+CD38− BM cells in FCS-supplemented medium negatively affected expansion of ELTC-CFC when compared with cells cultured under SF-conditions. The negative effect of the FCS-containing medium was much more evident on expansion of 12-week than of 5-week LTC-CFC. Similar to the cytokine concentration effect discussed above, the difference observed between the FCS-containing and SF-medium appeared to be unrelated to cell proliferation. However, whereas more than 50% of SF-expanded cells remained CD34+CD38−, less than 1% maintained this phenotype in the FCS-containing medium. Although our observations do not definitely prove that FCS has detrimental effects on stem cell self-renewal, they argue caution when exposing stem cells to FCS ex vivo.
In conclusion, we have demonstrated that human CD34+CD38− BM ELTC-CFC can be expanded after prolonged exposure to high concentrations of early acting cytokines (KF3 and KFM) under SF-conditions. The distinct and stringent requirements shown for expansion of BM ELTC-CFC when compared with LTC-IC support that the ELTC-IC assay detects a more primitive stem cell, which could prove to be a more reliable predictor for LTRC. Ongoing efforts are devoted towards comparing the identity, expansion potential, and retroviral-mediated transduction of ELTC-IC and SRC, because the SRC assay has also been implicated to detect a more primitive progenitor than the traditional LTC-IC.10 32 The ELTC-IC and SRC assays might prove to play a complimentary role towards development of more efficient ex vivo stem cell expansion and gene therapy.
ACKNOWLEDGMENT
The authors gratefully acknowledge the Department of Hematology (Lund University Hospital) for performing the human BM aspirations and thank our BM volunteers for their donations. We are indebted to Dr Per Nilsson and Stefan Johnsson for performing the stroma irradiations and to Per Anders Bertilsson and Sverker Segrén for expert assistance with the cell sorting. We thank Ingbritt Åstrand-Grundström, Eva Gynnstam, Irene Persson, and Lilian Wittman for technical assistance and animal care. We are also grateful to Drs Ian K. McNiece, Janet Nichol, and Graham Molineux for generously supplying MGDF, stem cell factor, and other cytokines and to Dr Stewart Lyman for providing FL for these studies. We also thank Dr Stefan Karlsson for helpful discussions and reviewing the manuscript.
Supported by grants from the Berta Kamprad Foundation; the Crafoord Foundation; the Georg Danielsson Foundation; the Gunnar, Arvid and Elisabeth Nilsson Foundation; the John and Augusta Persson Foundation; the Medical Faculty, University of Lund; the O and E and Edla Johansson Foundation; the Royal Physiographic Society in Lund; the Swedish Cancer Society; the Swedish Child Cancer Fund; the Harald and Greta Jeansson's Foundation; the Syskonon Svensson's Foundation; the Thelma Zoega's Foundation; and the Tobias Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sten E.W. Jacobsen, MD, PhD, Stem Cell Laboratory, Department of Molecular Medicine and Gene Therapy, Department of Internal Medicine, Institute for Laboratory Medicine, University Hospital of Lund, 221 85 Lund, Sweden; e-mail:sten.jacobsen@molmed.lu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal