Abstract
Activation of specific cytokine receptors promotes survival and proliferation of hematopoietic progenitor cells but their role in the control of differentiation is unclear. To address this issue, the effects of human interleukin-3 (hIL-3) and human granulocyte-macrophage colony-stimulating factor (hGM-CSF) on hematopoietic development were investigated in hematopoietic progenitor cells. Murine multipotent factor-dependent cell-Paterson (FDCP)-mix cells, which can self-renew or differentiate, were transfected with the genes encoding the unique and/or shared βc human hIL-3 receptor (hIL-3 R) or hGM-CSF receptor (hGM R) subunits by retroviral gene transfer. Selective activation of hIL-3 R,βc or hGM R,βc transfects by hIL-3 and hGM-CSF promoted self-renewal and myeloid differentiation, respectively, over a range of cytokine (0.1 to 100 ng/mL) concentrations. These qualitatively distinct developmental outcomes were associated with different patterns of protein tyrosine phosphorylation and, thus, differential signaling pathway activation. The cell lines generated provide a model to investigate molecular events underlying self-renewal and differentiation and indicate that the subunits act in combination with the hβc to govern developmental decisions. The role of the subunit in conferring specificity was studied by using a chimeric receptor composed of the extracellular hIL-3 R and intracellular hGM R subunit domains. This receptor promoted differentiation in response to hIL-3. Thus, the subunit cytosolic domain is an essential component in determining cell fate via specific signaling events.
RECEPTORS OF THE CYTOKINE receptor superfamily are present on primitive hematopoietic progenitor cells1,2 and activation by their cognate cytokines regulates hematopoietic cell survival and proliferation. The receptors for interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) are members of a subfamily of cytokine receptors and are composed of α and β subunits. They contain common structural motifs found in many other cytokine receptors.3 The α subunits that bind IL-3 or GM-CSF are related but unique to each of the receptors. They bind their cognate ligand with low affinity and interact with a common β subunit (βc) to form a functional high affinity receptor complex.4 The βc subunit lacks the ability to bind either of these cytokines independently, is structurally related to the IL-2 receptor β chain or gp130 subunit of the IL-6 receptor family, and is believed to elicit most, if not all, of the signaling events emanating from these receptors.5
IL-3 and GM-CSF both promote the survival and proliferation of multipotent cells; IL-3 stimulates the development of multilineage colonies from normal bone marrow, and GM-CSF promotes the production of granulocytes and macrophages.6-11 Thus, receptors for IL-3 and GM-CSF promote both overlapping and distinct biologic effects in hematopoietic cells. The specificity of the cytokine-mediated response may be conferred either by expression of the GM-CSF and/or IL-3 receptor α subunits on different cells or by differential effects elicited in response to receptor α subunit interaction with the β subunits. The precise role of the IL-3 and GM-CSF receptors in the regulation of hematopoietic progenitor cell differentiation is unclear.
We have investigated the function of the IL-3 and GM-CSF cytokine receptors in hematopoietic cell development by using the murine multipotential hematopoietic cell line, FDCP-mix (clone A4). These cells are nonleukemic, karyotypically normal, and their survival, proliferation, and development are subject to regulation by cytokines. Relatively high concentrations of murine IL-3 promote self-renewal12 and low concentrations of IL-3, in combination with other cytokines such as GM-CSF or erythropoietin, promote differentiation into granulocytes and macrophages or into mature erythroid cells.13,14 Removal of IL-3 results in cell death via apoptosis.15
IL-3 and GM-CSF are species specific; therefore, human IL-3 (hIL-3) and human GM-CSF (hGM-CSF) selectively activate hIL-3 and GM-CSF receptors only. The relative contributions of the hIL-3 receptor α (hIL-3 Rα), hGM-CSF Rα (hGM Rα), and hβc subunits in hematopoietic progenitor cell development could, therefore, be investigated by transfection into the murine FDCP-mix cell line and activation by addition of the cognate human cytokine. This experimental approach allowed the function(s) of the human receptor subunits to be studied in the context of a multipotential hematopoietic progenitor cell line. Transfection of mutated receptor subunits also enabled structure-function analysis of the human receptors to be performed. Here, we specifically address the effects on IL-3 and GM-CSF on development, unlike many previous studies on IL-3 and GM-CSF receptor function, which have been performed in leukemic or differentiation blocked cell lines16 17 and show a role for the α subunits in determining cell fate.
MATERIALS AND METHODS
FDCP-mix (clone A4) cells were routinely cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 5% (vol/vol) medium conditioned by the X63-Ag-653 cell line (used as a source of murine IL-3)18 and 20% (vol/vol) horse serum. For differentiation assays, cells were cultured in IMDM, 20% fetal calf serum (FCS), and the appropriate cytokine(s).13 In soft-gel assays, cells were cultured in IMDM, 20% (vol/vol) horse serum, 1% (wt/vol) bovine serum albumin (BSA), the appropriate cytokine, and 0.33% (vol/vol) agar.12 Colonies were incubated at 37°C in 5% CO2, 5% O2, and N2 for 7 days before analysis.
Cytokines.
Recombinant hIL-3 and hGM-CSF were gifts from Sandoz Pharma (Basel, Switzerland) and Glaxo (Greenford, UK), respectively. Recombinant mIL-3 (4 × 107 U/mg) and hG-CSF (108 U/mg) were obtained from R&D Systems (Abingdon, UK) and Chugai (Geneva, Switzerland), respectively. Murine (m) GM-CSF (1.25 × 107 U/mg) was a gift from Biogen (Geneva, Switzerland).
Transfection of FDCP-mix cells with subunits of the hIL-3 and hGM-CSF receptors.
Retroviral transfections were performed by using the pM5 vector containing the receptor subunit gene and an antibiotic resistance gene as a selectable marker.19 In the case of the α subunits, this was neomycin phosphotransferase (neo) and for the hβc, hygromycin phosphatase (hgr). All receptor subunits were cloned into the BamHI site of the pM5 retroviral vector. The chimeric receptor α subunit was produced by introducing an NheI restriction site (position 1093 for the hIL-3 Rα and 1142 for the hGM Rα) into the transmembrane domain by site-directed mutagenesis. The mutagenesis was performed on double-stranded DNA with the Chameleon ds mutagenesis kit (Stratagene, La Jolla, CA) in accordance with the manufacturer’s instructions. The chimeric receptor subunit was produced by ligating the extracellular domain of the hIL-3 Rα to the intracellular domain of the hGM Rα at the NheI site. All mutations and constructs were confirmed by DNA sequencing. Retroviral vectors containing the antibiotic resistance gene(s) were used as controls. The receptor constructs were lipofected into the GP+envAM12 murine fibroblast packaging cell line20 by using Lipofectamine (GIBCO, Paisley, UK). Retroviral transfection of FDCP-mix cells was performed by coculture with the packaging cell line for 48 hours. FDCP-mix cells were harvested, washed, and selected for antibiotic resistance by culturing in medium supplemented with 1 mg/mL G418 and/or 0.15 mg/mL hygromycin B, as appropriate. Polyclonal cell populations were labeled for flow cytometric analysis with antibodies directed against the extracellular domain of the receptor subunits (see below). After flow cytometric analysis, clonal populations were generated from single cells sorted into 96-well culture plates by using the Automatic Cell Deposition Unit facility of the fluorescence-activated cell sorter (FACS) Vantage flow cytometer (Becton Dickinson, Cowley, UK). For each set of experiments, data are shown from a clone representative of the multiple transfectants tested.
Analysis of human IL-3 and GM-CSF receptor expression.
Ectopic receptor expression was confirmed by flow cytometry by using a 2-step antibody labeling procedure. Cells expressing the hGM Rα were identified by using an anti-hGM Rα monoclonal antibody (Santa Cruz) and detected after incubation with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Becton Dickinson). For hIL-3 Rα and hβc, biotinylated anti-hIL-3 Rα and anti-hβc antibodies (Cambridge BioScience, Cambridge, UK) were used followed by incubation with FITC-avidin and streptavidin phycoerythrin (PE) (Becton Dickinson), respectively. Flow cytometric analysis was performed with a FACS Vantage flow cytometer (Becton Dickinson).
Scatchard analysis.
FDCP-mix cells were washed once in IMDM (4°C), centrifuged, and resuspended in phosphate-buffered saline (PBS), pH 3, for 1 minute to remove cytokine bound to receptors. The cells were then pelleted and resuspended in binding buffer (Dulbecco’s modified Eagle’s medium containing 0.02% sodium azide, 25 mmol/L HEPES, 0.1% [wt/vol] BSA, pH 7.4). Binding of [125I] hGM-CSF (specific activity, 1,820 Ci/mmol) or [125I] hIL-3 (specific activity, 543 Ci/mmol) was assayed.21 Nonspecific binding was calculated by using a 100-fold excess concentration of unlabeled hGM-CSF or hIL-3, as appropriate.
Measurement of proliferation.
DNA synthesis was used as a measure of proliferation and was performed by determining incorporation of [3H] thymidine as previously described.22 Briefly, cells were washed and incubated with cytokines (5 × 104 cells/sample unless otherwise stated) for 16 hours, pulsed for 4 hours with [3H] thymidine, harvested by using a DYNATECH cell harvester (DYNATECH, Billingshurst, UK), and the incorporated radioactivity measured by scintillation counting.
Morphologic analysis.
A morphologic analysis of cells in liquid culture was performed as described.12 Slides were prepared with a Shandon cytospin centrifuge (Shandon, Runcorn, UK) and stained with May-Grünwald-Giemsa stain. At least 100 cells were scored for each slide.
Granulocyte-macrophage differentiation assay of FDCP-mix cells.
FDCP-mix cells in the logarithmic growth phase were washed to remove growth factors and resuspended in IMDM supplemented with 20% (vol/vol) FCS, 0.01 ng/mL IL-3, and 10% (vol/vol) mouse lung conditioned medium.23 After 7 days in culture, cells were counted and cytospin preparations made.
Analysis of differentiation markers.
Cell-surface expression of Mac-1 (CD11b) was analyzed by flow cytometry. Cells were labeled by using anti–Mac-1 antibody (Pharmingen) followed by PE-conjugated anti-rat antibody (Becton Dickinson). Nonspecific labeling was assessed with primary antibodies of the corresponding isotype. Flow cytometric analysis was performed by using a FACS Vantage flow cytometer (Becton Dickinson).
Analysis of protein tyrosine phosphorylation.
Tyrosine phosphorylation of intracellular proteins was analyzed by Western blotting with a monoclonal mouse antiphosphotyrosine antibody (TCS, Botolph Claydon, UK) as previously described.22 Briefly, cell lysates were prepared in buffer containing 50 mmol/L Tris Acetate (pH 7.5), 1% (vol/vol) NP-40, 1 mmol/L EDTA, 1 mmol/L EGTA, 120 mmol/L NaCl, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL pepstatin A, benzamidine, antipain, aprotinin, TLCK, and TPCK. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose (Hybond C; Amersham), and immunoblotted by using a mouse monoclonal antiphosphotyrosine antibody. The protein-antibody complexes were visualized by ECL (Pierce, UK).
RESULTS
Retroviral mediated gene transfer of hIL-3 R and hGM R subunits into FDCP-mix cells.
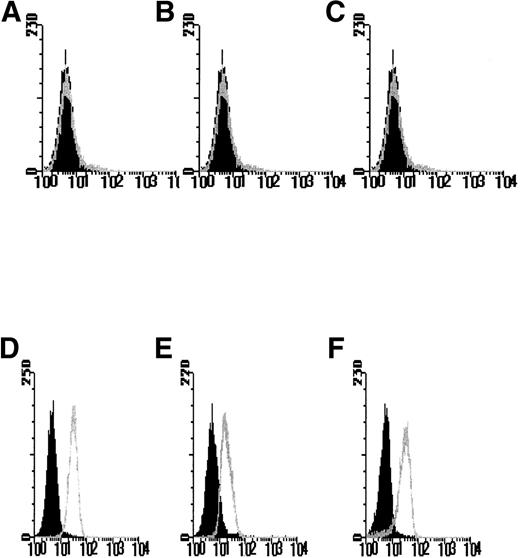
The use of 2 selectable markers and retroviral mediated-gene transfer allowed the generation of FDCP-mix cell lines expressing the hIL-3 or hGM-CSF receptor α subunit genes, both alone and in combination with the hβc subunit. After antibiotic selection, the polyclonal cell populations were analyzed for human receptor gene expression by flow cytometry with antibodies directed against the extracellular domain of the hIL-3 R and hGM R α and/or hβc subunits. Dual transfectants expressing both the hIL-3 Rα or hGM Rα together with hβc were assessed by using 2-color analysis with FITC- and PE-conjugated secondary antibodies to label the transfected human α and βcsubunits, respectively. Clonal cell populations were generated from cells identified and sorted on the basis of ectopic expression of hIL-3 R or hGM R subunits. Flow cytometric analysis was performed to verify ectopic expression of the human receptor subunit in the clonal populations obtained. No specific fluorescence labeling of the parental FDCP-mix cells was detected with anti–hIL-3 Rα, anti-hGM Rα, or anti-hβc antibodies (Figs 1A-C). However, in the appropriate clones, expression of hIL-3 Rα, hGM Rα, or hβc was evident as shown by a log increase in fluorescence (Figs 1D-F) relative to the parental cells. Cells transfected with the control retroviral vector containing the antibiotic resistance gene(s) showed a similar pattern of labeling to the parental FDCP-mix cells (data not shown).
FACS profiles of FDCP-mix cells transfected with hIL-3 and hGM-CSF receptor subunits. Cells were labeled with anti–hIL-3 and hGM-CSF receptor subunit antibodies in a 2-step procedure. The black histograms denote nonspecific fluorescence obtained with the secondary fluorescent reagent only and are overlaid with the histograms obtained after labeling with both the primary antireceptor subunit antibody and secondary reagent. Isotype control antibodies gave similar flow profiles to those obtained by using the secondary reagent only (data not shown). Results are shown for expression of the (A and D) hIL-3 R, (B and E) hGM R and (C and F) hβc receptor subunits by the (A-C) parental FDCP-mix cells and cells transfected with (D-F) hIL-3 R, hGM R, and hβc subunits and are representative of least 6 such experiments using different clones expressing hIL-3 R, hGM R, and hβc subunits, respectively. Similar profiles were obtained for cells coexpressing hβc subunits and either hIL-3 R or hGM R subunits.
FACS profiles of FDCP-mix cells transfected with hIL-3 and hGM-CSF receptor subunits. Cells were labeled with anti–hIL-3 and hGM-CSF receptor subunit antibodies in a 2-step procedure. The black histograms denote nonspecific fluorescence obtained with the secondary fluorescent reagent only and are overlaid with the histograms obtained after labeling with both the primary antireceptor subunit antibody and secondary reagent. Isotype control antibodies gave similar flow profiles to those obtained by using the secondary reagent only (data not shown). Results are shown for expression of the (A and D) hIL-3 R, (B and E) hGM R and (C and F) hβc receptor subunits by the (A-C) parental FDCP-mix cells and cells transfected with (D-F) hIL-3 R, hGM R, and hβc subunits and are representative of least 6 such experiments using different clones expressing hIL-3 R, hGM R, and hβc subunits, respectively. Similar profiles were obtained for cells coexpressing hβc subunits and either hIL-3 R or hGM R subunits.
To avoid biasing the differentiation or developmental potential of the cells before experimentation, cells were always cultured in mIL-3 rather than hIL-3 or hGM-CSF throughout the selection procedure. The investigation of the human receptor subunits’ effects in the control of differentiation required that the developmental potential of the FDCP-mix cells was maintained. The cell lines generated were checked and found capable of differentiation in response to cytokines that promote differentiation of the parental FDCP-mix cells.13
Characterization of the FDCP-mix hGM-CSF and hIL-3 receptor transfects.
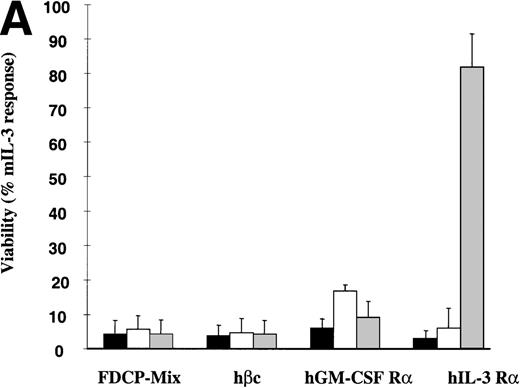
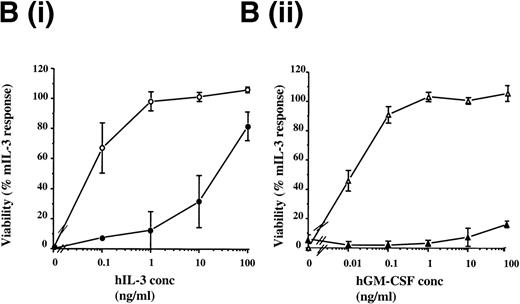
The hIL-3 and hGM-CSF responsiveness of the FDCP-mix hIL-3 R and hGM R subunit transfects were initially assessed by determining the effects of the human cytokines on cell viability. FDCP-mix cells expressing hGM Rα or hIL-3 Rα (hGM Rα cells and hIL-3 Rα cells) survived in response to the cognate human cytokine (Fig2A). The addition of hIL-3 promoted survival of hIL-3 Rα cells similar to that obtained in the presence of 10 ng/mL mIL-3, whereas hGM-CSF provided a relatively weak survival stimulus in hGM Rα cells. The relatively small response to hGM-CSF was not because of a discrete subpopulation of the FDCP-mix cells expressing the hGM Rα subunit; as flow cytometric analysis of this clonal population before experimentation indicated, there was a single population of cells expressing the hGM Rα subunit (Fig 1E). Notably, the response of the hIL-3 Rα and hGM Rα populations was only observed at relatively high concentrations (100 ng/mL) of the human cytokines (Fig 2B). This is consistent with previous reports indicating that there is a weak interaction between ectopic human α subunits and endogenous murine β subunits, resulting in formation of low affinity receptors.24-26
Effects of hIL-3 and hGM-CSF on survival of FDCP-mix cell transfects. (A) Cell viability was assessed either in the absence of cytokines (▪) or in the presence of 100 ng/mL hGM-CSF (□) or 100 ng/mL hIL-3 ( ). Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 2 experiments ± SEM. (B) Dose-response of FDCP-mix cells expressing (i) hIL-3 R (•) or hIL-3 R/βc (○) to hIL-3 (ii) hGM R (▴) or hGM R/βc (▵) to hGM-CSF. Cell viability was assessed in the presence of hIL-3 or hGM-CSF (0 to 100 ng/mL) by trypan blue exclusion after 48 hours culture. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained with at least 4 clones of each transfect.
). Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 2 experiments ± SEM. (B) Dose-response of FDCP-mix cells expressing (i) hIL-3 R (•) or hIL-3 R/βc (○) to hIL-3 (ii) hGM R (▴) or hGM R/βc (▵) to hGM-CSF. Cell viability was assessed in the presence of hIL-3 or hGM-CSF (0 to 100 ng/mL) by trypan blue exclusion after 48 hours culture. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained with at least 4 clones of each transfect.
Effects of hIL-3 and hGM-CSF on survival of FDCP-mix cell transfects. (A) Cell viability was assessed either in the absence of cytokines (▪) or in the presence of 100 ng/mL hGM-CSF (□) or 100 ng/mL hIL-3 ( ). Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 2 experiments ± SEM. (B) Dose-response of FDCP-mix cells expressing (i) hIL-3 R (•) or hIL-3 R/βc (○) to hIL-3 (ii) hGM R (▴) or hGM R/βc (▵) to hGM-CSF. Cell viability was assessed in the presence of hIL-3 or hGM-CSF (0 to 100 ng/mL) by trypan blue exclusion after 48 hours culture. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained with at least 4 clones of each transfect.
). Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 2 experiments ± SEM. (B) Dose-response of FDCP-mix cells expressing (i) hIL-3 R (•) or hIL-3 R/βc (○) to hIL-3 (ii) hGM R (▴) or hGM R/βc (▵) to hGM-CSF. Cell viability was assessed in the presence of hIL-3 or hGM-CSF (0 to 100 ng/mL) by trypan blue exclusion after 48 hours culture. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained with at least 4 clones of each transfect.
FDCP-mix cells expressing the hβc subunit (hβc cells) were nonresponsive to hIL-3 or human GM-CSF. However, cells coexpressing the hβc subunit together with the hIL-3 Rα subunit (hIL-3 Rα,βc cells) or hGM Rα subunit (hGM Rα,βc cells) responded to the cognate cytokine at concentrations for which cells singly transfected with hIL-3 Rα and hGM Rα cells were unresponsive (Fig 2B). The addition of hIL-3 and hGM-CSF promoted cellular proliferation of the hIL-3 Rα,βc and hGM Rα,βc transfects, respectively, as assessed by [3H] thymidine incorporation assays (Fig 3). The dissociation constant (kd) values for the receptors in FDCP-mix cells transfected with the hβc subunit together with either the hIL-3 Rα or hGM Rα were 269 pmol/L and 324 pmol/L, respectively, as determined by the Scatchard analysis (data not shown). These results are consistent with the presence of high affinity receptor sites.27-30 The hIL-3 R and hGM-CSF R were expressed at more than 1,000 receptors per cell with levels ranging between 1,200 to 17,000 receptors per cell. The same biologic response was stimulated by the cognate human cytokine in all cell lines over a range of cytokine concentrations (0.1 to 100 ng/mL) (Fig 6B). Thus, the transfected receptors were expressed at high levels relative to endogenous cytokine receptors on parental FDCP-mix cells and hematopoietic progenitor cells, which are present at 20 to approximately 100 receptors per cell.2,21 31
Effects of hIL-3 and hGM-CSF on proliferation of FDCP-mix cells coexpressing hIL-3 R,βc or hGM R,βc. Proliferation was assessed by determining [3H] thymidine incorporation after culturing for 16 hours at 37°C. FDCP-mix cells expressing (A) hIL-3 R /βcand (B) hGM R /βc were stimulated by hIL-3 (0.01 to 100 ng/mL) and hGM-CSF (0.1 to 100 ng/mL), respectively. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained with at least 4 clones of each transfect.
Effects of hIL-3 and hGM-CSF on proliferation of FDCP-mix cells coexpressing hIL-3 R,βc or hGM R,βc. Proliferation was assessed by determining [3H] thymidine incorporation after culturing for 16 hours at 37°C. FDCP-mix cells expressing (A) hIL-3 R /βcand (B) hGM R /βc were stimulated by hIL-3 (0.01 to 100 ng/mL) and hGM-CSF (0.1 to 100 ng/mL), respectively. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained with at least 4 clones of each transfect.
hGM-CSF and hIL-3 stimulate differential signaling events in transfected FDCP-mix cells.
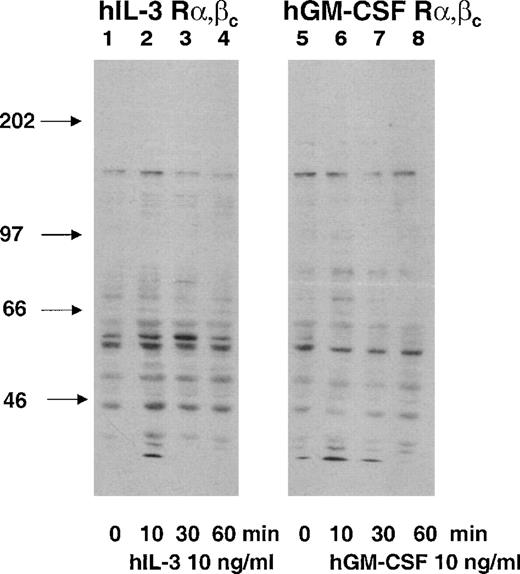
Cytokine receptors mediate their effects by promoting intracellular signaling pathways. Tyrosine phosphorylation of proteins is an initial signal transduction event in response to IL-3 and GM-CSF.32Tyrosine phosphorylation was assessed and compared by antiphosphotyrosine Western blotting of cell lysates prepared from the hGM Rα,βc and the hIL-3 Rα,βc cells after stimulation with their cognate cytokines. Cells were stimulated with hIL-3 or hGM-CSF at a concentration for which the singly transfected hIL-3 Rα and hGM Rα were nonresponsive (10 ng/mL). The effects of hIL-3 and hGM-CSF on the dual-transfected hGM Rα,βc and hIL-3 Rα,βc cell populations could, therefore, be attributed to interaction of the hIL-3 Rα and hGM Rα subunits with hβc and not endogenous murine β subunits (Figs 2 and 3). There were differences in the patterns of tyrosine phosphorylation observed in response to hIL-3 and hGM-CSF. The major differences were that a protein of molecular weight (Mr), approximately 80 kD, was tyrosine phosphorylated in response to hGM-CSF but not hIL-3 and an Mr 46-kD protein was more heavily phosphorylated in response to hIL-3 than hGM-CSF (Fig4). Next, it was determined whether or not the differences in hIL-3 and hGM-CSF signaling were associated with differential biologic responses.
Protein tyrosine phosphorylation in response to hIL-3 and hGM-CSF in hIL-3 R,βc and hGM R,βccells, respectively. Cells were washed and incubated growth-factor free for 4 hours before stimulation with 10 ng/mL hIL-3 or hGM-CSF as appropriate for 10, 30, and 60 minutes (lanes 2, 3, 4, and 6, 7, 8, respectively). Cell lysates were prepared and resolved by SDS-PAGE using a 7.5% gel before Western blotting using an antiphosphotyrosine antibody. Lanes 1 through 4 and 5 through 8 are cell lysates prepared from hIL-3 R,βc and hGM R,βc cells, respectively. Lane 1 and 5 are control samples (cytokine diluent only). Arrows indicate molecular weights of the molecular-weight markers. Similar results were obtained with at least 2 clones of each transfect.
Protein tyrosine phosphorylation in response to hIL-3 and hGM-CSF in hIL-3 R,βc and hGM R,βccells, respectively. Cells were washed and incubated growth-factor free for 4 hours before stimulation with 10 ng/mL hIL-3 or hGM-CSF as appropriate for 10, 30, and 60 minutes (lanes 2, 3, 4, and 6, 7, 8, respectively). Cell lysates were prepared and resolved by SDS-PAGE using a 7.5% gel before Western blotting using an antiphosphotyrosine antibody. Lanes 1 through 4 and 5 through 8 are cell lysates prepared from hIL-3 R,βc and hGM R,βc cells, respectively. Lane 1 and 5 are control samples (cytokine diluent only). Arrows indicate molecular weights of the molecular-weight markers. Similar results were obtained with at least 2 clones of each transfect.
The effects of hIL-3 and hGM-CSF on the clonogenic potential of FDCP-mix cells coexpressing human α subunits with hβc subunits.
To compare the effects of hIL-3 and hGM-CSF on development, their potential to stimulate proliferation and long-term expansion of the FDCP-mix receptor transfectants was assessed. The addition of hIL-3 promoted expansion of hIL-3 Rα,βc cells at a rate similar to that obtained in response to mIL-3, which is the cytokine in which FDCP-mix cells are routinely cultured (Table1). In contrast, although hGM-CSF was capable of stimulating [3H] thymidine incorporation over a short period (Fig 3), it promoted only limited population expansion compared with hIL-3 stimulation of hIL-3 Rα,βc cells (Table 1). Thus, although both hGM-CSF and hIL-3 promote proliferation of cells expressing hGM Rα,βc or hIL-3 Rα,βc, respectively, only hIL-3 was able to stimulate a persistent expansion or maintenance of the population.
Population Expansion of hGM R,βc Cells and hIL-3 R,βc Cells Continuously Cultured in the Presence of 10 ng/mL hGM-CSF and hIL-3, Respectively
| Days in Culture . | hIL-3 Rα,βc Cells . | hGM Rα,βc Cells . |
|---|---|---|
| 2 | 4.5 ± 1.5 | 1.1 ± 0.1 |
| 7 | 546 ± 25* | 4.3 ± 0.5 |
| Days in Culture . | hIL-3 Rα,βc Cells . | hGM Rα,βc Cells . |
|---|---|---|
| 2 | 4.5 ± 1.5 | 1.1 ± 0.1 |
| 7 | 546 ± 25* | 4.3 ± 0.5 |
Cells were seeded at an initial cell number of 5 × 104/mL and cell counts are presented after 2 and 7 days in culture. Data are expressed as fold increase in cell number. Results are the mean of three experiments ± SEM. Similar results were obtained for at least 2 clones of each transfect.
Cells were subcultured to 5 × 104/mL at day 4 to avoid overgrowth and death of the cultures.
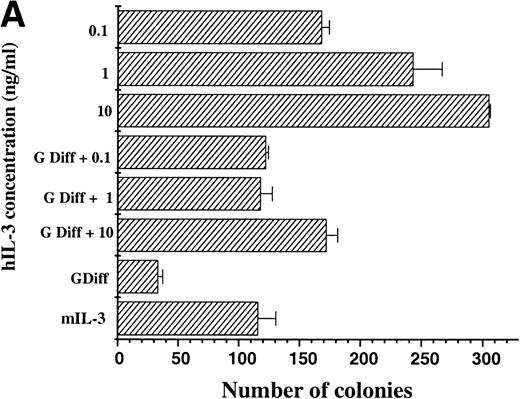
The maintenance of clonogenic potential of hIL-3 Rα,βcand hGM Rα,βc cells was assessed after 7 days liquid culture in the presence of the appropriate human cytokine, either alone or in combination with murine cytokines, which promote myeloid differentiation of FDCP-mix cells. This was assayed by plating cells into soft agar containing mIL-3 and determining the number of colonies formed (Fig 5). When hIL-3 Rα,βc cells were cultured in hIL-3 for 7 days before plating in mIL-3, it was apparent that hIL-3 was more potent than high concentrations of mIL-3 in maintaining clonogenic potential.12 13 Furthermore, culture of hIL-3 Rα,βc cells in granulocyte-macrophage differentiation conditions (which promote a loss in clonogenic potential) in the presence of hIL-3 had the effect of maintaining the clonogenic potential of hIL-3 Rα,βc cells (Fig 5A). Thus, hIL-3, via interaction with hIL-3 Rαβc complex, promoted prolonged stimulation of proliferation and suppression of clonogenic extinction in the presence of cytokines, which promote differentiation.
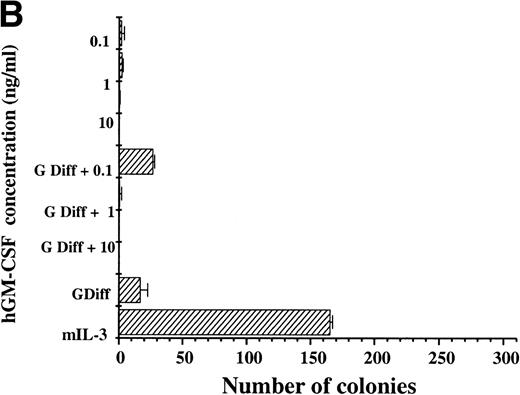
Clonogenic potential ability of (A) hIL-3 R,βc cells and (B) hGM R,βc cells. (A) hIL-3 R,βc cells and (B) hGM R,βccells were cultured in the presence of the cognate cytokine (0.1 to 10 ng/mL) alone or in combination with cytokines that promote granulocyte/macrophage development (G Diff) for 7 days before washing free of growth factors and plating (at a cell density of 2,000 cells/plate) in triplicate into soft agar containing 5% (vol/vol) mIL-3. Cells cultured in recombinant (r) mIL-3 (10 ng/mL) for 7 days before plating were used as the positive control. Data are from a single representative experiment of 3 and the values shown are the mean of triplicates ± SD. Similar results were obtained with at least 3 clones of each transfect.
Clonogenic potential ability of (A) hIL-3 R,βc cells and (B) hGM R,βc cells. (A) hIL-3 R,βc cells and (B) hGM R,βccells were cultured in the presence of the cognate cytokine (0.1 to 10 ng/mL) alone or in combination with cytokines that promote granulocyte/macrophage development (G Diff) for 7 days before washing free of growth factors and plating (at a cell density of 2,000 cells/plate) in triplicate into soft agar containing 5% (vol/vol) mIL-3. Cells cultured in recombinant (r) mIL-3 (10 ng/mL) for 7 days before plating were used as the positive control. Data are from a single representative experiment of 3 and the values shown are the mean of triplicates ± SD. Similar results were obtained with at least 3 clones of each transfect.
For hGM Rα,βc cells, hGM-CSF was unable to sustain the number of clonogenic cells compared with parallel cultures maintained in mIL-3 throughout the 7-day period. Culture of hGM Rα,βc cells with hGM-CSF together with granulocyte-macrophage differentiation conditions was also unable to support maintenance of colony-forming potential (Fig 5B). The hGM Rα,βc differed from hIL-3 Rα,βc in being unable to support long-term proliferation and maintenance of colony-forming cells.
Effects of human IL-3 and human GM-CSF on the development of FDCP-mix cells in the presence of ectopically expressed human receptor subunits.
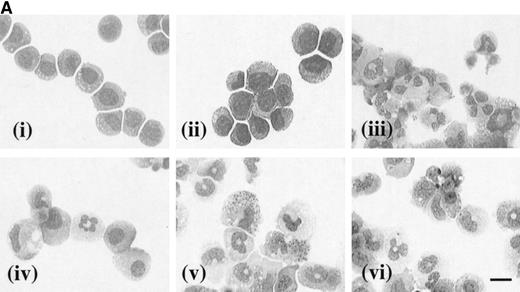
The effects of hGM-CSF and hIL-3 on the morphology of the receptor transfectants were assessed after 7 days in culture with the appropriate human cytokine. In the case of hIL-3 Rα,βccells cultured in the presence of hIL-3 (10 ng/mL), the vast majority of the cells had a blast cell morphology (Fig 6A [i]). Furthermore, culture of these cells with hIL-3 in combination with granulocyte-macrophage differentiation conditions resulted in maintenance of their blast cell morphology (Fig 6A [ii]). Thus, hIL-3 inhibited the acquisition of a mature cell phenotype, which is normally obtained in granulocyte-macrophage differentiation conditions and maintained the clonogenic potential of the hIL-3 Rα,βc cells even in the presence of developmental stimuli (Fig 5). The morphology of hIL-3 Rα,βc cells cultured in granulocyte-macrophage differentiation conditions is shown in Fig 6A [iii] and confirms that the cells were capable of differentiation.
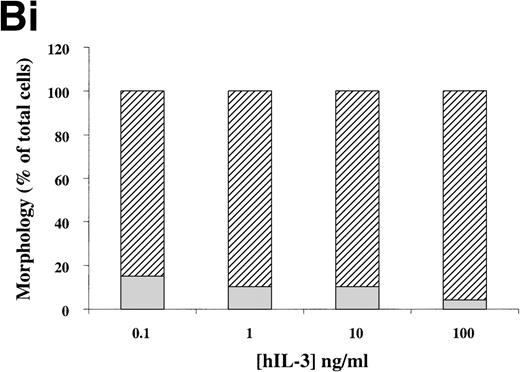
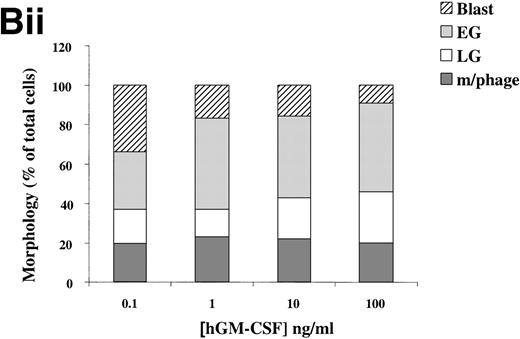
(A) Morphology of hIL-3 R,βc cells and hGM R,βc cells in culture. Cells expressing hIL-3 R,βc were cultured in (i) hIL-3 (10 ng/mL) alone or (ii) hIL-3 (10 ng/mL) in combination with murine cytokines that promote granulocyte-macrophage differentiation. Cells expressing hGM R,βc were cultured in (iv) hGM-CSF (10 ng/mL) alone or (v) hGM-CSF (10 ng/mL) in combination with granulocyte-macrophage differentiation conditions. Panels (iii) hIL-3 R,βccells and (vi) hGM-CSF R,βc cells show the morphology of cells cultured in granulocyte-macrophage differentiation conditions for comparison. Cytospin samples of cells were prepared after 7 days in culture and the morphology examined after May-Grunwald-Giemsa staining. Bar, 10 μm. Results are from an experiment representative of 3. Similar results were obtained with at least 3 clones of each transfect. (B) Dose-response of hIL-3 and hGM-CSF effects on morphology of hIL-3 R,βc cells and hGM R,βc cells respectively in culture. Cells expressing (i) hIL-3 R,βc or (ii) hGM R,βc were cultured in hIL-3 or hGM-CSF (0.1 to 100 ng/mL), respectively. Cytospin samples of cells were prepared after 7 days in culture and the morphology examined after May-Grünwald-Giemsa staining. Results are expressed as cell morphology (percentage of total cells scored). Cells were scored as blast, early granulocyte (EG), late granulocyte (LG), or macrophage (m/phage). Results are from a single experiment representative of 3. Similar results were obtained with at least 3 clones of each transfect.
(A) Morphology of hIL-3 R,βc cells and hGM R,βc cells in culture. Cells expressing hIL-3 R,βc were cultured in (i) hIL-3 (10 ng/mL) alone or (ii) hIL-3 (10 ng/mL) in combination with murine cytokines that promote granulocyte-macrophage differentiation. Cells expressing hGM R,βc were cultured in (iv) hGM-CSF (10 ng/mL) alone or (v) hGM-CSF (10 ng/mL) in combination with granulocyte-macrophage differentiation conditions. Panels (iii) hIL-3 R,βccells and (vi) hGM-CSF R,βc cells show the morphology of cells cultured in granulocyte-macrophage differentiation conditions for comparison. Cytospin samples of cells were prepared after 7 days in culture and the morphology examined after May-Grunwald-Giemsa staining. Bar, 10 μm. Results are from an experiment representative of 3. Similar results were obtained with at least 3 clones of each transfect. (B) Dose-response of hIL-3 and hGM-CSF effects on morphology of hIL-3 R,βc cells and hGM R,βc cells respectively in culture. Cells expressing (i) hIL-3 R,βc or (ii) hGM R,βc were cultured in hIL-3 or hGM-CSF (0.1 to 100 ng/mL), respectively. Cytospin samples of cells were prepared after 7 days in culture and the morphology examined after May-Grünwald-Giemsa staining. Results are expressed as cell morphology (percentage of total cells scored). Cells were scored as blast, early granulocyte (EG), late granulocyte (LG), or macrophage (m/phage). Results are from a single experiment representative of 3. Similar results were obtained with at least 3 clones of each transfect.
The loss of clonogenic potential seen in the hGM Rα,βccells, despite their initial marked proliferative response to hGM-CSF, suggested that the cells may have undergone maturation in response to this human cytokine. Analysis of cell morphology after 7 days in culture with hGM-CSF showed that the cells acquired a mature myeloid cell morphology (Fig 6A [iv]), resembling that observed for cells cultured in granulocyte-macrophage differentiation conditions shown in Fig 6A (vi). Furthermore, cells cultured in hGM-CSF plus granulocyte-macrophage differentiation conditions led to the formation of mature cells (Fig 6A [v]). The effects of hIL-3 or hGM-CSF on the morphology of the hIL-3 Rα,βc and hGM Rα,βc cells, respectively, were similar at all concentrations of human cytokine tested (0.1 to 100 ng/mL). This indicated that the differential effects of hIL-3 and hGM-CSF were not simply due to the dose of the cytokine used (Fig 6B).
To further characterize the developmental status of these cells, the expression of markers for granulocyte-macrophage development was assessed with flow cytometry. There was no significant change in expression of the myeloid lineage marker, MAC-1, in the hIL-3 Rα,βc cells cultured in hIL-3 (P ≤ .05, n = 2). However, there was a 2.3-fold increase in the level of expression of MAC-1 in hGM Rα,βc cells cultured in hGM-CSF. This confirmed the differential effects of hGM-CSF and hIL-3, respectively, on hGM Rα,βc and hIL-3 Rα,βc cells and suggested that the specificity of the response may be conferred by the α subunit of these heterodimeric receptors.
A chimeric α receptor subunit consisting of the extracellular domain from the IL-3 Rα subunit and the intracellular domain of the GM Rα subunit promoted myeloid development in FDCP-mix cells.
To investigate whether or not the signal specificity of the hGM-CSF receptor is generated by the unique α subunit, a chimeric hIL-3/GM Rα was generated. This chimeric α subunit was composed of the extracellular domain of the hIL-3 Rα subunit ligated to the cytosolic domain of the hGM Rα subunit. The fusion was made in the transmembrane region to avoid possible interference with known functional sequences proximal to the membrane. Thus, the chimeric hIL-3/GM Rα contained the binding site for hIL-3, which is present in the external domain of the hIL-3 Rα33 34 and the cytosolic domain of the hGM Rα subunit. Expression of the chimeric hIL-3/GM Rα subunit was detected by flow cytometry by using anti–hIL-3 Rα antibody. The results are shown in Fig7A and the flow cytometric profile resembled that obtained for hIL-3Rα expressing cells (Fig 1D) with a log increase in fluorescence relative to the nonspecific binding obtained with the secondary antibody only. hIL-3, but not hGM-CSF, promoted proliferation of these cells comparable in magnitude with that observed in hGM Rα cells in response to hGM-CSF (Fig 7B). Furthermore, exposure to hIL-3 led to a change in the phenotype of the cells from blast cells to a more mature cell phenotype (Fig 7C [i]), a response that was more similar to that observed for hGM Rα cells (Fig 7C [iii]) than hIL-3 Rα cells (Fig7C [ii]) cultured in hGM-CSF or hIL-3, respectively. Thus, replacing the hIL-3 Rα subunit cytosolic domain with the hGM Rα subunit cytosolic domain led to differential responses to hIL-3 in this cell line compared with hIL-3 Rα cells, which maintain a blast cell phenotype and undergo marked proliferation and population expansion in the presence of hIL-3. The developmental response resembled that obtained for hGM Rα cells, which undergo myeloid differentiation in response to hGM-CSF, indicating that the specificity is conferred by the cytosolic domain of the α subunit.
(A) Flow cytometric profile of FDCP-mix hIL-3/GM R cells. Expression of the chimeric receptor was determined by flow cytometry by using an anti–hIL-3 R antibody as outlined in Fig 1. (B) Effects of hIL-3 on proliferation of FDCP-mix cells expressing (i) chimeric hIL-3/hGM R, (ii) hIL-3 R, and (iii) hGM R subunits. Proliferation was assessed by determining [3H] thymidine incorporation stimulated by hIL-3 and hGM-CSF (1 to 100 ng/mL) after 16 hours in culture. Cells were plated at 4 × 105 per sample for the chimeric hIL-3/GM R cells and the hGM R cells. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained for at least 3 clones of each transfect. (C) Effects of hIL-3 on morphology of FDCP-mix cells expressing (i) chimeric hIL-3/hGM R (ii) hIL-3 R, and (iii) hGM R subunits. Morphology was assessed with May-Grünwald/Giemsa cytospin preparations of cells cultured in either (i), (ii) 100 ng/mL hIL-3, or (iii) 100 ng/mL hGM-CSF for 7 days as appropriate; (i) hIL-3/GM R cells, (ii) hIL-3 R, and (iii) hGM R cells. Bar, 10 μm. Similar results were obtained for at least 3 clones of each transfect.
(A) Flow cytometric profile of FDCP-mix hIL-3/GM R cells. Expression of the chimeric receptor was determined by flow cytometry by using an anti–hIL-3 R antibody as outlined in Fig 1. (B) Effects of hIL-3 on proliferation of FDCP-mix cells expressing (i) chimeric hIL-3/hGM R, (ii) hIL-3 R, and (iii) hGM R subunits. Proliferation was assessed by determining [3H] thymidine incorporation stimulated by hIL-3 and hGM-CSF (1 to 100 ng/mL) after 16 hours in culture. Cells were plated at 4 × 105 per sample for the chimeric hIL-3/GM R cells and the hGM R cells. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained for at least 3 clones of each transfect. (C) Effects of hIL-3 on morphology of FDCP-mix cells expressing (i) chimeric hIL-3/hGM R (ii) hIL-3 R, and (iii) hGM R subunits. Morphology was assessed with May-Grünwald/Giemsa cytospin preparations of cells cultured in either (i), (ii) 100 ng/mL hIL-3, or (iii) 100 ng/mL hGM-CSF for 7 days as appropriate; (i) hIL-3/GM R cells, (ii) hIL-3 R, and (iii) hGM R cells. Bar, 10 μm. Similar results were obtained for at least 3 clones of each transfect.
DISCUSSION
The requirement of specific cytoplasmic domains of the hβc for cell viability, proliferation, and differentiation signaling has been well-defined in vitro.16,17,26,35-37 The role of the βcsubunit in vivo has also been investigated in transgenic mice null for βc, βIL-3 or expressing a constitutively activated hβc38-42 (see below). However, to date, little information on the role of the α subunits of the GM-CSF and IL-3 receptors in differentiation has been available. The differential responses of murine multipotent FDCP-mix cells to mIL-3 (self-renewal) and mGM-CSF (weak proliferation and myeloid differentiation) intrigued the investigators.13Furthermore, constitutive mGM-CSF expression in FDCP-mix cells after retroviral transfection with the mGM-CSF gene, stimulated differentiation that could be blocked by mIL-3.43 Such differential effects must reside in receptor-mediated signaling events. Because murine hematopoietic cells express two β subunits, of which only one can interact with mGM Rα, we decided a clear structure/function analysis of the receptors could only be performed by ectopically expressing human receptors and mutants in FDCP-mix cells. Expression of hIL-3 and hGM-CSF receptor α and βcsubunits in FDCP-mix cells allowed both analysis and direct comparison of their effects on hematopoietic cell development. The IL-3 and GM-CSF cytokines are species specific; thus, addition of hIL-3 and hGM-CSF selectively activated the ectopically expressed hIL-3 R and hGM R, facilitating structure-function analysis of wild type and mutant receptor subunits.
FDCP-mix cells transfected with the hIL-3 Rα or hGM Rα subunits alone only responded to relatively high concentrations of their cognate cytokines (Fig 2B). These results indicate the formation of low-affinity receptors because of interaction with the endogenous murine βc and βIL-3 as reported previously.24-26 Coexpression of hβc subunits together with hIL-3 Rα or hGM Rα led to formation of high-affinity receptors (Fig 2B), although cells transfected with hβcsubunits alone were unable to respond to hIL-3 or hGM-CSF (Fig 2A). These data are consistent with previous reports indicating that the βc subunit alone is unable to bind IL-3 or GM-CSF but acts to convert the initial low-affinity ligand binding by the α subunit to a high-affinity receptor complex.4
The hIL-3 Rα,βc and hGM Rα,βc both promoted survival and proliferation of FDCP-mix cells but their developmental effects were distinct. The dual-transfected hIL-3 Rα,βc cells survived and proliferated in response to hIL-3 and maintained their clonogenic potential. Coaddition of hIL-3 with a combination of cytokines that promote granulocyte-macrophage development of the hIL-3 Rα,βc cells “countermanded” the differentiation response (Figs 5 and 6). The hIL-3 Rα,βc, thus, promoted self-renewal and maintenance of primitive morphology. In contrast, hGM-CSF promoted myeloid development of cells cotransfected with hGM Rα and hβc subunits (Fig 6). Thus, the human receptors for hIL-3 and hGM-CSF promote differential effects in FDCP-mix cells, self-renewal, and myeloid differentiation. The developmental responses observed were similar throughout a wide dose-range of human cytokine demonstrating that the effects are not concentration dependent. By culturing hIL-3 Rα,βc and hGM Rα,βccells in concentrations of hIL-3 or hGM-CSF, at which the single hIL-3 Rα or hGM Rα are nonresponsive (Figs 2 and 3), the human IL-3 Rα and hGM Rα subunits interact with hβc and not endogenous murine β subunits. The differences in the biologic effects observed with the hIL-3 Rα,βc and hGM Rα,βc can, thus, be attributed to results from interaction of the hIL-3 Rα or hGM Rα subunits with the hβc subunits.
Cells transfected with either the hIL-3 Rα or hGM Rα alone also gave similar results when cultured with their respective cognate cytokines, with hIL-3 promoting self-renewal and hGM-CSF promoting myeloid differentiation (Fig 7). Interestingly, the hGM Rα1 and α2 subunit isoforms have previously been shown to promote myeloid differentiation in response to hGM-CSF in FDCP-1 cells.25 However, the effects of activation of these hGM Rα isoforms on clonogenic potential were not determined.
We have shown also that hIL-3 and hGM-CSF stimulate differential cellular signaling events; they initiate different tyrosine phosphorylation responses (Fig 4). To our knowledge, this is the first demonstration of differential developmental and cellular signaling by these cytokines in primitive hematopoietic progenitor cells. Specific activation of hIL-3 and hGM-CSF receptors by the addition of their cognate cytokines to FDCP-mix cells provides a model system for the analysis of the molecular pathways associated with hematopoietic development. The magnitude of the responses elicited by activation of the hGM Rα,βc is much greater than that of the mGM-CSF receptor in FDCP-mix cells. This is probably because of overexpression of the hGM R compared with approximately 20 mGM receptors/cell13 and facilitates analysis of the signaling events associated with GM-CSF-mediated differentiation. Although the signal transduction pathways for survival and proliferation in response to hGM-CSF are relatively well defined, their roles in differentiation remain to be determined. For example, tyrosine phosphorylation of the SHP-2, JAK2, Shc, Erk, and STAT5 signaling proteins correlates with hGM-CSF-mediated proliferation but is not required for differentiation.37,44 Evidence for the involvement of specific signaling pathways in differentiation comes from several groups, including the demonstration that activation of the PKCα isoform determines lineage commitment in bipotential granulocyte-macrophage colony forming cells.45 46 The model we have developed will now permit differentiation signaling in multipotent cells to be investigated.
Is there a role for the unique α subunit in hIL-3 and hGM-CSF receptor function? Results obtained with a chimeric hGM R, composed of the extracellular domain of hGM Rα and the cytoplasmic domain of the hβc subunit, have indicated that the cytoplasmic domain of GM Rβc can functionally replace that of the hGM Rα subunit to promote proliferation.47 However, studies on deletion mutants of α subunits of the IL-3 subfamily of receptors have showed that their cytoplasmic domains play a key role in receptor mediated signal transduction.34,48-50 For example, deletion of the cytoplasmic domain of IL-3 Rα subunit resulted in a high-affinity hIL-3 R that was unable to stimulate proliferation or promote tyrosine phosphorylation of the βc subunit and STAT5 signaling proteins.34 Consistent with such data, the GM Rα also plays a direct role in activation of the JAK/STAT pathway.51 Recent studies on truncation mutants of the hGM Rα subunits expressed in IL-3–dependent murine FDCP-1 cells indicate that specific regions of the α subunit are required for survival proliferation and maturation.44 In this current study, the role of the α subunit in self-renewal/differentiation was investigated by transfection of a chimeric receptor composed of the extracellular domain of the hIL-3 Rα and the intracellular domain of the hGM Rα into FDCP-mix cells. Cells expressing the chimeric hIL-3/GM Rα construct responded to hIL-3 and not hGM-CSF. The cells resembled the hIL-3 Rα cells, in that the response was observed only at relatively high concentrations of hIL-3, which is consistent with the formation of a low-affinity receptor. The biologic response more closely resembled that of hGM Rα than hIL-3 Rα in that the chimeric hIL-3/GM Rα provided a weak survival and proliferation stimulus and promoted myeloid differentiation (Fig 7). These results suggest that the cytosolic domain of the α subunit may confer the specificity of the biologic response.
How do these results compare to data derived from experiments on the function of GM-CSF and IL-3 receptors in transgenic mice? A series of knock-out mice have been generated that are defective for βc, βIL-3 or both βc and IL-3 ligand.38-41 Hematopoiesis is apparently normal in these mice, although βc and βc -IL-3 null animals have a pulmonary alveolar proteinosis-like disease,38,41,52resembling that observed in GM-CSF ligand-deficient mice.53The βc null bone marrow does, however, display decreased ability to initiate white blood cell recovery on transplantation into irradiated recipient mice.39 Interestingly, expression of the hβc in transgenic mice resulted in a myeloproliferative disorder resembling polycythemia vera.42Thus, there is a clear role for β subunits from in vivo experiments. The α subunits are required for signal response coupling with the β subunit.4,32 However, there is no direct evidence on the role of the α subunits in self-renewal or differentiation; indeed the complexity of hematopoietic regulatory mechanisms in vivo would argue against addressing this issue using whole animal experiments. Therefore, we decided to investigate the receptor subunit functions in the context of the multipotent FDCP-mix cell line. Although it has been shown that the GM Rα subunit can alone promote glucose transport in oocytes,54 GM-CSF is unable to mediate glucose transport in neutrophils from mice deficient in the βcsubunit.38 Our data suggest that the hIL-3 and hGM-CSF receptor α subunits play a role in conferring signal specificity and eliciting different biologic responses within the context of the αβc receptor subunit complex.
It is well known that IL-3 stimulates normal marrow cells to form some blast colonies (corresponding with the data on the effects of transfection of the hIL-3 receptor into FDCP-mix cells) but it also promotes granulocyte-macrophage colony formation.10 This, in part, reflects the age structure of hematopoiesis where a spectrum of IL-3–responsive cells from the multipotent to late myeloid progenitors are present. Thus, normal bone marrow gives a heterogeneous response to IL-3 with some cells undergoing self-renewal. In FDCP-mix cells just such a spectrum of cells at different stages of differentiation can be generated by culture on stromal cells from long-term marrow cultures. Some FDCP-mix cells retain their multipotent phenotype and others differentiate to form mature granulocytes and macrophages. Thus, the similarities between primary hematopoietic cells and FDCP-mix are noteworthy, yet IL-3 is plainly not exclusively a self-renewal factor for hematopoietic progenitor cells. The FDCP-mix cells were first derived from murine long-term bone marrow cultures. IL-3 was required to prevent the death of suspension cells removed from these cultures. FDCP-mix cells self-renew indefinitely when cultured in high concentrations of IL-3, presumably this is a feature of the cells that allowed their isolation and cloning.
Nonetheless, FDCP-mix can be obtained in large quantities for biochemical studies, important clues to the molecular mechanisms stimulated by cytokines in primitive hematopoietic cells can be derived, and the information can be used to study enriched progenitor cell populations, which can be isolated from bone marrow. Further work is now needed to extend these observations that the cytoplasmic domain of the α subunit plays a role in conferring signal specificity for self-renewal or differentiation. This current study indicates that signaling pathways activated by interaction of hGM Rα or hIL-3 Rα and the βc receptor after cytokine activation are different and regulate self-renewal/differentiation decisions.
ACKNOWLEDGMENT
Thanks to Professor T.M. Dexter (Paterson Institute for Cancer Research) for the use of laboratory facilities and for helpful discussions with regard to this work.
Supported by the Biotechnology and Biological Science Research Council (UK), Cancer Research Campaign, and the Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Anthony D. Whetton, PhD, Leukaemia Research Fund Cellular Development Unit, Department of Biomolecular Sciences, UMIST, Manchester M60 1QD, United Kingdom.

![Fig. 3. Effects of hIL-3 and hGM-CSF on proliferation of FDCP-mix cells coexpressing hIL-3 R,βc or hGM R,βc. Proliferation was assessed by determining [3H] thymidine incorporation after culturing for 16 hours at 37°C. FDCP-mix cells expressing (A) hIL-3 R /βcand (B) hGM R /βc were stimulated by hIL-3 (0.01 to 100 ng/mL) and hGM-CSF (0.1 to 100 ng/mL), respectively. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained with at least 4 clones of each transfect.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1504/5/m_blod41727003ax.jpeg?Expires=1769091071&Signature=t6WrEyyVuOY-ZTeEGnoW9LAQ6ph9XZcM91QEjIvCXbLyHqVeW2WIb-l3zPj3E6LGBrozL7DRAZ9qqJ1v~gy-~V8B8qk3K~yC13hJYsTs6FX2SEOcF4fuzyhcRuk7Uag7Ou6wJIozdKK8w1mjmx6S~OqPzJ8Y1Xdju~JD3kAqryQDPPKLeRcpk79H~yBGZfGobYRBB6Sy58BbOtj-5e4MiU1kZXteNXrk-Dhg9uqunVR9gA1QL9Vjojb16q2VBTvFAirAfcjgoQImrhXnqe8vC3WL9hiHFhVHcIR~zFyjfLPgspImrsL-BYWRxn3icL5Bp3qx1qPF7bj8LsZqWC06ug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effects of hIL-3 and hGM-CSF on proliferation of FDCP-mix cells coexpressing hIL-3 R,βc or hGM R,βc. Proliferation was assessed by determining [3H] thymidine incorporation after culturing for 16 hours at 37°C. FDCP-mix cells expressing (A) hIL-3 R /βcand (B) hGM R /βc were stimulated by hIL-3 (0.01 to 100 ng/mL) and hGM-CSF (0.1 to 100 ng/mL), respectively. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained with at least 4 clones of each transfect.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1504/5/m_blod41727003bx.jpeg?Expires=1769091071&Signature=k33b7tDsE4T8x4vLBFZrcKnRreO04Cosh0kzhNPK56SvoOT0OW4G0f2GqOcJqDFFI~wg2JvY2jEYT02m9pgsTaFS2pyHCKjf9frCzkAhsTS8~NaPSuoWUeelE17v6Dhk~cBT~sVKW1TTP6VsIqyHKdZ2ajkCxU5j6kFPQuOP0BYM1~RhKrlg8kKgfjtlz7C6cNImLhJuSJXIyY1f0QI~y~q11zEwUXtOmzDbDcEnhGTT8jDouTFpZpnXZ9NUG4G6qnI41eL~IgEWgIJtr-cxRKwobZzWeMZDaECdfgj0BwiGINTK~3XawtCCnZ83828mw9e4h5rwXy08-B7758v2nA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. (A) Flow cytometric profile of FDCP-mix hIL-3/GM R cells. Expression of the chimeric receptor was determined by flow cytometry by using an anti–hIL-3 R antibody as outlined in Fig 1. (B) Effects of hIL-3 on proliferation of FDCP-mix cells expressing (i) chimeric hIL-3/hGM R, (ii) hIL-3 R, and (iii) hGM R subunits. Proliferation was assessed by determining [3H] thymidine incorporation stimulated by hIL-3 and hGM-CSF (1 to 100 ng/mL) after 16 hours in culture. Cells were plated at 4 × 105 per sample for the chimeric hIL-3/GM R cells and the hGM R cells. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained for at least 3 clones of each transfect. (C) Effects of hIL-3 on morphology of FDCP-mix cells expressing (i) chimeric hIL-3/hGM R (ii) hIL-3 R, and (iii) hGM R subunits. Morphology was assessed with May-Grünwald/Giemsa cytospin preparations of cells cultured in either (i), (ii) 100 ng/mL hIL-3, or (iii) 100 ng/mL hGM-CSF for 7 days as appropriate; (i) hIL-3/GM R cells, (ii) hIL-3 R, and (iii) hGM R cells. Bar, 10 μm. Similar results were obtained for at least 3 clones of each transfect.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1504/5/m_blod41727007ax.jpeg?Expires=1769091071&Signature=cJaJYJhCS2rQaAjGo~Ra4YeFFpgrW4Zu02q0flLzUFanRtmt2oKyVjTIdrkm3Qnv1SUw6VyWOzxOuxi~011MnayOkJKoNcjgeZUgoMYQ1tOp4Rfqe2oUWuZ9LKhwAtgCq~zRyv4qdGvfPART~seOtZMOeefinjuiB9AKY4rIT8MSB7qXj4u9RqfEjB1X2KLKM3A42L7HSdjIhKUZbJB5qGA3dQ5YUu5d-G1siDOANx0QQ7Pyv~osQvznYa-HTVAQPEhjkWaBK8WQUn0lglCNRIQ68en1s5PnllekrYetB3I9ykD90ZWXVzmTdvjrkKf8HgvgZZah~lKBys~-Uh8WNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. (A) Flow cytometric profile of FDCP-mix hIL-3/GM R cells. Expression of the chimeric receptor was determined by flow cytometry by using an anti–hIL-3 R antibody as outlined in Fig 1. (B) Effects of hIL-3 on proliferation of FDCP-mix cells expressing (i) chimeric hIL-3/hGM R, (ii) hIL-3 R, and (iii) hGM R subunits. Proliferation was assessed by determining [3H] thymidine incorporation stimulated by hIL-3 and hGM-CSF (1 to 100 ng/mL) after 16 hours in culture. Cells were plated at 4 × 105 per sample for the chimeric hIL-3/GM R cells and the hGM R cells. Results are expressed as percentage of control (10 ng/mL mIL-3) and are the mean values from at least 3 experiments ± SEM. Similar results were obtained for at least 3 clones of each transfect. (C) Effects of hIL-3 on morphology of FDCP-mix cells expressing (i) chimeric hIL-3/hGM R (ii) hIL-3 R, and (iii) hGM R subunits. Morphology was assessed with May-Grünwald/Giemsa cytospin preparations of cells cultured in either (i), (ii) 100 ng/mL hIL-3, or (iii) 100 ng/mL hGM-CSF for 7 days as appropriate; (i) hIL-3/GM R cells, (ii) hIL-3 R, and (iii) hGM R cells. Bar, 10 μm. Similar results were obtained for at least 3 clones of each transfect.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1504/5/m_blod41727007cy.jpeg?Expires=1769091071&Signature=MYVidHZ2pk0RNp~wrOcPSMSi8dwrXsvQRHiu~KJY9kO2RoU3ftZHJ7LeKTWqSoIkD8ASRKjCb8veMFGMbOq49RPaMtzymplOYbx~witX0DWpnOjyL~lIlKLF30e8TjrIxK~9aM7uLuuTTaLPzROGEUST4ky6LKJEDiaP~luV~pw52xXqqlOdf4H7EuPsWeUfAESoKB3u0zY8iat4KPE5aTA4MDqmaklLImuRoVBhgqIEKQkNZHdZSvgGjxmlgMF6wJ8KG6mNbMMHwJRoxqjQvY~U~oSF33cbhkkUHnVnWF-03WdBbyeyw2oO2tiXvNV8CFmqNiof2b-dvfpjwlaUzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal