Abstract
Little is known about the physiologic role of phosphatidylinositol 3-kinase (PI-3K) in the development of erythrocytes. Previous studies have shown that the effects of the PI-3K inhibitor wortmannin on erythropoietin (EPO)-dependent cell lines differed depending on the cell type used. Wortmannin inhibited EPO-induced differentiation of some cell lines without affecting their proliferation; however, the EPO-induced proliferation of other cell lines was inhibited by wortmannin. In neither case were signs of apoptosis observed. We have previously reported that signaling in highly purified human colony forming units-erythroid (CFU-E), generated in vitro from CD34+ cells, differed from that in EPO-dependent cell lines. In the current study, we examined the effects of a more specific PI-3K inhibitor (LY294002) on human CFU-E. We found that LY294002 dose-dependently inhibits the proliferation of erythroid progenitor cells with a half-maximal effect at 10 μmol/L LY294002. LY294002 at similar concentrations also induces apoptosis of these cells, as evidenced by the appearance of annexin V–binding cells and DNA fragmentation. The steady-state phosphorylation of AKT at Ser-473 that occurs as a result of PI-3K activation was also inhibited by LY294002 at similar concentrations, suggesting that the effects of LY294002 are specific. Interestingly, the acceleration of apoptosis by LY294002 was observed in the presence or absence of EPO. Further, deprivation of EPO resulted in accelerated apoptosis irrespective of the presence of LY294002. Our study confirms and extends the finding that signaling in human primary cultured erythroid cells is significantly different from that in EPO-dependent cell lines. These data suggest that PI-3K has an antiapoptotic role in erythroid progenitor cells. In addition, 2 different pathways for the protection of primary erythroid cells from apoptosis likely exist: 1 independent of EPO that is LY294002-sensitive and one that is EPO-dependent and at least partly insensitive to LY294002.
ERYTHROPOIETIN (EPO) is a glycoprotein hormone essential for normal erythropoiesis.1-4 Signaling through the EPO receptor (EPO-R) regulates the proliferation, differentiation, and survival of erythroid progenitor cells. Homodimerization of the receptor in response to EPO binding transiently activates the receptor-associated protein tyrosine kinase Jak2.5-8 It has been reported that activation of Jak2 is accompanied by tyrosine phosphorylation of numerous proteins, including Jak2 itself, STAT proteins, SHP-1, Shc, Vav, the EPO-R, and the classic phosphatidylinositol 3-kinase (PI-3K), although tyrosine kinases other than Jak2 may also phosphorylate these proteins.4,6-22 The classic PI-3K is a heterodimeric enzyme composed of a regulatory p85 subunit and a catalytic p110 subunit that phosphorylates phosphoinositides at the D3 position of the inositol ring.23 Published data have implicated PI-3K and its downstream target, the serine/threonine kinase AKT, in a pathway that conveys survival signals within various systems.24-26Activation of PI-3K results in the generation of 2 lipid products (PI-3,4-P2 and PI-3,4,5-P3), which serve as second-messenger molecules and activate AKT. Activated AKT may phosphorylate the proapoptotic factor BAD on a serine residue, resulting in its dissociation from BCL-XL and its association with 14-3-3.24 Released BCL-XL may then suppress cell-death pathways that involve the activity of APO-1, cytochrome c, and the caspase protease cascade.26 Such a mechanism may function in human erythroid cells, as it has been reported that these cells express BCL-XL and its expression is positively regulated during the final stage of erythropoiesis.27,28 Another possible target of activated AKT may be glycogen synthase kinase-3 (GSK-3), and it was reported that phosphorylation of GSK-3 by activated AKT may promote the survival of Rat-1 and PC12 cells.29
Little is known about the physiologic significance of PI-3K in the survival, proliferation, and differentiation of erythroid progenitors. Wortmannin, an inhibitor of PI-3K, was reported to inhibit the proliferation of HCD-57 and DA-3 cells in response to EPO.18,23 More recently, Sui et al30 reported that wortmannin inhibited the proliferation of human erythroid precursors expanded in vitro. The mechanism involved in the antiproliferative effects was unknown. Moreover, in certain cells, wortmannin had no antiproliferative effects. However, in some of the studies cited, high concentrations of wortmannin were used that probably had nonspecific effects. Lysophosphatidic acid (LPA) has been shown to be a major survival factor for murine macrophages. While high concentrations of wortmannin were necessary to inhibit the survival effects of LPA,31 LY294002, a more specific inhibitor of PI-3K,32 opposed the action of LPA at appropriate concentrations. We postulate that the use of wortmannin and different cell lines could explain the controversy regarding the role of PI-3K in erythropoiesis.
We previously used primary cultured human erythroid progenitor cells33 to gain insight into the physiologic role of PI-3K activation to support proliferation and differentiation of erythroid progenitors. Using these cells, we found that EPO induces tyrosine phosphorylation of Jak2, STAT5A, and STAT5B. In the present study, we show that LY294002 induces apoptosis in human erythroid progenitors in a time- and dose-dependent manner, and that it blocks phosphorylation of AKT at concentrations similar to those required for the induction of apoptosis. Our results suggest that PI-3K may be essential for the survival of human erythroid precursor cells by preventing apoptosis.
MATERIALS AND METHODS
Reagents.
HEPES, sodium dodecyl sulfate (SDS), 2-mercaptoethanol (2-ME), sodium orthovanadate, bovine serum albumin (BSA), chicken egg albumin, Iscove’s modified Dulbecco’s medium (IMDM), propidium iodide (PI), protein A–Sepharose, Triton X-100, and Tris were purchased from Sigma (St Louis, MO). Polyvinylidene difluoride (PVDF) membranes (pore size, 0.45 μm) were from Millipore Corp (Bedford, MA). SDS-polyacrylamide gel electrophoresis (SDS-PAGE) molecular standards and enhanced chemiluminescence reagents including secondary antibodies were purchased from Amersham (Arlington Heights, IL). Insulin (porcine sodium; activity, 26.3 USP U/mg) was purchased from Calbiochem and Behring Diagnostics (La Jolla, CA). Antibodies against AKT and phosphorylated AKT were from Upstate Biotechnology Inc (Lake Placid, NY) and New England Biolabs (Beverly, MA). Nitroblue tetrazolium chloride and 5-bromo-4 chloro-3-indolyl phosphate p-toluidine salt were from GIBCO-BRL (Gaithersburg, MD). Horse tendon type I collagen was from Nycomed (Munich, Germany).
Recombinant human EPO (180,000 U/mg) was kindly donated by Chugai Pharmaceutical Co (Tokyo, Japan). Recombinant human interleukin-3 ([IL-3] 108 chronic myelogenous leukemia U/mg) was from Amgen Biologicals (Thousand Oaks, CA). Recombinant human stem cell factor (SCF) was kindly donated by Kirin Brewery Co (Tokyo, Japan). Vitamin B12 and folic acid were from Sankyo Pharmaceutical Co (Tokyo, Japan) and Takeda Pharmaceutical Co (Osaka, Japan), respectively. Fetal calf serum (FCS), penicillin, and streptomycin were from Flow Laboratories Inc (McLean, VA). LY294002 was purchased from BIOMOL Research Laboratories Inc (Plymouth Meeting, PA).
Ex vivo generation of erythroid progenitor cells.
Human erythroid progenitor cells were generated ex vivo as previously described.33 In brief, recombinant human granulocyte colony-stimulating factor ([G-CSF] Chugai Pharmaceutical Co and Kyowa Hakko Pharmaceutical Co, Tokyo, Japan) was administered to healthy subjects who previously signed consent forms approved by the Hokkaido University School of Medicine and the Hokkaido Red Cross Blood Center Committee for the Protection of Human Subjects, as described previously.34 The mobilized peripheral blood (PB) CD34+ cells were isolated using immunomagnetic beads.35,36 The cells were then cryopreserved and stored until use in liquid nitrogen. The frozen PB CD34+ cells were thawed, suspended in IMDM containing 30% FCS and 100 U/mL DNase, and then centrifuged at 400g for 5 minutes at 4°C. The cells were washed twice with IMDM containing 20% FCS and then resuspended in IMDM containing 0.3% deionized BSA.37 The cells were next cultured in liquid phase as described elsewhere.33 38 In brief, cells at 0.5 × 104 to 2.0 × 104cells/mL were suspended in a mixture containing 20% FCS, 10% heat-inactivated pooled human AB serum, 1% BSA, 10 μg/mL insulin, 10 μg/mL vitamin B12, 15 μg/mL folic acid, 100 U/mL IL-3, 100 ng/mL SCF, and 4 U/mL EPO in the presence of 5 × 10−5 mol/mL β-ME, 50 U/mL penicillin, 50 U/mL streptomycin, and IMDM in a 50-mL polystyrene flask (Corning Coster Corp, Cambridge, MA). After incubation for 8 days at 37°C in a 5% CO2/95% O2 atmosphere, the cells were collected, washed twice with IMDM containing 0.3% BSA (day 8 cells).
Semisolid culture of progenitors.
Day 8 cells were incubated in triplicate at a concentration of 500 cells/mL in flat-bottom 48-well tissue culture plates (Linbro; Flow Laboratories) in 0.25 mL serum-containing or serum-free fibrin clots with EPO at 2 U/mL.38-41 After 7 days of incubation at 37°C in a 5% CO2/5% O2 incubator, the clots were fixed and stained with benzidine-hematoxylin.37 The aggregates consisting of 8 to 49 hemoglobinized cells were defined as colony-forming units–erythroid (CFU-E), while aggregates consisting of 2 to 7 hemoglobinized cells were defined as small erythroid. Erythroid colony-forming cells (ECFCs) were defined as cells that yield colonies of 2 to 49 hemoglobinized cells after 7 days of culture of day 8 cells.40
Liquid suspension culture of progenitors.
Day 8 cells were incubated at concentrations of 1 × 105to 5 × 105 cells/mL in flat-bottom 24- to 96-well tissue culture plates (Linbro; Flow Laboratories) in serum-free medium with EPO at 2 U/mL.39 After incubation at 37°C in a 5% CO2/5% O2 incubator for the indicated periods, the cells were collected and washed twice with IMDM containing 0.3% BSA before the subsequent colony assay of these cells.
Immunoprecipitation, gel electrophoresis, and Western blotting.
After starvation, day 8 cells were stimulated with EPO. Cells were lysed by adding an equal amount of lysis buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L PMSF, 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, and 2% Triton X-100 vol/wt, pH 7.4). After 20 minutes on ice, the lysates were centrifuged at 10,000× g (at 4°C) for 20 minutes. The supernatant was removed and precleared with preimmune serum and protein A–Sepharose (40 μL of 50% slurry) for 1 hour. Anti-AKT polyclonal antibody was then added and the preparation was incubated for 2 to 3 hours on ice. Protein A–Sepharose (40 μL of 50% slurry) was added and followed by 1 hour of incubation. The immune complexes were washed 3 times with 1 mL cold washing buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L PMSF, 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, and 1% Triton X-100 vol/wt, pH 7.4) and resuspended in Laemmli sample buffer (10% glycerol, 1% SDS, 5% 2-ME, 50 mmol/L Tris HCl (pH 6.8), and 0.002% bromophenol blue) with 10 mmol/L EGTA and 1 mmol/L sodium orthovanadate. After boiling at 95°C for 5 minutes, 1-dimensional electrophoresis was performed on SDS 10% or 7.5% to 15% polyacrylamide gels.42 Separated proteins were electrophoretically transferred from the gel onto PVDF membranes or nitrocellulose in a buffer containing Tris (25 mmol/L), glycine (192 mmol/L), and 20% methanol at 0.2 amps for 12 hours at room temperature. To block residual protein binding sites, membranes were incubated in TBST (Tris-buffered saline [TBS], 10 mmol/L Tris, and 150 mmol/L NaCl, pH 7.6, with 0.1% Tween 20) with 10% chicken egg albumin. The blots were washed with TBST and incubated overnight with primary antibodies at a final concentration of 1.0 μg/mL in TBST. The primary antibody was removed, and the blots were washed 4 times in TBST and incubated with horseradish peroxidase–conjugated secondary antibodies diluted 1:3,000 in TBST. The blots were then washed 4 times in TBST. Antibody reactions were quantified by chemiluminescence according to the manufacturer’s instructions.
Flow cytometry.
Day 8 cells were cultured at a concentration of 5 × 105/mL in 1.0 mL serum-free medium supplemented with 2 U/mL EPO with or without 50 μmol/L LY294002. After incubation for 24 hours, the cells were collected, washed twice with staining medium (phosphate-buffered saline [PBS] containing 3% FCS and 0.005% NaN3), counterstained with PI and annexin V conjugated with FITC (Apoptosis Detection Kit; R&D Systems Inc, Minneapolis, MN), and then analyzed by FACS Vantage (Becton Dickinson, Franklin Lakes, NJ).
DNA fragmentation.
DNA fragmentation was measured by quantitation of cytosolic oligonucleosome-bound DNA using an enzyme-linked immunosorbent assay (ELISA) kit (Cell Death Detection ELISA; Boehringer, Mannheim, Germany) according to the manufacturer’s instructions. Briefly, day 8 cells were incubated in 0.5 mL serum-free medium in the presence of 2 U/mL EPO with or without 50 mmol/L LY294002 at a concentration of 1 × 105 cells/mL. In the other set of experiments, cells were incubated for 24 hours with various concentrations of LY294002 (0, 1, 10, 20, 50, and 100 μmol/L). After incubation for the indicated periods, the cells were collected and washed twice with PBS and the cytosolic fraction (13,000gsupernatant) was extracted. The diluted cytosolic fraction equivalent to 200 cultured cells was used as the Ag source in a sandwich ELISA with a primary anti-histone Ab coated to the microtiter plate and a secondary anti-DNA Ab coupled to peroxidase. From the absorbance values, the percentage of fragmentation, in comparison to controls, was calculated according to the following formula:
Platelet preparation.
Washed platelets were prepared from citrated whole blood as previously described.43
RESULTS
Characteristics of expanded cells.
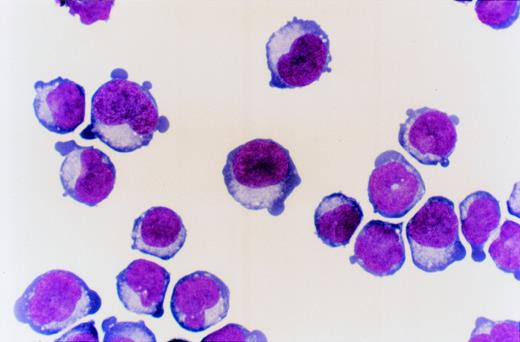
PB CD34+ cells cultured for 8 days in serum-containing medium with SCF, IL-3, and EPO generated cells that predominantly consist of erythroid cells (day 8 cells; Fig1). The ECFCs in day 8 cells predominantly consist of mature erythroid progenitor cells for which the maturation level is equivalent to CFU-E as described elsewhere.33
PB CD34+ cells cultured for 8 days with SCF, IL-3, and EPO (day 8 cells). May-Grünwald staining (original magnification ×1,000).
PB CD34+ cells cultured for 8 days with SCF, IL-3, and EPO (day 8 cells). May-Grünwald staining (original magnification ×1,000).
Inhibition of erythroid colony growth by LY294002.
To gain insight into the role of PI-3K signaling in human erythroid colony growth, day 8 cells were incubated in serum-free fibrin clots with 2 U/mL EPO in the presence of various concentrations of LY294002, a specific PI-3K inhibitor. The inhibition of erythroid colony growth by LY294002 occurred in a dose-dependent manner, and half-maximal inhibition occurred at 10 μmol/L LY294002 (P < .001). Maximal inhibition occurred at about 50 μmol/L (Table1).
Effect of PI-3K Inhibitor on Erythroid Colony Growth
| LY294002 Concentration (μmol/L) . | Total . | CFU-E . | Small Erythroid . |
|---|---|---|---|
| 0 (IMDM) | 76.3 ± 3.2 | 72.0 ± 3.0 | 4.3 ± 1.2 |
| 0 (0.1% DMSO) | 91.7 ± 1.5 | 83.7 ± 2.3 | 8.0 ± 3.6 |
| 1 | 78.3 ± 14.5 | 68.3 ± 9.4 | 10.0 ± 6.3 |
| 10 | 36.3 ± 4.6* | 30.3 ± 3.5† | 6.0 ± 2.6 |
| 20 | 34.7 ± 5.5† | 23.3 ± 2.0† | 8.0 ± 2.6 |
| 50 | 3.0 ± 2.0† | 0† | 3.0 ± 2.0 |
| 100 | 0† | 0† | 0 |
| LY294002 Concentration (μmol/L) . | Total . | CFU-E . | Small Erythroid . |
|---|---|---|---|
| 0 (IMDM) | 76.3 ± 3.2 | 72.0 ± 3.0 | 4.3 ± 1.2 |
| 0 (0.1% DMSO) | 91.7 ± 1.5 | 83.7 ± 2.3 | 8.0 ± 3.6 |
| 1 | 78.3 ± 14.5 | 68.3 ± 9.4 | 10.0 ± 6.3 |
| 10 | 36.3 ± 4.6* | 30.3 ± 3.5† | 6.0 ± 2.6 |
| 20 | 34.7 ± 5.5† | 23.3 ± 2.0† | 8.0 ± 2.6 |
| 50 | 3.0 ± 2.0† | 0† | 3.0 ± 2.0 |
| 100 | 0† | 0† | 0 |
Day 8 cells were plated into 0.25 mL serum-free fibrin clots at a concentration of 250 cells/clot with EPO at 2 U/mL. After 7 days of incubation, the clots were fixed and stained with benzidine-hematoxylin. The mean ± SD of triplicates is shown. Statistical analysis was performed using Scheffe’s F post hoc test.
P < .01, decrease v 0.1% DMSO.
P < .001, decrease v 0.1% DMSO.
Inhibition of proliferation and survival of erythroid progenitor cells by LY294002.
To investigate the effect of LY294002 on the viability and proliferation of erythroid progenitor cells, day 8 cells were incubated in liquid phase in serum-free medium with 2 U/mL EPO in the presence of various concentrations of LY294002 (Table 2). After 7 days of culture, the cells incubated with neither LY294002 nor 0.1% DMSO increased in number by 27.8 ± 2.8-fold with 88.0% ± 5.3% viability, resulting in a 24.5 ± 3.5-fold increase in the number of viable cells. The presence of 0.1% DMSO, a vehicle of LY294002, did not affect the proliferation or viability of erythroid progenitor cells. The proliferation of day 8 cells was markedly inhibited by LY294002 in a dose-dependent manner and was accompanied by a decrease in viability. Inhibition of the proliferation and survival of day 8 cells was evident at concentrations of 10 μmol/L (P < .001 for total cells and P < .01 for viable cells) and 20 μmol/L LY294002 (P < .01), respectively, the maximum being about 50 μmol/L, which suggests that a blockage of PI-3K signaling by LY294002 inhibits the proliferation and differentiation of erythroid progenitor cells with an accompanying decrease in cell viability. These findings suggest that the inhibition of erythroid progenitor cells by LY294002 could be an early event during the course of erythroid proliferation and differentiation.
Effect of PI-3K Inhibitor on the Proliferation and Survival of Human Erythroid Progenitors
| LY294002 (μmol/L) . | Total Cells (-fold) . | Viability (%) . | Viable Cells (-fold) . |
|---|---|---|---|
| 0 (IMDM) | 27.8 ± 2.8 | 88.0 ± 5.3 | 24.5 ± 3.5 |
| 0 (0.1% DMSO) | 26.8 ± 3.6 | 89.5 ± 2.6 | 24.0 ± 3.5 |
| 1 | 26.2 ± 2.2 | 90.0 ± 3.2 | 23.7 ± 2.8 |
| 10 | 10.4 ± 0.5† | 84.5 ± 2.6 | 8.8 ± 0.6* |
| 20 | 4.8 ± 0.4† | 70.0 ± 2.1* | 3.8 ± 0.3† |
| 50 | 1.8 ± 0.4† | 7.5 ± 3.1† | 0.1 ± 0.1† |
| 100 | 1.3 ± 0.2† | 0† | 0† |
| LY294002 (μmol/L) . | Total Cells (-fold) . | Viability (%) . | Viable Cells (-fold) . |
|---|---|---|---|
| 0 (IMDM) | 27.8 ± 2.8 | 88.0 ± 5.3 | 24.5 ± 3.5 |
| 0 (0.1% DMSO) | 26.8 ± 3.6 | 89.5 ± 2.6 | 24.0 ± 3.5 |
| 1 | 26.2 ± 2.2 | 90.0 ± 3.2 | 23.7 ± 2.8 |
| 10 | 10.4 ± 0.5† | 84.5 ± 2.6 | 8.8 ± 0.6* |
| 20 | 4.8 ± 0.4† | 70.0 ± 2.1* | 3.8 ± 0.3† |
| 50 | 1.8 ± 0.4† | 7.5 ± 3.1† | 0.1 ± 0.1† |
| 100 | 1.3 ± 0.2† | 0† | 0† |
Day 8 cells were cultured in serum-free liquid medium with EPO at 2 U/mL. After 7 days of incubation, the cells were counted. Viability was determined by the trypan blue dye–exclusion test. The mean ± SD of quadriplicates is shown. Expansion-fold is the number of cells after 7 days of culture/the number of initial cells. Statistical analysis was performed using Scheffe’s F post hoc test.
P < .01, decrease v 0.1% DMSO.
P < .001, decrease v 0.1% DMSO.
Time-course study of the effect of LY294002 on erythroid progenitor cells.
To elucidate the early events initiated by LY294002, the time course of the inhibitory effect of LY294002 on the proliferation and differentiation of erythroid progenitor cells was investigated. Day 8 cells were suspended in liquid phase in serum-free medium with 2 U/mL EPO in the presence of 50 μmol/L LY294002 and cultured for 0, 4, 8, 24, and 48 hours, and the number and viability of the cells were assessed. To determine if the inhibitory effect of LY294002 is reversible, the cells were collected and washed twice with IMDM containing 0.3% BSA and then cultured in serum-containing fibrin clots with 2 U/mL EPO for 7 days, the objective being to assess the colony-forming ability after exposure to LY294002.
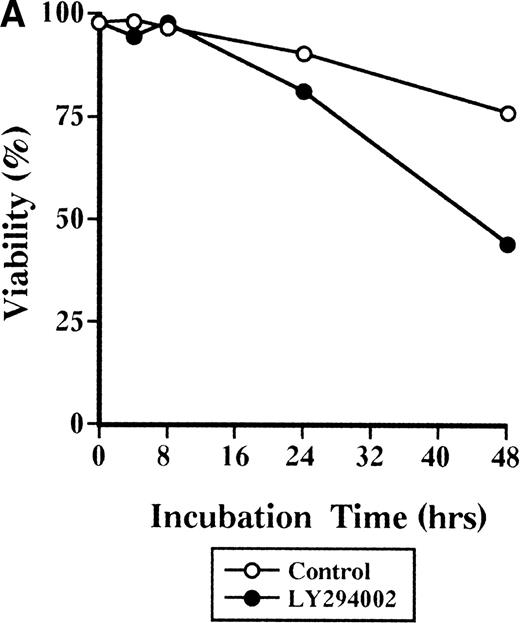
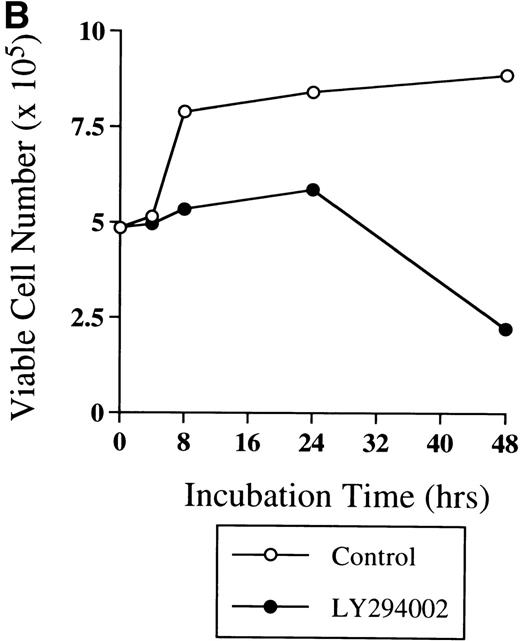
The viability of cells cultured with LY294002 decreased to 44% at 48 hours, while that of cells incubated without LY294002 remained at 75% (Fig 2A). In addition, the viability of cells cultured without LY294002 was close to 90% for up to 7 days of culture (Table 2). Among the viable cells, those incubated without LY294002 markedly increased from 4.85 × 105 to 8.8 × 105 during 48 hours of incubation (Fig 2B); however, cells incubated with LY294002 did not appear to increase until 24 hours and decreased to 2.20 × 105 during 48 hours (Fig 2B). When the reversibility of the inhibitory effect of LY294002 on the proliferation and differentiation of erythroid progenitor cells was assessed by erythroid colony formation (Fig 2C), the decrease in erythroid colony growth caused by LY294002 was evident in a time-dependent manner. The statistically significant decrease of erythroid progenitor cells was noted after 24 hours’ exposure (from 100% ± 6% to 18% ± 4%,P < .001), while erythroid progenitor cells in cells incubated without LY294002 continuously increased for up to 48 hours (195% ± 17%). Therefore, inhibition of erythroid progenitor cells by LY294002 occurs as an early event in the course of proliferation and differentiation in a time-dependent manner, and the inhibition is irreversible.
Time-course study of the effect of LY294002 on the proliferation and survival of erythroid progenitor cells. Day 8 cells that contained 69% ± 4% erythroid progenitor cells were suspended at a concentration of 5 × 105/mL in 1.0 mL serum-free medium supplemented with 2 U/mL EPO with (•) or without (○) 50 μmol/L LY294002. After incubation and at the indicated periods, the cells were collected, washed twice in IMDM containing 0.3% BSA, and then plated into serum-containing fibrin clots with 2 U/mL EPO. After 7 days of incubation, the clots were fixed and stained with benzidine-hematoxylin. (A) Viability; (B) absolute number of viable cells; (C) percent expression for the number of erythroid colonies in relation to the value of 0 hours as 100%. The mean ± SD of triplicates is shown for C. +++P < .001, decrease v control (○); ***P < .001, decreasev 0 hours; #P < .05,###P < .001, increase v 0 hours.
Time-course study of the effect of LY294002 on the proliferation and survival of erythroid progenitor cells. Day 8 cells that contained 69% ± 4% erythroid progenitor cells were suspended at a concentration of 5 × 105/mL in 1.0 mL serum-free medium supplemented with 2 U/mL EPO with (•) or without (○) 50 μmol/L LY294002. After incubation and at the indicated periods, the cells were collected, washed twice in IMDM containing 0.3% BSA, and then plated into serum-containing fibrin clots with 2 U/mL EPO. After 7 days of incubation, the clots were fixed and stained with benzidine-hematoxylin. (A) Viability; (B) absolute number of viable cells; (C) percent expression for the number of erythroid colonies in relation to the value of 0 hours as 100%. The mean ± SD of triplicates is shown for C. +++P < .001, decrease v control (○); ***P < .001, decreasev 0 hours; #P < .05,###P < .001, increase v 0 hours.
Titration of the effect of LY294002 on erythroid progenitor cells.
To titrate the inhibitory effect of LY294002 on erythroid progenitor cells, day 8 cells were suspended in liquid phase in serum-free medium with 2 U/mL EPO in the presence of various concentrations of LY294002 (0, 1, 10, 20, 50, and 100 μmol/L) and cultured for 4 and 24 hours, and the number and viability were assessed. To investigate whether the irreversibility of the inhibitory effect of LY294002 depends on the dose of LY294002, the cells were collected and washed twice with IMDM containing 0.3% BSA and then cultured in serum-containing fibrin clots with 2 U/mL EPO for 7 days.
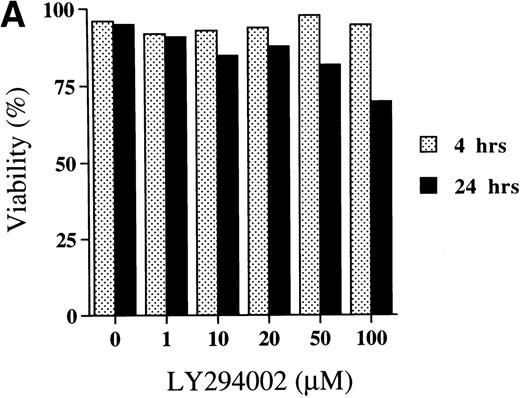
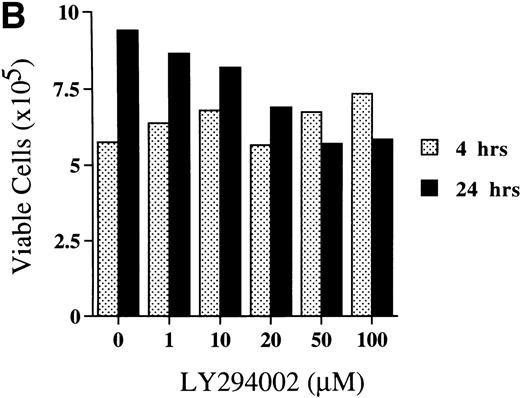
The viability of cells cultured for 4 hours was not affected by LY294002 at the concentrations tested. However, when day 8 cells were incubated for 24 hours with LY294002, viability decreased in a dose-dependent manner (Fig3A).
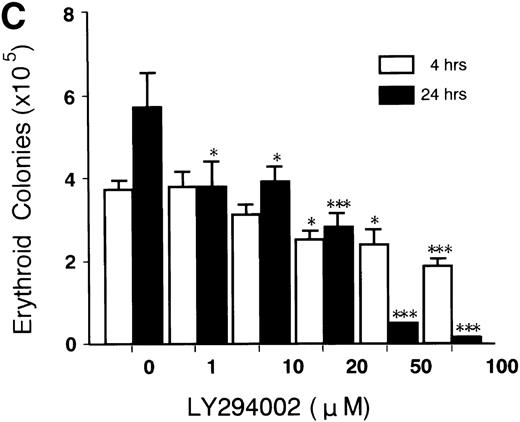
Titration of the effects of LY294002 on the proliferation and survival of erythroid progenitor cells. Day 8 cells that contained 64% ± 6% erythroid progenitor cells were suspended at a concentration of 5 × 105/mL in 1.0 mL serum-free medium supplemented with 2 U/mL EPO with various concentrations of LY294002 as indicated. After incubation for 4 or 24 hours, the cells were collected and washed twice in IMDM containing 0.3% BSA and then plated into serum-containing fibrin clots with 2 U/mL EPO. After 7 days of incubation, the clots were fixed and stained with benzidine-hematoxylin. (A) Viability; (B) absolute number of viable cells; (C) percent expression for the number of erythroid colonies relative to the value in 0.1% DMSO as 100%. The mean ± SD of triplicates is shown for C. Cells incubated for 4 or 24 hours with 0.1% DMSO contained 69% ± 4% and 65% ± 9% erythroid colony-forming cells, respectively. *P < .05, ***P< .001, decrease v control.
Titration of the effects of LY294002 on the proliferation and survival of erythroid progenitor cells. Day 8 cells that contained 64% ± 6% erythroid progenitor cells were suspended at a concentration of 5 × 105/mL in 1.0 mL serum-free medium supplemented with 2 U/mL EPO with various concentrations of LY294002 as indicated. After incubation for 4 or 24 hours, the cells were collected and washed twice in IMDM containing 0.3% BSA and then plated into serum-containing fibrin clots with 2 U/mL EPO. After 7 days of incubation, the clots were fixed and stained with benzidine-hematoxylin. (A) Viability; (B) absolute number of viable cells; (C) percent expression for the number of erythroid colonies relative to the value in 0.1% DMSO as 100%. The mean ± SD of triplicates is shown for C. Cells incubated for 4 or 24 hours with 0.1% DMSO contained 69% ± 4% and 65% ± 9% erythroid colony-forming cells, respectively. *P < .05, ***P< .001, decrease v control.
Among the viable cells, those incubated with LY294002 for 4 hours were not affected by any of the concentrations tested (Fig 3B). After 24 hours of incubation of day 8 cells without LY294002, the number of viable cells increased from 5.75 × 105 (after 4 hours) to 9.40 × 105. The presence of LY294002 for 24 hours decreased the number of viable cells in a dose-dependent manner.
Figure 3C shows the significant decrease in erythroid colony growth of cells exposed to LY294002 in a dose-dependent manner in both cultures for 4 and 24 hours. The decrease of erythroid colony growth in cells exposed to LY294002 for 24 hours was more prominent than that found in cells exposed for 4 hours, which indicates that the inhibition of erythroid proliferation and differentiation is augmented by prolonged exposure to LY294002. It is of note that the significant inhibition of erythroid progenitor growth occurred (Fig 3C) during periods in which the viability of cells and the number of viable cells were not affected (Fig 3A and B; incubation for 4 hours).
LY294002 induces apoptosis and DNA fragmentation in erythroid progenitor cells.
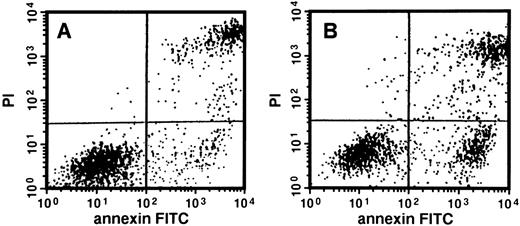
A decrease in erythroid progenitor growth with intact viability after exposure to LY294002 suggests that an early event in the induction of an internal suicide program (apoptosis) could be responsible for the mechanism. Because apoptosis is commonly associated biochemically with the loss of phospholipid asymmetry on the cell surface and DNA fragmentation, we examined these 2 issues. The loss of phospholipid asymmetry on the cell surface was examined using FITC-conjugated annexin V and FACS (Fig 4). When day 8 cells were incubated for 24 hours, 22.8% of cells incubated with LY294002 at 50 μmol/L were detected as annexin V+/PI−, in contrast to 9.8% found in the control.
Apoptosis of erythroid progenitor cells by LY294002. Day 8 cells that contained 71% ± 5% erythroid progenitor cells were suspended at a concentration of 5 × 105/mL in 1.0 mL serum-free medium supplemented with 2 U/mL EPO with (B) or without (A) 50 μmol/L LY294002. After incubation for 24 hours, cells were collected, washed twice, counterstained with PI and annexin V conjugated with FITC, and then analyzed using FACS Vantage.
Apoptosis of erythroid progenitor cells by LY294002. Day 8 cells that contained 71% ± 5% erythroid progenitor cells were suspended at a concentration of 5 × 105/mL in 1.0 mL serum-free medium supplemented with 2 U/mL EPO with (B) or without (A) 50 μmol/L LY294002. After incubation for 24 hours, cells were collected, washed twice, counterstained with PI and annexin V conjugated with FITC, and then analyzed using FACS Vantage.
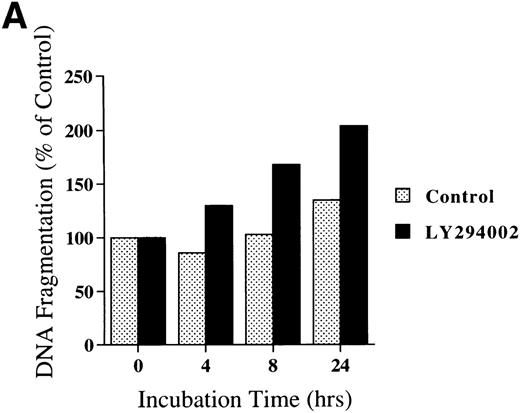
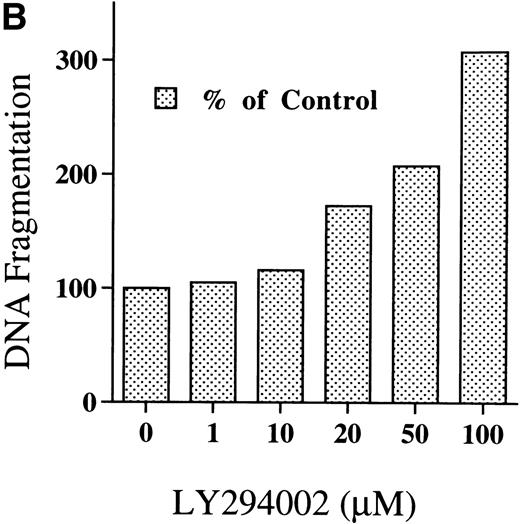
Chromatin fragmentation into oligonucleosomes was determined using an ELISA specific for cytosolic histone-bound DNA (Fig 5). LY294002-induced DNA fragmentation was found after 4 hours of incubation and continuously increased in a time-dependent manner (Fig5A). For day 8 cells cultured for 24 hours, LY294002-induced DNA fragmentation was found for a concentration of 20 μmol/L and increased in a dose-dependent manner (Fig5B).
DNA fragmentation in erythroid progenitor cells challenged with LY294002. Day 8 cells that contained 62% ± 13% erythroid progenitor cells were suspended at a concentration of 5 × 105/mL in 0.5 mL serum-free medium supplemented with 2 U/mL EPO in the presence of 50 μmol/L LY294002 (A) or with various concentrations of LY294002 (B). After incubation for the indicated periods (A) or for 24 hours (B), the cells were collected and washed twice with PBS. DNA fragmentation was determined by quantifying the amount of oligonucleosome-bound DNA in the 20,000× gsupernatant of cell lysates. Data represent the mean from 2 determinations.
DNA fragmentation in erythroid progenitor cells challenged with LY294002. Day 8 cells that contained 62% ± 13% erythroid progenitor cells were suspended at a concentration of 5 × 105/mL in 0.5 mL serum-free medium supplemented with 2 U/mL EPO in the presence of 50 μmol/L LY294002 (A) or with various concentrations of LY294002 (B). After incubation for the indicated periods (A) or for 24 hours (B), the cells were collected and washed twice with PBS. DNA fragmentation was determined by quantifying the amount of oligonucleosome-bound DNA in the 20,000× gsupernatant of cell lysates. Data represent the mean from 2 determinations.
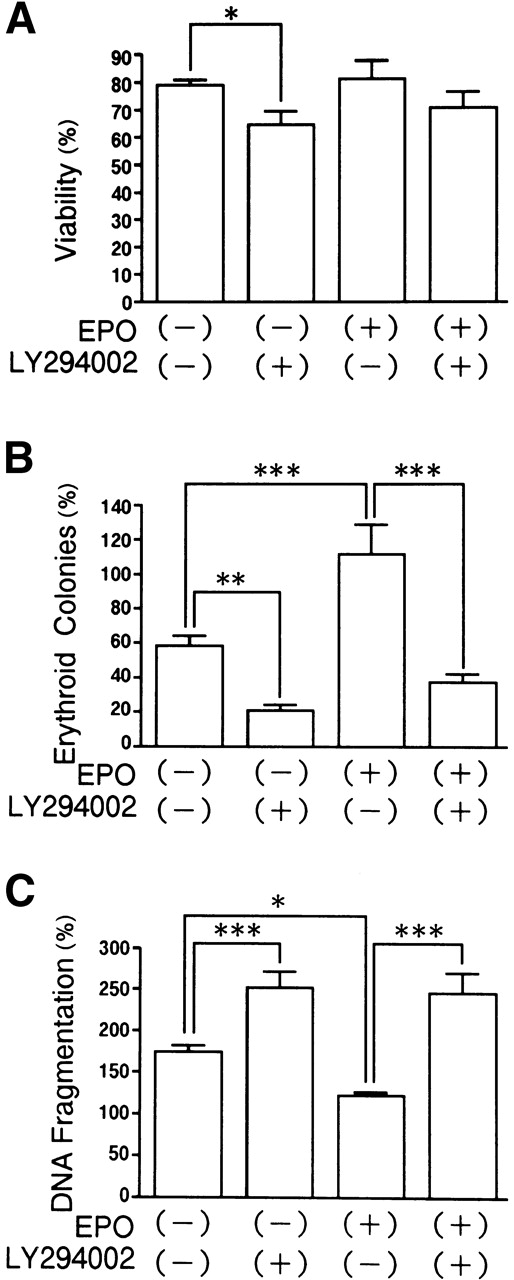
Deprivation of EPO by itself causes apoptosis in erythroid precursor cells.1-4 Therefore, the effects of LY294002 with or without EPO on the viability and survival of erythroid progenitor cells and DNA fragmentation were examined (Fig 6). When day 8 cells were incubated for 24 hours, LY294002 decreased the viability of the cells cultured without EPO (Fig 6A; P < .05). Deprivation of EPO decreased the number of erythroid progenitor cells, which was further accelerated by the presence of LY294002 (Fig 6B). Deprivation of EPO resulted in accelerated DNA fragmentation irrespective of the presence of LY294002. LY294002 accelerated the DNA fragmentation of cells cultured without EPO (Fig 6C; P < .001). These data suggest that the effects of LY294002 on apoptosis in erythroid precursor cells cannot be fully explained by the blockade of EPO-induced signal transduction.
Effects of LY294002 with or without EPO on the viability and survival of erythroid progenitor cells and DNA fragmentation. Day 8 cells that contained 61% ± 3% erythroid progenitor cells were suspended at a concentration of 1 × 106/mL in 1.0 mL serum-free medium with or without 2 U/mL EPO or 50 μmol/L LY294002. After incubation for 24 hours, cells were collected and washed twice in IMDM containing 0.3% BSA and then plated into serum-containing fibrin clots with 2 U/mL EPO. After 7 days’ incubation, the clots were fixed and stained with benzidine-hematoxylin. Data represent the mean ± SD of quadruplicates. (A) Viability; (B) percent expression for the number of erythroid colonies relative to the value of fresh day 8 cells as 100%; (C) DNA fragmentation using fresh day 8 cells as controls. *P < .05, **P < .01, ***P < .001.
Effects of LY294002 with or without EPO on the viability and survival of erythroid progenitor cells and DNA fragmentation. Day 8 cells that contained 61% ± 3% erythroid progenitor cells were suspended at a concentration of 1 × 106/mL in 1.0 mL serum-free medium with or without 2 U/mL EPO or 50 μmol/L LY294002. After incubation for 24 hours, cells were collected and washed twice in IMDM containing 0.3% BSA and then plated into serum-containing fibrin clots with 2 U/mL EPO. After 7 days’ incubation, the clots were fixed and stained with benzidine-hematoxylin. Data represent the mean ± SD of quadruplicates. (A) Viability; (B) percent expression for the number of erythroid colonies relative to the value of fresh day 8 cells as 100%; (C) DNA fragmentation using fresh day 8 cells as controls. *P < .05, **P < .01, ***P < .001.
LY294002 inhibits phosphorylation of the PI-3K–dependent kinase AKT.
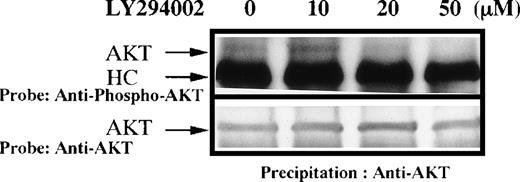
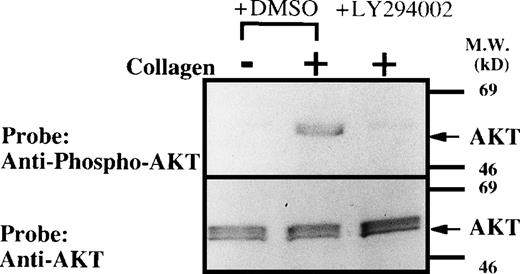
To determine if PI-3K activity in situ was indeed inhibited by LY294002 in primary cultured human erythroid progenitor cells, we next examined the effect of LY294002 on the steady-state phosphorylation of AKT at Ser-473, a recognized downstream event of PI-3K activation. AKT was modestly and constitutively phosphorylated at Ser-473 in human erythroid progenitor cells, and phosphorylation of AKT was blocked by treatment of the cells with LY294002 at concentrations similar to those which suppressed erythroid growth or induced apoptosis (Fig7). We acknowledge that the degree of AKT phosphorylation was small. However, it should be noted that we have tried to measure steady-state levels of AKT phosphorylation instead of induced levels. In preliminary experiments, we could not find any steady-state phosphorylation over the background levels by simple Western blotting on 3 different occasions. However, we confirmed that the reagents work well, because of the results of the following experiments. Human washed platelets were prepared and stimulated by type 1 horse tendon collagen (50 μg/L) as previously described.43 Whole-cell lysates (107cells/lane) were subjected to SDS-PAGE followed by Western blotting to detect AKT phosphorylation using the same system. As expected from the previous report,44 collagen induced a clear increase in AKT phosphorylation and the increase was abolished by LY294002 (50 μmol/L), suggesting that the detection system and LY294002 are appropriate (Fig 8). Similar amounts of AKT were detected on each lane (lower panel). In both panels, the bands appear broad, probably due to the presence of different isoforms of AKT.26
(A) Phosphorylation of AKT in human erythroid cells stimulated by EPO (10 U/mL). Day 8 cells were washed once in IMDM and lysed by adding an equal amount of a buffer containing 2% Triton X-100 after incubation for 1 hour in the presence of DMSO or various concentrations of LY294002. AKT was immunoprecipitated with specific AKT antisera. Immune complexes were resuspended in SDS-sample buffer and divided into two. Phosphorylation of AKT was detected using an anti-phospho-AKT antibody (A), and total AKT was detected using an anti-AKT polyclonal antibody (B). Bands were visualized by chemiluminescence.
(A) Phosphorylation of AKT in human erythroid cells stimulated by EPO (10 U/mL). Day 8 cells were washed once in IMDM and lysed by adding an equal amount of a buffer containing 2% Triton X-100 after incubation for 1 hour in the presence of DMSO or various concentrations of LY294002. AKT was immunoprecipitated with specific AKT antisera. Immune complexes were resuspended in SDS-sample buffer and divided into two. Phosphorylation of AKT was detected using an anti-phospho-AKT antibody (A), and total AKT was detected using an anti-AKT polyclonal antibody (B). Bands were visualized by chemiluminescence.
Collagen-induced phosphorylation of AKT in human platelets. Washed platelets were pretreated with DMSO or LY294002 (50 μmol/L) as indicated for 15 minutes. Platelets were then treated with horse tendon type I collagen (50 μg/mL) or buffer (control) for 5 minutes. Whole-cell extracts (107 cells/lane) were subjected to SDS-PAGE and Western blot analysis with an anti-phospho-AKT (A) or AKT antibody (B).
Collagen-induced phosphorylation of AKT in human platelets. Washed platelets were pretreated with DMSO or LY294002 (50 μmol/L) as indicated for 15 minutes. Platelets were then treated with horse tendon type I collagen (50 μg/mL) or buffer (control) for 5 minutes. Whole-cell extracts (107 cells/lane) were subjected to SDS-PAGE and Western blot analysis with an anti-phospho-AKT (A) or AKT antibody (B).
DISCUSSION
We examined the role of PI-3K in the proliferation and viability of human erythroid progenitors expanded in vitro. Because these cells, unlike genetically engineered murine cell lines expressing the EPO-R or murine and human leukemic cell lines, regularly undergo full terminal differentiation and become reticulocytes, these studies are more likely to reflect actual physiologic situations. However, we acknowledge the possible presence of artifacts arising from in vitro culture.30 32
We found that LY294002, a specific PI-3K inhibitor, dose- and time-dependently inhibits the proliferation and survival of human erythroid progenitor cells. The inhibition of erythroid progenitor growth by LY294002 was irreversible, and half-maximal inhibition was observed at 10 μmol/L LY294002 and was maximal at about 50 μmol/L. One of the mechanisms involved in this inhibitory effect is the induction of apoptosis in the erythroid progenitor cells as observed at 20 μmol/L. The effect was dependent on the dose and the exposure time, as evidenced by the appearance of annexin V–binding cells and DNA fragmentation. Phosphorylation of AKT at Ser-473, a recognized downstream event of PI-3K activation, was inhibited by LY294002 at similar concentrations for suppressing proliferation and inducing apoptosis, suggesting that the effects of LY294002 may be specific. These results suggest that PI-3K has antiapoptotic effects on erythroid progenitor cells and such effects may be mediated through downstream molecules such as AKT. One of the possible pathways that are inhibited by LY294002 includes the ras/MAP kinase pathway. Sui et al30 have reported that wortmannin inhibited EPO- or SCF-induced activation of MAP kinase and that PD98059, an MEK inhibitor, inhibited the proliferation of primary erythroid cells. In preliminary experiments, we also observed that PD98059 inhibited the proliferation of erythroid cells, consistent with the report by Sui et al.30
Although antagonistic effects of wortmannin on EPO-induced cell proliferation have been reported,18,23,30 Kubota et al16 noted that wortmannin prevents EPO-induced differentiation but not proliferation of these cells. Miura et al15 demonstrated that the C-terminus–deleted EPO-R, which lacks the ability to associate with PI-3K and to activate it, is still mitogenically active in 32D cells, an IL-3–dependent cell line, and hence PI-3K is not required for growth signal transduction from EPO-R. Taken together, these data suggest that in cells that proliferate or differentiate in response to EPO, the results of PI-3K activation may vary depending on the cell lines used in each study. Furthermore, in these reports, wortmannin did not induce apoptosis in erythroid-like cells. Thus, to our knowledge, our data suggest for the first time that PI-3K may have a role in protecting human erythroid cells from apoptosis. In view of a recent report on murine macrophages,31 the use of LY294002 instead of wortmannin may be one reason that our study found induction of apoptosis in erythroid cells by inhibition of PI-3K while the other studies did not. This view is strengthened by the report by Sui et al,30 as they did not observe induction of apoptosis in human erythroid precursors by wortmannin. Indeed, in preliminary experiments, we also found that greater than 10 μmol/L wortmannin is required to induce apoptosis in human erythroid cells. Such a high dose of wortmannin probably has nonspecific effects. Interestingly, insulin (or insulin-like growth factor [IGF]), EPO and c-kit ligand all activate PI-3K in various cells.15-29 In our system, we regularly add insulin; our previous studies have shown that albumin (fraction V) contains IGF-1, which may induce PI-3K activation.26,37These factors also have proliferative and antiapoptotic effects on human erythroid precursors. The steady-state phosphorylation of AKT at Ser-473 observed in our current studies may reflect, in part, the steady-state activation of PI-3K,26 which may in turn be induced by a combination of these factors. It is possible that EPO supports the viability of cells by stimulating activation of PI-3K, but that even without EPO some other factor maintains partial activation of PI-3K and thus prolongs cell viability without EPO. Although more studies are necessary to elucidate the factors responsible for activation of PI-3K in primary cells, it seems likely that EPO protects erythroid cells from apoptosis in a pathway that is not directly related to PI-3K. Although such conclusions seem heretical in view of previous studies using cell lines, it was recently reported that SCF, but not EPO, induces activation of AKT in primary erythroid cells.45 On the other hand, deprivation of EPO facilitated apoptosis in the presence and absence of LY294002 (Fig6).
A potential major limitation to the interpretation of our study is the use of the PI-3K inhibitor LY294002, which may or may not be absolutely specific for PI-3K. However, irrespective of the specificity of LY294002, our studies revealed the presence of at least 2 different pathways in the protection from apoptosis in primary erythroid cells. One is LY294002-sensitive, and this pathway may not be directly related to the presence of EPO. The other pathway is apparently EPO-dependent and at least partly insensitive to LY294002, and the molecular basis of this pathway remains to be determined. Very recently, abnormalities of PTEN, which dephosphorylates D3-polyphosphoinositides, were noted in hematopoietic cell lines and some primary specimens.46 The reduced levels of PTEN expression were associated with enhanced AKT phosphorylation.46 The levels of D3-polyphosphoinositides may partially regulate the survival of normal erythroid cells (and possibly cells of different lineage), and their deregulation may lead to malignant transformation of hematopoietic cells.
ACKNOWLEDGMENT
We thank Dr C.I. Civin for the generous gift of the monoclonal antibodies, without which this study could not have been performed. We also thank Chugai Pharmaceutical Co, Kirin Brewery Co, Sankyo Pharmaceutical Co, and Takeda Pharmaceutical Co for the generous gift of recombinant human growth factors and reagents. Finally, we thank S. Okazaki for manuscript preparation, M. Ohara for helpful comments, and M. Kitayama and I. Sato for technical assistance.
Supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan (K.S., A.O., and Y.I.), a research grant from the Idiopathic Disorders of Hematopoietic Organs Research Committee of the Ministry of Health and Welfare of Japan (K.S.), and the Ryoichi Naito Foundation for Medical Research (A.O.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ken-ichi Sawada, MD, Department of Medicine II, Hokkaido University School of Medicine, N-15, W-7, Sapporo, Hokkaido 060-8638, Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal