Abstract
We investigated the mechanisms of sickle cell disease (SCD) hematopoietic/erythropoietic defects using bone marrow, spleen, and/or peripheral blood from the transgenic SAD mouse model, which closely reproduces the biochemical and physiological disorders observed in human SCD. First, the erythropoietic lineage late precursors (polychromatophilic normoblasts to the intramedullary reticulocytes) of SAD mouse bone marrow were significantly altered morphologically. These anomalies resulted from high levels of hemoglobin polymers and were associated with increased cell fragmentation occurring during medullary endothelial migration of reticulocytes. Secondly, analysis of bone marrow erythropoiesis in earlier stages showed a marked depletion in SAD erythroid burst-forming units (BFU-E; of ∼42%) and erythroid colony-forming units (CFU-E; of ∼23%) progenitors, despite a significant increase in their proliferation, suggesting a compensatory mechanism. In contrast to the bone marrow progenitor depletion, we observed (1) a high mobilization/relocation of BFU-E early progenitors (∼4-fold increase) in peripheral blood of SAD mice as well as of colony-forming units–granulocyte-macrophage (CFU-GM) and (2) a 7-fold increase of SAD CFU-E in the spleen. Third, and most importantly, SAD bone marrow multipotent cells (spleen colony-forming units [CFU-S], granulocyte-erythroid-macrophage-megakaryocyte colony-forming units [CFU-GEMM], and Sca+Lin−) were highly mobilized to the peripheral blood (∼4-fold increase), suggesting that peripheral multipotent cells could serve as proliferative and autologous vehicles for gene therapy. Therefore, we conclude the following. (1) The abnormal differentiation and morphology of late nucleated erythroid precursors result in an ineffective sickle erythropoiesis and likely contribute to the pathophysiology of sickle cell disorders; this suggests that transfer of normal or modified SCD bone marrow cells may have a selective advantage in vivo. (2) A hematopoietic compensatory mechanism exists in SAD/SCD pathology and consists of mobilization of multipotent cells from the bone marrow to the peripheral blood and their subsequent uptake into the spleen, an extramedullary hematopoietic site for immediate differentiation. Altogether, these results corroborate the strong potential effectiveness of both autologous and allogeneic bone marrow transplantation for SCD hematopoietic therapy.
SICKLE CELL DISEASE (SCD) is characterized by hemoglobin polymerization and sickling of mature red blood cells (RBCs). Polymerization of hemoglobin S with consequent sickling of RBCs is a pivotal pathogenetic event in SCD. Intense molecular and biochemical studies on SCD have been performed in the last decades and have provided important insights into the RBC defects. In contrast, there has been a relative paucity in understanding the in vivo bone marrow pathophysiology of this disorder. Knowledge of the molecular and cellular mechanisms in SCD bone marrow is critical for the selection or development of appropriate gene therapy applications.
Previously, erythroid precursors in SCD have been studied by means of peripheral blood smears1-3 or bone marrow aspirates4 of humans with SCD. In vitro erythropoietic studies on peripheral blood of SCD patients identified an increase in erythroid5 in association with increased γ-globin/α-globin chain ratio compared with controls.6,7 Other investigators, in contrast, have reported that the levels of erythroid progenitors were inversely correlated with fetal hemoglobin levels in peripheral blood.8-11 Although this correlation was not observed in peripheral blood from children, a strong inverse relationship was noticed between the number of erythroid progenitors and age.12 Because there is usually no indication for a bone marrow biopsy in human SCD, there has been virtually no direct in vivo evaluation of the erythropoietic efficiency by analysis of precursor morphology and function. However, crucial insights may be gained from the development of animal models of SCD.
The generation of transgenic animal models of SCD provides the opportunity to determine the in vivo cellular pathophysiology of the sickle erythron. Over the last decade, several attempts have been undertaken to produce animal models,13,14 some of which are good genetic representatives.15,16 Although these latter mouse models exhibit several characteristics of severe SCD, they are also associated with thalassemic features. Because no thalassemic feature is observed in the transgenic SAD mouse,17,18 this model that we developed is phenotypically one of the best for SCD studies. The SAD mouse was generated by coexpression of the human α2-globin gene and a modified βS-globin gene, βSAD, both linked to the β-globin locus control region, to produce a sickle hemoglobin. RBCs of the highest expressing transgenic line, SAD-1, and of compound heterozygous SAD-1/βd3 mice [SAD-1 mated to Hbbsingle/Hbbd3(th) mice; previously β-thal/SAD]19 contain 19% and 26% of the hemoglobin SAD, respectively.17 The hematologic parameters of SAD mice show anemia in fetuses and neonates. SAD RBCs display the typical SCD characteristics of hemoglobin polymerization and sickling in vivo. Quite uniquely, the SAD mouse has previously been shown to reproduce the most severe systemic microvascular occlusions and hemolytic and renal complications of human SCD,18 20 3 key features of SCD not reported to be present simultaneously in the other mouse models. The SAD transgenic mouse has an average lifespan of approximately 15 months, and the cause of death of SAD mice could be ascribed to either acute vasoocclusive events in young mice or to specific chronic organ damage in older mice, both of which are typical features of SCD.
In this study, we demonstrate that the SAD and SAD/βd3mouse models are characterized by in situ ineffective erythropoiesis into SCD bone marrow. In association with such in vivo precursors anomalies, analysis of the proliferation, differentiation, and maturation potential of the erythroid/hematopoietic cells demonstrated a major mobilization and a systemic expansion of the multipotent cell population. Our data also uncovered the existence of a hematopoietic compensatory mechanism in SAD mice SCD (SAD/SCD), consistent with a response to erythropoietic stress. The study of the erythron and hematopoietic lineages in the SAD murine model have provided insights into the in vivo pathophysiologic role of sickle hemoglobin polymerization and may have significant implications on the development of gene therapy strategies.
MATERIALS AND METHODS
Morphologic Studies
SAD (Hbbs/s SAD-1 [n = 5]), SAD/βd3(Hbbs/Hbbd3(th) SAD-1 [n = 3]), and age-matched control mice (Hbbs/s [n = 4], Hbbs/Hbbd3(th) [n = 2]) were identified by hemoglobin analysis.17 All animals were maintained on a high folate, thiamin, and B12 diet and under normal oxygen tension in room air. Because the aim of this study was to analyze the in vivo characteristics of the SAD erythroid precursors, no attempts were made to deoxygenate the tissues before fixation. Samples of vertebral bone were processed for electron microscopy immediately upon death. Several biopsies of vertebral bone marrow were obtained per animal and promptly fixed in 2.5% buffered glutaraldehyde and postfixed in 1% osmium tetroxide, which previously had been shown not to induce artefactual hemoglobin polymerization.21 After postosmication which facilitates cellular classification of the erythroid lineage, tissues were dehydrated and embedded in Epon 812. Thin sections were stained with uranyl acetate and lead citrate. All morphologic studies were performed by a pathologist without knowledge of the specific genetic origin of the tissues.
Hematopoietic Progenitor Studies
Clonogenic assays.
Clonogenic assays were performed on SAD and control C57BL/6 mice. For each animal, the percentage of spleen weight per total body weight was determined. Progenitor cell analyses were performed on 3 hematopoietic tissues: bone marrow, peripheral blood, and spleen. Bone marrow cells, peripheral blood mononuclear cells, and spleen single-cell suspensions were plated at a density of 105 cells/mL, 106cells/mL, and 5 × 105 cells/mL, respectively, in 1% methylcellulose/Iscove’s modified Dulbelco’s medium (IMDM), as previously described.18 All experimental samples were performed in duplicate for each animal. Colony-forming units-erythroid (CFU-E) were counted after 2 days in culture, whereas burst-forming units-erythroid (BFU-E), colony-forming units–granulocyte-macrophage (CFU-GM) and colony-forming units-macrophage (CFU-M) were counted at 7 days and colony-forming units–granulocyte-erythroid-macrophage-megakaryocyte (CFU-GEMM) were counted on day 11. Results were expressed as the mean ± standard deviation (SD) from all animals analyzed.
In vitro thymidine suicide.
In vitro thymidine suicide assays were performed on 5 × 106 cells from bone marrow or spleen using 50 μCi methyl-3H-thymidine or unlabeled thymidine, as described.22 After incubation, cells were plated at a density of 1.5 × 105 cells/mL for bone marrow and 5 × 105 cells/mL for spleen in IMDM-methylcellulose media as described above.
Day-12 spleen colony-forming unit (CFU-S12) evaluation.
CFU-S12 evaluation in peripheral blood of SAD (n = 10) and C57BL/6J (n = 4) was performed with 2 × 106 nucleated blood cells. Cells were injected into the tail vein of irradiated C57BL/6-Gpi1a mice (at 900 rads). CFU-S colonies were counted on day 12. Colonies were picked and analyzed at random to verify presence of the SAD transgene and glucosephosphate isomerase (GPI).
Flow cytometry.
Flow cytometry analyses were performed on blood samples from SAD and C57BL/6 mice collected by cardiac puncture in Hank’s balanced salt solution containing 1% heparin. After lysis of RBCs, nucleated cells were washed and resuspended in phosphate-buffered saline (PBS) containing 2% fetal calf serum (FCS) and 0.1% NaN3. Cells were first incubated in a blocking solution containing human Igs and anti-Fc receptor antibodies (clone no. 2.4G2) and then stained with antimouse monoclonal antibodies: fluorescein isothiocyanate (FITC)-labeled antibodies to CD4 (clone GK1.5), CD8 (clone 53-6.72) (gifts from Dr P. Hugo, Procrea Bioscience, Montreal, Quebec, Canada); Ter-119, MAC-1 (CD11b), Gr-1 (Ly-6G), B220, and biotinylated-Sca-1 (Pharmingen, Mississauga, Canada). Anti–Sca-1 was detected by streptavidin-RED670 (Life Technologies, Burlington, Ontario, Canada). Samples were analyzed on a Coulter EPICS XL 4 colors with system II software (Coulter, Hialeah, FL).
Splenectomy.
Splenectomy was performed under sterile conditions on C57BL/6 and SAD mice as described.23 Briefly, mice were anesthesized with avertin. After a small incision was made, the spleen was removed after ligation with surgical silk of the major blood vessels running around the spleen. No specific features were observed on gross pathologic examination. Splenectomized SAD and control mice had unchanged hematocrit levels and buffy coats.
Statistical Methods
Unpaired 2-sample Student’s t-test and the Fisher’s exact test were used for statistical analysis; P < .05 was considered significant.
RESULTS
Morphologic Studies of SAD Erythropoiesis/Hematopoiesis
To investigate the erythropoietic/hematopoietic mechanisms in SCD, we first have undertaken several morphologic studies in the SAD transgenic model. Histologic and ultrastructural analysis of bone marrow showed a large proportion of nucleated erythroid precursors (pronormoblast, normoblast); the marked erythroid hyperplasia showed a reversal of the usual 1:3 erythroid:myeloid ratio to an erythroid increase of at least 1:1 in all transgenic SAD and SAD/βd3 mice. Identification of the erythroid cell type was based on ultrastructural morphologic changes in both the pattern of nuclear chromatin and electron density of cytoplasm.24,25 Pronormoblasts and basophilic normoblasts, which are identified by their characteristic open chromatin pattern, large cell size, and abundance of cytoplasmic organelles, were frequently found in mitosis, consistent with a state of accelerated erythropoiesis (data not shown). These early erythroid precursors showed no pathologic alterations and did not contain hemoglobin polymers. In contrast, polychromatophilic normoblasts, identified by increased chromatin condensation and higher cytoplasmic density, were the earliest erythroid precursors to demonstrate hemoglobin polymers, which is probably related to their higher intracellular hemoglobin content compared with the earlier differentiation stages (Fig 1A). Polymers were identified in 21% ± 5% of polychromatophilic normoblasts in the marrow matrix of SAD mice (Table 1). Cytoplasmic polymers were arranged in small bundles, most often limited to one region of the cytoplasm (Fig 1A and B). In late normoblasts, the cell membrane was frequently stretched and deformed by underlying polymer bundles. Interestingly, polymers were present not only in the cytoplasm, but also in the interchromatin regions of the nucleus (Fig 1A, inset). Polymer fibers were occasionally seen extending from cytoplasm into the nucleus, apparently through the nuclear pores at sites where invagination of the nuclear envelope was observed (Fig 1B), and upon enucleation, polymer fibers were sometimes retained in extruded nuclei (Fig 1A). Large conglomerates of polymer fibers (Fig 1C and D) were also identified in 43% ± 10% of the more mature erythroid reticulocytes in the marrow matrix of SAD mice (Table 1). Reticulocytes (Fig 1A through D) were distinguished from erythrocytes by their larger cell size and clear cytoplasm and the presence of cytoplasmic organelles such as ribosomes and mitochondria. During migration of reticulocytes from the marrow matrix through the sinusoidal endothelium to enter the circulation, large amounts of polymers were detected in the reticulocyte portion that remained in the extravascular matrix while the engaged intravascular portion was often devoided of polymers (Fig1C), consistent with the differential oxygen levels between both sides.26 Alternatively, this peculiar distribution pattern of polymers could be related to a mechanical effect whereby polymer fibers could be retained in the extravascular portion during the passage. These SAD reticulocytes passing through the narrow fenestrae of the sinusoidal barrier also showed marked morphologic deformation. Moreover, the occurrence of frequent cellular fragments highly suggestive of reticulocyte fragmentation were observed in sites adjacent to the egressing polymerized and deformed reticulocytes. These types of fragments were never seen in nontransgenic control animals. Most likely, the observed fragmentation suggests that hemoglobin polymerization caused an increased cell fragility. Furthermore, a majority of the medullary macrophages were involved in active phagocytic clearance of the fragmented as well as intact polymerized reticulocytes. Whereas cells from the erythroid lineage present several anomalies, cells from the myeloid and megakaryocytic lineages appeared to be ultrastructurally normal in SAD mice.
(A) Polychromatophilic normoblast and reticulocyte in bone marrow of SAD mouse. Parallel bundles of polymer fibers are noted in the cell periphery (arrow) of polychromatophilic normoblast and in cytoplasm of reticulocyte (arrowhead; original magnification × 6,372). Inset shows polymer bundles (star) in the cytoplasm as well as in the interchromatin regions of the extruding nucleus (original magnification × 18,720). (B) Polychromatophilic normoblast in bone marrow of SAD mouse showing cytoplasmic and nuclear polymer bundles with indentation of the nuclear envelope (original magnification × 10,000). (C) Reticulocyte in bone marrow of SAD/βd3 mouse migrating through narrow gap in sinusoidal endothelium (SE). Polymers are restricted to the extravascular portion of the cytoplasm (arrow) compared with the intravascular portion (arrowhead) of a markedly deformed reticulocyte. An erythroid cell fragment (black star) is present next to migrating reticulocyte. A dense chromatin-like cap, probably a retained nuclear fragment, is seen involving part of the cell periphery of a polymerized reticulocyte (white star). No polymers are seen in intravascular erythrocytes. (original magnification × 7,500). (D) Reticulocyte traversing a wide gap in the sinusoidal barrier (SE), showing pointed extensions with polymer bundles oriented in the direction of the flow (arrow). A large geometric conglomerate of polymer bundles is seen in adjacent extravascular reticulocytes (arrowhead). Reticulocyte fragments (black star) are present next to reticulocyte in process of migrating through a narrow gap. Polymers are absent in the intravascular erythrocyte (original magnification × 3,000).
(A) Polychromatophilic normoblast and reticulocyte in bone marrow of SAD mouse. Parallel bundles of polymer fibers are noted in the cell periphery (arrow) of polychromatophilic normoblast and in cytoplasm of reticulocyte (arrowhead; original magnification × 6,372). Inset shows polymer bundles (star) in the cytoplasm as well as in the interchromatin regions of the extruding nucleus (original magnification × 18,720). (B) Polychromatophilic normoblast in bone marrow of SAD mouse showing cytoplasmic and nuclear polymer bundles with indentation of the nuclear envelope (original magnification × 10,000). (C) Reticulocyte in bone marrow of SAD/βd3 mouse migrating through narrow gap in sinusoidal endothelium (SE). Polymers are restricted to the extravascular portion of the cytoplasm (arrow) compared with the intravascular portion (arrowhead) of a markedly deformed reticulocyte. An erythroid cell fragment (black star) is present next to migrating reticulocyte. A dense chromatin-like cap, probably a retained nuclear fragment, is seen involving part of the cell periphery of a polymerized reticulocyte (white star). No polymers are seen in intravascular erythrocytes. (original magnification × 7,500). (D) Reticulocyte traversing a wide gap in the sinusoidal barrier (SE), showing pointed extensions with polymer bundles oriented in the direction of the flow (arrow). A large geometric conglomerate of polymer bundles is seen in adjacent extravascular reticulocytes (arrowhead). Reticulocyte fragments (black star) are present next to reticulocyte in process of migrating through a narrow gap. Polymers are absent in the intravascular erythrocyte (original magnification × 3,000).
Quantification of Erythroid Precursors With Hemoglobin Polymers
| Mice . | n . | % of Cells With Polymers . | |
|---|---|---|---|
| Normoblasts . | Reticulocytes . | ||
| Controls | 6 | 0 | 0 |
| SAD | 5 | 21 ± 5 | 43 ± 10* |
| SAD/βd3 | 3 | 36 ± 6† | 62 ± 14*,† |
| Mice . | n . | % of Cells With Polymers . | |
|---|---|---|---|
| Normoblasts . | Reticulocytes . | ||
| Controls | 6 | 0 | 0 |
| SAD | 5 | 21 ± 5 | 43 ± 10* |
| SAD/βd3 | 3 | 36 ± 6† | 62 ± 14*,† |
Data represent the mean ± SD. P values determined by the Student’s t-test.
.01 < P < .05 v normoblasts.
.01 < P < .05 v SAD.
To determine whether the polymerization characteristics of the erythroid cell lineage were affected by the intracellular sickle hemoglobin concentrations, we have compared the SAD mice erythrons (19% hemoglobin SAD) with those of the SAD/βd3 mice (26% hemoglobin SAD).17 Previous analysis of mature RBCs of SAD/βd3 mice showed balanced hemoglobin chains and did not show any of the ultrastructural features described in human β-thalassemia, such as Heinz bodies, remnants of membranes or organelles, or iron accumulation.18 In comparison with SAD mice, polymers were observed in a significantly larger proportion of differentiating erythroid cells in SAD/βd3 mice: 36% ± 6% for late normoblasts and 62% ± 14% for reticulocytes (Table 1). Higher levels of hemoglobin SAD in erythroid cells thus appear to induce a higher number of cells with polymer fibers. However, the polymer structures were similar in diameter and length for both SAD and SAD/βd3 mice, as previously documented.18
Functional Analysis of SAD Erythropoiesis/Hematopoiesis
To determine whether these histopathologic features of hemoglobin polymers and morphological alterations affecting late erythroid precursors cells have functional consequences on more primitive progenitor cells of the SAD erythroid lineage, we have analyzed the progenitor cells from bone marrow and spleen in normal oxygenated steady-state condition. We have evaluated the proliferation and differentiation potential of these progenitors using culture assays that give rise to differentiated colonies that represent initial hematopoietic progenitors. Sixteen independent experiments were performed using femur bone marrow from 33 SAD mice and 30 C57BL/6 mice. A similar amount of nucleated cells per femur were obtained from C57BL/6 (9.3 × 106 ± 2.1 × 106cells) and SAD (9.7 × 106 ± 2.3 × 106 cells). A general decrease in the number of CFU-E from SAD mice as compared with C57BL/6 mice was detected by colony assays (Table 2). An even more important difference was observed in the number of SAD BFU-E early progenitors: all SAD mice showed a very significant decrease in colony numbers compared with C57BL/6 mice. Nevertheless, the morphology of CFU-E and of BFU-E colonies was very similar in SAD and control mice as defined by size, shape, and color. In contrast, we detected no significant difference in the numbers of CFU-GM and CFU-M between SAD and C57BL/6 mice, indicating that only the erythroid lineage was affected (Table2).
Alterations of Hematopoiesis in Bone Marrow and Spleen of SAD Mice
| . | n . | CFU-E . | BFU-E . | CFU-GM . | CFU-M . | CFU-GEMM . |
|---|---|---|---|---|---|---|
| Bone marrow | ||||||
| No. of progenitors/femur | ||||||
| C57BL/6 | 30 | 9,800 ± 3,800 | 2,100 ± 900 | 2,900 ± 600 | 1,400 ± 400 | 400 ± 100 |
| SAD | 33 | 7,700 ± 3,200 | 1,200 ± 800 | 3,200 ± 900 | 1,400 ± 400 | 500 ± 100 |
| P value | .024 | .00021 | .45 | .97 | .16 | |
| % Proliferating progenitors | ||||||
| C57BL/6 | 19* | 55 ± 17 | 54 ± 18 | 40 ± 12 | 35 ± 15 | 39 ± 21 |
| SAD | 19* | 67 ± 13 | 75 ± 13 | 40 ± 17 | 42 ± 17 | 55 ± 16 |
| Pvalue | .022 | .00039 | .89 | .25 | .021 | |
| Spleen | ||||||
| No. of progenitors/spleen | ||||||
| C57BL/6 | 8 | 1,000 ± 500 | 2,600 ± 1,100 | 1,900 ± 1,000 | 400 ± 400 | 500 ± 300 |
| SAD | 12 | 8,000 ± 6,800 | 1,900 ± 1,400 | 2,400 ± 1,100 | 600 ± 600 | 700 ± 300 |
| P value | .0043 | .26 | .45 | .57 | .39 | |
| % Proliferating progenitors | ||||||
| C57BL/6 | 6* | 49 ± 23 | 28 ± 16 | 36 ± 6 | ND | ND |
| SAD | 7* | 72 ± 12 | 54 ± 21 | 54 ± 21 | ND | ND |
| P value | .085 | .026 | .33 | NA | NA |
| . | n . | CFU-E . | BFU-E . | CFU-GM . | CFU-M . | CFU-GEMM . |
|---|---|---|---|---|---|---|
| Bone marrow | ||||||
| No. of progenitors/femur | ||||||
| C57BL/6 | 30 | 9,800 ± 3,800 | 2,100 ± 900 | 2,900 ± 600 | 1,400 ± 400 | 400 ± 100 |
| SAD | 33 | 7,700 ± 3,200 | 1,200 ± 800 | 3,200 ± 900 | 1,400 ± 400 | 500 ± 100 |
| P value | .024 | .00021 | .45 | .97 | .16 | |
| % Proliferating progenitors | ||||||
| C57BL/6 | 19* | 55 ± 17 | 54 ± 18 | 40 ± 12 | 35 ± 15 | 39 ± 21 |
| SAD | 19* | 67 ± 13 | 75 ± 13 | 40 ± 17 | 42 ± 17 | 55 ± 16 |
| Pvalue | .022 | .00039 | .89 | .25 | .021 | |
| Spleen | ||||||
| No. of progenitors/spleen | ||||||
| C57BL/6 | 8 | 1,000 ± 500 | 2,600 ± 1,100 | 1,900 ± 1,000 | 400 ± 400 | 500 ± 300 |
| SAD | 12 | 8,000 ± 6,800 | 1,900 ± 1,400 | 2,400 ± 1,100 | 600 ± 600 | 700 ± 300 |
| P value | .0043 | .26 | .45 | .57 | .39 | |
| % Proliferating progenitors | ||||||
| C57BL/6 | 6* | 49 ± 23 | 28 ± 16 | 36 ± 6 | ND | ND |
| SAD | 7* | 72 ± 12 | 54 ± 21 | 54 ± 21 | ND | ND |
| P value | .085 | .026 | .33 | NA | NA |
Bone marrow cells were plated at 105 cells/mL and at 1.5 × 105 cells/mL for cycling experiments, whereas spleen cells were plated at 5 × 105 cells/mL for both experiments. Values shown are mean ± SD; n = number of independent mice analyzed. P values determined by the Student’s t-test.
Abbreviations: ND, not determined; NA, not applicable.
The percentage of proliferating progenitors was assayed only on the last (consecutive) mice analyzed that correspond to a subgroup of the mice used to quantify the numbers of progenitors/femur or spleen.
Because both the number of CFU-E and BFU-E of SAD mice were significantly decreased compared with those of controls, we sought to evaluate the proliferative potential of these progenitors and the probable consequence on the sickle phenotype. Table 2 shows that the percentages of cycling CFU-E and BFU-E of SAD mice (n = 19) were increased compared with controls (n = 19) and reached levels at which more than two thirds of the individual pools of erythroid progenitors are in a proliferating state. Data from 8 thymidine suicide assays demonstrated that, in addition to the CFU-E and BFU-E progenitors of SAD mice, cycling of the multipotent CFU-GEMM was also significantly increased (Table 2). No increase in proliferative status was observed for SAD CFU-GM and CFU-M progenitors in bone marrow.
Because the spleen was considerably enlarged in SAD mice, we investigated its role in SAD erythropoiesis. The spleen/body weight of SAD mice (n = 24; 0.52% ± 0.37%) was increased 2-fold compared with C57BL/6 controls (n = 19; 0.26% ± 0.09%). Correlating with the spleen enlargement, the histomorphologic studies of the SAD mouse showed extensive splenic sequestration of RBCs, erythrophagocytosis, and hematopoiesis. The absence of severe anemia in SAD mice observed at the adult stage17 suggests the existence of a compensatory mechanism from the spleen. To verify this hypothesis, the potential of proliferation and differentiation of splenic erythroid precursors were analyzed. The number of nucleated cells was similar for the control (69.8 × 106 ± 21.9 × 106 cells; n = 8) and SAD mice (68.3 × 106 ± 20.3 × 106 cells; n = 12), indicating that the 2-fold increase of spleen/body weight of SAD mice most likely resulted from high levels of erythrocyte sequestration and congestion. Because morphologic studies showed enrichment in foci of erythroid progenitors, we evaluated the number of splenic CFU-E and BFU-E by colony assays. In all experiments, SAD mouse spleen contained a significant increased numbers of CFU-E (2- to 70-fold) compared with C57BL/6 mouse spleen (Table 2). However, the number of BFU-E in SAD mouse spleen was similar (or slightly decreased) to that of C57BL/6 (Table 2). Furthermore, thymidine suicide assay showed that cycling of CFU-E and BFU-E of SAD mice (n = 7) was increased as compared with C57BL/6 (n = 6), which indicated a strong proliferation pressure on the erythroid lineage.
To determine the importance of the spleen erythropoiesis in SAD mice as a potential compensatory mechanism, we performed splenectomy on control C57BL/6 (n = 11) and SAD (n = 8) male mice. Strikingly, 7 of 8 SAD mice died within few days (mean, 5.5 days) after the splenectomy, whereas all splenectomized control animals survived several weeks without any evidence of adverse effect (P = .00016, Fisher’s exact test). Although the exact cause of death was impossible to define, these results suggest that extramedullary erythropoiesis is essential for survival of SAD mice and that SAD mice are under intense hematopoietic/erythropoietic stress.
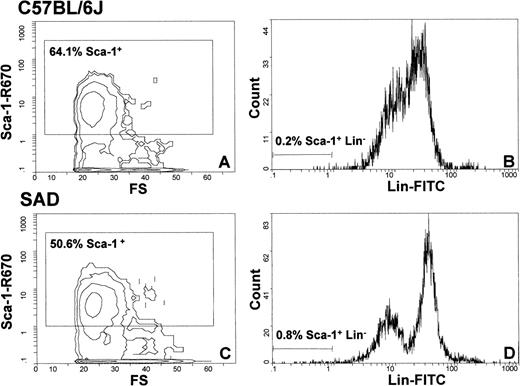
Because the SAD bone marrow CFU-GEMM, BFU-E, and CFU-E showed an increase in cycling, whereas no significant increase in colony numbers was observed, we set out to determine whether SAD bone marrow progenitors could be mobilized (or partially relocated) to the circulation and could then generate extramedullary sites of erythropoiesis by colonization of organs such as the spleen. Thus, to verify our hypothetical mechanism, we determined the levels of hematopoietic/erythropoietic nucleated progenitor cells in the peripheral blood of SAD mice (n = 13) compared with C57BL/6 (n = 13) control mice. Whereas SAD CFU-E were not mobilized into the circulation, we did indeed observe an increase of 3- to 5-fold in the numbers of CFU-GEMM and BFU-E from SAD mouse blood culture compared with C57BL/6 (Table 3). The increase in BFU-E was consistent with the increased BFU-E observed in SCD patients. Furthermore, analysis of splenectomized SAD mice (n = 5) showed an additional increase in the number of circulating multipotent CFU-GEMM (4.7 ± 2.1/mL; P < .02) compared with nonsplenectomized SAD and control mice (Table 3), supporting a mechanism of progenitor uptake by the spleen. Moreover, fluorescence-activated cell sorter (FACS) analysis of SAD peripheral blood (n = 5) demonstrated a significant increase (∼2-to 4-fold) in the Sca-1+Lin− compartment that contains the hematopoietic multipotent cell population (Fig 2). The number of primitive hematopoietic cells, CFU-S12, in peripheral blood was then determined for SAD mice (n = 10) and showed a significant increase of 6.0 ± 3.5 CFU-S12 compared with 1.7 ± 1.0 CFU-S12 for control mice (n = 4; P = .004). In parallel, there was an approximately 4-fold increase in the number of peripheral blood CFU-GM/M (Table 3). The increase in these macrophage precursors correlated with the morphologic observation of high levels of erythrophagocytosis in the spleen. These results suggested that the SAD bone marrow multipotent cells (CFU-S, CFU-GEMM, and Sca-1+Lin−), BFU-E, and CFU-GM/M become mobilized to the circulation, are then taken up by the spleen or other extramedullary sites, and, upon uptake (homing), are immediately differentiated into CFU-E, as shown in our model (Fig 3).
Mobilization of Hematopoietic Progenitors in Peripheral Blood
| Mice . | n . | CFU-E (per mL) . | BFU-E (per mL) . | CFU-GM/M (per mL) . | CFU-GEMM (per mL) . |
|---|---|---|---|---|---|
| C57BL/6 | 13 | 0 | 3.12 ± 2.46 | 3.19 ± 3.43 | 0.54 ± 0.66 |
| SAD | 13 | 0 | 15.74 ± 11.49 | 12.76 ± 10.80 | 1.93 ± 1.37 |
| P value | NA | .0019 | .0085 | .0041 |
| Mice . | n . | CFU-E (per mL) . | BFU-E (per mL) . | CFU-GM/M (per mL) . | CFU-GEMM (per mL) . |
|---|---|---|---|---|---|
| C57BL/6 | 13 | 0 | 3.12 ± 2.46 | 3.19 ± 3.43 | 0.54 ± 0.66 |
| SAD | 13 | 0 | 15.74 ± 11.49 | 12.76 ± 10.80 | 1.93 ± 1.37 |
| P value | NA | .0019 | .0085 | .0041 |
Values shown are mean ± SD; n = number of independent mice analyzed. P values determined by the Student’s t-test.
Abbreviation: NA, not applicable.
Flow cytometry analysis of peripheral blood cells of C57BL/6 (A and B) and SAD (C and D) mice with Sca-1 and lineage-specific antibodies. The enclosed boxes depicted in (A) and (C) represent the total Sca-1+ cells from the entire cell population analyzed. (B) and (D) subdivide the Lin- and Lin+ cells from the total Sca-1+ cell population. The quantity of Sca-1+Lin−multipotent progenitor cells in SAD (0.8%) is increased by 4-fold compared with control mouse (0.2%).
Flow cytometry analysis of peripheral blood cells of C57BL/6 (A and B) and SAD (C and D) mice with Sca-1 and lineage-specific antibodies. The enclosed boxes depicted in (A) and (C) represent the total Sca-1+ cells from the entire cell population analyzed. (B) and (D) subdivide the Lin- and Lin+ cells from the total Sca-1+ cell population. The quantity of Sca-1+Lin−multipotent progenitor cells in SAD (0.8%) is increased by 4-fold compared with control mouse (0.2%).
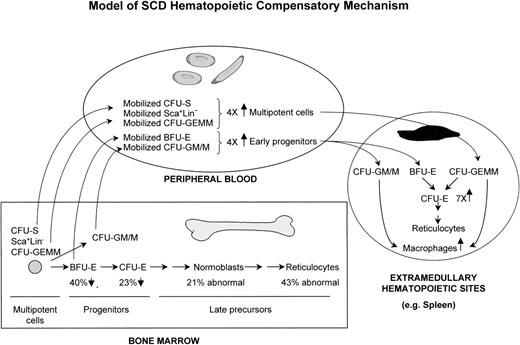
Model of SAD/SCD hematopoietic compensatory mechanism. A significant proportion of multipotent and early progenitors of bone marrow are mobilized to the peripheral blood and homed to several extramedullary hematopoietic sites, with spleen being a major site in SAD mice.
Model of SAD/SCD hematopoietic compensatory mechanism. A significant proportion of multipotent and early progenitors of bone marrow are mobilized to the peripheral blood and homed to several extramedullary hematopoietic sites, with spleen being a major site in SAD mice.
DISCUSSION
An in vivo understanding of the consequences of hemoglobin polymer formation on the properties of the sickled erythroid cells is critical for the comprehension of SCD pathophysiology and, ultimately, for the design of more effective therapeutic strategies. Herein is the first in vivo demonstration in a transgenic mouse model of human SCD that alterations resulting from hemoglobin polymers occurred not only in RBCs, but also in erythroid precursors, leading to an ineffective erythropoiesis, which most likely plays a role in the pathophysiology of SCD. The present studies on the earlier erythroid and multipotent progenitors differentiation, proliferation, and tissue localization have shown the existence of a model of compensatory hematopoietic mechanism in the pathophysiology of SCD that proceeds through several events: (1) a significant increased proliferation of bone marrow multipotent and erythroid progenitor cells consecutive to a severe depletion of the erythroid progenitor pools; (2) a significant mobilization/relocation of bone marrow multipotent and erythroid progenitors to the peripheral blood; (3) a colonization by such peripheral blood progenitors at extramedullary hematopoietic sites, eg, spleen; and (4) a high rate of proliferation/differentiation in the extramedullary sites for additional and complementary supply to the erythrocyte pool.
Our morphologic studies directly show ineffective erythropoiesis, ie, destruction of abnormal erythroid cells before complete maturation and release from bone marrow. Previously, the differentiation and maturation of nucleated erythroid cells had been considered to be unaffected in SCD.4,27 In contrast, our study shows that, even in physiologic conditions, morphologic anomalies can occur not only in RBCs, but also in bone marrow late nucleated erythroid precursors from the polychromatophilic normoblasts to the reticulocytes. Consequent to intramedullary hemoglobin polymerization, normoblasts and reticulocytes showed severe deformity and stretching of the cell membrane that can induce permanent membrane alterations. Intringuingly, hemoglobin polymers also occurred in the nucleus of late normoblasts. Furthermore, because intranuclear polymers were present in late normoblasts of SAD mice, it is likely that, during the process of nuclear expulsion, a variable loss of hemoglobin polymers (and therefore hemoglobin) may occur from cell to cell and may explain the variable intracellular hemoglobin concentration and heterogeneous cell density of RBCs observed in SAD mice18 and in human SCD.28 29 Hemoglobin loss could be further amplified through the cellular fragmentation of reticulocytes. In parallel, fragmented normoblasts and reticulocytes undergo increased phagocytosis associated with iron/ferritin deposits in intramedullary macrophages. We thus propose that, due to the SAD/SCD ineffective erythropoiesis, normal bone marrow transfer to an SAD/SCD host should result in a strong selective advantage of normal donor erythroid precursor cells compared with the recipient sickle cell precursors. Consequently, in bone marrow transfer protocols, a small proportion of normal murine cells should be sufficient to reconstitute most of the mature RBC population.
Consistent with our results in a murine model, the occurrence of ineffective erythropoiesis is compatible with 4 observations in human SCD. First, polymers have been identified in intramedullary reticulocytes in human marrow aspirates.4 Second, sickling of nucleated erythroid precursors can be induced by in vitro deoxygenation.30 Third, despite the limitations of the ferrokinetic measurements, the increased plasma iron turnover in human SCD is suggestive of a defective erythropoiesis.31,32Fourth, 1 SCD patient that received an allogeneic bone marrow transplantation resulting in a chimeric engraftment of only 10% to 20% donor cells became asymptomatic with a majority of normal circulating RBCs.33 In light of our results, this recovery suggests that a selective enrichment or outgrowth of the normal erythroid cells over the defective sickle erythroid cells probably occurred due to their effective maturation and differentiation in the chimeric bone marrow and the better survival of the RBCs in the circulation. Consequently, our murine SCD results and the human SCD findings suggest that complete ablation of defective endogeneous bone marrow is not an absolute requirement for a successful phenotypic cure and that milder (less cytotoxic) conditioning regimens (eg, irradiation) hold better promise than previously expected for curative host engraftment.
In addition to ineffective erythropoiesis, the characterization of primitive hematopoietic progenitor cells have uncovered the mobilization of multipotent cells in the circulation and the existence of a compensatory hematopoietic mechanism in SAD mice (Fig 3). A first compensatory response is detected in the marrow, most likely in an attempt to restore homeostasis and to counterbalance the ineffective erythropoiesis and the short half-life of sickle RBCs. This is demonstrated by the increased proliferation of SAD multipotent cells (CFU-GEMM) and erythroid progenitor cells (BFU-E and CFU-E). Furthermore, an increased differentiation into pronormoblasts and reticulocytes is supported by the reduced number of erythroid progenitors and by the larger population of derived pronormoblasts observed histologically. These bone marrow cellular responses of cell proliferation and differentiation are consistent with the role attributed to erythropoietin in the erythropoietic lineage,34 which is elevated in SAD mice.18 In parallel to the bone marrow response, an increase of several fold in SAD peripheral blood of the multipotent cells (CFU-S, CFU-GEMM, and Sca-1+Lin−) and the highly proliferative early erythroid progenitors (BFU-E) are evidence of a major mobilization from the bone marrow. The relevance of these SAD results to human SCD is substantiated by a similar approximately 4-fold increase in progenitor mobilization to the circulation (unpublished data). Consequent to this mobilization, a significant fraction of these circulating hematopoietic cells are probably homed to the spleen, where they sustain a second level of compensatory erythropoietic/hematopoietic response. This splenic compensatory response is evidenced by (1) an increased proliferation of the homed early BFU-E and CFU-GM progenitors, (2) an increase in the multipotent CFU-GEMM progenitor pool, and (3) an increase in the population of CFU-E and macrophages that must be generated from the differentiation of splenic multipotent CFU-GEMM, early BFU-E, and CFU-GM progenitors (Fig 3 and Table 2). Clearly, the marked increase in the CFU-E cell population must derive from the earlier progenitors, because CFU-E are not present in peripheral blood. Importantly, the critical compensatory role played by the spleen was demonstrated herein for extramedullary hematopoiesis, by the increase in circulating multipotent CFU-GEMM in splenectomized SAD mice and for the survival of SAD mice, and by the short-term death of splenectomized SAD mice. In human SCD, the presence of erythroid foci in the spleen and other organs/tissues suggests that such extramedullary compensatory mechanism may also occur in patients.35-40
Mobilization of the SAD multipotent and early progenitor cells in peripheral blood can be triggered in response to a change in hematopoietic microenvironment and/or in progenitor properties in the marrow. Although the molecular mechanism underlying hematopoietic mobilization is poorly understood, it has been shown that administration of specific cytokines in mice is associated with increased number of circulating progenitors.41,42Interestingly, SAD mice have been shown to have increased levels of several cytokines such as interleukin-1,43 which in association with other cytokines has previously been shown to induce mobilization. The SAD/SCD mouse model thus provides a naturally occurring state to define the molecular mechanisms that regulate mobilization/homing occurring in a clinically relevant disease, SCD.
In conclusion, chronic SAD/SCD displays ineffective erythropoiesis throughout differentiation and maturation. This suggests that normal donor cells in allogeneic bone marrow transplantation may have a strong selective advantage. Furthermore, the SAD/SCD systemic studies have shown a dynamic compensatory erythropoietic/hematopoietic mechanism altering the bone marrow, peripheral blood, and extramedullary progenitor pools. Importantly, because of the characteristic cell pool expansion, increased proliferation, and mobilization of SAD multipotent progenitors demonstrated herein, gene targeting to the sickled hematopoietic stem cells in SAD mice and possibly in SCD patients should be more efficient than expected, with major impact for significant bone marrow hematopoietic reconstitution and, thus, correction of SAD/SCD phenotype.
ACKNOWLEDGMENT
The authors are very grateful to Drs Muriel Aubry and George Atweh for helpful review of the manuscript and to Caroline Lagacé for technical assistance.
Supported by the Medical Research Council of Canada. M.T. is a chercheur-boursier of the FRSQ.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marie Trudel, PhD, 110 ouest ave. des Pins, Montreal, Quebec, Canada H2W1R7; e-mail: trudelm@IRCM.qc.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal