Abstract
The highly polymorphic Rh system is encoded by 2 homologous genesRHD and RHCE. Gene rearrangements, deletions, or point mutations may cause partial D and CE antigens. In this study, a newRHD variant, DAR, and a new RHCE variant, ceAR, are described in 4 Dutch African Blacks. Serologically, DAR showed weaker reactions with a monoclonal antibody and polyclonal antiserum against D. The DAR phenotype was characterized by complete loss of at least 9 of 37 Rh D epitopes. Erythrocytes expressing ceAR were all typed as VS−, V+. DNA analysis showed a partial D allele with only 3 mutations: C602G (exon 4), T667G (exon 5), and T1025C (exon 7). The ceAR allele carried G48C (exon 1), a hybrid exon 5 (A712G, C733G, A787G, and T800A), and A916G (exon 6). To study the frequency of these variants, 326 South-African Blacks was screened genomically. Of the 326 donors, 16 (4.9%) carried the DAR allele, 20 (6.1%) the ceAR allele, and 14 (4.3%) both mutated alleles. Five of these donors (1.5%) had the DAR phenotype, indicating that they carried the DAR allele homozygously or next to a D-negative allele. Immunogenicity of the D antigen for individuals with the DAR phenotype was proven, because 1 of the 4 Dutch individuals produced allo-antibodies against D after multiple transfusions with D-positive blood. In a multiethnic society, the prevalence of this D phenotype will increase and is therefore relevant in transfusion practice and in prevention of hemolytic disease of the newborn.
THE RHESUS (Rh) BLOOD-GROUP system is clinically important, because antibodies against Rh antigens are involved in hemolytic disease of the newborn, hemolytic transfusion reactions, and autoimmune hemolytic anemia.
The Rh system is complex; as many as 45 different antigens have been serologically defined.1,2 These antigens are carried by nonglycosylated, nonphosphorylated polypeptides. The Rh polypeptides are predicted to have 12 transmembrane-spanning domains with intracellular N- and C-termini, resulting in 6 extracellular loops on which the Rh antigens are located.3,4 Two highly homologous genes, RHCE and RHD, encode the Rh antigens. Both genes are localized on chromosome 1p34.3-p36.1 and are inherited together.5RHCE gives rise to the C/c and E/e polymorphisms. RHD encodes the RhD antigen. Total or partial deletion of the RHD gene can result in the D-negative phenotype.6-10 In non-Whites, it has been found that D negativity can appear in individuals carrying the complete RHDgene.11 12
Partial D phenotypes, characterized by loss of epitopes, can arise from replacement of RHD exons by their RHCE counterparts, as has been shown in DIIIb, DIIIc, DIVb, DVa, DVI, DFR, and DBT and by point mutations in the RHD gene that occur in DII, DIVa, DVII, DHMi, DNU, and DHR. Frequencies of DVII, DVI, DIV, DV, DII-like, and DFR are 1:900, 1:6,800, 1:10,000, 1:30,000, 1:30,000, and 1:60,000, respectively, as established with serological methods in a White population.15 Alloantibodies may be produced against missing epitopes in individuals expressing rhesus D variants when exposed to the complete antigen by blood transfusion or during pregnancy.
Three types of RHCE variants have been described.16Single point mutations are found in VS, V, Cw, Cx, and Rh:26. RHCE exon replacements, in which exons of different alleles of RHCE are exchanged, were found in rGr and a variant in which exon 1 and intron 1 of the c-allele are replaced by the corresponding part of the C-allele. Finally, replacement of RHCE exons by their RHDequivalents may occur, as is found in D−−, Dc−, RoHar, RN, and partial E. As inRHD variants, these exon replacements or mutations not only result in loss of epitopes, but may also account for the formation of new epitopes.
In the present report, we describe a new partial D antigen, called DAR, expressed in 4 unrelated Dutch women of African Black origin. In these 4 individuals, a variant RHCE gene, called ceAR, was also found. That more African Blacks are carrier of the mutatedRHD gene was suggested by the fact that 3 of these 4 women were noticed in a routine pregnancy screening. Thereafter, blood was sent for confirmation of the rhesus typing to the Central Laboratory for Blood Transfusion (CLB; Amsterdam, The Netherlands). We also screened 326 African Black donors from the South African Blood Transfusion Service (Johannesburg, South Africa) for DAR andceAR.
MATERIALS AND METHODS
Samples
EDTA anticoagulated blood samples were obtained from 4 unrelated African Black women (identification numbers 3308, 3424, 3895, and 4413). Three samples were noticed because of weak D expression during routine pregnancy screening and were sent to the CLB for confirmation. A blood sample of individual 4413 was referred to our lab because antibodies were detected. Red blood cells (RBCs) of these individuals were Rh phenotyped according to standard protocols with monoclonal antibody (MoAb) MS-201 (CLB) recognizing D epitope 6/7 (9-epitope model; equal to D-epitope 12 in the 37-epitope model), a polyclonal reagent (anti-D with bromelain as enhancer; CLB), and MoAbs (all obtained from CLB) recognizing C (MS 24), c (MS 32), E (MS 260), or e (MS 21, MS 63). Polyclonal reagents 97-501639 (patient serum) and Q-sera were used to phenotype VS and V, respectively. The presence of the low incidence antigen DW, thus far only found in DVa, was tested with an anti-Rh23 by C. Green and G. Daniels (International Blood Group Reference Laboratory, Bristol, UK). Phenotyping for partial D was performed with a panel of selected MoAbs with known epitope specificity (Third International Workshop on Monoclonal Antibodies against Red Cell and Related Antigens, 1996, Nantes, France).17 RBCs of donor 3424 were sent to the IBGRL (Bristol, UK) for confirmation.
In the blood sample of individual 4413, erythrocyte antibodies were present with the specificity of anti-D, -C, -E, -Fya, -Jka, -M, and -Sla. This individual suffered from sickle cell anemia and received multiple transfusions over the years. Adsorption-elution techniques were used to determine whether the anti-D antibodies were alloantibodies or autoantibodies.
Blood samples of 326 South-African Black donors were randomly collected by the South African Blood Transfusion Service in Johannesburg (courtesy of J. Hooydonk, Johannesburg, South Africa). Seven donors were serologically typed as RhD negative, and all other donors were RhD positive.
cDNA Sequence Analysis
White blood cell-reduced RBCs were enriched with reticulocytes as described before.18,19 RNA was isolated from the reticulocyte-enriched fraction.20 cDNA was obtained by reverse transcriptase-polymerase chain reaction (RT-PCR; using Superscript II RNAse H-RT; GIBCO BRL, Gaithersburg, MD), and full-length amplification was performed with consensus primers as described in Table 1. PCR products were ligated into pGEM-T vector (system I; Promega, Madison, WI), and the vectors were introduced into competent Escherichia coli by electroporation.21 Inserts were cycle-sequenced automatically (ABI-PRISM 377, DNA sequencer; Perkin-Elmer, Norwalk, CT) on both strands.
Nucleotide Sequences and Positions of Primers
| Primer . | . | Sequence . | Annealing Time and Temperature . | Amplicon . | Specificity . |
|---|---|---|---|---|---|
| R-15 | Sense | 5′tatctagagacggacacaggATGAGC3′ | 1 min, 60°C | Exon 1 | Consensus |
| R31 | Sense | 5′CGCTGCCTGCCCCTCTGC3′ | Exon 1, C specific | C specific: nt 48 | |
| R147 | Antisense | 5′TTGATAGGATGCCACGAGCCCC3′ | Consensus | ||
| R496 | Sense | 5′CACATGAACATGATGCACA3′ | 1 min, 55°C | Exon 4 to 5 | D specific: nt 514 |
| Rex5AD2 | Antisense | 5′cacCTTGCTGATCTTACC3′ | Dspecific: nt 787 | ||
| R581 | Sense | 5′ACGGAGGATAAAGATCAGAG3′ | 1 min, 55°C | Intron 4, CE specific | CE specific: nt 602 |
| R667 | Antisense | 5′CTCAGCAGAGCAGAGTTGAC3′ | CE specific: nt 667 | ||
| Rex5S2 | Sense | 5′cctctctggccccaggCGCC3′ | 1 min, 55°C | Exon 5 | Consensus |
| Rex5A | Antisense | 5′cagcgccctgctcac3′ | Consensus | ||
| R678 | Sense | 5′CTGCTGAGAAGTCCAATCC3′ | 1 min, 55°C | Exon 5, CE-D hybrid | CEspecific: nt 707 |
| Rex5AD2 | Antisense | 5′cacCTTGCTGATCTTACC3′ | D specific: nt 787 | ||
| R678 | Sense | 5′CTGCTGAGAAGTCCAATCC3′ | 1 min, 55°C | Exon 5 to 6, CE-D hybrid | CE specific: nt 707 |
| R933 | Antisense | 5′GTACTTGGCTCCCCCGAC3′ | Dspecific: nt 916 | ||
| R716 | Sense | 5′TCAACACCTACTATGCTG3′ | 1 min, 55°C | Intron 5 | VS and D specific: nt 733 |
| R870 | Antisense | 5′AGAAGGGATCAGGTGACACG3′ | Consensus | ||
| Rex6S | Sense | 5′gctatttctttgcag3′ | 30 s, 48°C | Exon 6 | Consensus |
| Rex6A | Antisense | 5′tgtctagtttcttca3′ | α taq added | Consensus | |
| R973 | Sense | 5′AGCTCCATCATGGGCTACAA3′ | 1 min, 67°C | Exon 6 to 7,D-CE hybrid | D specific: nt 992 |
| R1044 | Antisense | 5′CACCAGCAGCACAATGTAGG3′ | CEspecific: nt 1025 | ||
| R973 | Sense | 5′AGCTCCATCATGGGCTACAA3′ | 1 min, 55°C | Exon 7 | D specific: nt 992 |
| R1068 | Antisense | 5′ATTGCCGGCTCCGACGGTATC3′ | Dspecific: nt 1068 | ||
| R-15 | Sense | 5′tatctagagacggacacaggATGAGC3′ | 1.5 min, 55°C | Full-length cDNA | Consensus |
| R1339 | Antisense | 5′gcgtttctcacgtacaaatgc3′ | Consensus |
| Primer . | . | Sequence . | Annealing Time and Temperature . | Amplicon . | Specificity . |
|---|---|---|---|---|---|
| R-15 | Sense | 5′tatctagagacggacacaggATGAGC3′ | 1 min, 60°C | Exon 1 | Consensus |
| R31 | Sense | 5′CGCTGCCTGCCCCTCTGC3′ | Exon 1, C specific | C specific: nt 48 | |
| R147 | Antisense | 5′TTGATAGGATGCCACGAGCCCC3′ | Consensus | ||
| R496 | Sense | 5′CACATGAACATGATGCACA3′ | 1 min, 55°C | Exon 4 to 5 | D specific: nt 514 |
| Rex5AD2 | Antisense | 5′cacCTTGCTGATCTTACC3′ | Dspecific: nt 787 | ||
| R581 | Sense | 5′ACGGAGGATAAAGATCAGAG3′ | 1 min, 55°C | Intron 4, CE specific | CE specific: nt 602 |
| R667 | Antisense | 5′CTCAGCAGAGCAGAGTTGAC3′ | CE specific: nt 667 | ||
| Rex5S2 | Sense | 5′cctctctggccccaggCGCC3′ | 1 min, 55°C | Exon 5 | Consensus |
| Rex5A | Antisense | 5′cagcgccctgctcac3′ | Consensus | ||
| R678 | Sense | 5′CTGCTGAGAAGTCCAATCC3′ | 1 min, 55°C | Exon 5, CE-D hybrid | CEspecific: nt 707 |
| Rex5AD2 | Antisense | 5′cacCTTGCTGATCTTACC3′ | D specific: nt 787 | ||
| R678 | Sense | 5′CTGCTGAGAAGTCCAATCC3′ | 1 min, 55°C | Exon 5 to 6, CE-D hybrid | CE specific: nt 707 |
| R933 | Antisense | 5′GTACTTGGCTCCCCCGAC3′ | Dspecific: nt 916 | ||
| R716 | Sense | 5′TCAACACCTACTATGCTG3′ | 1 min, 55°C | Intron 5 | VS and D specific: nt 733 |
| R870 | Antisense | 5′AGAAGGGATCAGGTGACACG3′ | Consensus | ||
| Rex6S | Sense | 5′gctatttctttgcag3′ | 30 s, 48°C | Exon 6 | Consensus |
| Rex6A | Antisense | 5′tgtctagtttcttca3′ | α taq added | Consensus | |
| R973 | Sense | 5′AGCTCCATCATGGGCTACAA3′ | 1 min, 67°C | Exon 6 to 7,D-CE hybrid | D specific: nt 992 |
| R1044 | Antisense | 5′CACCAGCAGCACAATGTAGG3′ | CEspecific: nt 1025 | ||
| R973 | Sense | 5′AGCTCCATCATGGGCTACAA3′ | 1 min, 55°C | Exon 7 | D specific: nt 992 |
| R1068 | Antisense | 5′ATTGCCGGCTCCGACGGTATC3′ | Dspecific: nt 1068 | ||
| R-15 | Sense | 5′tatctagagacggacacaggATGAGC3′ | 1.5 min, 55°C | Full-length cDNA | Consensus |
| R1339 | Antisense | 5′gcgtttctcacgtacaaatgc3′ | Consensus |
The sequences of the oligonucleotides are given in capital letters when exon sequences are indicated and in small letters when intron sequences are indicated.
Genomic DNA Analysis
Genomic DNA was isolated from peripheral blood leukocytes with a DNA isolation kit (Puregene, Minneapolis, MN).
Sequence analysis.
On genomic DNA, exon-specific PCRs were used. All primers are listed in Table 1. Exons 4 to 5 (including intron 4) and 7 were amplified withRHD-specific primers (R496/Rex5AD2 and R973/R1068, respectively) and cycle-sequenced automatically (ABI-PRISM 377, DNA sequencer). Exon 5 and exon 6 were amplified with consensus primers (Rex5S2/Rex5A and Rex6S/Rex6A, respectively); PCR products were subcloned and sequenced.
PCR assays.
Five PCR allele-specific primer amplifications (ASPAs) were designed specifically for detection of mutations. Primerset R31/R147 and internal control primer R-15 (all 3 primers located in exon 1) were used to recognize the C-specific nucleotide at position 48.22 An ASPA specific for CE nucleotides at position 602 and 667 (primerset R581/R667) was developed to amplify intron 4. We applied an exon 5 ASPA, using a CE-specific sense primer (R678) and a D-specific antisense primer (Rex5AD2), to detect a hybrid exon 5. An exon 5 to 6 ASPA was used to amplify intron 5 with the CE-specific sense primer R678 in exon 5 and the D-specific antisense primer R933 in exon 6. An exon 6 to 7 ASPA (primerset R973/R1044) with a D-specific sense primer in exon 6 and a CE-specific antisense primer in exon 7 was developed to detect the CE-specific mutation inD-exon 7.
RHD-specific multiplex PCR.
RHD exons 3, 4, 5, 6, 7, and 9 were amplified withRHD-sequence specific primers in a 1-reaction mixture assay as described before.23
Restriction fragment length polymorphism of RH intron 5.
To determine the origin of the intron 5 of the ceAR allele, intron 5 was amplified with sense primer R716 (specific for nt 733G, present in the CE allele of VS+ individuals and in the D allele) and antisense primer R870 (consensus primer). This product was D-specifically digested with restriction enzyme Apa I (New England Biolabs Inc, Beverly, MA) and analyzed by electrophoresis in a 1% agarose gel.
Southern blot analysis.
Ten micrograms of DNA from all donors was digested with the endonuclease BamHI and, after electrophoresis, transferred to a nitrocellulose membrane. Blots were hybridized with a32P-labeled RH full-length cDNA (kindly provided by Dr D. Anstee, IBGRL). The results were visualized by autoradiography.
PCR Conditions
All PCR assays were performed in a Perkin-Elmer Cycler Model 480 on 200 ng of cDNA or gDNA in a total volume of 50 μL. Reaction mixtures contained 50 ng of each primer, 0.2 mmol/L of each dNTP (Pharmacia, Uppsala, Sweden), and 2 U of Taq DNA polymerase (Promega) in the appropriate buffer supplemented with 1.5 mmol/L MgCl2.
PCR conditions were 1 cycle of 5 minutes at 95°C, followed by 35 cycles of 1 minute at 95°C, with an annealing time and temperature as described in Table 1 and, depending on the size of the expected product, the extension time at 72°C varied between 45 seconds and 2.5 minutes. Extension was completed during 5 minutes at 72°C.
RESULTS
Serology
Individuals 3308, 3424, 3895, and 4413 were serologically typed as C−, c+, E−, and e+, VS−, and V+. RBCs of these 4 individuals showed weaker reactions with anti-D MoAb MS-201 and polyclonal anti-D antiserum than did normal Rh (D)-positive control cells. Therefore, with restricted screening protocols, these donors might be considered as expressing weak D. However, extensive serological studies of all 4 individuals showed a new partial D pattern (Table 2) in which 9 of the 37 epitopes were completely missing and 6 of the 37 epitopes showed different results with several MoAbs. These results were confirmed by Joyce Poole's laboratory of the IBGRL. RBCs of these 4 donors did not carry the low incidence anti- gen DW.
Epitope Models (9 and 37) With the Reaction Pattern of the New Partial D Tested With MoAbs Described in the Nantes Workshop (1996)
| Epitope Model . | Nantes . | DAR . | |
|---|---|---|---|
| 1-9 . | 1-37 . | ||
| 1 | 1 | LHM169/81 | ± |
| 1 | 2 | LHM70/45, LHM174/102 | − |
| 2 | 3 | LOR12-E2, LORE | ± |
| 2 | 4 | LOR28-7E6 | − |
| 3 | 5 | LOR11-2D9, LHM76/55, H41.11B7, H41 | + |
| 4 | 6 | LOR17-6C7 | + |
| 5 | 7 | CAZ7-4C5 | − |
| HIRO-6 | ± | ||
| 5 | 10 | AUB-2F7/Fiss, MAR-1F8 | − |
| C205-29, CLAS1-126, BS229, BS231 | ± | ||
| 5 | 11 | 819 | − |
| 6/7 | 12 | 175-2, LOR17-8D3, P3AF6, RUM-1, MS201, P3x61 | ± |
| 6/7 | 13 | D-89/47, 17010C9, LOR28-21D3, H2D5D2F5 | ± |
| F5S | + | ||
| 6/7 | 15 | D-90/7, D-90/17, NOI, SAL20-12D5, LHM50/2B, LHM169/80, BIRMA-DG3, BIRMA-D56, L87.1G7, D10 | ± |
| 6/7 | 17 | HeM-92, NaTH53-2A7, P3F17, HIRO-I, HS114 | − |
| B9A4B2, NaTH28-3C11, BS232 | ± | ||
| 6/7 | 18 | LHM59/20, T3D2F7, P3F20 | − |
| HG/92, LHM50/3.5, LHM59/25, HM10 | ± | ||
| ID6-H8 | + | ||
| 6/7 | 21 | VOL-3F6 | − |
| D-90/12, BRAD5 | ± | ||
| HIRO-2 | + | ||
| 8 | 22 | LHM59/19 | − |
| 9 | 23 | P3x21223B10 | − |
| BIRMA-D6, P3G6, MS26, HIRO-4, HIRO-7, HIRO-8 | ± | ||
| 31 | NOU | − | |
| 32 | ZIG-189 | − | |
| 33 | NaTH87-4A5 | − | |
| 34 | LORA | − | |
| 35 | SALSA-12 | − | |
| 36 | NAU3-2E8, BTSN6, LHM76/59 | ± | |
| NAU6-4D5 | + | ||
| 37 | 822, HIRO-3 | + | |
| Epitope Model . | Nantes . | DAR . | |
|---|---|---|---|
| 1-9 . | 1-37 . | ||
| 1 | 1 | LHM169/81 | ± |
| 1 | 2 | LHM70/45, LHM174/102 | − |
| 2 | 3 | LOR12-E2, LORE | ± |
| 2 | 4 | LOR28-7E6 | − |
| 3 | 5 | LOR11-2D9, LHM76/55, H41.11B7, H41 | + |
| 4 | 6 | LOR17-6C7 | + |
| 5 | 7 | CAZ7-4C5 | − |
| HIRO-6 | ± | ||
| 5 | 10 | AUB-2F7/Fiss, MAR-1F8 | − |
| C205-29, CLAS1-126, BS229, BS231 | ± | ||
| 5 | 11 | 819 | − |
| 6/7 | 12 | 175-2, LOR17-8D3, P3AF6, RUM-1, MS201, P3x61 | ± |
| 6/7 | 13 | D-89/47, 17010C9, LOR28-21D3, H2D5D2F5 | ± |
| F5S | + | ||
| 6/7 | 15 | D-90/7, D-90/17, NOI, SAL20-12D5, LHM50/2B, LHM169/80, BIRMA-DG3, BIRMA-D56, L87.1G7, D10 | ± |
| 6/7 | 17 | HeM-92, NaTH53-2A7, P3F17, HIRO-I, HS114 | − |
| B9A4B2, NaTH28-3C11, BS232 | ± | ||
| 6/7 | 18 | LHM59/20, T3D2F7, P3F20 | − |
| HG/92, LHM50/3.5, LHM59/25, HM10 | ± | ||
| ID6-H8 | + | ||
| 6/7 | 21 | VOL-3F6 | − |
| D-90/12, BRAD5 | ± | ||
| HIRO-2 | + | ||
| 8 | 22 | LHM59/19 | − |
| 9 | 23 | P3x21223B10 | − |
| BIRMA-D6, P3G6, MS26, HIRO-4, HIRO-7, HIRO-8 | ± | ||
| 31 | NOU | − | |
| 32 | ZIG-189 | − | |
| 33 | NaTH87-4A5 | − | |
| 34 | LORA | − | |
| 35 | SALSA-12 | − | |
| 36 | NAU3-2E8, BTSN6, LHM76/59 | ± | |
| NAU6-4D5 | + | ||
| 37 | 822, HIRO-3 | + | |
The serology was performed as described before.24 This pattern was found in all 9 individuals expressing DAR homozygously or next to a D-negative allele. These results were confirmed by Joyce Poole's laboratory of the IBGRL.
Abbreviations: −, negative MoAb reaction; ±, positive MoAb reaction, but weaker than that of normal Rh (D)-positive control cells; +, positive MoAb reaction.
The antibodies present in the serum of patient 4413 were characterized with the adsorption-elution technique. It was shown that antibodies directed against D were present, among other antibodies. With agglutination studies it was shown that these antibodies did not react with her own erythrocytes or with the erythrocytes of other donors with the DAR phenotype, but did react with erythrocytes from normal D-positive donors, indicating that these antibodies are allo-anti-D.
cDNA Sequence Analysis
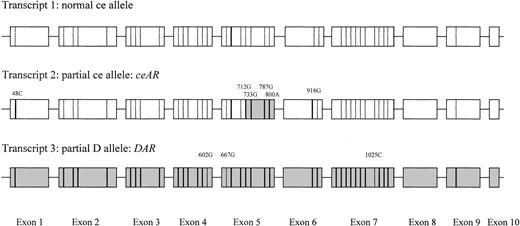
Sequencing of cDNA from 1 individual (identification no. 3424) showed the presence of 3 different transcripts (Fig 1). At least 3 clones per different transcript were completely sequenced: (1) a normal cetranscript; (2) a ce-like transcript carrying G48C (Trp16Cys) in exon 1; A712G (Met238Val), C733G (Leu245Val), A787G (Arg263Gly) and T800A (Met267Lys) in exon 5; and A916G (Ile306Val) in exon 6; and (3) a D-like transcript carrying C602G (Thr201Arg) in exon 4, T667G (Phe223Val) in exon 5, and T1025C (Ile342Thr) in exon 7.
Transcripts as found by sequencing cDNA. Nucleotides derived from the RHCE sequence are represented by a thin line, whereas nucleotides derived from the RHD-specific sequence are represented by a fat line. RHD-specific exons or exon parts are represented by a gray rectangle, whereas those of RHCE are given in white.
Transcripts as found by sequencing cDNA. Nucleotides derived from the RHCE sequence are represented by a thin line, whereas nucleotides derived from the RHD-specific sequence are represented by a fat line. RHD-specific exons or exon parts are represented by a gray rectangle, whereas those of RHCE are given in white.
Genomic DNA Analysis
To confirm the mutations found in cDNA of individual 3424, as well as to show the presence of the mutations in the other 3 individuals, 3308, 3895, and 4413, analysis on genomic DNA was performed. All of these results were in full concordance with the cDNA analysis.
From individual 4413, not only the exons of interest, but also all exons were amplified from genomic DNA and subsequently cycle-sequenced. No other mutations were found.
The mutations found in the ce-like transcript were confirmed on genomic DNA by an ASPA that recognized the C-specific nucleotide at position 48; by sequence analysis of ce-exon 5 that showed the mutations A712G, C733G, A787G, and T800A; and by sequence analysis ofce-exon 6 that showed A916G. The ce origin of intron 5 was indicated by a remaining uncut part, after D-specific digestion with Apa I of the 1,791-bp product, obtained afterD/VS-specific amplification of intron 5.
The mutations found in the D-like transcript were detected by sequence analysis of RHD-exon 4 to 5 and exon 7. Beside the mutations C602G and T667G, sequence analysis showed a normalD-intron 424 and the T1025C mutation in D-exon 7. To show that only the mutated D transcript was present, in the absence of a normal D allele, the RHD-specific multiplex was performed. The 200-bp internal control fragment from the β-actin gene was amplified from all 4 DNA samples. Amplification products from exons 3, 6, 7, and 9 were detected, whereas exons 4 and 5 were not amplified.
Southern blot analysis.
The BamHI digestion pattern of the genomic DNA from the 4 individuals (3308, 3424, 3895, and 4413) showed no difference in the Rh patterns compared with those of a normal D-positive donor.
Screening of the South-African Black Donors
In 56 of the 326 South-African Black donors (17.2%), the RHCEintron 4 PCR demonstrated the 2 CE-specific nucleotides in the exon 4 and 5 of the RHD gene, with a D intron 4 in between. All 56 of these donors were serologically RhD positive. In 16 of these 56 donors, the presence of T1025C was proven by the D-CEhybrid exon 6 to 7 PCR, indicating the presence of the DARallele.
Because a linkage between the DAR and the ceAR allele was assumed, we tested all 326 donors for the presence of theceAR allele. In 20 of the 326 donors (6.1%), theCE-D hybrid exon 5 PCR and the CE-Dhybrid exon 5 to 6 PCR gave positive results, indicating the presence of a ceAR allele. Of those 20 donors, 14 were also carriers of the DAR allele. Thus, 2 donors carried the DAR allele without the ceAR allele and 6 donors carried the ceARallele without the DAR allele.
Five of the 14 donors carrying the DAR and ceAR allele (1.5%) had the deviant RHD multiplex PCR pattern, missing exon 4 and 5. This indicates that these 5 donors were either homozygous for the DAR or carried DAR on 1 allele and lacked RHD on the other. The results found on gDNA of these 5 donors were serologically confirmed and gave with all 5 the same 37-epitope pattern as shown in Table 2.
In DNA of the 40 donors carrying only the C602G and T667G mutations in exon 4 and 5 of the RHD gene and not the T1025C mutation in exon 7, the CE-D hybrid exon 5 PCR and theCE-D hybrid exon 5 to 6 PCR gave negative results, indicating the absence of the ceAR allele. One of these 40 donors showed the deviant RHD multiplex PCR pattern, missing exons 4 and 5.
DISCUSSION
In this study, a newly discovered D variant named DAR that occurs frequently in African Blacks is described. This new D variant consists of a D allele with 3 point mutations on polymorphic sites, in which D-specific nucleotides are replaced byCE-specific ones. These mutations are located in exon 4 (nt 602), exon 5 (nt 667), and exon 7 (nt 1025). The 4 probands also had a variant ce allele, called ceAR. This allele had a C-specific mutation on nt 48 in exon 1, a hybrid exon 5 in which the polymorphic sites between nt 712 and nt 800 were replaced by D-specific nucleotides, and a D-specific point mutation on polymorphic site nt 916 in exon 6.
This new D variant was previously described by us as ARRO-I.25 At that time, sequence analysis was not completed. Further analysis showed the mutation in exon 7 and the mutant ce-allele in these individuals.
Serologically, this new D variant showed weaker reactions with a monoclonal anti-D and with polyclonal antiserum used for routine screening, indicating weak D expression. The genomic features of the new variant have much resemblance with the recently described weak D type 4, in which the mutations C602G and T667G are also present, but the mutation T1025C is not.26 However, with extensive serological testing, the D characteristics of the DAR phenotype gave a different, not previously described pattern of serological reactions with MoAbs. The loss of epitopes, as well as the finding of allo-anti-D formation in an individual with the DAR phenotype, indicates that we are dealing with a new qualitative D variant with low expression. In 1 of the 40 South African Black donors in whom PCR-based analysis showed the mutation in exon 4 and 5 but not in exon 7, the expression was not masked by the presence of a normal D gene. This donor is expected to present the weak D type 4 phenotype. Preliminary serological results of this donor suggest a quantitative instead of a qualitative loss of epitopes, as can be expected from the results of Wagner et al.26 This is an intriguing observation in view of the effect of the mutation in exon 7 for the loss of epitopes. An explanation for this phenomenon can perhaps be found in the fact that the mutations in exon 4 and 5 do not change the polarity of the amino acids in the protein. In contrast, the transmembranal mutation in exon 7 causes the incorporation of a hydrophilic amino acid (Thr) instead of a hydrophobic amino acid (Ile). This might result in a severe change in the conformation of the Rh protein, explaining the described loss of epitopes. In the future, transfection studies will be performed to study this phenomenon in detail.
The loss of so many D epitopes in DAR is the result of only 3 mutations in RHD, because from the variant ce-allele only addition of D epitopes can be expected. The mutations at the 3′ end of exon 4 and the 5′ end of exon 5 of the D allele are due to point mutations. This is suggested by the presence of a normal D intron 4, as shown by sequence analysis. However, aberrant nucleotides may occasionally be introduced if incorrect mismatch repair of the heteroduplex DNA takes place during gene conversion.27 So, the DAR mutations on the 3′ side of exon 4 and the 5′ side of exon 5 also could be due to the occurrence of heteroduplex repair, rather than to spontaneous point mutations. The same phenomenon has been described for the glycophorins A and B, which are also encoded by highly homologous genes.27
The variant ce-gene, ceAR, is characterized by a mutation in exon 1, a hybrid exon 5, and a mutation in exon 6. The presence of G48C (Trp16Cys), the C-specific nucleotide in exon 1, without expression of this antigen, frequently occurs in African Blacks.28 The D-specific nucleotide found in exon 6 of the CE allele is probably caused by a point mutation or might be due to heteroduplex repair (see above), because restriction site analysis suggested a normal ce-intron 5. Besides theceAR allele, a complete ce-allele was also found, so it was not possible to test the loss of ce-epitope expression. All 4 individuals were expressing the rare VS−, V+ phenotype. Daniels et al29 has previously described that the VS mutation (G733, in exon 5) surrounded byD-specific nucleotides in exon 5 and the V-specific nucleotide 1006G (Gly336) provided the VS−, V+serotype. In this study, RHCE exon 6 was not sequenced. Therefore, 4 VS−, V+ samples of African Black donors (provided by Dr G.L. Daniels, IBGRL) were sequenced, and, indeed, these samples also showed the mutation in exon 6 as well as the C mutation (G48C). These results suggest that the most common genetic basis of the VS−, V+ phenotype is theceAR variant. Furthermore, these 4 donors also carried the DAR allele.
The 4 original probands in whom this new serological reaction pattern was found all proved to be of African Black origin. No Whites carrying this variant have been found so far during routine screening. This suggested that more African Blacks might be carriers of these genes. Therefore, a group of 326 South-African Blacks was screened by genomic PCR. We found that 4.9% of this group are carriers of a DARallele. Five donors (1.5%) had the gene homozygous or in combination with a D-negative allele, as was shown by the absence of the amplification products from exons 4 and 5 in the multiplex PCR. These donors also had the same serological partial D pattern as was shown in the 37 D-epitope model. The fact that so many South-African Blacks are carriers of this gene suggests that these donors have an evolutionary advantage, as described for Duffy involving malaria.30 So far, no correlations between Rh phenotypes and the occurrence of malaria have been found. However, these studies have been performed on immunologically recognized antigens, whereas this new variant is primarily recognized at the DNA level. The frequency of the DAR phenotype (1.5%) in the African Black population is much higher than the frequency of D variants in the White population (0.1% to 0.001%) and therefore might have an impact on monoclonal reagent design. Testing the African Blacks for the presence of the ceAR showed that 6.1% were carriers of this allele.
The finding that the first 4 individuals tested expressed both variant alleles suggested that these 2 genes were inherited en bloc. By screening the 326 African Blacks, we expected to find only donors carrying both mutated alleles or both normal alleles. The screening results did not confirm this idea, because, besides donors with both variant alleles, also donors with only the DAR or ceARallele were found. Nevertheless, the incidence of the combination is much higher than is expected to occur by chance, indicating linkage ofDAR and ceAR.
Individuals with the DAR phenotype may form anti-D antibodies when exposed to a complete D antigen. Despite the loss of so many epitopes, a complete D antigen does not seem to be highly immunogenic for individuals expressing DAR. Otherwise, the highly frequent DAR variant should have been recognized much earlier. A possible explanation for this low responsiveness may be that the footprints of most anti-Rh (D) antibodies are related to one another, as was published recently.31 It is postulated that, in the allo-immune response against the Rh (D) antigen in different individuals, a similar and restricted pathway is used. It is tempting to speculate that, in this variant, the apparent low immunogenicity of the complete D antigen is due to the fact that the most common pathway could not be used, because these B cells have been clonally deleted or have become anergic to avoid self-reactivity. Probably a less-common pathway has produced the anti-Rh (D) made by the multitransfused donor 4413.
The clinical significance of these anti-D antibodies in this individual is not yet clear. Nevertheless, pregnant women and recipients of blood transfusions expressing the DAR variant should be regarded as D negative. As donors, people expressing DAR should be carefully distinguished from D-negative donors by the use of selected reagents, because allo-immunization is likely to occur when administering erythrocytes expressing the DAR variant to D-negative recipients. Therefore, donors expressing DAR should be regarded as D positive. Hemolytic disease of the newborn could occur in fetuses expressing DAR when carried by an immunized D-negative mother or in fetuses with the complete D antigen carried by mothers with the DAR phenotype. Therefore, in the future, anti-D monoclonals for immunoprophylaxis should be guaranteed to cover this new variant, especially because of the high incidence of this new variant in a multiethnic society in which D negativity is found more frequently than in the original African Black population. In addition, the chance of getting a population with an even higher frequency of people expressing DAR is increased, because the expression will not be masked by the presence of a normal D allele.
ACKNOWLEDGMENT
J. Hooydonk (South African Blood Transfusion Service) was very helpful with the collection of samples from the African Black population. The authors thank him for the collaboration. We are grateful to J. Poole, C. Green, and G. Daniels from the IBGRL for their very kind collaboration on this manuscript. We thank M.A.M. Overbeeke, A.E.G.Kr. von dem Borne, and D. Roos for their comments on the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to P.A. Maaskant-van Wijk, PhD, Bloodbank Rotterdam Location Dordrecht, Laboratory for Transfusion Science, Albert Schweitzerplaats 5, 3318 AS Dordrecht, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal