Abstract
Using surface markers, we identified two bone marrow (BM) subsets enriched for TdT+ cells on the brink of CD45R acquisition. These two populations, Lin−c-kitLo and Lin−c-kit−, consisting of 35.4% and 7.4%, respectively, TdT+ cells, generated B-lineage cells in overnight cultures. Approximately half of the c-kitLoB-lineage precursors were bipotential, yielding myeloid and lymphoid progeny, whereas most that were c-kit− gave rise only to lymphocytes. Analysis of B-lineage progression during a finite culture period showed that the most mature precursors were concentrated in the Lin−c-kit− population. Moreover, a majority of the earliest CD45R+ pro-B cells in BM, identified as CD45R+ CD43+ BP-1−CD25− natural killer (NK)1.1−sIgM−, were also c-kit−. These c-kit− cells, like their c-kitLocounterparts, expressed TdT, proliferated in response to interleukin (IL)-7, and generated sIgM+ cells. These data suggest that TdT expression is initiated as c-kit downregulation begins in Lin− cells, with progressive loss of c-kit during B-lineage differentiation. CD45R expression is initiated during the transition from c-kitLo to c-kit− with many cells losing c-kit before acquiring CD45R. The ability to isolate highly enriched populations of viable CD45R− precursors will be instrumental in characterizing the earliest B-lineage cells.
IN THE ADULT MOUSE, self-renewing hematopoietic stem cells (HSC) reside in bone marrow (BM) in which they generate all blood lineages, including B lymphocytes (for review see Morrison et al1). Enrichment of murine HSC and multipotential progenitor activity has been achieved in sorted BM cells that lack surface markers of the various blood lineages (Lin−) but express the receptor for stem cell factor, c-kit (c-kit+).2 Recently, common lymphoid progenitors (CLP) that can generate T- and B-lymphoid and natural killer (NK), but not myeloid cells, were identified.3 This population has no, or limited, capacity for self-renewal. CLP resemble stem cells in being Lin−, but they lack Thy-1 and express the receptor for interleukin (IL)-7 as well as low levels of c-kit and Sca-1. B-lineage cells can be isolated from BM on the basis of CD45R4 5 and CD196 expression, but Lin− precursor populations poised to enter the B-lymphocyte lineage are poorly resolved.
The stages of B-lineage differentiation in which sequential heavy- and light-chain gene rearrangements occur have been designated the pro-B and pre-B stages, respectively (for review see Loffert et al7). Using combinations of cell surface and intracellular differentiation markers, three schemes for B-cell development have been proposed.8-10 Although these models define intermediate stages of maturation, the earliest stages of B-lineage commitment are not well characterized.
Park and Osmond8 used expression of terminal deoxynucleotide transferase (TdT) and cytoplasmic μ heavy chains (cμ), in addition to CD45R to identify early pro-B cells (TdT+ CD45R− cμ−), intermediate pro-B cells (TdT+ CD45R+cμ−), late pro-B cells (TdT−CD45R+ cμ−), and pre-B cells (TdT− CD45R+ cμ+) in murine BM. TdT adds nontemplated nucleotides at junctions of gene segments during immunoglobulin heavy-chain gene rearrangement,11 12whereas cμ is the protein product of the productively rearranged immunoglobulin heavy-chain gene. This model is clearly indicative of differentiation within the B lineage and identifies a putative population of Lin− B-lymphocyte precursors. However, assessment of TdT and cμ expression requires cell fixation and permeabilization, precluding subsequent exploration of cellular function. Thus, the fate of TdT+ CD45R−cμ− early pro-B cells is unknown.
Hardy et al9 characterized pro-B cells by coexpression of CD45R and CD43. Populations presumed to be developmentally sequential pro-B subsets were resolved on the basis of successive expression of heat-stable antigen (HSA, CD24) and BP-1. In this model, Fraction A (CD45R+CD43+CD24−BP-1−) B-lineage precursors give rise to Fraction B (CD45R+CD43+CD24+BP-1−) and then Fraction C (CD45R+CD43+CD24+BP-1+).9 After a report that Fraction A contained cells expressing an NK cell marker,6 Hardy et al9 more extensively characterized this population and identified a possible CD45R− precursor to Fraction A that expressed AA4.1.13 From AA4.1+CD43+ CD24− BM, Hardy et al9resolved fraction A0(CD45R−CD4+), fraction A1(CD45R+CD4+), and fraction A2(CD45R+CD4−). These were postulated to represent sequential populations, primarily on the basis of progressive expression of transcripts for B-lineage–associated genes.13 However, the lineage potential of Fraction A0 and verification of the successive nature of these populations have not been reported.
Rolink et al14 designated B-lineage precursor populations on the basis of gene rearrangement and expression, partially enriching subsets using surface markers. In their model, CD45R−c-kitLo/+ precursors are thought to have immunoglobulin genes in germline configuration.15,16 These pro-B cells give rise to CD45R+ c-kit+CD25− pre-B I cells that initiate heavy-chain gene rearrangement. Further differentiation yields CD45R+c-kit− CD25+ pre-B II cells that rearrange light-chain genes. In this model, TdT is lost and cμ acquired as the cells enter the pre-B II stage.10,14,16,17However, CD45R− precursors isolated on the basis of c-kit included non–B-lineage (Mac-1+ Gr-1+, TER-119+, CD3+) cells, almost all of which lack c-kit.18 Such cells selectively diluted B-lineage precursor activity measured in the c-kit− subset.15For this reason, details are lacking about when c-kit is downregulated and how this corresponds to B-lineage commitment.
Lack of resolution of the earliest B-cell precursor has significantly hampered investigations of the regulatory processes influencing lineage commitment. Park and Osmond’s8 early studies describing a putative B-lineage precursor population (CD45R−TdT+) likely included such cells but, because assessment of TdT required fixation, their functional characteristics could not be examined in tissue culture.11 12 Therefore, our first goal was to isolate a population of viable BM cells enriched for those B-lymphoid precursors poised to enter the B lineage. Subsequent culture of those cells and concomitant analysis of murine BM showed that many B-lineage cells lose c-kit earlier than previously believed.
MATERIALS AND METHODS
Mice.
BALB/c mice were used for sorting Lin− cells and CD45R+ cells were isolated from C57/BL6 mice. Mice were of either sex and ranged in age from 5 to 15 weeks. Mice were obtained from either the Oklahoma Medical Research Foundation Laboratory Animal Resource Center (Oklahoma City, OK), Charles Rivers Breeding Laboratories (Wilmington, ME), or Taconic Farms (Germantown, NY). Animals not bred in our facility were maintained at least 1 week after shipping before experimentation.
Antibodies.
TER-119 and Gr-1 (RB6-8C5) purchased from PharMingen (San Diego, CA), and 10× concentrated anti–Mac-1 culture supernatants (M1/70 hybridoma obtained from American Type Culture Collection, Rockville, MD) were used for enrichment of Lin− cells and early CD45R+ pro-B cells. For enrichment of Lin− cells, 10× concentrated CD45R culture supernatant (14.8 hybridoma developed in our laboratory4) was also used. To sort Lin− subsets the following antibodies were used: Mac-1 fluorescein isothiocyanate (FITC) (M1/70) (Boehringer Mannheim Corp, Indianapolis, IN), CD3 phycoerythrin (PE) (29B) (GIBCO BRL, Gaithersburg, MD) or CD3 FITC (145-2C11), Gr-1 FITC (RB6-8C5), CD19 FITC (1D3), CD8 FITC or CD8 PE (53-6.7), TER-119 PE, CD45R PE (RA3-6B2), and biotinylated c-kit (2B8) shown by Streptavidin CyChrome (PharMingen). To sort early CD45R+ pro-B cells CD45R allophycocyanin (APC) or CD45R FITC (RA3-6B2), NK1.1 PE (PK136), BP-1 PE (6C3) (PharMingen), goat antimouse immunoglobulinM(IgM) PE (Southern Biotechnology Associates, Birmingham, AL), CD25 PE (PC61 5.3) (Caltag Laboratories, Burlingame, CA), and biotinylated CD43 (purified from S7 hybridoma supernatant and biotinylated in our laboratory using standard protocols, hybridoma purchased from American Type Culture Collection) were used. APC-labeled antibody to c-kit (2B8) purchased from PharMingen was added when sorting c-kit+ and c-kit− subsets. The following antibodies were used for surface staining of cultured cells: CD45R PE or CD45R APC (RA3-6B2), CD19 FITC, CD19 PE, or biotinylated CD19 (1D3), CD24 FITC (M1/69), BP-1 PE (6C3), c-kit PE, or c-kit APC (2B8) purchased from PharMingen and goat antimouse IgM purchased from Southern Biotechnology Associates. Biotinylated reagents were using either Streptavidin CyChrome (PharMingen) or Streptavidin-Red613 (GIBCO BRL). Polyclonal FITC-labeled goat antimouse IgM purchased from Zymed (San Francisco, CA) was used for cμ staining. For TdT staining, polyclonal rabbit anti-TdT and goat antirabbit FITC from Supertechs (Bethesda, MD) were used. Appropriate isotype control antibodies were used at the same concentration to set gates. Supernatant from the anti-Fc receptor hybridoma, 2.4G2, (American Type Culture Collection) was used to reduce nonspecific staining of cultured cells.
Cell sorting.
BM was harvested and suspended in staining wash (phosphate-buffered saline without Ca2+ or Mg2+[PBS−] containing 3% heat-inactivated fetal bovine serum [FBS]). For sorts of Lin− subsets and early CD45R+ pro-B cells, whole BM was enriched for desired populations by immunomagnetic bead depletion. In short, BM was incubated with purified monoclonal antibodies on ice for 25 minutes, washed two times, and incubated with goat antirat Ig-coated magnetic beads (Perseptive Biosystems, Framingham, MA) for 25 minutes at 4°C on a rocking table. Beads were removed from cell-bead suspensions by four rounds of incubation with a magnetic separator. Enriched BM suspensions were then incubated simultaneously with primary antibodies for 25 minutes, washed two times, incubated with secondary-labeled Streptavidin for 25 minutes (to detect the biotinylated antibody), washed once, and resuspended in staining wash. CD45R primary antibodies were added along with secondary antibodies for Lin−sorts to insure that there was little opportunity for capping and shedding of this surface marker. Cells were kept on ice until sorted on the FACStarPlus (Becton Dickinson, San Diego, CA) cell sorter and samples were kept chilled during sorting.
Immunofluorescence staining.
Cells in stromal cell cocultures were harvested with 2 mmol/L EDTA in PBS−. After harvesting, wells were visually examined for complete removal of adherent cells. Cells in stromal-free cultures were harvested by washing wells with medium. For surface staining, harvested cells were incubated with 50% 10× concentrated 2.4G2 hybridoma supernatant in 4% rat serum for 15 minutes before staining to prevent nonspecific binding of antibodies. Cells were then incubated with primary antibodies simultaneously for 20 minutes, washed two times, incubated with secondary antibody for 20 minutes, washed once, and resuspended in staining wash. Intranuclear staining for TdT and cμ was performed as described by Melchers et al.14 For TdT staining, whole BM or sorted cells were first stained for surface markers as described above and then fixed with 1% paraformaldehyde in PBS− (pH 7.4) for 5 minutes on ice, or 0.25% paraformaldehyde in PBS− (pH 7.4) overnight. Fixed cells were permeabilized by incubating with 70% methanol for 30 minutes on ice, and then incubated with goat serum to prevent nonspecific antibody binding. Cells were washed once and then incubated with anti-TdT for 45 minutes to 1 hour at room temperature, washed twice, incubated with secondary antibody for 30 minutes on ice, washed twice, and resuspended in staining wash for flow cytometric analysis. Preliminary experiments showed that permeabilization did not affect surface-marker staining, except in the case of APC fluorochrome-labeled antibodies. Consequently, PE- or FITC-labeled antibodies were used for this purpose. For cμ staining, cells were harvested and stained for surface IgM as described above and fixed with 4% paraformaldehyde in PBS− (pH 7.4) for 10 minutes on ice, or 0.25% paraformaldehyde in PBS− (pH 7.4) overnight. Cells were then permeabilized by incubating with PBS−containing 0.2% Tween 20 at room temperature for 20 minutes, labeled by incubating with FITC-labeled anti-IgM at room temperature for 30 minutes, washed two times, and resuspended in staining wash. Gates for surface, intracellular, and intranuclear staining were set with appropriate isotype controls. After staining, all samples were kept on ice until analyzed on the FACScan, FACSCalibur, or FACStarPlus flow cytometer (Becton Dickinson). Forward and side scatter were used to gate out larger stromal cells in samples containing cells grown in stromal cell cocultures.
Cell culture and clonal assays.
All liquid cell cultures used Optimem medium (GIBCO BRL) containing 2.5% (single-cell sorts of Lin− cells) or 1.5% (all other cultures) FBS (HyClone, Logan, UT), 2 mmol/L glutamine, 5 × 10−5 mol/L 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin. Stromal cells were plated 1 or 2 days before initiation of stromal cell cocultures at 5,000 cells per well in 24-well plates for bulk cultures and at 500 cells per well in 96-well plates for clonal assays of single sorted cells. Lin−cells were plated on S17 stromal cells (a generous gift from Dr K. Dorshkind) and CD45R+ cells were plated on ST2 stromal cells (a generous gift from Dr S-I. Nishikawa) and supplemented with IL-7 (Endogen, Woburn, MA) at 1 ng/mL. T-25 flasks with a subconfluent layer (∼50% confluency) of ST2 stromal cells were used to assess the ability of c-kit+ and c-kit− early CD45R+ pro-B cells to generate sIgM+ cells. These cultures were not supplemented with IL-7 because removal of IL-7 has been reported to promote differentiation to the sIgM+state.19 Stromal cell free cultures contained 50% ST2 stromal cell conditioned medium and were supplemented with flt ligand at 100 ng/mL, stem cell factor at 20 ng/mL (R & D Systems, Minneapolis, MN) and IL-7 at 1 ng/mL. Liquid cultures were fed every 3 to 4 days.
Semi-solid agar assays were performed as follows. Sorted c-kit+ or c-kit− early CD45R+pro-B cells were plated at 4.3 × 103 or 1 × 104 cells per mL in colony-forming unit (CFU) medium consisting of McCoy’s Modified 5A medium containing 15% FBS, 2 mmol/L L-glutamine, 100 U penicillin/mL, 100 μg streptomycin/mL and 5 × 10−5 mol/L 2-ME, supplemented with 1 mmol/L sodium pyruvate solution, 1.5% sodium bicarbonate solution (7.5%), 0.8% minimal essential medium (MEM) essential amino acids (50×), 0.4% MEM nonessential amino acids (100×), and 1.6% MEM vitamin solution. Medium was warmed to 37°C, cells were added to medium and then mixed with a 1/10 volume of boiled 3% bacto agar (Difco, Ann Arbor, MI) in water, which had cooled to approximately 40°C. Then 1 mL aliquots of medium were quickly plated in 35-mm tissue culture dishes and allowed to gel for about 20 minutes at room temperature. After 6 days of incubation at 37°C in a fully humidified atmosphere of 5% CO2 in air, colonies (aggregates of greater than 30 cells) were counted using a dissecting microscope.
RESULTS
Expression of TdT corresponds to reduced c-kit on Lin− precursors.
We have devised a strategy for sorting viable early B-lineage precursors on the brink of CD45R acquisition. This strategy permits functional analysis of sorted precursors in cell culture and accordingly, we have followed the fate of these cells during in vitro lineage commitment. Enrichment for CD45R− B-lymphoid precursors was accomplished by sorting Lin− cells whose surface c-kit levels best correlated with TdT expression.
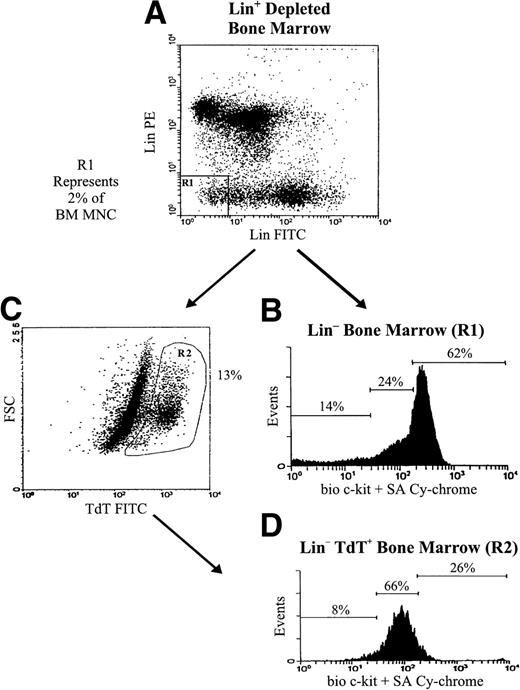
We sorted Lin− (Mac-1−, Gr-1−, Ter-119−,CD45R−, CD19−, CD8−, CD3−) cells, thereby removing myeloid, erythroid, and lymphoid lineage cells that make up about 98% of BM mononuclear cells (BM MNC). Figure 1 shows the analysis of this population when costained for surface c-kit and intranuclear TdT. Lin− BM was divided into c-kitHi, c-kitLo, and c-kit− populations (Fig 1B) that made up 1.4%, 0.56%, and 0.33%, respectively, of BM MNC (Table 1). Gating on TdT+ cells within Lin− BM (Fig 1C) showed that the majority of Lin− TdT+ cells were c-kitLo(Fig 1D). Indeed, TdT+ cells made up 35% of the Lin−c-kitLo population, giving an estimated 116-fold enrichment for Lin−TdT+ cells when compared with BM MNC (Table 1). A smaller fraction of Lin−c-kit− cells were TdT+ (7.4%). TdT+ cells comprised 5.7% of the Lin−c-kitHi population, and were among cells with the lowest c-kit expression within the subset (Fig 1D, and Table 1). Lin−c-kit− and Lin−c-kitHi subsets had 24-fold and 19-fold enrichments, respectively, for Lin−TdT+ cells when compared with BM MNC (Table 1). Thus, expression of TdT in the Lin− population corresponded to reduced surface c-kit with the majority of Lin− TdT+ cells being c-kitLo.
Surface c-kit expression identifies Lin− BM populations enriched for TdT+ cells. Lin−BM, representing approximately 2% of BM mononuclear cells, was sorted from whole BM depleted of Lin+ cells by magnetic separation (A), and costained for intranuclear TdT and surface c-kit as described in Materials and Methods. Within costained Lin−BM, c-kit−, c-kitLo, and c-kitHipopulations were identified (B). Lin− BM was gated for TdT+ cells (C). (D) shows expression of c-kit in gated Lin− TdT+ cells. The frequencies of these subsets in BM are shown in Table 1.
Surface c-kit expression identifies Lin− BM populations enriched for TdT+ cells. Lin−BM, representing approximately 2% of BM mononuclear cells, was sorted from whole BM depleted of Lin+ cells by magnetic separation (A), and costained for intranuclear TdT and surface c-kit as described in Materials and Methods. Within costained Lin−BM, c-kit−, c-kitLo, and c-kitHipopulations were identified (B). Lin− BM was gated for TdT+ cells (C). (D) shows expression of c-kit in gated Lin− TdT+ cells. The frequencies of these subsets in BM are shown in Table 1.
Frequencies of Lin− Populations in Bone Marrow
| Sorted Population* . | Frequency (% of BM MNC) . | Frequency of TdT+ Cells (% of BM MNC) . | TdT+ Cells in Sorted Population (%) . | Fold Enrichment for Lin−TdT+ Cells . |
|---|---|---|---|---|
| Lin− c-kitHi | 1.40 ± .09 | 0.080 | 5.7 ± 1.3 | 19 |
| Lin− c-kitLo | 0.56 ± .09 | 0.200 | 35.4 ± 5.1 | 116 |
| Lin− c-kit− | 0.33 ± .03 | 0.024 | 7.4 ± 1.2 | 24 |
| Sorted Population* . | Frequency (% of BM MNC) . | Frequency of TdT+ Cells (% of BM MNC) . | TdT+ Cells in Sorted Population (%) . | Fold Enrichment for Lin−TdT+ Cells . |
|---|---|---|---|---|
| Lin− c-kitHi | 1.40 ± .09 | 0.080 | 5.7 ± 1.3 | 19 |
| Lin− c-kitLo | 0.56 ± .09 | 0.200 | 35.4 ± 5.1 | 116 |
| Lin− c-kit− | 0.33 ± .03 | 0.024 | 7.4 ± 1.2 | 24 |
Lin− cells were stained, gated, and sorted as shown in Fig 1. Bone marrow frequencies of Lin−c-kit+, Lin− c-kitLo, and Lin− c-kit− cells reflect means from three or four independent experiments with marrow pooled from 9 or 10 mice. Frequencies of TdT+ populations within bone marrow were calculated from percentages of TdT+ cells within the indicated subsets. Fold enrichment of Lin−TdT+ cells within each subset is relative to the frequency among bone marrow mononuclear cells (0.304%).
Lin−c-kit− and Lin−c-kitLo subsets generate B-lineage cells in overnight culture.
The data presented in Fig 1 suggested that the B-lineage precursors generated from Lin−c-kitHi stem cells might begin to downregulate c-kit as they acquire TdT. To determine which of our precursor populations was able to generate B-lineage cells most rapidly, we looked for appearance of CD45R+ and CD19+ cells after intervals of culture. Though acquired before CD19,20 CD45R is a less exclusive B-lineage marker than CD19.6 Stromal cell free cultures were used to ensure that stromal cell debris was not inadvertently included in lymphocyte light-scatter gates and to preclude the possibility that stromal adherent B-lineage cells might be excluded from analysis.
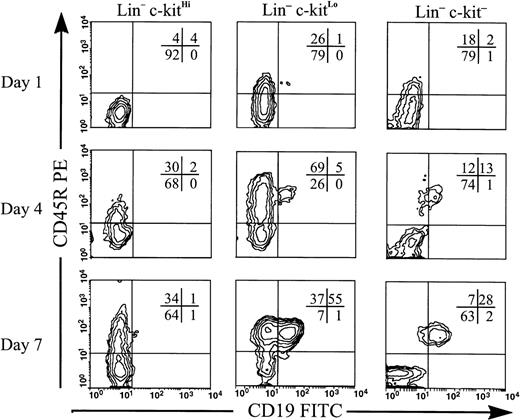
After overnight culture, Lin−c-kitLo and Lin−c-kit−, but not Lin−c-kitHi precursors, generated cells that expressed CD45R at low levels (Fig 2). By day 4, progeny from both Lin−c-kit− and Lin−c-kitLo BM subsets included cells that expressed only CD45R and others that coexpressed CD45R and CD19. By day 7, a majority of these B-lineage cells coexpressed CD45R and CD19. A substantial population of cells in Lin−c-kitHi cultures began to express CD45R by day 4, but few double-positive cells were observed even by day 7.
Lin−c-kitLo and Lin−c-kit− cells generate B-lineage cells in overnight culture. Lin−c-kitHi, Lin−c-kitLo, and Lin−c-kit− cells were sorted from BM and placed in stromal cell free culture containing IL-7, FL, SCF, and stromal cell conditioned medium. Cells were harvested at days 1, 4, and 7. Costaining is shown for cells within lymphocyte light scatter and percentages of cells in each quadrant are given. C-kitHiand c-kitLo cultures were initiated with equal numbers of cells, but due to low frequency, half as many cells were used to initiate c-kit− cultures. Representative data are shown from one of two independent experiments using pooled marrow from 10 mice.
Lin−c-kitLo and Lin−c-kit− cells generate B-lineage cells in overnight culture. Lin−c-kitHi, Lin−c-kitLo, and Lin−c-kit− cells were sorted from BM and placed in stromal cell free culture containing IL-7, FL, SCF, and stromal cell conditioned medium. Cells were harvested at days 1, 4, and 7. Costaining is shown for cells within lymphocyte light scatter and percentages of cells in each quadrant are given. C-kitHiand c-kitLo cultures were initiated with equal numbers of cells, but due to low frequency, half as many cells were used to initiate c-kit− cultures. Representative data are shown from one of two independent experiments using pooled marrow from 10 mice.
Thus, we found that Lin− cells that are c-kitLo and c-kit− include B-lineage precursors that acquire CD45R after overnight culture. Lin−c-kitHi cells were able to generate the B lineage but required more time to do so. In addition, heterogeneity with respect to CD19 expression on CD45R+cells was observed in progeny of both Lin−c-kitLo and Lin−c-kit− populations (Fig 2). This heterogeneity was most noticeable in the Lin−c-kitLo cultures in which B-lineage cells from day 7 retained a distinct CD19− population that was not evident in day-7 Lin−c-kit− cultures.
Reduction of c-kit in Lin− precursor populations corresponds to increasing maturity in B-lineage progeny.
The above studies suggested that cells poised to enter the B lineage were abundant among Lin−c-kitLo and Lin−c-kit− cells but not the Lin−c-kitHi population. The c-kitLo precursor population included some cells less mature than those that were c-kit−. However, it was not clear if a progressive loss of c-kit corresponded to increased maturity in Lin− B-lymphocyte precursors. We reasoned that the maturity of B-lineage cells produced from cultures in a finite period of time would reflect the maturity of precursors in each starting population. To determine the maturity of culture-generated B-lineage cells, we examined expression of CD24 and BP-1, markers identified by Hardy et al9 as being successively acquired by CD45R+ CD43+ pro-B cells during differentiation.
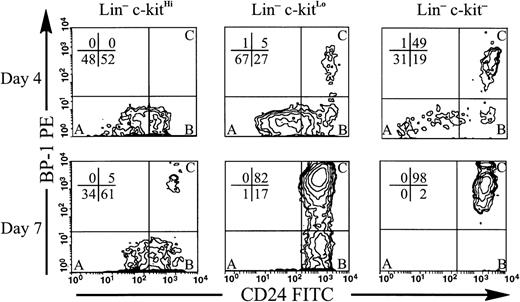
Lin−c-kitLo and Lin−c-kit− subsets generated B-lineage cells that were more mature than those from cultures initiated with Lin−c-kitHi cells. In a representative experiment shown in Fig 3, all B-lineage cells generated from day-4 Lin−c-kitHi cultures expressed a Fraction A (CD24− BP-1−) or Fraction B (CD24+ BP-1−) surface phenotype. In contrast, the majority of those in the Lin−c-kit− cultures, and a smaller population in Lin−c-kitLo cultures, had acquired BP-1, placing them in Fraction C. By day 7, the majority of B-lineage progeny from both Lin−c-kit− and Lin−c-kitLo cultures had reached Fraction C. However, almost all of those from Lin−c-kitHi precursors were still BP-1−. Furthermore, some of the B-lineage progeny generated in Lin−c-kitLo, and more of those in Lin−c-kit− cultures, had lost CD43 expression by day 7, whereas all of those within Lin−c-kitHi cultures retained this marker (data not shown). Thus, changing patterns of CD24, BP-1, and CD43, as well as CD45R and CD19, in cultured cells, show that Lin−c-kitLo and Lin−c-kit− precursors give rise to more mature B-lineage progeny than Lin−c-kitHi cells.
Reduced expression of c-kit by precursor populations corresponds to increasing maturity of B-lineage progeny . Lin−c-kitHi, Lin−c-kitLo, and Lin−c-kit− cells were sorted from BM and placed in stromal cell free culture containing IL-7, FL, SCF, and stromal cell conditioned medium. Cells were harvested at days 4 and 7. Costaining is shown for CD45R+ CD43+ cells within lymphocyte light scatter. The lower left quadrant contains cells in Fraction A, the lower right quadrant cells in Fraction B, and the upper right quadrant cells in Fraction C as identified by Hardy et al.9 Percentages of cells in each quadrant are shown. C-kitHi and c-kitLo cultures were initiated with equal numbers of cells, but because of low frequency, half as many cells were used to initiate c-kit− cultures. Representative data are shown from one of two independent experiments using pooled marrow from 10 mice. Similar trends in maturity were observed in numerous experiments with cells harvested at time points from day 2 to 7.
Reduced expression of c-kit by precursor populations corresponds to increasing maturity of B-lineage progeny . Lin−c-kitHi, Lin−c-kitLo, and Lin−c-kit− cells were sorted from BM and placed in stromal cell free culture containing IL-7, FL, SCF, and stromal cell conditioned medium. Cells were harvested at days 4 and 7. Costaining is shown for CD45R+ CD43+ cells within lymphocyte light scatter. The lower left quadrant contains cells in Fraction A, the lower right quadrant cells in Fraction B, and the upper right quadrant cells in Fraction C as identified by Hardy et al.9 Percentages of cells in each quadrant are shown. C-kitHi and c-kitLo cultures were initiated with equal numbers of cells, but because of low frequency, half as many cells were used to initiate c-kit− cultures. Representative data are shown from one of two independent experiments using pooled marrow from 10 mice. Similar trends in maturity were observed in numerous experiments with cells harvested at time points from day 2 to 7.
If a uniform cohort of precursors representing the same degree of lineage commitment and maturity were present in any of our three starting populations, it might be expected to move synchronously through subsequent differentiation stages in culture. The Lin−c-kit− subset was the most homogeneous in this respect, with a majority of B-lineage progeny reaching the most mature phenotype more quickly than in the other precursor populations. Yet, even in this population, some cells took longer than others to acquire CD19 (Fig 2) or reach the Fraction C stage (Fig 3). Lin−c-kitHi cultures produced the most immature B-lineage cells at all time points. However, as shown in Fig 3, these precursors included both Fraction A and Fraction B cells at days 4 and 7. In some experiments, a small population of Fraction C cells were also present in day-7 cultures initiated with these cells (Fig 3). The Lin−c-kitLo population was the most heterogeneous with respect to maturity. At both day 4 and day 7 some B-lineage progeny from Lin−c-kitLocultures clearly overlapped those from the less mature Lin−c-kitHi and the more mature Lin−c-kit− precursors. However, many B-lineage cells generated in Lin−c-kitLo cultures seemed to represent intermediates in maturity. These cells only reached Fraction C by day 7, unlike the progeny of Lin−c-kit−cells. Furthermore, they had not progressed beyond Fraction A or Fraction B by day 4. These data reflect a trend in maturity observed in numerous experiments in which we assessed expression of CD19 and BP-1 at time points from days 2 to 7 (data not shown).
Therefore, although all precursor populations exhibited heterogeneity, the majority of B-lineage cells produced in Lin−c-kit− cultures reached the more mature phenotype sooner than those generated in Lin−c-kitLo or Lin−c-kitHi cultures. Likewise, the majority of B-lineage progeny from Lin−c-kitLo cultures attained the more mature phenotype before those produced by Lin−c-kitHi cultures. This pattern is generally consistent with a progressive loss of c-kit corresponding to increased maturity in Lin− B-lineage precursors, though downregulation of c-kit is not precisely synchronized with degree of maturity.
Reduction of c-kit in precursor populations corresponds to increased lymphoid-lineage restriction.
During the progression from stem cell to B lineage, there is a progressive loss of the potential to generate the other hematopoietic cells. The above studies suggested that heterogeneity in maturity existed in each of our Lin− B-lineage precursor populations. As a measure of the relative distribution of more and less mature B-lineage precursors, we examined the frequencies of bipotential (lymphoid/myeloid) and lymphoid-restricted cells within our precursor populations.
The Lin−c-kitHi population, which is enriched for stem cells and multipotential progenitors, has been shown to generate B-lineage cells in bulk coculture with stromal cells and IL-7.21 We found that these cultures also supported differentiation of myeloid cells. Single cells from each of our Lin− precursor populations were sorted directly onto stromal cells supplemented with IL-7 and assessed for growth at 10 to 13 days. Randomly selected clones were stained for expression of CD45R, CD19, and Mac-1. This allowed determination of cloning frequencies for Lin− precursors capable of generating only B-lineage (CD45R+ CD19+) cells, only myeloid-lineage (Mac-1+) cells, or cells of both lineages. Results from these experiments are shown in Table 2.
Cloning Frequencies of B-Lineage Precursor Populations
| Sorted Population* . | Wells Plated . | Clones Assessed for Lineage Potential . | Cloning Frequency (% of clones with phenotype) . | ||||
|---|---|---|---|---|---|---|---|
| Total . | Total B Lineage . | B Only . | B + M Bi-potential . | M Only . | |||
| Lin− c-kitHi | 560 | 92 | 1/4.7 | 1/100 | 1/166 | 1/250 | 1/5.8 |
| (3.3%) | (2.2%) | (94.5%) | |||||
| Lin− c-kitLo | 560 | 158 | 1/3.5 | 1/17 | 1/35 | 1/33 | 1/4.5 |
| (10.1%) | (10.8%) | (79.1%) | |||||
| Lin− c-kit− | 554 | 131 | 1/3.1 | 1/34 | 1/41 | 1/210 | 1/3.4 |
| (7.6%) | (1.5%) | (90.9%) | |||||
| Sorted Population* . | Wells Plated . | Clones Assessed for Lineage Potential . | Cloning Frequency (% of clones with phenotype) . | ||||
|---|---|---|---|---|---|---|---|
| Total . | Total B Lineage . | B Only . | B + M Bi-potential . | M Only . | |||
| Lin− c-kitHi | 560 | 92 | 1/4.7 | 1/100 | 1/166 | 1/250 | 1/5.8 |
| (3.3%) | (2.2%) | (94.5%) | |||||
| Lin− c-kitLo | 560 | 158 | 1/3.5 | 1/17 | 1/35 | 1/33 | 1/4.5 |
| (10.1%) | (10.8%) | (79.1%) | |||||
| Lin− c-kit− | 554 | 131 | 1/3.1 | 1/34 | 1/41 | 1/210 | 1/3.4 |
| (7.6%) | (1.5%) | (90.9%) | |||||
Single Lin− c-kitHi, Lin−c-kitLo, and Lin− c-kit− cells were sorted directly onto S17 stromal cells along with IL-7. Randomly selected clones were harvested after 10 to 13 days and assessed by flow cytometry. Clones were assessed for CD45R, CD19, and Mac-1 (CD11b). Clones contained only CD45R+ CD19+ B-lineage cells, Mac-1+ cells, or cells of both types. Total cloning frequencies reflect wells with growth relative to total wells plated. These numbers were multiplied by flow cytometry results for the analyzed clones to yield cloning frequencies for each type of progenitor. Results were combined from two independent experiments using marrow pooled from three mice.
The three precursor populations had similar total cloning frequencies (ranging from 1/3.1 to 1/4.7). Cells capable of giving rise to the B lineage, including lymphoid-only cells and bipotential B-lineage precursors, were more frequent in the Lin−c-kitLo population (1/17) than in the Lin−c-kit− subset (1/34) or the Lin−c-kitHi subset (1/100). Among clonable Lin−c-kitLo B-lineage precursors, bipotential cells and lymphoid-only cells were equally common (each type of precursor represented 10% of clonable cells). In contrast, clonable Lin−c-kit−B-lineage precursors were much more frequently lymphoid-only (7.6% of cells that cloned) than bipotential (1.5% of cells that cloned). Both types of B-lineage precursors were rare in the Lin−c-kitHi population, though Lin−c-kitHi BM generated B-lineage cells readily in bulk cultures (Fig 2). Myeloid-only precursors were common in all three subsets, though their frequency was highest in Lin−c-kitHi cells.
These data showed that loss of c-kit corresponds with increasing lymphoid restriction in Lin− B-lineage precursors. Although more mature lymphoid-committed cells represented about half of the Lin−c-kitLo B-lineage precursors, they comprised most of those that were Lin−c-kit−. This was consistent with our observations that precursors giving rise to the most mature B-lineage progeny in a finite culture period were concentrated in the Lin−c-kit− subset (Fig 2 and 3). Taken together, our data suggest a model of B-lineage differentiation in which TdT expression begins as c-kit is downregulated in Lin−c-kitHi cells, with progressive loss of c-kit corresponding to increasing maturity and lymphoid commitment (see Fig 7 below).
Many of the earliest CD45R+ cells generated in culture lack c-kit.
B-lineage precursors form an expanding pool of cells within BM and we would expect to see a much larger population of c-kit− TdT+ precursors than those found in the Lin− category. Both the distribution of TdT+ cells (Table 1) and the frequencies of lymphoid-committed cells (Table 2) suggest that the majority of CD45R− lymphoid-committed B-lineage precursors are contained within the Lin−c-kitLo subset of BM. Because both the Lin−c-kitLo and Lin−c-kit− populations contained B-lineage precursors on the verge of acquiring CD45R, we wondered if acquisition of CD45R might be occurring at the transition from c-kitLo to c-kit−. If this were the case, then most of the earliest lymphoid-committed, c-kit−B-lineage precursors would have been eliminated from our stringently gated Lin− population because they had begun to express CD45R.
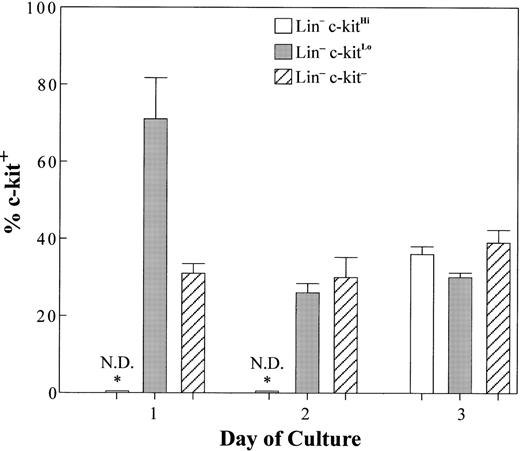
Expression of c-kit has been thought to be a feature of early B-lineage precursors and pro-B cells.10 14-17 However, we showed that progressive loss of c-kit corresponded to increased maturity (Fig 3) and lymphoid commitment (Table 2) in Lin− B-lineage precursors. This raised the interesting question of whether c-kit is lost from many B-lineage cells before or during CD45R acquisition, much earlier than previously believed. Therefore, we examined c-kit on early CD45R+ cells generated in culture from Lin− precursor populations (Fig 4). Lin−c-kitHi cultures did not generate CD45R+ cells until day 3 of culture and all of them were CD19− (Fig 2 and data not shown). About two thirds of these cells totally lacked c-kit (Fig 4) and the remainder expressed the marker at low levels. Thus, the majority of newly generated B-lineage cells from cultures initiated with very early Lin−c-kitHi precursors no longer expressed c-kit.
Many newly generated CD45R+ cells lack c-kit . Lin−c-kitHi, Lin−c-kitLo, and Lin−c-kit− cells were sorted from BM and placed in stromal cell free culture containing IL-7, FL, SCF, and stromal cell conditioned medium. Cells were harvested at days 1 to 3 and percentages of CD45R+ CD19− cells expressing c-kit at each time point generated from each starting population are represented in the bar graph. (CD45R+cells were found to express c-kit at low levels.) Data reflect means±SE from three independent experiments with pooled marrow from 8 or 10 mice. The asterisks indicate that no B-lineage cells were generated before day 3 in cultures initiated with c-kit+cells.
Many newly generated CD45R+ cells lack c-kit . Lin−c-kitHi, Lin−c-kitLo, and Lin−c-kit− cells were sorted from BM and placed in stromal cell free culture containing IL-7, FL, SCF, and stromal cell conditioned medium. Cells were harvested at days 1 to 3 and percentages of CD45R+ CD19− cells expressing c-kit at each time point generated from each starting population are represented in the bar graph. (CD45R+cells were found to express c-kit at low levels.) Data reflect means±SE from three independent experiments with pooled marrow from 8 or 10 mice. The asterisks indicate that no B-lineage cells were generated before day 3 in cultures initiated with c-kit+cells.
Cultures initiated with Lin− cells that were c-kit− or c-kitLo produced CD45R+ CD19− cells by day 1 (Fig 2) and CD45R+ CD19+ cells by day 2 (data not shown). However, more of the cells in Lin−c-kit− than in Lin−c-kitLo cultures coexpressed CD19 at each time point (Fig 2 and data not shown). Of those CD45R+CD19− progeny generated at day 1, approximately one third from Lin−c-kitLo and two thirds from Lin−c-kit− precursors completely lacked c-kit (Fig 2). At time points beyond day 1, we gated on CD45R+ CD19− cells to insure that we were assessing the earliest B-lineage cells and found that approximately two thirds of them were c-kit− (Fig 4) . The more mature CD45R+ CD19+ populations were similar in this respect (data not shown). It is interesting to note that a third of the B-lineage progeny of Lin−c-kit− precursors expressed c-kit at low levels (Fig 4), apparently reacquiring this marker along with CD45R.
In summary, two thirds of the earliest CD45R+CD19− B-lineage cells generated in culture had already lost, or were losing, c-kit as they acquired CD45R, whereas the remaining third expressed c-kit at low levels. A similar pattern of c-kit expression was observed on the more mature CD45R+CD19+ B-lineage precursors. Thus, it appears that many B-lineage precursors acquired CD45R during the transition from c-kitLo to c-kit−. However, these surface marker changes were not perfectly synchronized; some cells lost c-kit before CD45R was acquired and others afterwards. A fraction of B-lineage cells generated from Lin−c-kit− cultures reacquired c-kit, raising questions about modulation of this marker in the pro-B compartment.
The majority of early CD45R+ pro-B cells in murine BM are c-kit−.
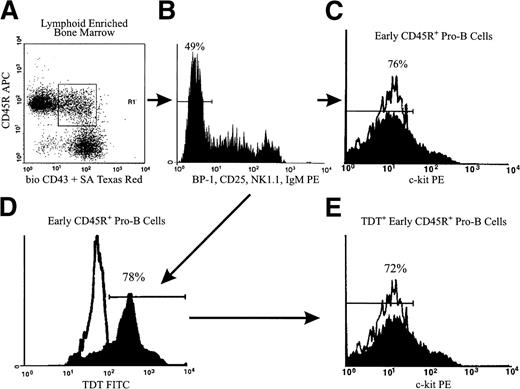
After determining that most early CD45R+ cells generated in culture were c-kit−, we wanted to see if this was also the case for comparable cells in marrow. We isolated the earliest CD45R+ pro-B cells that had not progressed to Hardy’s Fraction C or Melchers’ Pre-B II, while excluding NK precursors or NK cells that expressed CD45R. To achieve this, we sorted CD45R+ CD43+ cells that lacked expression of BP-1, CD25, NK1.1, and surface IgM (Fig5A,B). We then assessed expression of c-kit in this population that we refer to as early CD45R+ pro-B cells. Over three fourths of these cells lacked c-kit, whereas the remainder expressed the marker at low levels (Fig 5C and Table 3). This parallels the pattern observed in early B-lineage cells produced in culture (Fig 4).
The majority of early CD45R+ pro-B cells in BM are c-kit−. The boxed region of Panel A shows CD45R+CD43+ cells in lymphoid-enriched BM, from which BP-1− CD25− NK1.1−sIgM− (B) cells were sorted. These gating criteria allowed isolation of early CD45R+ pro-B cells that were subsequently stained for surface c-kit, fixed, permeabilized, and stained for intranuclear TdT. Expression of c-kit (C) and TdT (D) in this costained population is shown. Panel E shows c-kit expression in the gated TdT+ cells within CD45R+CD43+ BP-1− CD25−NK1.1− sIgM− cells. The frequencies of these subsets in BM are shown in Table 3. Means are from two independent experiments performed on pooled marrow from five or seven mice.
The majority of early CD45R+ pro-B cells in BM are c-kit−. The boxed region of Panel A shows CD45R+CD43+ cells in lymphoid-enriched BM, from which BP-1− CD25− NK1.1−sIgM− (B) cells were sorted. These gating criteria allowed isolation of early CD45R+ pro-B cells that were subsequently stained for surface c-kit, fixed, permeabilized, and stained for intranuclear TdT. Expression of c-kit (C) and TdT (D) in this costained population is shown. Panel E shows c-kit expression in the gated TdT+ cells within CD45R+CD43+ BP-1− CD25−NK1.1− sIgM− cells. The frequencies of these subsets in BM are shown in Table 3. Means are from two independent experiments performed on pooled marrow from five or seven mice.
Frequencies of Early CD45R+ Pro-B Subsets in Bone Marrow
| Population3-150 . | % of Early CD45+ Pro-B Cells . | Frequency (% of BM MNC) . |
|---|---|---|
| Pro-B (CD45R+CD43+) | — | 4.5 |
| Early CD45R+ Pro-B (CD45R+ CD43+BP-1− CD25− sIgM−) | 100 | 2.2 |
| Early CD45R+ Pro-B subsets | ||
| c-kit+ | 24 | 0.53 |
| c-kit− | 76 | 1.7 |
| TdT+ | 78 | 1.7 |
| TdT+ c-kit+ | 22 | 0.48 |
| TdT+ c-kit− | 56 | 1.2 |
| Population3-150 . | % of Early CD45+ Pro-B Cells . | Frequency (% of BM MNC) . |
|---|---|---|
| Pro-B (CD45R+CD43+) | — | 4.5 |
| Early CD45R+ Pro-B (CD45R+ CD43+BP-1− CD25− sIgM−) | 100 | 2.2 |
| Early CD45R+ Pro-B subsets | ||
| c-kit+ | 24 | 0.53 |
| c-kit− | 76 | 1.7 |
| TdT+ | 78 | 1.7 |
| TdT+ c-kit+ | 22 | 0.48 |
| TdT+ c-kit− | 56 | 1.2 |
CD45R+CD43+ BP-1−CD25−NK1.1− sIgM− cells (early CD45R+ pro-B cells) were sorted, stained, gated, and assessed for marker expression as shown in Fig 6. Frequencies of subsets were calculated from the frequency of CD45R+CD43+ pro-B cells among bone marrow mononuclear cells (4.5%) with 49% being early CD45R+ pro-B cells.
Park and Osmond8,22,23 proposed that TdT is an intranuclear marker of the earliest pro-B cells. Examining TdT+ cells that express the early CD45R+ pro-B cell-surface phenotype provides another way of investigating c-kit expression. We sorted early CD45R+ pro-B cells and then costained for TdT and c-kit. About three fourths of early CD45R+ pro-B cells expressed TdT (Fig 5D and Table 3). We gated on the TdT+cells within the population and found that over two thirds were c-kit− (Fig 5E and Table 3). Therefore, c-kit− cells comprised the majority of the TdT+ early CD45R+ pro-B population.
Of TdT+ early pro-B cells, c-kit+ and c-kit− cells formed 0.48% and 1.2%, respectively, of BM MNC (Table 3). When Lin− TdT+ cells (Table 1) and early CD45R+ TdT+ pro-B cells (Table 3) were combined, we found that cells expressing c-kit (including c-kitHi and c-kitLo cells) represented 0.76% whereas c-kit− cells represented 1.2% of BM MNC. Thus, the c-kit− population of early B-lineage cells is larger, as predicted by the progressive loss of c-kit with increased maturity in B-lineage precursors.
Both c-kit− and c-kit+ cells contribute to expansion of the pro-B compartment and give rise to B lymphocytes.
A substantial minority of early CD45R+ pro-B cells either retained or reacquired c-kit. It is therefore important to ask if this receptor conferred any special growth or differentiation advantage. We examined the role of c-kit+ and c-kit−early pro-B cells in expansion of the pro-B compartment by looking at the ability of these cells to proliferate in culture. Single c-kit+ or c-kit− early CD45R+pro-B cells were sorted directly onto stromal cells supplemented with IL-7. The average cloning frequency of c-kit+ cells (1/4) was higher in this assay than that of c-kit− cells (1/21) (Table 4). Using the frequencies of c-kit+ and c-kit− cells in the early CD45R+ pro-B compartment of BM MNC (Table 3), we calculated that the frequency of clonable c-kit+ cells was about one and one half times that of c-kit− cells (Table 4).
c-kit+ and c-kit− Early CD45R+ Pro-B Cells Proliferate in Response to IL-7
| Subset of Early CD45R+ Pro-B Cells4-150 . | Single-Cell Sort on Stroma With IL-7 . | Semi-Solid Agar With IL-7 Only . | ||||
|---|---|---|---|---|---|---|
| Wells Plated . | Cloning Frequency . | Frequency of Clonable Precursors (% of BM MNC) . | CFU IL-7 (colonies per 105 cells) . | Cloning Frequency . | Frequency of Clonable Precursors (% of BM MNC) . | |
| c-kit+ | 768 | 1/4 | 0.13 | 2072 ± 300 | 1/48 | 0.011 |
| c-kit− | 768 | 1/21 | 0.08 | 1121 ± 178 | 1/89 | 0.019 |
| Subset of Early CD45R+ Pro-B Cells4-150 . | Single-Cell Sort on Stroma With IL-7 . | Semi-Solid Agar With IL-7 Only . | ||||
|---|---|---|---|---|---|---|
| Wells Plated . | Cloning Frequency . | Frequency of Clonable Precursors (% of BM MNC) . | CFU IL-7 (colonies per 105 cells) . | Cloning Frequency . | Frequency of Clonable Precursors (% of BM MNC) . | |
| c-kit+ | 768 | 1/4 | 0.13 | 2072 ± 300 | 1/48 | 0.011 |
| c-kit− | 768 | 1/21 | 0.08 | 1121 ± 178 | 1/89 | 0.019 |
CD45R+ CD43+ BP-1−CD25− NK1.1− sIgM− cells (early CD45R+ pro-B cells) were sorted into c-kit+ and c-kit− subsets and assessed for proliferation potential on ST2 stromal cells (8 to 10 days) or in semisolid agar cultures (day 6). Data shown reflect combined results from two independent experiments performed on pooled marrow from five mice. Triplicate or six replicates of semi-solid agar cultures were plated in each experiment. Frequencies of clonable precursors in bone marrow were calculated from the incidences of each subset (Table 3).
In semi-solid agar assays, in which only IL-7 was present, the cloning frequency of the c-kit+ early CD45R+ pro-B population (1/48) was only about twice that of the c-kit− cells (1/89) (Table 4). When the distribution of the c-kit+ and c-kit− cells within the early CD45R+ pro-B population of BM MNC was taken into account (Table 3), we calculated that almost twice as many of the B-lineage cells clonable in IL-7 alone were c-kit−(Table 4).
These data show that c-kit+ early CD45R+ pro-B cells had a cloning advantage in the combined presence of IL-7, factors produced by stromal cells (including SCF, the ligand for c-kit), and stromal cell contact. However, this advantage was greatly reduced in semi-solid agar assays in which stromal cells were not present. Given the abundance and cloning frequency of c-kit− cells within the early CD45R+ pro-B compartment, the contribution of these cells to the expanding B-cell compartment is likely close to that of c-kit+ cells.
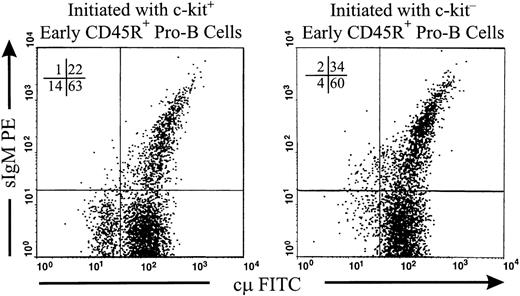
We next addressed the question of whether c-kit−early CD45R+ pro-B cells could generate cμ+pre-B and surface IgM+ B cells. Intracellular staining showed that both c-kit− and c-kit+ cells with the early CD45R+ pro-B surface phenotype were negative for cμ (data not shown) . We separated early CD45R+ pro-B cells from BM into c-kit− and c-kit+populations and placed them in coculture with stromal cells. After 6 days, the majority of cells generated from either c-kit− or c-kit+ populations expressed cμ and more than one fourth of these cells expressed surface IgM (Fig 6).
C-kit+ and c-kit− early CD45R+ pro-B cells generate sIgM+ cells. C-kit+ and c-kits− subsets of CD45R+ CD43+ BP-1−CD25− NK1.1− sIgM− cells were sorted from lymphoid-enriched BM and placed in coculture with ST2 stromal cells. Cells were harvested at day 6 and costained for surface IgM and cytoplasmic μ chains as described in Materials and Methods. Costaining is shown for cells within lymphocyte light scatter gates. Percentages of cells falling in each quadrant are given. The data are representative of either two (c-kit+) or three (c-kit−) similar independent experiments performed on pooled marrow from 5 or 7 mice.
C-kit+ and c-kit− early CD45R+ pro-B cells generate sIgM+ cells. C-kit+ and c-kits− subsets of CD45R+ CD43+ BP-1−CD25− NK1.1− sIgM− cells were sorted from lymphoid-enriched BM and placed in coculture with ST2 stromal cells. Cells were harvested at day 6 and costained for surface IgM and cytoplasmic μ chains as described in Materials and Methods. Costaining is shown for cells within lymphocyte light scatter gates. Percentages of cells falling in each quadrant are given. The data are representative of either two (c-kit+) or three (c-kit−) similar independent experiments performed on pooled marrow from 5 or 7 mice.
Thus, early CD45R+ pro-B cells that lack c-kit performed Ig gene rearrangement and generated cμ+ and surface IgM+ B-lineage cells in culture. Taken together, these data support a role for both c-kit− and c-kit+pro-B cells in expansion of the pro-B compartment and the production of sIgM+ cells in murine BM.
DISCUSSION
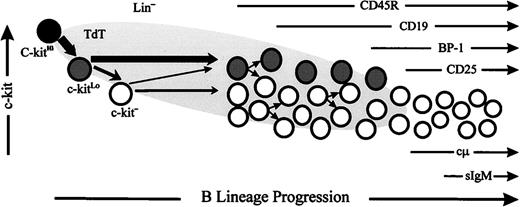
A major goal of our studies was to identify and enrich for viable B-lineage precursors on the verge of acquiring CD45R. Simultaneous staining for intranuclear TdT and surface c-kit in Lin− cells showed that c-kit density could be used to resolve three populations containing B-lineage precursors. Lin− populations with reduced or absent c-kit expression were enriched for TdT+ cells and B-lineage precursors poised to acquire CD45R. We observed a general trend in which progressive loss of c-kit corresponded to increased maturity and lymphoid commitment among these precursors. We also found that many of the earliest CD45R+ precursors, including those capable of proliferating and generating sIgM+ cells, had completely lost c-kit. These studies suggest that TdT expression is initiated as c-kit downregulation begins in Lin− cells, with progressive loss of c-kit concomitant with B-lineage differentiation. CD45R expression is apparently initiated during the transition from c-kitLo to c-kit− with many cells losing c-kit before acquiring CD45R. However, this loss is not precisely synchronized with maturity, because a minority of B-lineage cells retain or reacquire c-kit during the early pro-B stage.
c-kit as a marker for early (Lin−) lymphocyte precursors.
Studies by Park and Osmond8,22 identified a small population (0.09% to 0.5% of nucleated marrow cells) of CD45R− TdT+ cells and used a variety of kinetic analyses to conclude that they probably represent very early B-lineage precursors. More recently, 84% of TdT+ cells in human BM have been shown to express the B-lymphoid–specific marker CD79a.29 We reasoned that it might be possible to enrich for a viable population of very early B-lineage precursors by sorting Lin− cells whose surface properties correlated with TdT expression. Approximately 0.3% of nucleated marrow cells were identified as CD45R− TdT+ using our staining conditions and low-level c-kit expression on these cells allowed us to identify a population over 100-fold enriched for TdT+ cells (Table 1).
Lin−c-kitLo and Lin−c-kit− cells sorted from BM were able to generate substantial populations of CD45R+B-lineage cells during overnight coculture (Fig 2). In addition, reduced levels of c-kit expression in B-lineage precursors corresponded with increased lymphoid restriction (Table 2) and increased maturity of B-lineage progeny (Fig 2 and 3). Therefore, we conclude that there is a general trend of increasing maturity with progressive loss of c-kit in precursors to the B lineage and that most cells poised to acquire CD45R lack or express low levels of c-kit (Fig 7).
Kinetics of c-kit downregulation, TdT expression and acquisition of CD45R in B-lineage differentiation . Our studies suggest that TdT expression is initiated as c-kit downregulation begins in Lin− cells, with progressive loss of c-kit during B-lineage differentiation and that CD45R expression is initiated during the transition from c-kitLo to c-kit− with many cells losing c-kit before acquiring CD45R. However, this loss is not precisely synchronized with maturity and a minority of B-lineage cells retain or reacquire c-kit during the early pro-B stage.
Kinetics of c-kit downregulation, TdT expression and acquisition of CD45R in B-lineage differentiation . Our studies suggest that TdT expression is initiated as c-kit downregulation begins in Lin− cells, with progressive loss of c-kit during B-lineage differentiation and that CD45R expression is initiated during the transition from c-kitLo to c-kit− with many cells losing c-kit before acquiring CD45R. However, this loss is not precisely synchronized with maturity and a minority of B-lineage cells retain or reacquire c-kit during the early pro-B stage.
Neither the transmembrane tyrosine kinase receptor, c-kit, nor its ligand, stem cell factor, are essential for B lymphopoiesis.18,24-26 However, c-kit has been used extensively as a marker of stem cells and early precursor populations2,27,28 and antibodies to c-kit have been used to sort early cells with B-lineage potential.10,15Confusion arises from several technical aspects of previous studies.15 For example, one study examined B-lineage precursor activity in CD45R− populations that were c-kitHi, c-kitLo, and c-kit−. Almost no cells (<1/10,000) with potential for B-lymphocyte formation were found in the c-kit− subset.15 This contrasts markedly with our finding that 1/34 Lin−c-kit− cells give rise to B-lineage cells (Table 2). This difference is due to the fact that myeloid and erythroid cells were removed from our Lin− suspensions. These abundant, nonlymphoid cells are primarily c-kit− and dilute the B-lineage potential of Lin−c-kit− cells if not removed. Our studies show that Lin− BM can be resolved into c-kitHi, c-kitLo, and c-kit− categories, all of which contain precursors to the B lineage (Table 1). We propose a differentiation sequence based on sequential sorting experiments and differences in apparent maturity of B-lineage precursors in each subset (Fig 7).
Expression of c-kit on recently formed CD45R+precursors.
CD45R has long been used as a marker for B-lineage lymphocytes and remains valuable for discriminating early precursors in BM, despite its presence on cells of other lineages.4-6 Cells that are committed to B-lymphocyte formation eventually acquire other markers, such as CD19, and differentiation is accompanied by substantial replication.20 We devised two experimental approaches to determine whether c-kit was present on very early CD45R+cells. Lin− subsets were isolated on the basis of c-kit density and used to generate CD45R+ cells in culture. We found that most of the newly formed B-lineage cells were c-kit− either by the time they acquired CD45R+, or within 1 day of that event (Fig 4). A second strategy exploited previous findings that early B-lineage cells display CD43, but lack BP-1 and CD25.9,10,14,16,17 This made it possible to isolate early pro-B (CD45R+ CD43+BP-1− CD25−NK1.1− sIgM−) cells from freshly harvested BM while excluding NK-lineage cells. A majority of these early precursors, including those that also expressed TdT, lacked c-kit (Fig 5 and Table 3). Our findings are consistent with those of Rico-Vargas et al,25 which showed that over half of all TdT+ cells are c-kit−. Both the c-kit+ and c-kit− subsets of early CD45R+ pro-B cells could generate sIgM+ cells (Fig 6) and proliferate in response to IL-7 (Table 4). We conclude that c-kit is absent from a majority of recently formed B-cell precursors (Fig 7). However, loss of this marker does not clearly demarcate cells with unique potential for expansion or differentiation.
Comparison with widely used models of B-lineage differentiation.
The model illustrated in Fig 7 outlines a complex pattern of c-kit loss and it is important to relate our findings to previously proposed differentiation models. Kondo et al3 recently described a category of common lymphocyte progenitors (CLP; Lin−IL-7R+ Thy-1− Sca-1Loc-kitLo) that have the potential to generate B, T, and NK cells, but not myeloid cells. We found that TdT expression correlates with B-lymphocyte potential and CLP may or may not overlap the TdT+ subset of Lin−c-kitLocells we studied. We used culture assays that only support the growth of B-lymphoid and myeloid cells. Therefore, some of the early lymphoid-committed B-lineage precursors identified here may also have NK- and/or T-lineage potential. The CLP population experimentally defined by Kondo et al3 is only one tenth the size of our Lin−c-kitLo Tdt+ subset (Table 1) and might be partially included within it. However, only Thy-1− cells were studied as CLP whereas we found that the majority of Lin−c-kitLoTdT+ cells were Thy-1Lo (unpublished observations). Therefore, TdT+ cells may represent a type of B-lymphocyte precursor within the Lin−c-kitLo subset that is more mature than the CLP and on the verge of acquiring CD45R.
Li et al13 proposed a sequence in which a putative CD45R− precursor, A0 (AA4.1+CD43+ CD24− CD4Lo), gives rise to developmentally sequential subsets of Fraction A (CD45R+ CD43+ CD24−BP-1−) precursors.13 Although we found small numbers of CD4Lo cells within all three of our Lin− subsets, none of the TdT+ cells expressed CD4 (unpublished data). Therefore, the relationship of Li’s A0 fraction to our precursor populations is unclear. We isolated a population of early CD45R+ pro-B cells that was highly enriched for TdT expression (78%+) by removing sIgM+-, CD25+-, BP-1+–, and NK-lineage cells (Fig 5 and Table 3). These cells gave rise to B cells in culture and presumably represent very early precursors. They must be a subset of Li’s Fraction A and Fraction B (CD45R+CD43+ CD24+ BP-1−) which are reported to contain 11% and 26% TdT+ cells, respectively.30
Melchers et al10,14,16,17 designate subsets of B-lineage precursors on the basis of Ig gene rearrangement and expression. In their terminology, pro-B cells have germline Ig genes, but are likely to give rise to B-lineage cells. They use c-kit as an experimental means for enrichment of either (CD45R−c-kit+) pro-B cells or (CD45R+c-kit+) pre-B I cells. In this model, the loss of c-kit and TdT corresponds with appearance of cμ and marks entry into the pre-B II stage. However, we find that most of the earliest CD45R+cells have already lost c-kit, including a majority of those that are TdT+. Furthermore, it is clear from our studies that downregulation of c-kit is not perfectly synchronized with important B-lineage differentiation events. For example, both c-kitLoand c-kit− subsets of our early pro-B cells respond in stromal cell cocultures and semi-solid agar assays driven by IL-7 (Table 4). Previous studies by Melchers et al10,14,16,17were not designed to assess the cloning capacity of c-kit− cells at this early stage in B-lineage development. Lymphocyte precursors in their studies were greatly diluted by more mature nonresponding cells and they reported a cloning frequency of less than 1/1,00015 for the total CD45R+ c-kit− population. In contrast, we observed a cloning frequency of 1/21 for the c-kit−subset of early CD45R+ pro-B cells in a similar assay (Table 4). It is also important to note that c-kit is transiently re-expressed by some B-lineage cells generated from Lin−c-kit− cell precursors (Fig 4and 7). These observations argue against using c-kit as a developmental milestone for B lymphopoiesis during the pro-B stage.
Our studies did not address, and do not preclude, functions for c-kit or the related receptor flt-3/flk-2 on B-lineage precursors. Rather, we rigorously tested the utility of c-kit as a marker that can discriminate stages of differentiation. Lineage-marker–negative populations could be resolved into three categories with respect to c-kit and considerable enrichment was obtained for TdT+lymphocyte precursors on that basis (Fig 1 and Table 1). In addition, the loss of c-kit corresponded with increased lymphoid restriction (Table 2) and apparent maturity in these Lin−lymphocyte precursors (Fig 2 and 3). However, we doubt that all cells with similar differentiation potential are uniformly positive or negative for this marker. The problem becomes more acute when attempting to use c-kit to discriminate pro-B and pre-B cell subsets. The ability to enrich for viable B-lineage precursors poised to acquire CD45R and for a population of early CD45R+ cells highly enriched for TdT expression should facilitate studies of early B-lineage differentiation events and resolve some of these issues. Furthermore, experimental models using these cells can address important questions regarding the roles of hormones, growth factors, and growth factor receptors in regulating B-lineage commitment and progression.
ACKNOWLEDGMENT
The authors thank Dr Glennda Smithson for helpful discussions and Viji Dandapani and Jim Henthorn for help with flow cytometry and cell sorting. We are grateful to Drs Lisa Borghesi, Linda Thompson, and Kim-Sue Tudor for manuscript suggestions and Shelli Wasson for manuscript preparation.
Supported by Grants No. AI20069 and AI33085 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal