GENE THERAPISTS have a special fondness for hematology. Initially, this was due to the many inherited diseases that could potentially be cured by ex vivo genetic modification of hematopoietic stem cells. Once the early murine leukemia virus (MLV) vectors were found to transduce only a small percentage of primate stem cells, the search began for other types of vectors that might work more efficiently, and adeno-associated virus (AAV) vectors were among the first alternatives to be investigated. Although a substantial amount of work with AAV vectors has occurred over the last several years, the bulk of evidence suggests that they transduce hematopoietic stem cells poorly, and that they will not replace retroviral vectors in stem cell gene transfer applications. Instead, it is applications involving the production of secreted proteins that now appear especially promising for AAV vectors, where they are delivered by direct injection rather than ex vivo cell manipulation. Recent animal studies have shown that AAV vectors encoding factor IX can lead to long-term, therapeutic clotting factor levels after in vivo administration, setting the stage for what may well be a cure for hemophilia B in the near future. Animal experiments have also shown that AAV vectors expressing erythropoietin can produce dramatic increases in hematocrit levels. Here we will review the role of AAV vectors in the field of hematology, including the basic biology of AAV, vector transduction properties, their use in hematopoietic cells, and their potential for delivering secreted proteins such as clotting factors and cytokines.
BIOLOGY OF AAV
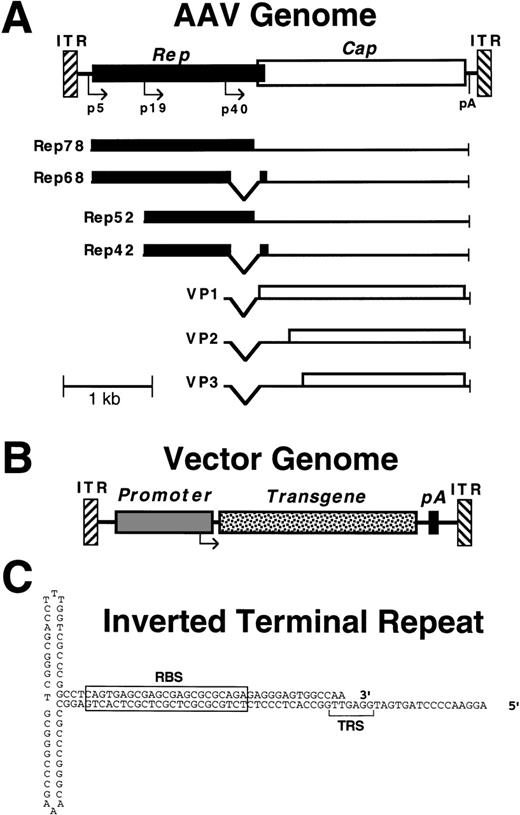
AAV is a dependent parvovirus with an ≈4.7-kb single-stranded linear genome that contains two open reading frames (rep andcap).1 The genome encodes 4 replication (Rep) proteins and 3 capsid proteins (VP1-3) (Fig1). The inverted terminal repeats (ITRs) can pair to form characteristic T-shaped hairpins, which are the only sequences required in cis for replication and packaging. For a productive infection to occur, AAV requires coinfection with a helper virus such as adenovirus, which allows the viral genome to replicate episomally, and leads to synthesis of the AAV proteins. In the absence of helper virus, the AAV genome can integrate into the host cell chromosome, where it remains in a latent proviral state until infection with helper virus occurs, at which point the proviral genome excises and replicates, and a productive infection resumes.
Structure of wild-type and vector AAV genomes. (A) Map of the wild-type AAV genome, including Rep (solid) and Cap (open) reading frames, promoters (p5, p19, and p40), polyadenylation site (pA), and inverted terminal repeats (ITR). The viral transcripts encoding the different Rep and Cap (VP1-3) proteins are shown below the genome. The smaller Rep proteins, VP2 and VP3, are translated from internal initiation sites. (B) Map of a typical AAV vector, showing replacement of the viral Rep and Cap genes with a transgene cassette (promoter, transgene cDNA, and polyadenylation site). (C) Secondary structure of the AAV ITR, with the locations of the Rep binding site (RBS) and terminal resolution site (TRS) indicated.
Structure of wild-type and vector AAV genomes. (A) Map of the wild-type AAV genome, including Rep (solid) and Cap (open) reading frames, promoters (p5, p19, and p40), polyadenylation site (pA), and inverted terminal repeats (ITR). The viral transcripts encoding the different Rep and Cap (VP1-3) proteins are shown below the genome. The smaller Rep proteins, VP2 and VP3, are translated from internal initiation sites. (B) Map of a typical AAV vector, showing replacement of the viral Rep and Cap genes with a transgene cassette (promoter, transgene cDNA, and polyadenylation site). (C) Secondary structure of the AAV ITR, with the locations of the Rep binding site (RBS) and terminal resolution site (TRS) indicated.
Several different serotypes of AAV have been identified by serological analysis,2-5 and DNA sequencing has shown significant differences in their capsid genes that presumably account for the distinct serological profiles.6-11 Antibodies against AAV types 1-3 and 5 are frequently found in human serum samples.12-14 Virtually all the AAV vectors developed to date were based on AAV type 2, which has implications for gene therapy, because there is a high prevalence of neutralizing antibodies against this serotype in human populations.14,15 The use of other vector serotypes has the potential to overcome this immunity, and may allow for repeat vector administration.8 Differences in tissue tropism could also influence which cell types are susceptible to transduction by each vector serotype.8-10 None of the AAV serotypes have been shown to be pathogenic.
A fascinating feature of the AAV life cycle is the frequent integration of proviral genomes at a common site on human chromosome 19 (19q13-qter).16-18 Although no significant homology exists between this site-specific integration locus and AAV, the locus does contain a binding site for the AAV Rep protein, which is required for the integration reaction.19-21 A number of research groups have sought to capitalize on this feature by designing vectors that integrate at chromosome 19.22-26 It is not clear how useful this approach will be, because the integration reaction is not predictable, causes chromosomal rearrangements, and occurs at about the same rate as random integration. Most AAV vectors do not include therep gene (which would significantly limit the packaging capacity), and they do not integrate at the chromosome 19 locus.27-32
AAV TRANSDUCING VECTORS
AAV vectors typically contain a transgene expression cassette flanked by the viral ITRs (Fig 1). The conventional method for generating vector stocks consists of cotransfection of a plasmid containing the vector genome with a trans-acting helper construct forrep and cap expression. The transfection is performed in the presence of adenovirus, and 2 to 3 days later the cells are lysed and the vector is harvested and purified. A major factor in AAV research has been the difficulty in preparing high-quality vector stocks, because the cotransfection method is cumbersome and time-consuming. Recent improvements in vector production include replacing adenovirus infection with a transfected construct containing a select set of adenovirus genes,33 split rep andcap expression cassettes to eliminate replication-competent AAV (which frequently contaminates stocks),34 and the development of higher titer packaging cell lines.35,36Vector purification is critical, because residual helper virus could significantly influence transgene expression levels,37,38and the cellular debris contaminating crude preparations contains transfected plasmids and transgene expression products that can mimic true transduction events.39 Today’s standards require that AAV vector purification protocols include isopycnic centrifugation on CsCl gradients (or perhaps column purification methods that are being developed), and heat inactivation of remaining adenovirus.
An important consideration in analyzing vector stocks is the method used for titering. Although functional titers (the amount of transgene expression produced by a stock) are useful in predicting vector performance, the number of vector particles required to produce a single transgene expression event can vary from <100 to >10,000 depending on the vector, cell line, and/or production methods. To obtain a reproducible titer measurement, one should determine vector particle numbers directly by quantifying the number of vector genomes present in purified vector preparations. We recommend performing alkaline Southern blot analysis of DNA released from vector virions, with quantitation of the signal from intact, full-length, single-stranded vector genomes.36
Figure 2 outlines the steps involved in transduction by AAV vectors. The vector virion interacts with cellular receptor(s) to enter the cell. An early candidate for the AAV type 2 receptor was a 150-kD glycoprotein that bound to virus particles40; however, subsequent studies have not identified this protein. More recently, heparan sulfate proteoglycan was shown to play a role in the binding and infection of AAV type 2,41 as was fibroblast growth factor receptor 1 (FGFR1)42 and αVβ5 integrin.43 Heparan sulfate is proposed to function as a primary receptor, with FGFR1 and αVβ5 integrin acting as coreceptors. The exact roles and interactions of these (and possibly other) molecules in virus internalization have not been elucidated. Serotypes other than AAV type 2 may bind different molecules for entry, because they have characteristic host ranges and competition experiments suggest they use distinct receptors.8-10 After internalization, the particle is rapidly transported to the nucleus and the vector genomes are released. Little is known about these events, although it is clear that entering vector genomes and capsid proteins can be efficiently delivered to the nucleus, even in nondividing cells.27 44
Transduction by AAV vectors. The different steps required for transduction by AAV vectors are indicated, including an initial interaction with a variety of possible receptor and coreceptor molecules on the cell surface, virion internalization, nuclear entry and release of the single-stranded vector genomes, followed by second-strand DNA synthesis, hybridization of complementary input genomes, and/or chromosomal integration before gene expression can occur from a double-stranded template. Potential secondary structures of episomal vector genomes are shown, as are vector-encoded RNA molecules (bent arrows). Question marks indicate a lack of detailed understanding of the particular step involved. For further details see main text.
Transduction by AAV vectors. The different steps required for transduction by AAV vectors are indicated, including an initial interaction with a variety of possible receptor and coreceptor molecules on the cell surface, virion internalization, nuclear entry and release of the single-stranded vector genomes, followed by second-strand DNA synthesis, hybridization of complementary input genomes, and/or chromosomal integration before gene expression can occur from a double-stranded template. Potential secondary structures of episomal vector genomes are shown, as are vector-encoded RNA molecules (bent arrows). Question marks indicate a lack of detailed understanding of the particular step involved. For further details see main text.
Once in the nucleus, the entering single-stranded genomes are assumed to be transcriptionally inactive, and additional events are required before transgene expression occurs. Early experiments with selectable markers showed that AAV vectors can stably integrate into host chromosomes.45-48 Southern blot, polymerase chain reaction (PCR), and fluorescence in situ hybridization (FISH) analyses suggested that vector integration occurred at random locations other than the site-specific integration locus of chromosome 19.27,28,30,32 Cloning and sequencing of chromosomal junction fragments from cells transduced with neo shuttle vectors has shown that most vector genomes integrate by nonhomologous recombination at random locations as single-copy proviruses with terminal deletions.29,31 When preintegration sites were analyzed, provirus insertion was found to be associated with chromosomal translocations and/or deletions,31 raising safety issues regarding the types of mutations that may be caused by AAV vectors. It is not known if integration requires a double-stranded or single-stranded vector molecule, nor if the vector deletions occur before or during the recombination reaction (Fig 2). Because the vector does not encode specific integration proteins, the reaction must use host cell enzymes, and it requires a chromosomal break. Only a small percentage of entering vector genomes ultimately integrate,27 which is also true of wild-type viral genomes,49-51 presumably due to limiting host cell factors. It should be noted that these detailed integration studies were performed on cultured cells undergoing selection in vitro, and the results may not apply to all cell types. Several studies suggest that vector proviruses can be found as large concatamers after in vivo administration (see below).52-55
In addition to transduction by integration, transgene expression can occur transiently from episomal AAV vector genomes.56,57Double-stranded, episomal vector transcription templates could be formed either by second-strand synthesis, or hybridization of complementary input genomes, because either strand is packaged in the virion (Fig 2). AAV vectors can also transduce cells by modifying homologous chromosomal sequences in a gene-targeting process.58 Thus, the deceptively simple AAV vector genome can transduce cells in several ways, and it is not always clear which mechanisms are involved in a particular experiment. Further complicating factors are related to various host cell conditions that can dramatically affect transduction rates, including S phase,27 exposure to agents that induce DNA repair functions,59,60 protein phosphorylation levels,61,62 expression of helper virus gene products,37,38 and transgene silencing.63 Some of these conditions presumably affect the cellular DNA synthesis or repair processes important for transduction, including second-strand synthesis and/or provirus integration. The ultimate fate of entering vector genomes will depend on the type of cell being transduced, its metabolic and proliferative state, and the transduction conditions used.
TRANSDUCTION BY AAV VECTORS IN VIVO
Long-term transgene expression has been produced by AAV vectors in several tissues after in vivo administration.52-54,64-75Attempts to determine the structure of the AAV vector genome in target tissues transduced in vivo have typically shown the presence of high-molecular-weight concatamers.52-55,74,76 Conversion of input single-stranded vector genomes to high-molecular-weight double-stranded concatamers is associated with an increase in transgene expression,55,76 which can take several weeks after in vivo administration until steady-state levels are reached.52,70,72,73,76,77 A similar, slow increase in transgene expression can also occur in vitro, especially with quiescent cells.27 45
Long-term transgene expression could be produced by integrated concatamers, or by stable concatameric episomes (especially in nondividing cell populations), and there is evidence for both scenarios.55,76,78 Recently, Miao et al55studied the kinetic fate of AAV vector genomes during the process of liver transduction in mice. The single-stranded DNA genomes disappeared over a 5-week period with a concomitant increase in double-stranded, high-molecular-weight forms. Pulse-field gel and FISH analyses were used to establish that the vector concatamers were integrated in about 5% of cells. This correlates well with the number of cells shown to express vector-encoded RNA or protein.70 In muscle experiments, Duan et al76 observed circular, monomeric vector episomes that were converted to larger multimers over an 80-day period, and the stability of these episomes was enhanced by the AAV ITRs. It is not known if the multimeric circles ultimately integrate. Further studies are needed to clarify the relationship between episomal and integrated concatameric forms, and whether tissue-specific factors influence transduction pathways.
Vector genome concatamers could be formed by replication or ligation and/or recombination of input monomers (which are often present at high multiplicity). In the many cases where stationary phase cells have been transduced in vivo, vector genome replication would have been limited to those cells undergoing unscheduled DNA synthesis by host enzymes, while vector genome ligation and recombination may have been promoted by the high multiplicities of infection (MOIs) used for in vivo experiments. The concatamer formation consistently observed in vivo stands in contrast to the formation of single-copy, integrated proviruses often observed in vitro.29 31 Potential factors that could favor concatamer formation include the use of normal, nonproliferating cells that do not dilute input vector genomes, a long time course in the absence of selection, and/or the presence of replication-competent AAV particles in vector stocks.
TRANSDUCTION OF HEMATOPOIETIC CELLS BY AAV VECTORS
Several research groups have reported transduction of various types of hematopoietic cells by AAV vectors. The evidence is clear that transformed, hematopoietic cell lines can be transduced,30,46,79-81 although the transduction rates are significantly lower than those of many nonhematopoietic cell types infected at the same MOI.8,57,82 In the case of primary hematopoietic cells, the efficacy of AAV vectors is controversial, as illustrated by studies with hematopoietic progenitors. Several investigators have concluded that progenitors can be efficiently transduced by AAV vectors.83-92 It is difficult to reconcile some of these studies with recent reports showing that transduction of CD34+ progenitors requires extremely high MOIs of 106 to 108 vector particles/cell, and often leads to transient transgene expression.57 82 Still other investigators have been unable to transduce hematopoietic progenitors, and these experiments have not been published. For example, we could not detect transduction of hematopoietic progenitors by AAV vectors at MOIs up to 106 vector particles/cell (D.W.R. and R.K. Hirata, unpublished results).
Despite a decade of research in this field, only 2 transplantation studies have been published to determine if hematopoietic stem cells can be transduced by AAV vectors. Ponnazhagan et al91described murine transplantation experiments with AAV vectors and reported long-term persistence of vector DNA in bone marrow and spleen specimens as detected by PCR (including samples from 1 secondary transplant recipient). Although this is the strongest evidence to date for murine stem cell transduction, the cell types containing vector sequences were not identified and progenitor assays were not presented to document the persistence of transduced hematopoietic cells, making it difficult to accurately assess stem cell transduction rates. Furthermore, the MOIs of 1 and 10 used were 5 to 7 logs below what others have shown are required for progenitor transduction.57 82
Schimmenti et al93 recently described a transplantation study with rhesus monkeys, in which hematopoietic cells (including granulocytes and lymphocytes) containing vector sequences could be detected for over 15 months after reinfusion of CD34+ cells transduced at MOIs >1,000 physical AAV vector particles/cell. No transgene expression data were presented. The transduction rates were low (approximately 1 in 105 cells), suggesting that AAV vectors are less effective than retroviral vectors for the transduction of primate hematopoietic stem cells. Further studies will be required to resolve remaining questions, such as whether murine stem cells are transduced at higher rates than primate stem cells by AAV vectors (as appears true for retroviral vectors), and whether higher MOIs would improve stem cell transduction rates.
There are several possible explanations for the conflicting results obtained with primary hematopoietic cells, including differences in vector design, stock preparation, the cell types being analyzed, transduction conditions, and transgene assays. One possibility is that the stocks used in many of the experiments reporting high transduction rates were crude cell lysates, which contain cellular debris, adenovirus particles, transgene DNA, RNA, and protein molecules, in addition to recombinant AAV. These contaminating substances could have influenced transduction rates, or even produced false-positive results themselves in the assays used to monitor transgene expression.39 Another important factor is the cell population being studied. We have found that the bone marrow stromal cells present in hematopoietic cell preparations are transduced at high rates by AAV vectors (D.W.R. and R.K. Hirata, unpublished results), so one must be careful to exclude these (and other) contaminating cells from the analysis when assays other than progenitor colony formation are used. Variation in the transduction rates of CD34+ cells from different individuals must also be considered.94 The particular transgene assay being used can have significant effects on the apparent “transduction rate.” Assays that detect transient transgene expression are likely to produce higher transduction rates than those requiring stable integration.56,57,82 Assays that detect vector DNA sequences such as PCR or Southern analysis are much more sensitive than those requiring transgene expression, because most entering vector genomes remain as transcriptionally inactive, single-stranded molecules.27 At the very high MOIs used in some experiments, the surrounding culture media itself is likely to contain many vector particles. A careful consideration of these experimental factors can help in comparing the different published studies.
GENE THERAPY FOR HEMOPHILIA
One of the most promising applications of AAV vectors is the treatment of hemophilia. This is due in part to the well-understood genetics of the disease, easily measured clinical endpoints, and the availability of mouse95-97 and canine98-100 animal models. Patients with hemophilia are categorized by degree of severity, with less than 1% of normal clotting factor levels producing severe, 1% to 5% moderate, and 5% to 20% mild disease. Thus, reconstitution of as little as 1% of clotting factor can convert a severe phenotype to a moderate one, and there is a broad range of potentially therapeutic factor levels, which significantly improves prospects for successful gene therapy. For a recent review of hemophilia gene therapy see Herzog and High.101
Even though hemophilia A and B are indistinguishable clinically, their gene therapy strategies are not necessarily the same. The relative amount of factor VIII that needs to be synthesized is much less than factor IX. Based on the molecular weight, normal plasma concentrations, and differences in the volume of distribution, we estimate that this difference is at least 2 orders of magnitude. The length of the factor IX coding region is about 1.4 kb, which is well below the ≈4.5 kb packaging limit of AAV vectors, while the full-length factor VIII cDNA of about 7 kb exceeds the packaging limit. Even the B-domain–deleted factor VIII message is about 4.4 kb, making it very difficult to produce a high-titer AAV vector with a promoter and polyadenylation site. An alternative strategy is to split the factor VIII molecule into 2 chains, and use separate AAV vectors. This approach will require efficient delivery of both vectors in the proper ratio to target cells, as well as functional reassembly of the two factor VIII peptides. Although AAV vectors can transduce many tissues in vivo, the efforts for hemophilia to date include muscle and liver experiments, the latter being a natural site of factor VIII and IX production. Other tissues such as vascular endothelium may also be appropriate targets. Although factor IX can be produced in muscle cells (see below), it is not completely clear what the biological consequences are of ectopic clotting factor synthesis, especially as it relates to the functional activity of the protein and possible inhibitor formation. It appears less likely that factor VIII can be efficiently produced in muscle.102 103
PRECLINICAL STUDIES WITH AAV VECTORS FOR HEMOPHILIA B
Significant progress has been made with AAV vectors encoding factor IX. Koeberl et al68 were the first to show that AAV vectors could be used for the expression of human factor IX in vivo, by delivering the vector to mouse liver after gamma irradiation. Irradiation was used in these experiments to induce DNA repair functions that were found to increase transduction rates in vitro.59 The amount of factor IX produced was less than 1 ng/mL, or about 0.01% of the normal level of 5 μg/mL. Two groups shortly thereafter achieved therapeutic human factor IX levels after in vivo delivery of AAV vectors in mice. Herzog et al72delivered an intramuscular injection of 2 × 1011physical particles of an AAV vector containing the cytomegalovirus (CMV) enhancer-promoter driving the human factor IX cDNA and produced plasma factor IX levels as high as 300 ng/mL that lasted for the length of the study. Snyder et al70 administered 8.4 × 1010 particles of an AAV vector containing the human factor IX cDNA driven by a Moloney leukemia virus (MLV) long terminal repeat (LTR) promoter into the livers of mice via portal vein infusion, and produced up to 2,000 ng/mL of biologically active factor IX (or 40% of normal levels), an amount considered curative. With portal vein infusion, about 5% of hepatocytes were positive for AAV-mediated gene expression as determined by RNA in situ hybridization and immunohistochemical staining for a marker gene. By quantitative Southern blot analysis there was on average more than 1 AAV vector genome copy per cell. High-level factor IX gene expression of up to 1,000 ng/mL was also achieved by Nakai et al,104 who transduced mouse livers with 1 × 1011 particles of an AAV vector containing the elongation factor 1 α promoter via portal vein injection. Interestingly, no factor IX expression was observed when the CMV promoter was used in the liver70,104, as also observed with retroviral vectors,105 emphasizing the importance of promoter choice as related to target organ. In the more recent studies, high-level expression in liver and muscle was achieved without pretreatment of the target organ to increase transduction rates, perhaps because of the higher MOIs or different expression cassettes used.
Three recent studies have shown the therapeutic value of AAV-mediated gene transfer in murine and/or canine hemophilia B106-108(earlier studies had used normal mice transduced with the human gene). These animals have severe hemophilia with virtually no factor IX activity. In the first study, 2.5 × 1012 AAV vector particles expressing human factor IX from the CMV promoter were administered by intramuscular injection to a dog with hemophilia B.106 A 1-week partial reduction in the whole-blood clotting time was observed. The transient nature of the clinical improvement was likely caused by the inhibitor formed against the human factor IX protein. A second study showed up to 70 ng/mL of plasma factor IX after intramuscular delivery of 3 to 8 × 1012 particles/kg of an AAV vector expressing canine factor IX from the CMV promoter to hemophilia B dogs.107 This plasma level represents about 1% of normal and would be of therapeutic benefit to a severe hemophiliac. One of 5 animals had a transient inhibitor that resolved without sequelae. These animals had decreases in their whole-blood clotting and partial thromboplastin times.
In a liver study, AAV vectors expressing human factor IX from an MLV LTR promoter were infused into the tails or portal veins of hemophilia B mice.108 With a dose of 6 × 1010particles, the bleeding time was reduced to the normal range of 3 to 5 minutes from a pretreatment level of over 30 minutes and was associated with a plasma factor IX concentration of 250 to 1,800 ng/mL. This correlated with a human factor IX bioactivity of up to 100%. In the same study, a similar AAV vector encoding canine factor IX was given to hemophilia B dogs at about 1/10 the dose given to the mice (based on body weight; about 2 × 1011 particles/kg), which produced persistent expression of canine factor IX and reduced whole-blood clotting and partial thromboplastin times. One animal had 1% of the normal factor IX plasma level. The untreated animals have on average 5 spontaneous bleeding episodes per year, and at the time of publication, only 1 treated animal had experienced a spontaneous bleed, where 6 or 7 in total would have been expected. There were no inhibitors associated with the therapy.
There are a number of issues relating to muscle versus liver as target organs for AAV factor IX vectors. Although the muscle is more readily accessible than the liver, in humans the portal vasculature can be infused by nonsurgical, radiographic procedures, making either organ a reasonable approach for clinical trials. Most adult hemophiliacs have been infected with hepatitis viruses, so the complications of liver disease may influence hepatic therapy outcomes. However, newer factor replacement therapies have substantially decreased the incidence of hepatitis in the pediatric hemophilia population, making this less of an issue in the future. The potency of AAV-mediated gene expression appears to be in favor of the liver. The relative AAV vector dose required to achieve similar factor IX levels was 40 times greater in the muscle than the liver. This point is complicated by the fact that the vectors used were produced in different labs, and contained different expression cassettes. Moreover, it is important to realize that promoter optimization may allow for increased expression in liver and/or muscle. Other factors that could differ between these organs are their capacity for posttranslational protein modifications essential for clotting factor activity, antigen presentation properties, and natural rates of cellular turnover and replacement.
The clinical endpoints required to monitor gene therapy for hemophilia are straightforward, because the severity of the disease is directly related to the factor activity measured in the patient’s blood. A major remaining issue is related to inhibitor formation. To minimize this risk, the first patients to be treated should have a long history of parenteral protein replacement without inhibitor formation. However, this does not guarantee the absence of inhibitors, because antigen presentation from soluble proteins may be different than from proteins produced and secreted by cells, ectopic clotting factor production could generate a novel immune response, the pharmacokinetics of continuous factor expression versus periodic infusion could affect antibody formation, and there may also be immunologic consequences of the vector particle. It is possible that a patient receiving an AAV factor IX vector could develop a new inhibitor, and become more difficult to manage by clotting factor transfusions in the future, which needs to be considered in designing clinical trials. Although the data in hemophilia B dogs suggest that AAV vectors can express factor IX without leading to inhibitor formation, many of these questions can only be resolved by appropriate gene therapy trials in people. After the initial patients are treated, the treatment of patients prone to inhibitor formation will need to be addressed. Given the convincing preclinical data showing that long-term therapeutic factor IX levels can be achieved, successful gene therapy for hemophilia B with AAV vectors may soon become reality.
COMPARING VECTORS FOR THE TREATMENT OF HEMOPHILIA
Many different vector systems have been used in various tissues in preclinical studies for the treatment of hemophilia, but for a comparison to AAV vectors we will limit our discussion to in vivo studies of the muscle and liver. Retroviral and adenoviral vectors encoding factors VIII and IX have been used for gene transfer into muscle and liver.95,109-111 When recombinant retroviruses were infused into the portal vein of hemophilia B dogs,105partial hepatectomy was required to stimulate hepatocellular proliferation, a prerequisite for MLV retroviral transduction. Expression persisted for at least a year, enough to lower the whole blood clotting time by about 50%. However, only about 1% of hepatocytes contained proviral DNA and factor IX expression was in the range of 0.1% of normal and not considered therapeutic. Moreover, surgical partial hepatectomy would not be appropriate for clinical trials in humans. More recently, improved rates of retroviral gene transfer were achieved in mice using growth factors to induce liver regeneration in place of liver injury.112,113 Under some conditions, as many as 30% of the hepatocytes were transduced. In addition, there is some evidence to suggest lentiviral vectors will transduce nondividing cells, including hepatocytes.114These methods have yet to be assessed in preclinical studies of hemophilia, and one must be careful in extrapolating in vivo results with retroviral vectors tested in animals to humans, because many retroviral vectors are inactivated by human serum.115 116
First-generation recombinant adenoviruses have been used in mice to achieve therapeutic levels of factors VIII and IX.95,109,111,117-119 In some strains, persistent expression has been achieved with long-term correction of murine hemophilia. However, the same adenoviral canine factor IX vector that was shown to persist in mice produced only transient, supra-normal factor levels with concomitant correction of the bleeding diathesis in hemophilia B dogs, due to the toxicity and immunogenicity of the vector.111 The discrepancy between the mouse and dog is related to differences in the immune responses seen in different inbred strains of mice.120 Transient expression of human factor VIII in canine hemophilia A has been obtained using a recombinant adenovirus; however, the factor VIII–deficient dogs made inhibitors to the human protein.118 Newer generation, “gutted” adenoviral vectors containing fewer viral genes show decreased toxicity and immunogenicity, but their persistence has not yet been shown in preclinical studies of genetic diseases such as hemophilia.121 122
In the case of hemophilia B, AAV vectors show the greatest potential at present, with several studies documenting long-term, therapeutic factor IX levels in animals. The lack of significant inflammatory response caused by the vector, the potential for permanent genetic modification by chromosomal integration, and the ability to transduce quiescent cells are major advantages of AAV vectors. Although retroviral vectors integrate, only the newer vectors based on alternative retroviruses such as lentiviruses have the potential to transduce nonproliferating cells in liver or muscle tissue. Adenovirus vectors efficiently transduce these cell types; however, they do not integrate and can be associated with a significant immune response. The newer, “gutted” adenovirus vectors may prove more effective, and at least in quiescent cells, it is possible that an episomal vector genome could persist long-term. A major disadvantage of AAV vectors is their limited packaging capacity, which may prevent their use in delivering factor VIII or other genes with cDNAs over 4 kb. As research on gene therapy for hemophilia progresses, the advantages and disadvantages of these and newer vector systems will become apparent, and the choice of vector will ultimately depend on the particular clinical application.
GENE THERAPY FOR OTHER COAGULOPATHIES
There are other coagulation disorders being studied in preclinical gene therapy experiments. Factor X deficiency is a rare but serious bleeding diathesis. Retroviral-mediated hepatic gene transfer into rats has resulted in persistent levels of factor X that are 10% to 43% of normal.123 Although AAV vectors have not been tested for factor X deficiency, the coding sequence is similar in size to factor IX, making this an excellent candidate for AAV-mediated gene transfer.
Gene therapy could also be used to treat hypercoagulation disorders, such as protein C or S deficiency. Unlike the bleeding disorders, individuals hemizygous for either gene are symptomatic, with recurrent thromboses. It is believed that over 5% of individuals with recurrent thromboembolic events have inherited protein C or S deficiency. Because these individuals have 1 normal allele, clinical manifestations can occur with 50% of the normal protein level. Thus, very high transgene expression levels will be required to produce a clinical effect in heterozygous individuals. Rare, homozygous patients (about 1/500,000 births) can have neonatal purpura fulminans or disseminated intravascular coagulation, and might have marked clinical improvement with smaller amounts of gene reconstitution. Cai et al124have shown retroviral-mediated gene transfer of protein C into rat hepatocytes in vivo with up to 22% of the normal plasma concentration. The high level of protein produced may in part have been caused by the formation of nonneutralizing antibodies against the human protein that prolonged the half-life of protein C. Both the protein C and S cDNAs will fit in AAV vectors, so these diseases could also be candidates for AAV-mediated gene therapy. However, in addition to the very high transgene expression levels required, a therapeutic response could be difficult to assess in these patients, because it may require prolonged observation before a decrease in thomboses can be documented. This will complicate early clinical trials, and could delay the development of genetic therapies for these disorders.
AAV AND OTHER HEMATOLOGIC DISEASES
The success of preclinical studies for hemophilia B with AAV vectors suggests that they could be used for the production of a variety of secreted proteins. Although these include several cytokines that might be used to influence hematopoiesis, the best studied example is the use of AAV vectors for the production of erythropoietin to treat anemia. This protein is normally made in the kidney with small amounts being produced in the liver, and patients with chronic renal disease or possibly thalassemia might benefit from erythropoietin gene therapy. Several groups have shown sustained expression of erythropoietin in primates and/or rodents after intramuscular injection of AAV vectors.35,67,125-128 In these studies, persistent hematocrit elevations were produced and, in some cases, the levels were dangerously high and phlebotomy was required. Thus, the success and safety of erythropoietin therapy will require regulated transgene expression. Three studies have shown that such regulation is possible by codelivery of inducible transcription factors that act on the vector-encoded erythropoietin transgene. Systems based on tetracycline-regulated transactivation127,128 and rapamycin-regulated chimeric transcription factors126 have been used.
Although erythropoietin expression can clearly be regulated with appropriate doses of tetracycline or rapamycin, delivery of transcription factor genes raises additional safety concerns. It is difficult to predict what effects an exogenous transcription factor will have on the regulation of endogenous genes, either through direct binding at chromosomal loci, or through interactions with cellular proteins. Recombination of the vector-encoded transcription factor gene with cellular genes could also generate new fusion proteins that might influence gene expression. In addition, the regulation is not physiologic, so the maintenance of therapeutic transgene levels requires continuous clinical monitoring with frequent dose adjustments of the appropriate regulatory drug. These issues are especially relevant for erythropoietin gene therapy, because too much expression could produce fatal polycythemia, while safe and effective pharmacological administration of the erythropoietin protein is already possible. Some of these problems might be solved by using an endogenous promoter that responds in a physiologically relevant manner to anemia129 130; however, proper regulation could still be difficult because of chromosomal position effects. Appropriate preclinical safety and efficacy studies will need to be completed before these strategies are applied clinically.
CONCLUSIONS
The last few years have seen remarkable advances in the development of AAV vectors for gene therapy, especially for applications in hematology. Although early enthusiasm for their use in hematopoietic cells has not been followed by reports of potentially therapeutic stem cell transduction rates, their use in liver or muscle can clearly generate physiologically relevant levels of secreted clotting factors or cytokines. Several studies have shown that therapeutic factor IX levels can be produced by AAV vectors in animals, and there is every reason to believe the same will be true of humans. The next few years should see the first clinical trials of AAV vectors for hemophilia B and, hopefully, a permanent genetic cure for these patients.
Supported by grants from the March of Dimes Birth Defects Foundation and the US National Institutes of Health (Nos. HL53750 and HL53682).
REFERENCES
Author notes
Address reprint requests to David W. Russell, MD, PhD, Division of Hematology, Department of Medicine, Mailstop 357720, Room K-236A HSB, University of Washington, Seattle, WA 98195-7720; e-mail:drussell@u.washington.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal