Abstract
Recent studies have shown a discrepancy between the level of tissue factor (TF) expression and the level of TF procoagulant activity on the apical and basolateral surface domains of polarized epithelial cells. The present investigation was performed to elucidate possible reasons for the discordant expression of TF and its activity on the surface of polarized epithelial cells using a human intestinal epithelial cell line, Caco-2 and Madin-Darby canine kidney epithelial cells, type II (MDCK-II). Functional activity of coagulation factor VIIa (VIIa) in complex with TF was 6- to 7-fold higher on the apical than the basolateral surface in polarized Caco-2 cells. In contrast, no significant difference was found in the formation of TF/VIIa complexes between the apical and basolateral surface. Confocal microscopy of Caco-2 cells showed TF expression on both the apical and the basolateral surface domains. Studies with MDCK-II cells showed that the specific functional activity of TF expressed on the apical cell surface was 5-fold higher than on the basolateral surface. To test whether differential expression of TF pathway inhibitor (TFPI) on the apical and basolateral surface could account for differences in TF/VIIa functional activity, we measured cell-surface–bound TFPI activity in Caco-2 cells. Small but similar amounts of TFPI were found on both surfaces. Further, addition of inhibitory anti-TFPI antibodies induced a similar enhancement of TF/VIIa activity on both surface domains. Because the availability of anionic phospholipids on the outer leaflet of the cell membrane could regulate TF/VIIa functional activity, we measured the distribution of anionic phospholipids on the apical and basolateral surface by annexin V binding and thrombin generation. The results showed that the anionic phospholipid content on the basolateral surface, compared with the apical surface, was 3- to 4-fold lower. Mild acid treatment of polarized Caco-2 cells, which markedly increased the anionic phospholipid content on the basolateral surface membrane, increased the TF/VIIa activity on the basolateral surface without affecting the number of TF/VIIa complexes formed on the surface. Overall, our data suggest that an uneven expression of TF/VIIa activity between the apical and basolateral surface of polarized epithelial cells is caused by differences in anionic phospholipid content between the two surface domains and not from a polar distribution of TFPI.
TISSUE FACTOR (TF) is a cellular receptor for plasma coagulation factor VIIa and formation of TF/VIIa complexes on the cell surface triggers the coagulation cascade in vivo.1 The TF/VIIa complex efficiently activates coagulation factors IX and X. The resultant protease factor Xa (Xa) activates prothrombin to thrombin, which in turn converts fibrinogen into a fibrin matrix. TF pathway inhibitor (TFPI) functions as the primary regulator of TF/VIIa activity during hemostasis.1,2Feedback inhibition of the TF/VIIa complex is accomplished by the formation of a stable quaternary complex of TF/VIIa/ Xa/TFPI.2
Normally, TF is constitutively expressed on the surface of many extravascular cell types that are not in contact with the blood, such as fibroblasts, pericytes, smooth muscle cells, and epithelial cells, but not on the surface of cells that come in contact with blood, such as endothelial cells and monocytes.3,4 However, a number of pathophysiologic stimuli induces TF expression in both endothelial cells and monocytes.5 Studies on the cellular distribution of TF have shown a polar distribution of TF in human umbilical vein endothelial cells and Madin-Darby canine kidney epithelial cells (MDCK).6-9 Ryan et al6 reported that TF activity was not expressed on the luminal cell surface of tumor necrosis factor-α (TNF-α)–stimulated endothelial cells but rather associated with subendothelial extracellular matrix. In contrast, Mulder et al8 found that nearly all TNF-α–induced TF activity was expressed on the endothelial cell surface rather than on the subendothelial matrix. Further, these investigators8also suggested that TF activity in endothelial cells was predominantly located on the basolateral surface. However, Narahara et al7 found that TF activity of interleukin-1β–stimulated endothelial cells was almost exclusively located on the apical surface, whereas little activity was found on the basolateral surface. Camerer et al9 were the first to actually measure TF distribution on the apical and basolateral surface domains of polarized cells grown in a transwell system. They reported that in endothelial cells not only TF activity, but also TF antigen, was localized primarily on the apical surface. This study also showed that a large fraction of TF/VIIa complexes formed on the basolateral surface of MDCK cells was not functionally active, whereas a much smaller number of complexes formed on the apical surface was highly active. The reason for the discordant expression of TF and its activity on polarized cells is, at present, unknown.
In the present study, we investigate possible reasons for the discordant expression of TF and its activity on the surface of polarized epithelial cells. Our data suggest that anionic phospholipids are not symmetrically exposed on the outer leaflet of the apical and basolateral cell membrane domains of polarized epithelial cells and that this differential availability of anionic phospholipids may explain the polar expression of TF/VIIa functional activity.
MATERIALS AND METHODS
Reagents.
Iodo-Gen was from Pierce Biochemical Co (Rockford, IL); sodium [125I]iodide was from Amersham Corp (Arlington Heights, IL); Chromozym X and Chromozym TH were from Boehringer Mannheim (Indianapolis, IN); Dulbecco’s modified Eagle’s medium (DMEM) was from GIBCO (Grand Island, NY); fetal bovine serum (FBS), trypsin-EDTA, and penicillin-streptomycin were from BioWhittaker (Walkersville, MD). Tissue-culture flasks and plates were from Becton Dickinson (Bedford, MA). Transwell cell culture inserts were from Corning Costar (Acton, MA). Other chemicals, reagent grade or better, were from Sigma (St Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Proteins.
Recombinant human VIIa was purified as described.10 Human coagulation factor X and factor Va were obtained from Enzyme Research Laboratories (South Bend, IN). Factor Xa,11prothrombin,12 human brain TF apoprotein,13 and polyclonal rabbit anti-human TF IgG13 were prepared as described previously. TF apoprotein was reconstituted in 60% phosphatidylcholine, 40% phosphatidylserine vesicles as described.14 Annexin V, purified from human placenta, was a gift from Dr J.F. Tait (University of Washington, Seattle). Recombinant TFPI15 was used as an antigen to raise monospecific polyclonal anti-TFPI antiserum in rabbits. The antiserum was heat inactivated at 56°C for 30 minutes and the IgG fraction was separated by precipitation at 40% ammonium sulfate saturation followed by DEAE-Affi-Gel blue chromatography. The IgG had specific activity of more than 200 inhibitor units/mg protein.
Cell culture.
Caco-2 cells16 were maintained in DMEM (4.5 g/L glucose) with GlutaMAX 1 supplemented with 10% FBS, 1% (vol/vol) non-essential amino acids and antibiotics. MDCK-II cells16 were maintained in DMEM (4.5 g/L glucose) with GlutaMAX 1 supplemented with 5% FBS and antibiotics. Cells were seeded in 12-mm polycarbonate membrane transwells (0.4-μm pore size) at a density of 3 × 105 cells/cm2 (in parallel, cells were also seeded at a same density in a clear polyester membrane transwell for visual inspection). Medium was changed every second day. Cells were maintained in transwells for a time period of more than 3 days after establishment of tight junctions to obtain well differentiated monolayers. Establishment of epithelial cell integrity and tight junction formation was assessed by transepithelial electrical resistance (TER) measurements across the membrane using a MilliCell-ERS ohmmeter (Millipore, Bedford, MA). Tight junction formation was also demonstrated by the absence of radioactive tracer leakage from either side of the cell monolayer (125I-VIIa or 125I-annexin V). Cells were washed once on both sides with buffer A (10 mmol/L HEPES, pH 7.45, 150 mmol/L NaCl, 4 mmol/L KCl, and 11 mmol/L glucose) supplemented with 5 mmol/L EDTA and then washed twice with buffer B (buffer A supplemented with 5 mg/mL bovine serum albumin [BSA] and 5 mmol/L Ca2+) before they were used in the experiment. At well-differentiated monolayers, the cell number was 6 to 8 × 105 per transwell.
Radiolabeling of proteins.
VIIa and Annexin V were labeled using IODO-GEN–coated (Pierce) tubes and Na125I according to the manufacturer’s technical bulletin and as described previously.17 The labeling reaction was performed in tubes coated with 10 μg of IODO-GEN for 4 minutes on ice. The reaction was quenched by the addition of 1% KI, and free iodine was removed by extensive dialysis against 150 mmol/L NaCl, 10 mmol/L HEPES, pH 7.5. The concentration of the labeled proteins was determined by measurement of absorbance at 280 nm. Extinction coefficients (Ecm1%) of 13.2 and 6.0 were used for VIIa and annexin V, respectively.
Radiolabeled ligand binding to polarized cells.
Binding of 125I-VIIa to TF on the cell surface of polarized Caco-2 cells was performed essentially as described.17Cells in transwells were incubated for 2 hours at 4°C with 10 nmol/L of radiolabeled VIIa in buffer B added to each side in a final volume of 500 μL. At the end of the incubation, cells were quickly washed 3 times with ice-cold buffer B. Total surface bound radioactivity was eluted from the apical and basolateral cell surface by treating the cells with buffer A supplemented with 5 mg/mL BSA and 15 mmol/L EDTA. Radioactivity in eluates was measured by a gamma counter (Cobra; Packard Instrument Co, Meriden, CT). Nonspecific binding was determined in parallel duplicate wells in which the cells were preincubated for 30 minutes with rabbit anti-human TF IgG (100 μg/mL) before the addition of radioligand. TF-specific binding was determined by subtraction of nonspecific binding from total binding. At 10 nmol/L of 125I-VIIa, the nonspecific binding of factor VIIa to Caco-2 cell surfaces (to both apical and basolateral surface domains) was about 40% of the total binding. Binding of125I-VIIa to the cell surface of polarized MDCK-II cells was performed essentially as described above except that nonspecific binding was determined by adding 50-fold molar excess of unlabeled factor VIIa because anti-human TF antibodies failed to block factor VIIa binding to canine TF. Nonspecific binding of 125I-VIIa to cell membrane in MDCK-II cells was in the range of 35 to 45% of the total binding.
Binding of 125I-annexin V to Caco-2 cells was measured essentially as described.18 Cells were incubated for 2 hours at 4°C with 10 nmol/L of radiolabeled annexin V in buffer B. Total cell-surface–associated (Ca2+-dependent) radioactivity was recovered from the apical and basolateral surfaces by eluting with EDTA containing buffer and the eluates were counted in a gamma counter. Nonspecific binding was determined in parallel duplicate wells in which the cells were preincubated for 30 minutes with 50-fold molar excess of cold annexin V (500 nmol/L) before the addition of radioligand. Annexin-V nonspecific binding, on both apical and basolateral membranes, was 3% to 8% of the total binding.
Factor X activation assay.
Polarized cells in transwells, after removing media and washing the cells, were incubated with 10 nmol/L VIIa in buffer B (final volume, 500 μL) at 37°C for 15 minutes to allow VIIa binding to the cell-surface TF. Unbound ligand was then removed, and the monolayer was washed 3 times with buffer B. Activation of factor X on the monolayers was initiated by adding 300 μL of buffer B containing 175 nmol/L factor X. At various time intervals or at a fixed time (usually at 30 minutes), 20-μL aliquots were removed from apical and basolateral sides of the well and added to 80 μL of stopping buffer, TBS (50 mmol/L Tris, 150 mmol/L NaCl, pH 7.5) containing 5 mmol/L EDTA and 1 mg/mL BSA. The amount of Xa generated was determined in a chromogenic assay by transferring 50 μL of the above mixture to a microtiter plate well and adding 50 μL of Chromozym X (2.15 mmol/L) to the well. The absorbance at 405 nm was measured continuously in a microplate reader (Molecular Devices, Sunnyvale, CA), and the initial rates of color development were converted to Xa concentrations using an Xa standard curve (85 pmol/L to 11 nmol/L). The rate of TF/VIIa dependent activation of factor X on both the apical and basolateral surface was linear up to 1 hour.
Fluorescence confocal microscopy.
Polarized Caco-2 cells were maintained in transwells. Cells on transwell filters were fixed in 2% formaldehyde in phosphate buffer (pH 7.2) and washed thoroughly. Nonspecific binding was blocked by incubating the cell monolayer in blocking buffer (5% normal goat serum in phosphate-buffered saline (PBS) in the absence or presence of 0.2% saponin for permeabilization and increased accessibility of antibodies). Cells were incubated for 60 minutes with rabbit anti-human TF IgG (10 μg/mL) in blocking buffer on both sides of the cell monolayer. As controls, monolayers were incubated with either blocking buffer alone or preimmune IgG (10 μg/mL). After washing the cells in PBS, they were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG antibody (10 μg/mL) (Southern Biotechnology, Birmingham, AL) for 60 minutes. After thorough washing in PBS, transwell filters were rinsed briefly in distilled water and mounted in Fluoromount G (Southern Biotechnology) containing 2.5 mg/mL N-propyl-galleate (Sigma). Slides were examined with a Zeiss LSM 510 confocal laser scanning microscope using a C-Apochromat 63×, 1.2 water immersion objective (Carl Zeiss, Thornwood, NY), and the 488-nm line of the argon laser for excitation of FITC. The cells were scanned and images saved at 1024 × 1024-pixel/8-bit resolution before importing into Adobe Photoshop (Adobe Systems, Mountain View, CA) for compilation and direct printing.
Prothrombin activation assay.
Polarized cells in transwells were incubated with factor Va (20 nmol/L) and Xa (2 nmol/L) for 15 minutes at 37°C, followed by prothrombin (1,400 nmol/L). At various time points after the addition of prothrombin, 20 μL subsamples were removed in 80 μL ice-cold stopping buffer. The amount of thrombin generated in each subsample was determined by transferring 50 μL of the above mixture to a microtiter plate well and adding 50 μL of Chromozym TH (1.9 mmol/L) to the well. The initial rates of color development in milliOD/min at 405 nm were measured continuously with a microplate reader and the reading was converted to thrombin concentrations from a standard curve made with human α thrombin (4 mU/mL to 2 U/mL).
Elution of cell-bound TFPI and TFPI functional assay.
Cells were washed as described previously. Both compartments of polarized cells in transwells were treated with 500 μL of 0.1 mol/L glycine, pH 3.0, for 3 minutes. Eluates were removed and pH was adjusted to 7.8 with 1 mol/L Tris, and assayed for TFPI activity in a 2-step factor X activation assay. A 20-μL sample was added to a microtiter plate containing 40 μL of reagent A (TBS containing 1 mg/mL BSA, 90 pmol/L Xa, 3 nmol/L VIIa, 22 pmol/L human TF, 15 mmol/L CaCl2). After a 30-minute incubation at room temperature, 100 μL of reagent B (TBS containing 1 mg/mL BSA, 42 nmol/L factor X, 4 mmol/L CaCl2, and 0.86 μmol/L Chromozym X) was added to each sample. At the end of a 20- to 30-minute reaction period, the absorbance was read at 405 nm in a microplate reader. A TFPI standard curve was obtained using dilutions of 0.125% to 2% pooled normal human plasma or full-length recombinant TFPI (2.6 to 105 pmol/L).
RESULTS
Expression of TF/VIIa functional activity on polarized epithelial cells.
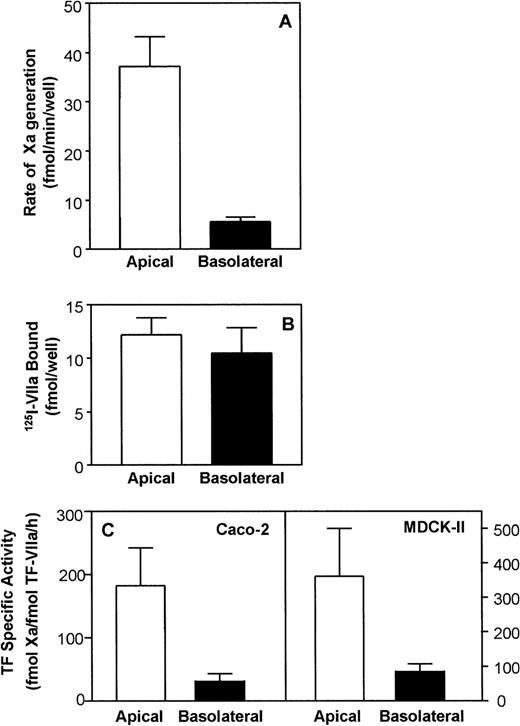
Caco-2 cells were grown to polarity in the transwell cell-culture system with distinct apical and basolateral cell-surface domains. Cell polarity was assessed by measuring TER and only well-differentiated cells with TER in the range of 400 to 600 Ωcm2 were used. In initial experiments, we added various concentrations of VIIa (0.1 to 40 nmol/L) to the apical and basolateral compartments, and the expression of TF/VIIa procoagulant activity was measured in a factor X activation assay. TF/VIIa activity was saturated at 10 nmol/L VIIa. As shown in Fig 1A TF/VIIa functional activity was expressed primarily on the apical side of the polarized Caco-2 cells, with a 6- to 7-fold higher activity than on the basolateral surface. Addition of inhibitory polyclonal anti-TF IgG completely inhibited the expression of TF/VIIa functional activity on both surface domains (data not shown). Similar experiments were also performed with polarized MDCK-II cells with TER in the range of 100 to 200 Ωcm2. In these cells, the apical surface exhibited a 2- to 3-fold higher TF/VIIa activity than the basolateral surface (data not shown).
Expression of cell-surface TF activity and antigen on polarized epithelial cells. (A) TF activity: The apical and basolateral surface domains of Caco-2 cells were incubated with VIIa (10 nmol/L) for 15 minutes at 37°C and TF/VIIa functional activity on each side was measured as the rate of Xa generation in the presence of 175 nmol/L factor X. Data are the mean ± SD of 4 independent experiments performed in duplicates. (B) Binding of 125I-VIIa to cell-surface TF on polarized Caco-2 cells. Both compartments of polarized Caco-2 cells were incubated with 10 nmol/L of125I-VIIa in the presence or absence of polyclonal anti-human TF IgG (100 μg/mL). VIIa bound to the apical (□) and basolateral (▪) surface was eluted by EDTA wash. The specific binding to TF was determined as described in Materials and Methods. Data are the mean ± SD of 5 independent experiments in duplicates. (C) Specific functional activity of TF/VIIa complexes. Specific functional activity of TF/VIIa complexes was calculated by relating the surface TF/VIIa activity with the number of VIIa molecules bound to cell-surface TF sites. Data are the mean ± SD of 4 independent experiments in duplicates.
Expression of cell-surface TF activity and antigen on polarized epithelial cells. (A) TF activity: The apical and basolateral surface domains of Caco-2 cells were incubated with VIIa (10 nmol/L) for 15 minutes at 37°C and TF/VIIa functional activity on each side was measured as the rate of Xa generation in the presence of 175 nmol/L factor X. Data are the mean ± SD of 4 independent experiments performed in duplicates. (B) Binding of 125I-VIIa to cell-surface TF on polarized Caco-2 cells. Both compartments of polarized Caco-2 cells were incubated with 10 nmol/L of125I-VIIa in the presence or absence of polyclonal anti-human TF IgG (100 μg/mL). VIIa bound to the apical (□) and basolateral (▪) surface was eluted by EDTA wash. The specific binding to TF was determined as described in Materials and Methods. Data are the mean ± SD of 5 independent experiments in duplicates. (C) Specific functional activity of TF/VIIa complexes. Specific functional activity of TF/VIIa complexes was calculated by relating the surface TF/VIIa activity with the number of VIIa molecules bound to cell-surface TF sites. Data are the mean ± SD of 4 independent experiments in duplicates.
Determination of TF/VIIa complex formation on polarized epithelial cells.
To determine the number of TF/VIIa complexes formed on the apical and basolateral surface of Caco-2 cells, we measured 125I-VIIa binding to both sides of the polarized cell monolayers. Polarized epithelial cells in transwells were exposed to125I-VIIa (10 nmol/L) in the presence and absence of anti-human TF IgG, and TF-specific VIIa binding was determined as described in Materials and Methods. As shown in Fig 1B, no significant difference was found in Caco-2 cells between the number of TF/VIIa complexes formed on the apical side and the number of TF/VIIa complexes formed on the basolateral surface. 125I-VIIa binding studies performed with MDCK-II cells showed that 2-fold more125I-VIIa was bound to the basolateral surface compared with the apical surface (data not shown).
The combined data of TF/VIIa activity and TF/VIIa complexes formed on the two cell-surface domains (see Fig 1C) provide strong evidence for the discordant expression of TF and the TF/VIIa functional activity in polarized epithelial cells. These data establish that TF/VIIa complexes formed on the basolateral surface of epithelial cells were less functionally active than TF/VIIa complexes formed on the apical surface.
Localization of TF by confocal immunofluorescence microscopy.
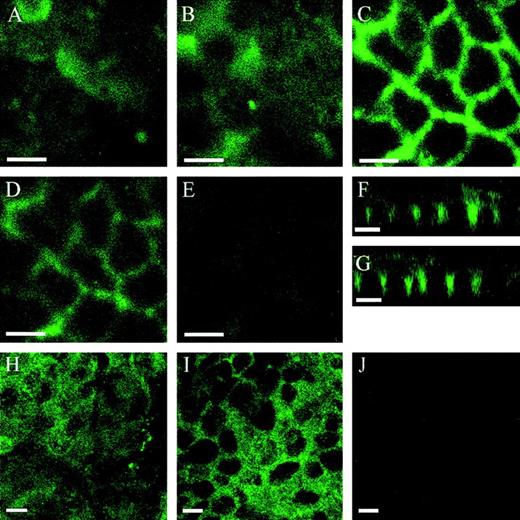
Confocal microscopy imaging of polarized Caco-2 cells showed TF expression on the apical as well as on the basolateral plasma membrane domain (Fig 2). Different levels of TF expression of individual cells within the monolayer combined with varying heights of the individual cells caused the TF staining on the apical membrane domain to appear heterogenous (Fig 2A and B). The basolateral TF had a reticular pattern of staining (Fig 2C) characteristic for basolateral antigens. The relatively strong lateral signal is most likely caused by interdigitated lateral plasma membranes. No TF staining was seen on the basal plasma membrane. Although the data from confocal microscopy do not allow quantitative comparisons of TF expression, they clearly showed TF antigen localization on both apical and basolateral membrane domains. Very little intracellular labeling was seen in permeabilized cells. Two different sources of polyclonal rabbit anti-human TF IgG antibodies gave the same results. Controls, including omission of primary antibodies or use of preimmune IgG, showed minimal staining.
TF localization on polarized Caco-2 cells. Cells were processed for indirect immunofluorescence with polyclonal rabbit anti-human TF IgG antibody followed by FITC-conjugated goat anti-rabbit antibody. Images were acquired by confocal microscopy as described in Materials and Methods. Cells were sectioned in the X-Y plane from the apical to basal membrane surfaces at 0.5-μm increments. Panels (A) through (G) represent nonpermeabilized cells. Panels (A) and (B) represent single focal X-Y planes at 2-μm increments around the apical membrane domain. Panels (C) and (D) represent single focal X-Y planes at 2-μm increments through the center of the cells. Panel (E) represents the basal membrane. Panels (F) and (G) show representative X-Z confocal views of nonpermeabilized cells. In panels (H) through (J), cells were permeabilized with 0.2% saponin. Panel (H) represents TF-specific staining of the apical membrane domain. Panel (I) represents mainly basolateral TF staining, 6 μm from plane in panel (H). Panel (J) represents TF staining of the basal membrane, 14 μm from plane in panel (H). Bars, 10 μm.
TF localization on polarized Caco-2 cells. Cells were processed for indirect immunofluorescence with polyclonal rabbit anti-human TF IgG antibody followed by FITC-conjugated goat anti-rabbit antibody. Images were acquired by confocal microscopy as described in Materials and Methods. Cells were sectioned in the X-Y plane from the apical to basal membrane surfaces at 0.5-μm increments. Panels (A) through (G) represent nonpermeabilized cells. Panels (A) and (B) represent single focal X-Y planes at 2-μm increments around the apical membrane domain. Panels (C) and (D) represent single focal X-Y planes at 2-μm increments through the center of the cells. Panel (E) represents the basal membrane. Panels (F) and (G) show representative X-Z confocal views of nonpermeabilized cells. In panels (H) through (J), cells were permeabilized with 0.2% saponin. Panel (H) represents TF-specific staining of the apical membrane domain. Panel (I) represents mainly basolateral TF staining, 6 μm from plane in panel (H). Panel (J) represents TF staining of the basal membrane, 14 μm from plane in panel (H). Bars, 10 μm.
TFPI expression on polarized Caco-2 epithelial cells.
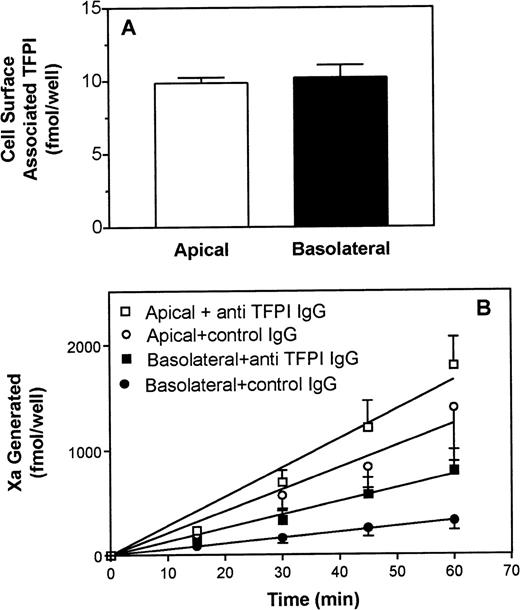
A polarized expression of TFPI, the primary inhibitor of the TF/VIIa complex, might explain the apparent discrepancy between expression of TF/VIIa functional activity on the apical and basolateral surface domains. To test this possibility, we examined the expression of TFPI on polarized cells. After removal of the media and washing, polarized epithelial cell monolayers on transwell membranes were treated with low pH buffer (0.1 mol/L glycine, pH 3.0) for 3 minutes to elute cell-surface–bound TFPI. The low pH eluate was assayed for TFPI activity in a TF/VIIa inhibition assay. We found small but similar amounts of TFPI in eluates derived from the apical and basolateral sides of Caco-2 cells (Fig 3A). These results suggest that Caco-2 cells synthesize low levels of TFPI and that the TFPI was evenly distributed between the apical and basolateral surface domains.
TFPI expression on polarized Caco-2 epithelial cells. (A) Cell-surface–associated TFPI: The apical and basolateral cell surfaces were treated with 500 μL 0.1 mol/L glycine pH 3.0 for 3 minutes to remove cell-surface–associated TFPI. Eluates were removed from the dish and pH was adjusted to 7.8 with 1 mol/L Tris and assayed for TFPI activity. Data are the mean ± SD of 4 independent experiments. (B) Effect of anti-TFPI on cell-surface TF/VIIa functional activity: Caco-2 cells grown in transwells were treated with control IgG (circles) or with polyclonal anti-TFPI antibodies (100 μg/mL) (squares) for 30 to 60 minutes on both the apical (open symbols) and basolateral (closed symbols) surfaces, and a Xa generation assay was performed as described in the legend to Fig 1. Data are the mean ± SD of 5 independent experiments.
TFPI expression on polarized Caco-2 epithelial cells. (A) Cell-surface–associated TFPI: The apical and basolateral cell surfaces were treated with 500 μL 0.1 mol/L glycine pH 3.0 for 3 minutes to remove cell-surface–associated TFPI. Eluates were removed from the dish and pH was adjusted to 7.8 with 1 mol/L Tris and assayed for TFPI activity. Data are the mean ± SD of 4 independent experiments. (B) Effect of anti-TFPI on cell-surface TF/VIIa functional activity: Caco-2 cells grown in transwells were treated with control IgG (circles) or with polyclonal anti-TFPI antibodies (100 μg/mL) (squares) for 30 to 60 minutes on both the apical (open symbols) and basolateral (closed symbols) surfaces, and a Xa generation assay was performed as described in the legend to Fig 1. Data are the mean ± SD of 5 independent experiments.
To further substantiate this conclusion, we tested the effect of inhibitory polyclonal anti-TFPI IgG on the expression of TF/VIIa functional activity on the apical and basolateral surface domains. Both surfaces of the polarized cells were preincubated with anti-TFPI IgG (or control IgG) for 60 minutes before they were used in determining cell-surface TF/VIIa functional activity. The results (Fig 3B) showed that addition of anti-TFPI IgG moderately enhanced the expression of TF/VIIa proteolytical activity on both the apical and basolateral surface. It is important to note that the addition of anti-TFPI IgG enhanced TF/VIIa activity to the same extent on both surfaces and the TF/VIIa functional activity on the basolateral surface was still substantially lower than the TF/VIIa activity on the apical surface. These data indicate that differential expression of TF/VIIa activity could not be abolished by neutralization of TFPI inhibitory activity.
Expression of anionic phospholipids in polarized epithelial cells.
Because the availability of anionic phospholipids on the outer leaflet of the cell membrane could regulate TF/VIIa functional activity,1 we evaluated the expression of anionic phospholipids on polarized epithelial cells. We used 2 different methods for assessment of anionic phospholipid expression on the outer leaflet of the apical and basolateral surface domains in Caco-2 cells: (1) ability of the cell surface to support prothrombin activation in the absence of exogenously added phospholipids, and (2) binding of radiolabeled annexin V to the cell surface.
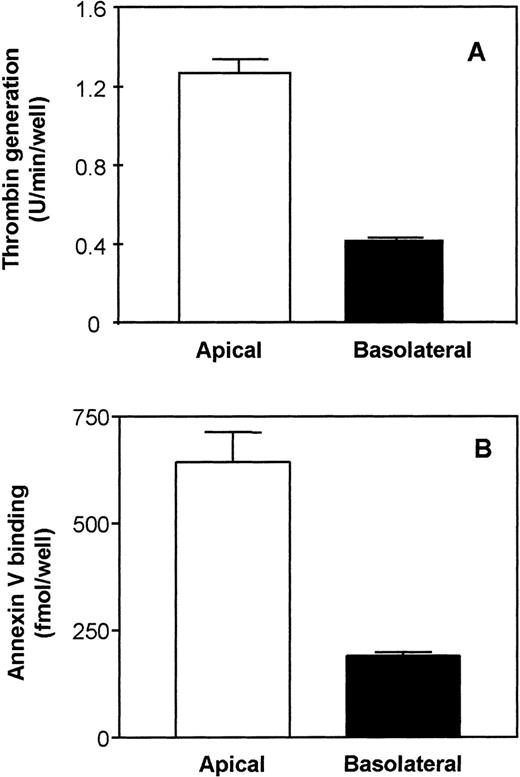
The initial rate of prothrombin activation on the basal surface was 3-fold lower than the initial rate observed on the apical surface (Fig 4A), suggesting a lower availability of anionic phospholipids on the basolateral surface. This observation was further supported by the annexin V binding data in Fig 4B, which showed a 3-fold lower annexin V binding to the basolateral surface compared with the apical surface (190 ± 10 fmol/well on the basolateral surface v 640 ± 70 fmol/well on the apical surface). A lower availability of anionic phospholipids on the basolateral surface compared with the apical surface was also observed in MDCK-II cells. These cells exhibited a 6- to 8-fold lower prothrombinase activity on the basolateral surface over the apical surface (data not shown).
Evaluation of anionic phospholipid availability by thrombin generation and annexin V binding on the surface of polarized Caco-2 epithelial cells. (A) Thrombin generation: Polarized cells in transwells were incubated with factor Va (20 nmol/L) and Xa (2 nmol/L) on both the apical and basolateral side for 15 minutes at 37°C before prothrombin (1,400 nmol/L) was added. At various time points, 20-μL aliquots were removed from the apical or the basolateral side and assayed for thrombin generated. Data are presented as initial rates of thrombin generation (n = 4). (B) Annexin V binding: Polarized Caco-2 cell layers were incubated with 10 nmol/L of125I-annexin V on the apical and basolateral side at 4°C. Cell-surface–associated radioactivity was removed from the apical (□) and basolateral (▪) surfaces by washing with an EDTA-containing buffer. Nonspecific binding was determined in parallel duplicate wells in which the cells were preincubated for 30 minutes at 4°C with 50-fold molar excess of cold annexin V (500 nmol/L) before adding 125I-annexin V (n = 3).
Evaluation of anionic phospholipid availability by thrombin generation and annexin V binding on the surface of polarized Caco-2 epithelial cells. (A) Thrombin generation: Polarized cells in transwells were incubated with factor Va (20 nmol/L) and Xa (2 nmol/L) on both the apical and basolateral side for 15 minutes at 37°C before prothrombin (1,400 nmol/L) was added. At various time points, 20-μL aliquots were removed from the apical or the basolateral side and assayed for thrombin generated. Data are presented as initial rates of thrombin generation (n = 4). (B) Annexin V binding: Polarized Caco-2 cell layers were incubated with 10 nmol/L of125I-annexin V on the apical and basolateral side at 4°C. Cell-surface–associated radioactivity was removed from the apical (□) and basolateral (▪) surfaces by washing with an EDTA-containing buffer. Nonspecific binding was determined in parallel duplicate wells in which the cells were preincubated for 30 minutes at 4°C with 50-fold molar excess of cold annexin V (500 nmol/L) before adding 125I-annexin V (n = 3).
Effect of mild acid wash on expression of TF/VIIa functional activity and anionic phospholipids in polarized Caco-2 cells.
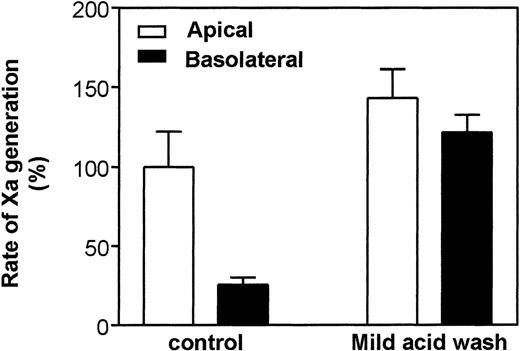
Polarized epithelial cells were treated with low pH buffer to elute cell bound TFPI (and possibly other extracellular components bound to the cell surface), and the formation of functionally active TF/VIIa complexes on the cell membrane was subsequently measured. Mild acid wash of cells slightly increased (1.4-fold) the TF/VIIa activity on the apical surface. However, the same acid treatment markedly enhanced (more than 5-fold) the expression of TF/VIIa functional activity on the basolateral surface, thus abolishing the difference in TF/VIIa activity between the basolateral and apical surface domains (Fig 5).
Effect of mild acid wash on TF/VIIa functional activity on polarized Caco-2 cells. Caco-2 cells in transwells were treated for 3 minutes with a mild acid wash (0.1 mol/L glycine, pH 3.0) or with buffer B (control). Cells were washed 3 times with buffer B after the acid treatment and then assayed for TF/VIIa activity as described in the legend to Fig 1. (Note: Polarized cell layers were tested for their integrity after the assay by measuring TER and flow of iodine labeled tracer across the cell layer. There was no loss of integrity after the mild acid wash.) Data are the mean ± SD of 3 independent experiments each in duplicates. Rate of Xa generation measured on the apical surface was taken as 100%.
Effect of mild acid wash on TF/VIIa functional activity on polarized Caco-2 cells. Caco-2 cells in transwells were treated for 3 minutes with a mild acid wash (0.1 mol/L glycine, pH 3.0) or with buffer B (control). Cells were washed 3 times with buffer B after the acid treatment and then assayed for TF/VIIa activity as described in the legend to Fig 1. (Note: Polarized cell layers were tested for their integrity after the assay by measuring TER and flow of iodine labeled tracer across the cell layer. There was no loss of integrity after the mild acid wash.) Data are the mean ± SD of 3 independent experiments each in duplicates. Rate of Xa generation measured on the apical surface was taken as 100%.
To test whether the increased expression of TF/VIIa functional activity on the basolateral surface after the mild acid wash is caused by an increase in number of TF/VIIa complexes formed on the cell surface, we determined the effect of mild acid wash on the number of TF/VIIa complexes formed on the membrane by binding studies with radiolabeled VIIa. The mild acid wash of cells did not increase TF-specific VIIa binding to the either apical or basolateral cell surface (data not shown).
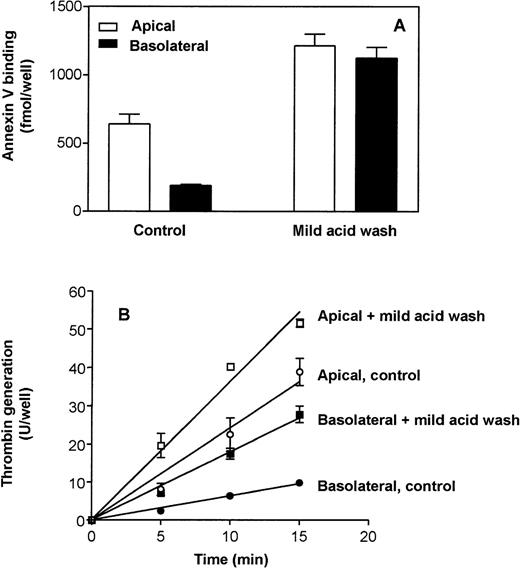
Next we examined the effect of the mild acid wash on the expression of anionic phospholipids measured by annexin V binding to the cell surface and by the prothrombinase activity on the cell surface. The annexin V binding to the basolateral surface was increased by 6-fold after the mild acid wash (190 ± 10 fmol/well in untreated cells to 1,120 ± 80 fmol/well in mild acid–treated cells) whereas only a 2-fold increase in annexin V binding was observed on the apical surface after the acid wash (640 ± 70 fmol/well in untreated cells to 1,220 ± 80 fmol/well acid-washed cells) (Fig 6A). Similar results were also obtained for prothrombinase activity assay (Fig 6B). The rate of thrombin generation was significantly increased (3-fold) on the basolateral surface after mild acid wash whereas a modest increase in rate of thrombin generation (1.5-fold) was observed on the apical surface (Fig 6B). After mild acid treatment we did not, however, obtain exactly the same rate of thrombin generation on the apical as on the basolateral surface, as might be expected from the annexin V binding data. The reason for this discrepancy is unknown, but one could speculate that factors additional to anionic phospholipids might affect the prothrombinase activity on cell surfaces.
Effect of mild acid wash on annexin V binding and thrombin generation on polarized Caco-2 cells. (A) Annexin V binding: The cells were treated with mild acid as described in the legend to Fig5. Cells were then incubated with 10 nmol/L of 125I-annexin V on the apical and basolateral side at 4°C. Total cell-associated radioactivity was removed from the apical and basolateral surfaces by washing with an EDTA-containing buffer. Nonspecific binding was determined in parallel duplicate wells in which the cells were preincubated for 30 minutes at 4°C with a 50-fold excess of cold annexin V (500 nmol/L) before adding 125I-annexin V. Specific binding (total-nonspecific) on the apical (□) and basolateral (▪) surfaces are presented (n = 4). (B) Thrombin generation: The polarized epithelial Caco-2 cells in transwells were subjected to a mild acid wash (0.1 mol/L glycine, pH 3.0) or to buffer B (control) on the apical or basolateral for 3 minutes. After washing, the cells were incubated with factor Va (20 nmol/L) and factor Xa (2 nmol/L) on both the apical and basolateral side for 15 minutes at 37°C before prothrombin (1,400 nmol/L) was added. At various time points, 20-μL samples were removed from the apical (open symbols) or the basolateral (closed symbols) side and assayed for the amount of thrombin generated (n = 4).
Effect of mild acid wash on annexin V binding and thrombin generation on polarized Caco-2 cells. (A) Annexin V binding: The cells were treated with mild acid as described in the legend to Fig5. Cells were then incubated with 10 nmol/L of 125I-annexin V on the apical and basolateral side at 4°C. Total cell-associated radioactivity was removed from the apical and basolateral surfaces by washing with an EDTA-containing buffer. Nonspecific binding was determined in parallel duplicate wells in which the cells were preincubated for 30 minutes at 4°C with a 50-fold excess of cold annexin V (500 nmol/L) before adding 125I-annexin V. Specific binding (total-nonspecific) on the apical (□) and basolateral (▪) surfaces are presented (n = 4). (B) Thrombin generation: The polarized epithelial Caco-2 cells in transwells were subjected to a mild acid wash (0.1 mol/L glycine, pH 3.0) or to buffer B (control) on the apical or basolateral for 3 minutes. After washing, the cells were incubated with factor Va (20 nmol/L) and factor Xa (2 nmol/L) on both the apical and basolateral side for 15 minutes at 37°C before prothrombin (1,400 nmol/L) was added. At various time points, 20-μL samples were removed from the apical (open symbols) or the basolateral (closed symbols) side and assayed for the amount of thrombin generated (n = 4).
DISCUSSION
Many cell types that do not normally come in contact with blood, such as epithelial cells lining the body cavities, constitutively express TF.4 Several types of epithelial cells form tight junctions separating the apical cell surface from the basolateral cell surface. Limited information is available on how TF is distributed and regulated in such polarized cells. In the present study, using epithelial cells grown on permeable filters where they polarize and form tight junctions, we investigated the expression and regulation of TF on the apical and basolateral cell-surface domains.
Binding studies with radiolabeled VIIa showed that TF antigen was evenly distributed between the apical and basolateral membrane domains of the polarized epithelial Caco-2 cells. Confocal microscopy also showed both apical and basolateral localization of TF receptors. However, measurement of functional TF/VIIa activity revealed a highly asymmetric distribution of the TF/VIIa activity, suggesting that complexes formed on the basolateral membrane surface were much less active than TF/VIIa complexes on the apical membrane surface. The discordant expression of TF and its activity observed on the surface of Caco-2 cells confirmed an earlier observation by Camerer et al9 on polarized MDCK-I cells. Our data with Caco-2 cells are, however, somewhat at variance with data reported by these investigators9 showing that 88% to 94% of surface TF was localized on the basolateral side of MDCK-I cells. We found an equal distribution of TF antigen between the apical and basolateral surface domains of Caco-2 cells. However, we should point out that we also found a basolateral predominance (70%) of canine TF antigen in MDCK-II cells. Camerer et al9 further reported that 73% to 83% of surface TF antigen on endothelial cells was exposed on the apical surface. The observed variance in TF antigen exposure between different cell types may partly rely on differences in TF antigen sorting in different cell types. In the absence of any known active sorting and endocytosis signal sequence in the cytoplasmic domain, it is possible that TF is subject to sorting by default pathways, which appear to differ between different cell types.19
In our present studies, most TF activity was found to be associated with the apical membrane surface of polarized epithelial cells. This is in accordance with earlier observations on both endothelial and epithelial cells,7,9 when the cells were grown on permeable supports and shown to establish tight junctions and polarized cell monolayers. Other reports6,8 on endothelial cell monolayers which suggested a different localization of TF activity were performed with cells grown on solid supports where the integrity of the cell monolayer might be questionable, and such monolayers may therefore not provide the best experimental model for studies of in vivoendothelial cell features especially when it comes to proteins with a polarized distribution.20
The discordant expression of TF and its activity observed on the surface of Caco-2 cells suggests that most of the TF/VIIa complexes formed on the basolateral surface were nonfunctional. These data extend earlier observations made with other cell types which showed that the majority of TF/VIIa complexes formed on the cell surface were nonfunctional or cryptic.17,21-26 A number of mechanisms have been proposed to account for the presence of nonfunctional TF/VIIa complexes. It has been postulated that a limited availability of anionic phospholipids on the outer leaflet of the cell membrane bilayer could limit the number of physiologically functional TF/VIIa complexes that could be formed.17,21,23 Camerer et al9 raised the possibility that a polar distribution of TF/VIIa inhibitors, such as TFPI, could account for the formation of nonfunctional TF/VIIa complexes on the basolateral surface membrane domain of epithelial cells. It has also been speculated that localization of TF to specialized plasma membrane microdomains, such as caveolae, might limit the function of TF.24 However, this later explanation is unlikely to account for the present findings in Caco-2 cells because these cells do not express caveolin-1 and are devoid of invaginated caveolar structures.16 A putative model for TF encryption has recently been proposed by Bach and Moldow.25 They suggested that TF exists as a dimer which binds VIIa but is unable to activate factor X, and that calcium influx into the cytosol (eg, induced by Ca2+ ionophores) triggers a calmodulin-dependent process that causes the dimers to dissociate into monomers capable of factor X activation.25 26 Because there were no specific data in the present study that could exclude this possibility, we cannot rule out the possibility that higher TF/VIIa functional activity observed on the apical surface of polarized cells could be due to the presence of a higher ratio of monomer TF on the apical surface compared with the basolateral surface.
TFPI is the primary inhibitor of the TF/VIIa functional activity. It is possible that an asymmetric distribution of TFPI, ie, sorting preferentially to the basolateral membrane, could limit the functional activity of TF/VIIa complexes formed on this surface of Caco-2 cells. Immunofluorescence studies of polar MDCK-I cell layers showed that TFPI was essentially localized on the basolateral surface.9However, our quantitative measurements of cell-bound TFPI and the analysis of the effect of anti-TFPI on TF/VIIa functional activity showed that a low level of TFPI is expressed in Caco-2 cells, where it is evenly distributed between the apical and basolateral cell-surface domains. Thus, it is unlikely that cell-bound TFPI plays a role in the discordant expression of TF and its activity observed in Caco-2 cells.
The proteolytic activity of TF/VIIa complexes formed in suspension with lipidated purified TF depends strongly on the presence of anionic phospholipids, such as phosphatidylserine (PS).1,23,27 This is also known to be the case for the procoagulant activity of TF on cellular membranes. Bach and Rifkin23 reported that treatment of pericytes with calcium ionophore, which enhances the expression of PS on the outer leaflet of cell membrane bilayer, enhanced the cell-surface TF/VIIa activation of factor X. Further, Le et al21 showed that treatment of fibroblasts with N-ethylmaleimide led to a 3- to 4-fold increase in the population of functional TF/VIIa complexes on the intact cells, and that this was accompanied by a substantial increase in the amount of anionic phospholipids present on the outer layer of the cell membrane. The increased TF/VIIa activity has also been observed on the surface of apoptotic cells, which typically expose elevated levels of PS in the exoplasmic leaflet.28 29 Our studies with polarized Caco-2 cells showed that the amount of anionic phospholipids on the basolateral surface was 3-fold lower than the amount of anionic phospholipids on the apical surface. Similar studies on polarized MDCK-II cells showed an 8-fold higher prothrombinase activity on the apical membrane domain compared to the basolateral domain. Thus, it is likely that differences in content of anionic phospholipids between the apical and the basolateral surface are a general phenomenon of polarized cells that could account for the observed difference in the functional state of TF/VIIa complexes formed on apical and basolateral surface domains.
The above conclusion is further supported by the data obtained from experiments in which polarized epithelial cells were mild acid–washed and then analyzed for expression of TF/VIIa functional activity and anionic phospholipid content. Our data showed that the mild acid treatment markedly increased the expression of TF/VIIa functional activity on the basolateral surface without affecting formation of TF/VIIa complexes. The increase in TF/VIIa activity on the basolateral cell surface was associated with an increase in the availability of anionic phospholipids on the basolateral cell membrane. This change in anionic phospholipid availability could be due to an increase in PS exposure on the outer leaflet of the membrane. Alternatively, the mild acid treatment could have led to dissociation of cell-membrane components that influence accessibility to anionic phospholipids. It is also possible that the mild acid wash might have removed or altered an unknown cell-surface component that plays a role, by a different mechanism, in regulating the expression of TF/VIIa functional activity on the cell surface.
Overall, the cumulative data of the present study strongly support the hypothesis that limited availability of anionic phospholipids on the basolateral surface of polarized epithelial cells is responsible for the nonfunctional property of TF/VIIa complexes formed on this surface domain.
ACKNOWLEDGMENT
We thank Dr Jan Stagsted (Institute of Medical Biochemistry, University of Aarhus, Aarhus, Denmark) for his advice and support during the course of this work. Dr Usha Pendurthi (UT Health Center at Tyler, TX) is greatly acknowledged for discussions and helpful suggestions. We also thank Todd Williams (UT Health Center at Tyler, TX) for his technical assistance, and Dr Fritz von Bülow (University of Copenhagen, Copenhagen, Denmark) for help with the confocal microscope.
Supported in parts by Grant No. HL 58869 from the National Heart Lung and Blood Institute. Supported in part by the The Danish Academy of Technical Sciences (ATV) (to C.B.H.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Carsten B. Hansen, PhD, Department of TF/VIIa Research, Novo Nordisk A/S, Novo Nordisk Park, 2760 Maalov, Denmark; e-mail: CBHa@novo.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal