Abstract
We have recently isolated a cDNA encoding a novel human receptor-type tyrosine phosphatase, termed PTP-RO (for a protein tyrosine phosphatase receptor omicron), from 5-fluorouracil–treated murine bone marrow cells. PTP-RO is a human homologue of murine PTPλ and is related to the homotypically adhering κ and μ receptor-type tyrosine phosphatases. PTP-RO is expressed in human megakaryocytic cell lines, primary bone marrow megakaryocytes, and stem cells. PTP-RO mRNA and protein expression are upregulated upon phorbol 12-myristate 13-acetate (PMA) treatment of the megakaryocytic cell lines CMS, CMK, and Dami. To elucidate the function of PTP-RO in megakaryocytic cells and its potential involvement in the stem cell factor (SCF)/c-Kit receptor pathway, COS-7 and 293 cells were cotransfected with the cDNAs of both the c-Kit tyrosine kinase receptor and PTP-RO. PTP-RO was found to be associated with the c-Kit receptor in these transfected cells and the SCF/Kit ligand induced a rapid tyrosine phosphorylation of PTP-RO. Interestingly, these transfected cells demonstrated a decrease in their proliferative response to the SCF/Kit ligand. In addition, we assessed the association of PTP-RO with c-Kit in vivo. The results demonstrated that PTP-RO associates with c-Kit but not with the tyrosine kinase receptor FGF-R and that PTP-RO is tyrosine-phosphorylated after SCF stimulation of Mo7e and CMK cells. Antisense oligonucleotides directed against PTP-RO mRNA sequences significantly inhibited megakaryocyte progenitor proliferation. Therefore, these data show that the novel tyrosine kinase phosphatase PTP-RO is involved in megakaryocytopoiesis and that its function is mediated by the SCF/c-Kit pathway.
TYROSINE PHOSPHORYLATION plays a crucial role in the regulation of signal transduction during diverse cell functions, including cell activation, proliferation, differentiation, survival, and metabolic homeostasis.1,2 Tyrosine phosphorylation is regulated by controlling the balanced and opposing actions of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPases). PTPases are classified into two groups, membrane-spanning receptor-type (R-PTPases) and cytosolic-type. Hematopoietic cells express a number of R-PTPases, such as CD45, PTPς, and PTPγ, which are highly expressed in hematopoietic tissues.3-6 CD45 is required for T-cell development and plays a positive role in both T- and B-cell receptor-mediated signaling.3 PTPς is expressed abundantly in immature thymus and is suggested to control T-cell differentiation.5PTPγ was shown to regulate hematopoietic differentiation using murine embryonic stem cells.6 The cytosolic PTPase, SHP-1, expressed primarily in hematopoietic cells, negatively regulates signaling in T- and B-cell antigen receptors and c-Kit in hematopoietic progenitor cells.3,4,7 Another cytosolic PTPase, SHP-2, although expressed ubiquitously, regulates T-cell signaling negatively as well as hematopoietic cell development positively.3,4,7 8 However, relatively little is known about the role of PTPases in signaling in hematopoietic stem cells and megakaryocytes.
To examine the involvement of PTPases in hematopoietic stem cell and in megakaryocytic cell growth and differentiation, we have characterized the expression of PTPases in 5-fluorouracil (5-FU)–treated murine bone marrow stem cells and have cloned the human cDNA of a novel R-PTPase termed PTP-RO,9 which is similar to the recently discovered hPTP-J.10 In addition, the mouse homologue of PTP-RO/PTP-J, termed PTPλ/Ftp1, was also cloned recently.11,12PTP-RO/PTP-J and PTPλ are related to the homotypically adhering κ and μ R-PTPases.13-19 The human PTP-RO cDNA clone encodes a polypeptide of 1,439 amino acids and the predicted molecular mass of the PTP-RO protein is 162 kD. PTP-RO/PTP-J and PTPλ proteins, as well as PTPκ and PTPμ, consist of an extracellular segment containing an MAM domain, an Ig domain, four fibronectin-type III (FN-III) repeats, a transmembrane segment, and two tandem intracellular phosphatase domains and are classified to be type IIB R-PTPases.20 Reverse transcription-polymerase chain reaction (RT-PCR) and Northern blot analyses showed that PTP-RO is expressed in human CD34+ stem cells as well as in various human tissues.9
In addition to homotypical binding, PTPκ, PTPμ, and PTPλ were reported to interact with the cadherin/catenin complex,11,21,22 which is essential for cadherin-mediated cell-cell adhesion and association with the cytoskeleton. Furthermore, β-catenin and E-cadherin were suggested to be substrates for PTPκ and PTPμ, respectively.21 22 These data suggest that PTPκ, PTPμ, and PTPλ are involved in the regulation of cadherin/catenin-mediated cell-cell adhesion. However, the function of type IIB R-PTPases in hematopoietic cells is largely unknown.
Stem cell factor (SCF) is a growth and differentiation factor for hematopoietic progenitor cells.23,24 SCF is the ligand for the transmembrane c-Kit receptor.25,26 Two forms of SCF, a membrane-bound and a secreted form, have been described.27-30 c-Kit subserves pivotal functions in promoting the development, survival, and proliferation of hematopoietic stem cells, neuronal crest-derived cells, and germ cells. Upon SCF stimulation, the c-Kit receptor associates with and/or phosphorylates other signal transduction molecules such as phosphatidylinositol 3′-kinase (PI-3 K), phospholipase C-γ1, Lyn, Csk homologous kinase (CHK), Janus kinase 2 (JAK2), signal transducers and activators of transcription 1 (STAT1), Tec kinase, and the tyrosine phosphatase SHP-1.29-37 Recently, SHP-1 was reported to bind and negatively modulate the c-Kit receptor by interaction with Tyrosine 569 in the juxtamembrane domain of the c-Kit receptor.38
In this report, we analyzed the expression of PTP-RO mRNA and protein in a hematopoietic stem cell line and in various megakaryocytic cell lines and elucidated the role of PTP-RO in SCF/c-Kit receptor signaling pathways in megakaryocytes. Our results demonstrated that PTP-RO expression is upregulated during differentiation of megakaryocytic cells and that PTP-RO is constitutively associated with the c-Kit receptor and is tyrosine-phosphorylated by the SCF/Kit ligand.
MATERIALS AND METHODS
Materials.
Phorbol 12-myristate 13-acetate (PMA) was purchased from Life Technologies, Inc (Bethesda, MD) and stored at a concentration of 1 mg/mL in dimethyl sulfoxide (Sigma, St Louis, MO) at −80°C. SCF was kindly provided by Amgen (Thousand Oaks, CA). Calf intestine alkaline phosphatase (CIAP) was purchased from Boehringer Mannheim (Indianapolis, IN).
Antibodies.
Polyclonal antibodies against PTP-RO were raised in rabbits immunized by injection of the N-terminus of the PTP-RO intracellular domain (residues: 770-930) fused to glutathione S-transferase (GST; R5702) and against the MAM domain of PTP-RO (residues: 21-189) fused to GST (D2712). Both anti–PTP-RO antibodies were purified by protein G-Sepharose (Pierce, Rockford, IL). Polyclonal anti-PTPλ antibodies against the PTPλ extracellular domain (αECD)11 were kindly provided by Dr Laurence A. Lasky (Genentech, Inc, South San Francisco, CA). Monoclonal and polyclonal anti-Flag antibodies were purchased from Kodak (Rochester, NY; M2) and Zymed (South San Francisco, CA), respectively. Antiphosphotyrosine antibody, 4G10, was kindly provided by Dr Brian J. Druker (Oregon Health Sciences University, Portland, OR). Another antiphosphotyrosine antibody, PY20, was purchased from Transduction Laboratories (Lexington, KY). Anti–β-catenin and anticadherin antibodies were purchased from Zymed and Transduction Laboratories, respectively. Anti–c-Kit antiserum against the extracellular domain was kindly provided by Amgen, and anti–c-Kit antibody against the C-terminus was purchased from Transduction Laboratories.
Cell lines.
CTS, CMS, and CMK cells were kindly provided by Dr Takeyuki Sato (Chiba University, Chiba, Japan). CTS, CMK, and Dami cells were maintained in RPMI-1640 medium (Mediatech [Washington, DC] or Life Technologies, Inc) containing 10% fetal calf serum (FCS; Life Technologies, Inc). CMS, COS-7, and 293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Mediatech or Life Technologies, Inc) containing 10% FCS. Mo7e cells were maintained in RPMI-1640 medium containing 20% FCS, 10 ng/mL interleukin-3 (IL-3; R&D Systems, Minneapolis, MN), and 10 ng/mL granulocyte-macrophage colony-stimulating factor (R&D Systems). Penicillin (100 IU/mL) and streptomycin (10 μg/mL) were added to all media.
CTS cells are a recently established human leukemia cell line derived from the peripheral blood of a 13-year-old girl suffering relapse of acute myeloblastic leukemia (AML) and have the characteristics of pluripotent stem cells.39 CMS cells have also been recently established as a megakaryoblastic cell line. CMK,40Dami,41 and Mo7e42 cells have authentic properties of megakaryocytic lineages.
Human bone marrow was obtained by aspiration from the iliac crests of normal donors after informed consent was obtained, as described previously.28,29 After two washes with sterile 1× phosphate-buffered saline (PBS), the cells were resuspended in RPMI-1640 medium with 7.5% platelet-poor plasma (PPP), penicillin/streptomycin (P/S), and L-glutamine; seeded onto T-75 tissue culture flasks (Corning, Corning, NY); and incubated at 37°C in 5% CO2. CD34+ bearing marrow progenitor cells were purified from heparinized bone marrow aspirates using immunomagnetic beads coated with anti-CD34 monoclonal antibody as described.28 The CD34+ cell population was 95% to 98% pure as judged by labeling with fluorescein-conjugated CD34 antibodies after an overnight recovery in RPMI plus 7.5% PPP.
Antisense oligonucleotide synthesis and cell treatment.
Modified 18-mer oligonucleotides were synthesized by Genosys Biotechnologies, Inc (The Woodlands, TX), precipitated, and resuspended in RPMI-1640. PTP-RO antisense AS1 (5′-CGTACTGGGCCTCCTTGAACA-3′) corresponded to nucleotides +4 to +24. All experiments were performed with the corresponding sense (5′-AGTACAGCCAGGCCCAGTACG-3′) and scrambled sequence controls. CD34+ cells were incubated at a concentration of 1 × 106 cells/mL in serum-deprived medium. Medium contained iron-saturated human transferrin (300 ng/mL), insulin (100 ng/mL), calcium chloride (28 μg/mL), deionized bovine serum albumin (2%), 6.14 mg of oleic acid, and 7.4 mg of dipalmitoyl lecithin in 10 mL of RPMI. Incubation medium was supplemented with recombinant human IL-3 (100 U/mL; R&D Systems). Oligonucleotides were used at a concentration of 10 mmol/L (70 μg/mL). After 16 hours of incubation at 37°C, 5 mmol/L of oligonucleotides was added. Cells were further incubated for an additional 6 hours and then washed in RPMI-1640 before plating.
Colony assays.
Cells were placed in the fibrin clot culture system as described.28 29 Cells were seeded at a concentration of 500 cells/0.5 mL in culture containing 10% PPP and IL-3 (100 U/mL). Cultures were incubated for 12 days. Fibrin clots were fixed for 5 minutes with 10% neutral formalin and reacted with platelet glycoprotein IIIa (GpIIIa) fluorescein-conjugated monoclonal mouse antibodies to human GpIIIa (1:1000 dilution; Dako, Carpinteria, CA) for 30 minutes. The numbers of positive megakaryocyte colony-forming units (CFU-MK) were counted.
Flow cytometric analysis of surface protein expression.
To detect the potential surface binding proteins that bind PTP-RO, we used flow cytometric analysis (FACS staining). Cells were washed with sterile PBS, and 1 × 106 cells, untreated or treated with PMA for 24 hours, were resuspended in 0.1 mL of PBS. Cells were incubated with 10 μL of the PTP-RO antibodies or with GpIIIa antibodies as a positive control, mouse IgG as a negative control (Immunotech Inc, Westbrook, ME), or PBS at 4°C for 20 minutes. Fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG or goat antirabbit IgG was added at a final dilution of 1:500 and followed by incubation for 20 minutes at 4°C. Cells were washed twice and resuspended in 0.5 mL of 1% (vol/vol) paraformaldehyde in PBS. Cells were then analyzed by flow cytometry.
Generation of a PTP-RO expression construct.
A pcDNA3/Flag expression vector was constructed by inserting a short DNA fragment encoding an 8-amino acid Flag peptide into a pcDNA3 expression vector (Invitrogen, San Diego, CA) at the EcoRI site.43 A PTP-RO cDNA was then subcloned into the pcDNA3/Flag vector to generate a PTP-RO expression vector (pcDNA3/PTP-RO-Flag), where the Flag sequence was added downstream of the PTP-RO cDNA.
Transfection analysis.
COS-7 and 293 cells were transfected using SuperFect (Qiagen, Valencia, CA) according to the manufacturer’s protocol. In the cotransfection system, pcDNA3/c-Kit44 and pcDNA3/PTP-RO-Flag were transfected simultaneously. In some experiments, pcDNA3.1(−)/Myc-His/lacZ (Invitrogen) was also transfected simultaneously to determine transfection efficiency. After 48 to 72 hours of transfection, cells were either lysed or serum-starved. The amounts of extract used for immunoprecipitation were normalized by measurement of β-galactosidase activity by anO-nitrophenyl β-D-galactopyranoside reporter assay (Promega, Madison, WI).
Immunoprecipitation and Western blotting.
Cells were washed with PBS and were lysed in modified RIPA buffer (50 mmol/L Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 mmol/L NaF, and 1 mmol/L Na3VO4) or in Triton X-100 lysis buffer (10 mmol/L Tris-HCl, pH 7.4, 1% Triton X-100, 50 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 mmol/L NaF, and 1 mmol/L Na3VO4). In some experiments, as indicated, cells were lysed in modified RIPA buffer without NaF and Na3VO4. After centrifugation at 14,000 rpm for 15 minutes, supernatant was used as total cell lysate. Protein concentrations were determined using a colorimetric protein assay kit (Bio-Rad, Hercules, CA).
Total cell lysates were incubated with different antibodies for 4 hours or overnight at 4°C. Protein G-Sepharose (Pierce) was added for 1 hour at 4°C. Precipitates were collected by centrifugation and washed four times with the modified RIPA buffer. Proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride (PVDF) membranes (Micron Separations, Inc, Westboro, MA). After blocking with nonfat dry milk (Carnation) or bovine serum albumin (Boehringer Mannheim), the membranes were probed with primary antibodies. Immunoreactive bands were visualized using horseradish peroxidase (HRP)-conjugated secondary antibodies and the enhanced chemiluminescent (ECL) system (Amersham Pharmacia, Piscataway, NJ) or Renaissance Western blot chemiluminescent reagent (NEN, Boston, MA). In some experiments, as indicated, total cell lysates were applied directly on SDS-PAGE gels.
Proliferation assay.
For proliferation assays, 104 cells were plated in 96-well microliter plates and cultured in RPMI plus 0.5% FCS for 4 hours. SCF/Kit ligand was added at a final concentration of 10 ng/100 μL, and cell proliferation was evaluated every 24 hours by a Cell Titer 96 nonradioactive assay (Promega) based on the conversion of a tetrazolium salt to formazan. Proliferation was quantitated by measuring the amount of formazan at 570 nm with a microtiter cell reader.
Northern blot analysis.
Poly(A+) RNA was isolated using an Oligo(dT)-cellulose column (Amersham Pharmacia or Life Technologies, Inc) or a Fast Track 2.0 Kit (Invitrogen). Six micrograms of poly(A+) RNA of each sample were run on a 1% agarose gel containing formamide and then transferred to a nylon membrane (Hybond-N; Amersham Pharmacia). Hybridization and washing were performed using standard procedures. Radiolabeled DNA probes of the PTP-RO 3′ untranslated region and 5′ region of the cDNA were generated by PCR. Blots were assessed with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or an actin-specific probe as a control.
RESULTS
Expression of PTP-RO mRNA and protein in stem cell and megakaryocytic cell lines.
We examined PTP-RO mRNA and protein expression in a hematopoietic stem cell line and various megakaryocytic cell lines by Northern analysis using purified Poly(A+) RNA and Western blot analysis using total cell lysates. Northern analysis showed the existence of one (4.4 kb) or two mRNAs (5.5 and 4.4 kb) in the CTS, CMK, CMS, and Dami cell lines (Fig 1). Western blot analysis of CMS, CMK, Dami, and Mo7e cells using two anti–PTP-RO antibodies against different regions of PTP-RO (the MAM domain and the intracellular domain) showed several PTP-RO proteins ranging from 190 to 250 kD (Fig 2). These results suggest that the megakaryocytic cell lines express several isoforms of PTP-RO, including the full-length PTP-RO. Additionally, Western blot analysis showed the presence of a possible proteolytic product of PTP-RO (∼100 kD).
Expression of PTP-RO mRNA in megakaryocytic cell lines. 6 μg of Poly(A+) mRNA extracted from the indicated human megakaryocytic cell lines (A) and 100 nmol/L PMA-treated (6 and 18 hours) CMK cells (B) were electrophoresed in a denatured 1% agarose-formaldehyde gel and transferred to nylon membranes. Hybridization was performed with a 32P-labeled PTP-RO probe (upper panel), followed by hybridization with a 32P-labeled GAPDH probe or actin probe (lower panel). The positions of PTP-RO, GAPDH, and actin mRNAs are indicated.
Expression of PTP-RO mRNA in megakaryocytic cell lines. 6 μg of Poly(A+) mRNA extracted from the indicated human megakaryocytic cell lines (A) and 100 nmol/L PMA-treated (6 and 18 hours) CMK cells (B) were electrophoresed in a denatured 1% agarose-formaldehyde gel and transferred to nylon membranes. Hybridization was performed with a 32P-labeled PTP-RO probe (upper panel), followed by hybridization with a 32P-labeled GAPDH probe or actin probe (lower panel). The positions of PTP-RO, GAPDH, and actin mRNAs are indicated.
Upregulation of PTP-RO protein expression in megakaryocytic cell lines. (A) Total cell lysates containing equal amounts of protein (100 μg) from CTS, CMK, Dami, and Mo7e cells were separated on 7.5% SDS-PAGE. Immunoblotting was performed using anti–PTP-RO antibodies (R5702; 7 μg/mL) or control preimmune antibody (7 μg/mL). (B and C) CMS, CMK, and Dami cells were cultured in RPMI-1640 or DMEM medium (referred to in Materials and Methods) containing 10% FCS in the presence of 100 nmol/L PMA at a concentration of 106 cells/mL. After culture for 0, 24, and 48 hours, cells were collected and lysed with the RIPA buffer. One hundred micrograms of protein from clarified cell lysates were analyzed by 4% to 12% SDS-PAGE followed by Western blotting using two anti–PTP-RO antibodies R5702 (7 μg/mL; B) and D2712 (8 μg/mL; C) as well as control preimmune antibodies (8 μg/mL). The positions of migration of full-length PTP-RO and putative proteolytic PTP-RO are indicated.
Upregulation of PTP-RO protein expression in megakaryocytic cell lines. (A) Total cell lysates containing equal amounts of protein (100 μg) from CTS, CMK, Dami, and Mo7e cells were separated on 7.5% SDS-PAGE. Immunoblotting was performed using anti–PTP-RO antibodies (R5702; 7 μg/mL) or control preimmune antibody (7 μg/mL). (B and C) CMS, CMK, and Dami cells were cultured in RPMI-1640 or DMEM medium (referred to in Materials and Methods) containing 10% FCS in the presence of 100 nmol/L PMA at a concentration of 106 cells/mL. After culture for 0, 24, and 48 hours, cells were collected and lysed with the RIPA buffer. One hundred micrograms of protein from clarified cell lysates were analyzed by 4% to 12% SDS-PAGE followed by Western blotting using two anti–PTP-RO antibodies R5702 (7 μg/mL; B) and D2712 (8 μg/mL; C) as well as control preimmune antibodies (8 μg/mL). The positions of migration of full-length PTP-RO and putative proteolytic PTP-RO are indicated.
The approximately 100-kD polypeptide was recognized by the R5702 antibodies (against the N-terminus of PTP-RO fused to GST), but not by the D2712 antibodies (against the MAM domain of PTP-RO fused to GST; see Materials and Methods for more details). This protein was also recognized by polyclonal anti-PTPλ antibodies (the mouse homologue of PTP-RO; αECD) against its extracellular domain (data not shown), suggesting that the approximately 100-kD protein is a proteolytic product containing the intracellular domain and the extracellular domain of PTP-RO without the MAM domain.
Upregulation of PTP-RO mRNA and protein expression in megakaryocytic cell lines upon PMA stimulation.
Our analyses confirmed that, in CMS cells, the primitive hematopoietic marker CD34 was downregulated, whereas the megakaryocyte-specific markers, CD42b (GPIb) and CD61 (GPIIIa), were upregulated by PMA treatment, as analyzed by flow cytometric analysis (data not shown). CTS, CMK, and Dami cells differentiate into mature megakaryocytes upon stimulation with PMA.39-41 Northern blot analysis demonstrated a time-dependent upregulation of PTP-RO mRNA expression upon PMA stimulation (Fig 1B). We have examined whether PTP-RO protein expression levels are also regulated by PMA treatment in these megakaryocytic cell lines. The full-length PTP-RO was significantly upregulated by PMA treatment (Fig 2B and C). Upregulation was observed after 24 hours of PMA treatment.
Immunofluorescence staining showed that more than 58% of the CMK cells stained positive with PTP-RO antibodies. Upon PMA treatment, more than 89% of the CMK cells were stained positive with PTP-RO antibodies. No staining was observed with control antibody. Taken together, these data along with the Western blot analysis indicate positive expression of PTP-RO on megakaryocytic cells, an expression that is upregulated upon PMA treatment.
Cell surface expression of PTPκ and PTPμ has been reported to be upregulated by cell-cell contact due to high cell density.22 45 To examine whether upregulation of PTP-RO is due to cell-cell contact, we seeded CMK cells at three independent concentrations (1 × 106, 3.3 × 105, and 1.1 × 105 cells/mL). After 48 hours, cells became approximately 100%, 90%, and 60% confluent, respectively. We analyzed PTP-RO protein expression by Western blotting. No significant difference in PTP-RO expression was observed among the three cell densities (data not shown), indicating that upregulation of PTP-RO was not due to cell-cell contact.
CMK cells became more adherent to culture flasks upon stimulation with PMA. Under normal conditions, without any treatments, CMK cells grow in in vitro culture in which approximately 70% of the cells are grown in suspension and approximately 30% are adherent. To examine whether cell adhesion is related to upregulation of PTP-RO, we compared PTP-RO protein expression of the nontreated CMK cells growing in suspension with that of the adherent CMK cells. Expression of PTP-RO was similar in both populations of CMK cells, indicating that PTP-RO upregulation was not due to cell adhesion (data not shown).
PMA suppresses CMK cell growth and induces cell differentiation.40 To analyze whether PTP-RO upregulation is due to cell growth suppression, we maintained CMK cells in serum-free medium for 48 hours and then harvested the cells. PTP-RO expression was not upregulated by serum starvation (data not shown). This suggests that PTP-RO upregulation by PMA treatment was not due to cell growth suppression, but to megakaryocyte differentiation.
Taken together, these results strongly suggest that PTP-RO upregulation is observed during megakaryocyte differentiation and is not mediated by cell-cell contact, cell adhesion, and/or cell growth suppression.
PTP-RO function in megakaryocytic cells is not related to its interaction with the cadherin/catenin complex.
To examine whether PTP-RO is involved in the cadherin/catenin complex-like PTPs κ, μ, and λ,11,21 22 we analyzed the expression of cadherin and β-catenin proteins in CTS, CMS, CMK, and Dami cells using Western blot analysis. Neither the cadherin nor β-catenin proteins were detected in CTS, CMK, and Dami cells (Fig 3). Interestingly, untreated CMS cells did not express cadherin and β-catenin, whereas PMA treatment did induce expression of cadherin as shown by Western blot analysis (Fig3B). β-Catenin was not observed in PMA-treated CMS cells. Taken together, the cadherin/catenin complex was not found in the CTS cells and the megakaryocytic Dami and CMK cell lines examined, suggesting that PTP-RO function is not related to its interaction with this well-known complex in megakaryocytes, contrary to observations in other cells such as epithelial cells.
A hematopoietic stem cell line and megakaryocytic cell lines do not express β-catenin and cadherin. Western blot analysis of total cell lysates (100 μg protein per lane) from CTS, CMS, CMK, and Dami cells untreated or PMA (100 nmol/L)-treated for 48 hours was performed using anti–β-catenin antibody (1 μg/mL; A) and anticadherin (1 μg/mL) antibody (B). COS-7 cells were used as a positive control. The positions of migration of β-catenin and cadherin are shown.
A hematopoietic stem cell line and megakaryocytic cell lines do not express β-catenin and cadherin. Western blot analysis of total cell lysates (100 μg protein per lane) from CTS, CMS, CMK, and Dami cells untreated or PMA (100 nmol/L)-treated for 48 hours was performed using anti–β-catenin antibody (1 μg/mL; A) and anticadherin (1 μg/mL) antibody (B). COS-7 cells were used as a positive control. The positions of migration of β-catenin and cadherin are shown.
Association of the c-Kit receptor with PTP-RO.
To address the role of PTP-RO in signal transduction and cell growth, we characterized the association of PTP-RO and the c-Kit tyrosine kinase receptor in two cell lines (Mo7e and CMK) known to express high levels of c-Kit receptor and to proliferate in response to SCF stimulation. CMK and Mo7e cells were starved and stimulated with 500 ng/mL SCF. After stimulation, cells were lysed and immunoprecipitated with anti–c-Kit antibodies against the extracellular domain of c-Kit. The precipitates were immunoblotted with antiphosphotyrosine antibodies. Tyrosine phosphorylation of the c-Kit receptor was observed upon SCF stimulation of CMK and Mo7e cells (Fig 4A and C). Next, the blots were reprobed with anti–PTP-RO antibodies (R5702). Interestingly, the approximately 190-kD protein, corresponding to the full-length PTP-RO, was observed specifically in the immunoprecipitates using anti–c-Kit receptor antibodies from both unstimulated and SCF-stimulated CMK and Mo7e cells (Fig 4A and C). These results suggest a constitutive association of PTP-RO and the c-Kit receptor in CMK and Mo7e cells.
In vivo association of c-Kit and PTP-RO in megakaryocytic cells. (A and B) CMK cells were starved in serum-free RPMI-1640 medium for 6 hours and incubated with or without 500 ng/mL SCF or FGF for 5 minutes at 37°C. Reaction was stopped by adding one-third volume of ice-cold PBS, followed by rapid centrifugation. After an additional wash with PBS, cells were lysed with the Triton X-100 lysis buffer. The clarified cell lysates were immunoprecipitated with either anti–c-Kit antiserum (Amgen; 10 μL), normal rabbit serum (NRS), or anti–FGF-R antiserum. The precipitates were immunoblotted with the 4G10 antibody (1:3,000; upper panel), anti–PTP-RO antibodies (R5702; 7 μg/mL; [A], middle panel; [B], lower panel), anti–c-Kit antibody (Santa Cruz, Santa Cruz, CA; 1 μg/mL; [A], lower panel) or anti–FGF-R antibody (Santa Cruz; [B], middle panel). (C and D) Mo7e cells were starved in RPMI-1640 medium containing 0.5% bovine serum albumin for 6 hours and stimulated with 500 ng/mL SCF for 10 minutes. Additional procedures were the same as in the case of CMK cells, except that cells were lysed with the modified RIPA buffer, and PY20 antibody (1:800) was used in (C) instead of the 4G10 antibody. Lysates were immunoprecipitated with anti–c-Kit or anti–PTP-RO antibodies (10 μL) or normal rabbit serum (NRS). The immunoprecipitates were immunoblotted with the 4G10 antibody (1:800), anti–PTP-RO antibodies (R5702), or anti–c-Kit antibodies, as indicated.
In vivo association of c-Kit and PTP-RO in megakaryocytic cells. (A and B) CMK cells were starved in serum-free RPMI-1640 medium for 6 hours and incubated with or without 500 ng/mL SCF or FGF for 5 minutes at 37°C. Reaction was stopped by adding one-third volume of ice-cold PBS, followed by rapid centrifugation. After an additional wash with PBS, cells were lysed with the Triton X-100 lysis buffer. The clarified cell lysates were immunoprecipitated with either anti–c-Kit antiserum (Amgen; 10 μL), normal rabbit serum (NRS), or anti–FGF-R antiserum. The precipitates were immunoblotted with the 4G10 antibody (1:3,000; upper panel), anti–PTP-RO antibodies (R5702; 7 μg/mL; [A], middle panel; [B], lower panel), anti–c-Kit antibody (Santa Cruz, Santa Cruz, CA; 1 μg/mL; [A], lower panel) or anti–FGF-R antibody (Santa Cruz; [B], middle panel). (C and D) Mo7e cells were starved in RPMI-1640 medium containing 0.5% bovine serum albumin for 6 hours and stimulated with 500 ng/mL SCF for 10 minutes. Additional procedures were the same as in the case of CMK cells, except that cells were lysed with the modified RIPA buffer, and PY20 antibody (1:800) was used in (C) instead of the 4G10 antibody. Lysates were immunoprecipitated with anti–c-Kit or anti–PTP-RO antibodies (10 μL) or normal rabbit serum (NRS). The immunoprecipitates were immunoblotted with the 4G10 antibody (1:800), anti–PTP-RO antibodies (R5702), or anti–c-Kit antibodies, as indicated.
The potential association of PTP-RO with another tyrosine kinase receptor was examined in CMK cells. Stimulation of CMK cells with FGF induced a rapid, but transient, tyrosine phosphorylation of a number of cellular proteins, including FGF-R. In contrast to the results with c-Kit, PTP-RO was not detected in FGF-R immunoprecipitates at any of the times examined after FGF stimulation (Fig 4B). These results indicate that PTP-RO selectively associates with c-Kit in megakaryocytic cells.
To determine whether c-Kit engagement in these cells is associated with the tyrosine phosphorylation of c-Kit and PTP-RO, the proteins were individually immunoprecipitated from resting and SCF-stimulated Mo7e and CMK cells, and their phosphorylation status was assessed by antiphosphotyrosine immunoblotting analysis. As shown in Fig 4C and D, PTP-RO and c-Kit are both tyrosine-phosphorylated after c-Kit engagement by SCF. These findings suggest that PTP-RO associates with the c-Kit receptor and is tyrosine-phosphorylated upon SCF/Kit ligand stimulation.
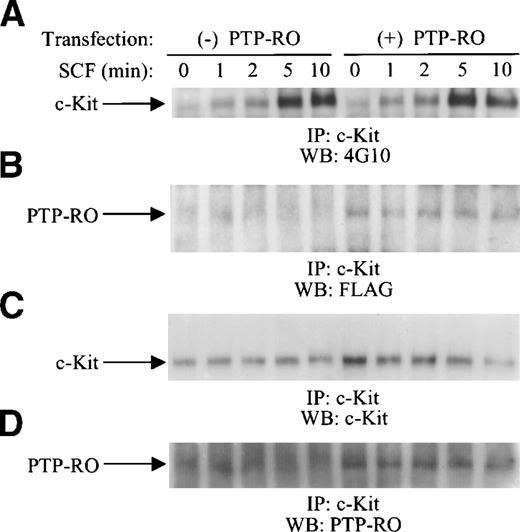
To further confirm the in vivo association of the c-Kit receptor with PTP-RO, we performed a transient transfection of both the c-Kit receptor and PTP-RO in COS-7 and 293 cells. An expression vector for the c-Kit receptor was transfected together with the construct for PTP-RO-Flag or a control vector, pcDNA3/Flag. pcDNA3.1(−)/Myc-His/LacZ, the expression vector for β-galactosidase, was also cotransfected to determine the transfection efficiency in these sets of experiments. After starvation, cells were stimulated with SCF and then lysed. The expression amount of the c-Kit receptor was normalized by measuring β-galactosidase activity. Cell lysates showing approximately the same β-galactosidase activity were immunoprecipitated with anti–c-Kit antibodies. SCF-induced tyrosine phosphorylation of c-Kit was observed in both c-Kit–transfected and PTP-RO/c-Kit–cotransfected COS-7 cells (Fig 5) and in 293 cells (data not shown), indicating that c-Kit was expressed as a functional receptor. Kinetic studies indicated that c-Kit phosphorylation levels were similar between PTP-RO–transfected and untransfected cells. The 190-kD protein corresponding to PTP-RO, because of its reactivity to anti-Flag antibody, was observed clearly only in the immunoprecipitates from the 293 cells (data not shown) and COS-7 cells expressing PTP-RO (Fig 5). These results indicate that PTP-RO was coimmunoprecipitated by anti–c-Kit antibodies, suggesting constitutive association of c-Kit and PTP-RO.
In vivo association of c-Kit and PTP-RO in transfected COS-7 cells. COS-7 cells were cotransfected with c-Kit and PTP-RO cDNAs or c-Kit cDNA and control vector together with pcDNA3.1(−)/Myc-His/LacZ. Cells were starved with DMEM containing 0.5% FCS for 15 hours, followed by serum-free DMEM for 4 hours. SCF stimulation (500 ng/mL) was performed for the indicated time points and then cells were lysed with the modified RIPA buffer. After measuring β-galactosidase activity, clarified total cell lysates containing equal amounts of c-Kit were immunoprecipitated with anti–c-Kit antiserum (10 μL; Amgen). The precipitates were immunoblotted with the 4G10 antibody (1:3,000; A), anti-Flag antibody (2 μg/mL; Zymed; B), anti–c-Kit antibody (1 μg/mL; Santa Cruz; C), or anti–PTP-RO antibodies (D).
In vivo association of c-Kit and PTP-RO in transfected COS-7 cells. COS-7 cells were cotransfected with c-Kit and PTP-RO cDNAs or c-Kit cDNA and control vector together with pcDNA3.1(−)/Myc-His/LacZ. Cells were starved with DMEM containing 0.5% FCS for 15 hours, followed by serum-free DMEM for 4 hours. SCF stimulation (500 ng/mL) was performed for the indicated time points and then cells were lysed with the modified RIPA buffer. After measuring β-galactosidase activity, clarified total cell lysates containing equal amounts of c-Kit were immunoprecipitated with anti–c-Kit antiserum (10 μL; Amgen). The precipitates were immunoblotted with the 4G10 antibody (1:3,000; A), anti-Flag antibody (2 μg/mL; Zymed; B), anti–c-Kit antibody (1 μg/mL; Santa Cruz; C), or anti–PTP-RO antibodies (D).
SCF induces tyrosine phosphorylation of PTP-RO.
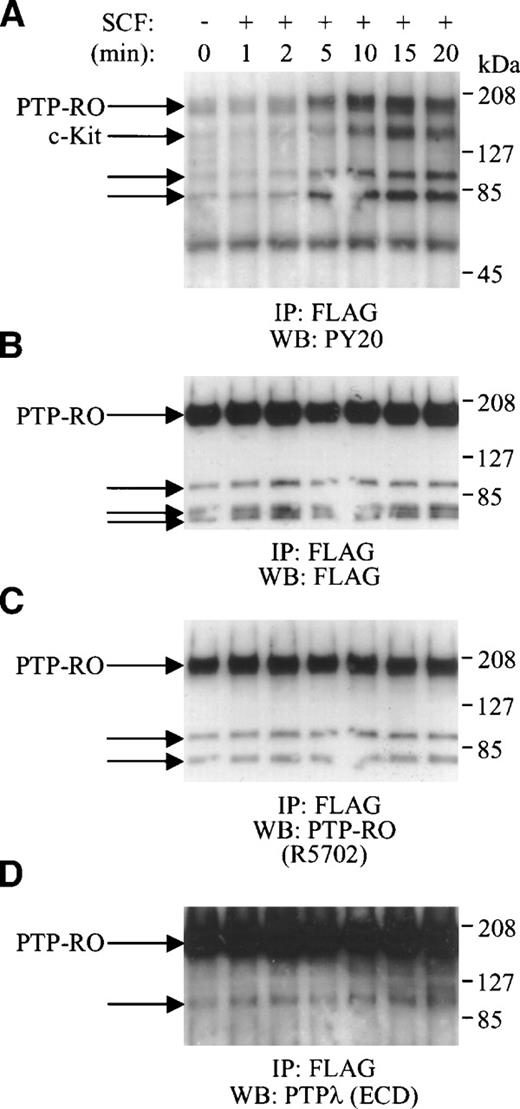
To examine whether PTP-RO phosphorylation is regulated by the SCF/c-Kit pathway, we used 293 cells and COS-7 cells cotransfected with c-Kit and PTP-RO-Flag cDNAs. After serum starvation, COS-7 transfectants were stimulated with SCF and then harvested at the indicated times (Fig 6). Cells were lysed and immunoprecipitated with anti-Flag antibody. The precipitates were then immunoblotted with the PY20 antibody. SCF stimulation induced the appearance of four tyrosine-phosphorylated bands with molecular masses of approximately 190, 145, 100, and 80 kD (Fig 6A). The 145-kD band corresponded to c-Kit. The phosphorylation level of all proteins increased at 5 minutes, peaked at 15 minutes, and then decreased at 20 minutes. To confirm the identity of these proteins, the membrane was stripped and reprobed with anti-Flag antibody (Fig 6B), anti–PTP-RO antibodies (R5702) against the N-terminus of the intracellular domain (Fig 6C), and anti-PTPλ antibodies (αECD) reactive to the extracellular domain of PTP-RO (Fig 6D). All antibodies recognized the 190-kD band as the full-length PTP-RO protein as well as the proteolytic products of PTP-RO. Similar results were obtained with transfected 293 cells (data not shown). These results indicate that SCF induced tyrosine phosphorylation of the full-length PTP-RO (190 kD) and the proteolytic forms of PTP-RO (100 and 80 kD). Interestingly, using the anti-Flag antibody, a non–tyrosine-phosphorylated 73-kD band was detected in addition to those of 190, 90, and 80 kD. Because the 73-kD polypeptide did not react with the R5702 antibodies, it must be another proteolytic product of PTP-RO containing only the intracellular domain possessing a deficiency in its N-terminus (Fig 7).
SCF-induced tyrosine phosphorylation of PTP-RO in COS-7 cells transfected with c-Kit and PTP-RO cDNAs. Serum-starved COS-7 cells expressing exogenous c-Kit and PTP-RO-cFlag were incubated with 500 ng/mL SCF for the indicated times. Cells were lysed with the modified RIPA buffer and immunoprecipitated with 5 μg of anti-Flag antibody (Zymed). The immunoprecipitates were used for Western blotting using the antiphosphotyrosine antibody, PY20 (1:800; A). The immunoblot was stripped and reprobed with anti-Flag antibody (M2; 1:2,000; B), anti–PTP-RO antibodies (R5702; 7 μg/mL; C), and anti-PTPλ antibodies (D), sequentially.
SCF-induced tyrosine phosphorylation of PTP-RO in COS-7 cells transfected with c-Kit and PTP-RO cDNAs. Serum-starved COS-7 cells expressing exogenous c-Kit and PTP-RO-cFlag were incubated with 500 ng/mL SCF for the indicated times. Cells were lysed with the modified RIPA buffer and immunoprecipitated with 5 μg of anti-Flag antibody (Zymed). The immunoprecipitates were used for Western blotting using the antiphosphotyrosine antibody, PY20 (1:800; A). The immunoblot was stripped and reprobed with anti-Flag antibody (M2; 1:2,000; B), anti–PTP-RO antibodies (R5702; 7 μg/mL; C), and anti-PTPλ antibodies (D), sequentially.
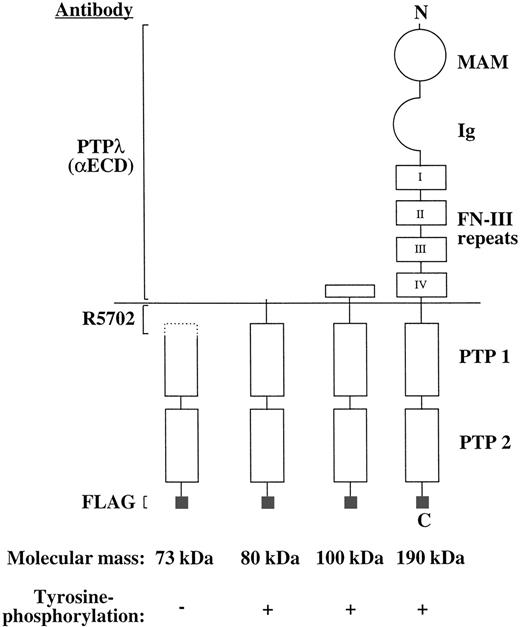
Schematic presentation of the full-length and proteolytic forms of PTP-RO in COS-7 cells. MAM, MAM domain; Ig, Ig domain; PTP 1 and 2, protein tyrosine phosphatase domains 1 and 2, respectively; N, N-terminus; C, C-terminus.
Schematic presentation of the full-length and proteolytic forms of PTP-RO in COS-7 cells. MAM, MAM domain; Ig, Ig domain; PTP 1 and 2, protein tyrosine phosphatase domains 1 and 2, respectively; N, N-terminus; C, C-terminus.
Effect of PTP-RO antisense oligonucleotides on in vitro megakaryocytopoiesis.
To address the role of PTP-RO in the regulation of megakaryocytopoiesis, we exposed purified bone marrow CD34+cells to PTP-RO antisense and sense oligonucleotides. This approach has been successfully used to address the function of C-myb in megakaryocytopoiesis. C-myb antisense oligonucleotide (5′-GTGCCGGGGTCTTCGGCC-3′) served as a positive control due to its known inhibitory effects on the generation of megakaryocyte colonies (CFU-MK).46 The CD34+ cells were isolated using immunomagnetic beads. CD34+ cells were incubated at a concentration of 1 × 106 cells/mL in serum-deprived medium containing growth factors and synthetic sense or antisense oligonucleotides. The generation of megakaryocyte colonies (CFU-MK) from CD34+ progenitor cells treated with PTP-RO antisense was reduced significantly (∼50%) compared with the sense-treated CD34+ and control untreated cells (Table 1). These results indicate that PTP-RO antisense oligonucleotides specifically inhibited in vitro megakaryocytopoiesis using primary bone marrow progenitor cells.
Effect of Oligonucleotide Treatment of Bone Marrow-Derived CD34+ Cells On In Vitro CFU-MK Colony Formation
| Treatment . | No. of Colonies . |
|---|---|
| Control | 32.0 ± 5.0 |
| PTP-RO sense | 35.0 ± 3.0 |
| PTP-RO scramble | 28.3 ± 2.0 |
| PTP-RO antisense | 16.3* ± 3.0 |
| c-myb antisense | 12.5 ± 2.0 |
| Treatment . | No. of Colonies . |
|---|---|
| Control | 32.0 ± 5.0 |
| PTP-RO sense | 35.0 ± 3.0 |
| PTP-RO scramble | 28.3 ± 2.0 |
| PTP-RO antisense | 16.3* ± 3.0 |
| c-myb antisense | 12.5 ± 2.0 |
Cells were seeded at a concentration of 500 cells.5 mL in plasma-clot cultures in 1% PPP and IL-3 (100 ng/mL). CFU-MK colonies were scored after 12 days. Results are shown as means ± SE of four different experiments (4 plates/experiment).
P < .5 for PTP-RO antisense compared with control PTP-RO sense and scramble.
DISCUSSION
In this report, we have shown that a type IIB R-PTPase, PTP-RO, was expressed in megakaryocytic cell lines and that its expression was upregulated during differentiation by PMA treatment. In addition, PTP-RO was constitutively associated with the c-Kit receptor and tyrosine-phosphorylated upon SCF stimulation of megakaryocytic cells. Full-length PTP-RO was expressed as multiple isoforms (Fig 2). Two main categories of PTP-RO isoforms were found: the high (∼220 to ∼250 kD) and the low (∼190 to ∼ 200 kD) molecular mass proteins. Isoforms of PTP-RO might be formed by alternative processing. Alternatively, these various PTP-RO isoforms might result from differences in their glycosylation. In addition to the full-length PTP-RO, we observed a putative proteolytic product of PTP-RO (∼100 kD), consistent with previous data about type IIB R-PTPases and other R-PTPases. It is a common phenomenon that R-PTPases are digested within the fourth FN-III repeat at a sequence, RLRR,11 that exists within PTP-RO sequences. Determining whether alternative processing is taking place and identifying whether different glycosylation forms of PTP-RO exist remain to be addressed in future studies.
In addition, we have observed that PMA treatment upregulates PTP-RO mRNA and protein expression in megakaryocytic cells (Figs 1B and 2B and C). This upregulation was related to megakaryocyte differentiation but not to cell-cell contact, cell adhesion, and cell growth suppression. Interestingly, our data are in contrast with hPTP-J expression in Jurkat cells, in which hPTP-J was downregulated by PMA stimulation.10 hPTP-J was also downregulated by calcium ionophore, A23187. It is likely that signal transduction mechanisms by PMA stimulation are different between T cells, such as Jurkat cells, and megakaryocytic cells. Transforming growth factor β (TGFβ), an inhibitor of proliferation of human keratinocyte cells, upregulated PTPκ expression.46 Cell-cell contact upregulated PTP κ and μ expression in mink lung epithelial cells and mammary carcinoma cells, respectively.22 45 Thus, the type IIB R-PTPases can be regulated by different mechanisms in various cell types.
Because type IIB R-PTPases such as PTPs κ, μ, and λ play a role in the regulation of cell-cell contact and adhesion mediated by the cadherin/catenin complex,11,21,22 47 we analyzed whether PTP-RO has similar functions. In the megakaryocytic cell lines, CMS, CMK, and Dami, expression of PTP-RO was upregulated by treatment with PMA. In parallel, PMA treatment induced these cells to aggregate (CMS) or to be adherent (CMK and Dami). However, neither β-catenin nor cadherin was expressed in these cell lines, except for in CMS cells treated with PMA (Fig 3). There are no reports showing that cadherin controls cell-cell adhesion without catenin. Thus, if PTP-RO regulates cell contact and adhesion in megakaryocytes, it may be due to the direct interaction of PTP-RO via its extracellular domain and not through the β-catenin/cadherin complex.
Interestingly, we have observed that PTP-RO is tyrosine-phosphorylated upon SCF stimulation and is associated with c-Kit independently of SCF stimulation (Figs 4, 5, and 6). Various proteins, such as CHK, the p85 subunit of PI-3 K, phospholipase C-γ1, Fyn, Lyn, Shc, Grap, SHP-1, and STAT1,31,32,34,36,37,48 have been reported to be associated with c-Kit upon SCF stimulation via their SH2 domains. Tec-kinase was constitutively associated with c-Kit via its SH3 domain.33 However, PTP-RO contains neither an SH2 nor an SH3 domain, suggesting that, unlike other signal transduction proteins, the binding mechanism of PTP-RO to c-Kit is not via SH2 and SH3 domains.
A 200-kD transmembrane glycoprotein was reported to be associated with c-Kit by SCF stimulation of Mo7e cells.49 Although 200 kD is very close to the molecular mass of PTP-RO, the 200-kD protein failed to associate with c-Kit without SCF stimulation, which is in contrast with our data concerning PTP-RO. Thus, our analysis suggested that the 200-kD protein is not PTP-RO. Importantly, in CMK and Mo7e cells, only the low molecular mass (∼200 kD) isoforms of PTP-RO were immunoprecipitated by anti–c-Kit antibody. This suggests that different full-length PTP-RO isoforms may play various roles such as some other R-PTPases, such as CD45 isoforms, which differently affect T-cell development.50
PTP-RO was tyrosine-phosphorylated by SCF stimulation, suggesting that PTP-RO is a signaling molecule involved in the SCF/c-Kit–mediated signal transduction pathway. Some R-PTPases, LAR, PTPδ, and DEP-1, were tyrosine-phosphorylated in TrkA-dependent and EGF-R–dependent manners, respectively.51,52 TrkA, the nerve growth factor (NGF) receptor, and EGF-R are receptor-type tyrosine kinases that have similar characteristics in signal transduction as c-Kit, such as dimerization, tyrosine phosphorylation, and binding to several SH2-containing proteins (ie, Shc, the p85 subunit of PI-3 K, and phospholipase C-γ1) upon ligand stimulation.53,54However, the mechanisms of tyrosine phosphorylation of DEP-1 by EGF and PTP-RO by SCF are likely to be different. Whereas relatively long-time stimulation (30 minutes) by EGF was necessary to tyrosine phosphorylate DEP-1,52 PTP-RO phosphorylation was observed after only 5 minutes of SCF stimulation (Fig 6). These results suggest that, whereas DEP-1 is not a direct target of EGF-R, PTP-RO might be a direct target of c-Kit.
At present, the biological importance of the tyrosine phosphorylation of PTP-RO is not known. One possibility is that the tyrosine phosphorylation of PTP-RO regulates its catalytic activity. There are some reports suggesting tyrosine phosphorylation-induced phosphatase activity. For instance, tyrosine phosphorylation of SHP-2, PTP1B, and CD45 correlated with an enhancement in their catalytic activity.55-57 Although we detected possible in vivo phosphatase activity of PTP-RO in COS-7 cells overexpressing PTP-RO (data not shown), we could not detect in vitro catalytic activity. Catalytic activity of PTP-RO may require modification of PTP-RO such as its tyrosine phosphorylation. The other possibility is that its tyrosine phosphorylation site(s) is a target(s) for other proteins. Our next interest is the identification of molecules that bind to the phosphorylated tyrosine residues of PTP-RO. CD45, upon phosphorylation on tyrosine by treatment with a phosphatase inhibitor, was reported to bind to Lck kinase.56 PTPα is tyrosine-phosphorylated constitutively in vivo and is associated with the adaptor protein, Grb2, via its SH2 domain.58 59 Although there are 30 tyrosine residues in the cytoplasmic region of PTP-RO, not all of them are known as potential SH2 binding sites.
A schematic presentation of the full-length and proteolytic forms of PTP-RO in COS-7 transfectants are shown in Fig 7. The immunoreactivity of these PTP-RO isoforms to three different antibodies and their tyrosine phosphorylation are also shown. All constructs contained the C-terminus of PTP-RO, because they were recognized by anti-Flag antibody. Because the 100- and 80-kD proteins bound to WGA-Sepharose, they should have been glycosylated, implying that they have the C-terminus of the extracellular domain of PTP-RO. The failure of PTP-RO to react with anti-PTPλ antibodies (αECD) of the 80-kD protein indicates that it contains only a very small part of the extracellular domain close to the membrane. The 73-kD protein has only an intracellular domain deficient in its N-terminus. Interestingly, only the 73-kD protein was not tyrosine-phosphorylated. This suggests that phosphorylated tyrosine residues exist within the epitope of the R5702 antibodies. Thus, within the amino acid residues 770 to 930, there is a possible epitope in which there are six tyrosine residues responsible for PTP-RO phosphorylation.
Our results demonstrating that the function of PTP-RO is mediated by the SCF/c-Kit pathway and that antisense oligonucleotides directed against PTP-RO mRNA sequences significantly inhibit megakaryocyte progenitor proliferation indicate that PTP-RO is involved in megakaryocytopoiesis. We propose a novel biological function of PTP-RO, a type IIB R-PTPase, in the regulation of SCF/c-Kit signaling in megakaryocytes. We suggest that PTP-RO negatively regulates c-Kit signaling and thereby mitigates the signaling events linking c-Kit engagement to hemopoietic cell proliferation and differentiation. Future studies will determine the binding site of PTP-RO to c-Kit and the molecular mechanisms by which c-Kit signaling is regulated by PTP-RO.
ACKNOWLEDGMENT
The authors thank Dr Brian J. Druker (Oregon Health Sciences University) for providing the antiphosphotyrosine antibody, 4G10; Dr Brian Bennett (Amgen Inc) for providing anti–c-Kit antiserum and SCF; Dr Laurence A. Lasky (Genentech, Inc) for providing anti-PTPλ antibodies; and Dr Takeyuki Sato (Chiba University) for providing CTS, CMS, and CMK cell lines. We also thank Yigong Fu for DNA sequencing. We thank Drs Shalom Avraham and Daniel Price for their much appreciated advice and reading of this manuscript. We are grateful to Tee Trac and Peter Park for typing this manuscript, Janet Delahanty for editing, and Nancy DesRosiers for preparation of the figures.
Supported in part by National Institutes of Health Grant No. HL 51456 and the Toray Research Center Inc.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hava Avraham, PhD, Division of Experimental Medicine, Harvard Institutes of Medicine-BIDMC, 4 Blackfan Circle, Boston, MA 02115; e-mail: havraham@caregroup.harvard.edu.




![Fig. 4. In vivo association of c-Kit and PTP-RO in megakaryocytic cells. (A and B) CMK cells were starved in serum-free RPMI-1640 medium for 6 hours and incubated with or without 500 ng/mL SCF or FGF for 5 minutes at 37°C. Reaction was stopped by adding one-third volume of ice-cold PBS, followed by rapid centrifugation. After an additional wash with PBS, cells were lysed with the Triton X-100 lysis buffer. The clarified cell lysates were immunoprecipitated with either anti–c-Kit antiserum (Amgen; 10 μL), normal rabbit serum (NRS), or anti–FGF-R antiserum. The precipitates were immunoblotted with the 4G10 antibody (1:3,000; upper panel), anti–PTP-RO antibodies (R5702; 7 μg/mL; [A], middle panel; [B], lower panel), anti–c-Kit antibody (Santa Cruz, Santa Cruz, CA; 1 μg/mL; [A], lower panel) or anti–FGF-R antibody (Santa Cruz; [B], middle panel). (C and D) Mo7e cells were starved in RPMI-1640 medium containing 0.5% bovine serum albumin for 6 hours and stimulated with 500 ng/mL SCF for 10 minutes. Additional procedures were the same as in the case of CMK cells, except that cells were lysed with the modified RIPA buffer, and PY20 antibody (1:800) was used in (C) instead of the 4G10 antibody. Lysates were immunoprecipitated with anti–c-Kit or anti–PTP-RO antibodies (10 μL) or normal rabbit serum (NRS). The immunoprecipitates were immunoblotted with the 4G10 antibody (1:800), anti–PTP-RO antibodies (R5702), or anti–c-Kit antibodies, as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/2/10.1182_blood.v94.2.539/5/m_blod41440004w.jpeg?Expires=1769917201&Signature=CZjlQ~NWEbAPMHwmDkuV5ab0GvmdhT729U106vHeGqXnGvTJcTCGMxxVt9x~YtT4SNj0ijK5D5de5IoT1hgD2oZtj8SnBOMVP50sGUVg2QpzEADrxzB7PlnvT3RxEFoyQtTENi1l6eN5nvPJ9cxhegnOy1puAwHhpYAbZy9v8Th9BytZXUAj86xYqyuSBsanWrjTTrPJzE6Dd6VUf98RtHe0LqQzNZKUcVroQbcC6WjXjnCz6tSaGeavSnY7S-ghrO4p8AYyw2F9SLbDSpwFEaZSZg7mk9ICSADO8x5wbqbWdy54JS9ODVCtYLeNa6iPq4X1yNKuIhCIWOlBOg2fJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal