Abstract
Human interleukin-5 (IL-5), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-3 are eosinophilopoietic cytokines implicated in allergy in general and in the inflammation of the airways specifically as seen in asthma. All 3 cytokines function through cell surface receptors that comprise a ligand-specific chain and a shared subunit (βc). Although binding of IL-5, GM-CSF, and IL-3 to their respective receptor chains is the first step in receptor activation, it is the recruitment of βc that allows high-affinity binding and signal transduction to proceed. Thus, βc is a valid yet untested target for antiasthma drugs with the added advantage of potentially allowing antagonism of all 3 eosinophil-acting cytokines with a single compound. We show here the first development of such an agent in the form of a monoclonal antibody (MoAb), BION-1, raised against the isolated membrane proximal domain of βc. BION-1 blocked eosinophil production, survival, and activation stimulated by IL-5 as well as by GM-CSF and IL-3. Studies of the mechanism of this antagonism showed that BION-1 prevented the high-affinity binding of125I–IL-5, 125I–GM-CSF, and125I–IL-3 to purified human eosinophils and that it bound to the major cytokine binding site of βc. Interestingly, epitope analysis using several βc mutants showed that BION-1 interacted with residues different from those used by IL-5, GM-CSF, and IL-3. Furthermore, coimmunoprecipitation experiments showed that BION-1 prevented ligand-induced receptor dimerization and phosphorylation of βc, suggesting that ligand contact with βc is a prerequisite for recruitment of βc, receptor dimerization, and consequent activation. These results demonstrate the feasibility of simultaneously inhibiting IL-5, GM-CSF, and IL-3 function with a single agent and that BION-1 represents a new tool and lead compound with which to identify and generate further agents for the treatment of eosinophil-dependent diseases such as asthma.
HUMAN INTERLEUKIN-5 (IL-5), IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are cytokines involved in hematopoiesis and inflammation.1 All 3 cytokines stimulate eosinophil production, function, and survival2-6 and therefore have the ability to influence inflammatory diseases, such as asthma, atopic dermatitis, and allergic rhinitis, in which the eosinophil plays a major effector role.7 IL-5, being the eosinophil-specific cytokine, has received most of the initial attention, with IL-5 mRNA and protein levels noted to be elevated in lung tissue and bronchoalveolar lavage fluid from symptomatic asthma patients.8-10 Correlations between IL-5 levels and allergen challenge and disease activity have also been seen. However, it is becoming apparent that not only IL-5, but also GM-CSF and IL-3 play a role in eosinophil production and activation in asthma, because there is evidence of both GM-CSF and IL-3 production at sites of allergic inflammation.11-17 The concomitant expression of these cytokines probably contributes to the total number of infiltrating eosinophils as well as to the degree of eosinophil activation. In addition, each of these cytokines may be responsible for the different phases and compartmentalization of the eosinophil infiltrate. Recent kinetic data from patients undergoing antigen challenge showed that IL-5 levels increased between days 2 and 7 postchallenge, whereas GM-CSF peaked at day 2 and remained elevated through to day 16. Furthermore, detection of GM-CSF extended beyond the site of allergen challenge.18
IL-5, GM-CSF, and IL-3 stimulate eosinophils and other cells by binding to cell surface receptors that comprise a ligand-specific α chain and a β chain that is shared by the 3 receptors (βc).1 Binding to each receptor α chain is the initial step in receptor activation; however, engagement of either α chain alone is not sufficient for activation to occur. Recruitment of βc by each ligand:α chain complex follows, a step that has 2 major functional consequences. Firstly, it allows the binding of IL-5, GM-CSF, and IL-3 to become essentially irreversible. Secondly, it leads to full receptor activation. βc is the major signaling component of these receptors, and its engagement leads to the activation of JAK-2, STAT-5, and other signaling molecules,19 culminating in the full plethora of cellular activities commonly associated with either IL-5, GM-CSF, or IL-3 stimulation, such as eosinophil adherence, priming for degranulation and cytotoxicity, and prolongation of viability.
To block or antagonize the activity of eosinophil-activating cytokines in vivo, 3 major approaches are being tried. One of them uses antibodies to the implicated cytokines. For example, antibodies to human IL-5 are being used in an animal model of allergen-induced asthma20,21 and have been shown to have a relatively long-lasting effect in preventing eosinophil influx into the airways and bronchial hyperresponsiveness. A second approach relies on IL-5 or GM-CSF mutants that can bind to their respective α chains with wild-type affinity but that have lost or shown reduced ability to interact with human βc. IL-5 mutants such as E13Q, E13K, and E13R and the human GM-CSF mutant E21R directly antagonize the functional activation of eosinophils by IL-5 or GM-CSF, respectively.22,23 However, at least in the case of E13K, eosinophil survival is not antagonized, and, in fact, this mutant is able to support eosinophil survival.24 A third approach involves the use of soluble receptor α chains that can sequester circulating cytokines.25 However, this carries the risk of a cytokine:receptor α chain complex potentially interacting with surface-expressed βc and triggering receptor activation. The common theme among these approaches is that they tackle a single receptor system involving either IL-5, GM-CSF, or IL-3, leaving the other 2 eosinophil-acting cytokines unaffected. Although the concomitant administration of IL-5 and GM-CSF antagonists may be considered, this could be clinically impracticable.
An alternative approach to blocking eosinophil-activating cytokines involves targeting βc. Although βc does not directly bind IL-5, GM-CSF, or IL-3 alone, it does associate with these cytokines complexed to the appropriate receptor α chain. Recent studies have identified the major binding sites of βc for the IL-5:IL-5Rα, GM-CSF:GM-CSFRα, and IL-3:IL-3Rα complexes. Significantly, these sites are used by all 3 complexes and comprise the predicted B-C loop and F-G loops in the membrane proximal domain of βc.26-28 Thus, targetting βc is not only desirable, but also feasible, with the potential to allow the simultaneous inhibition of IL-5, GM-CSF, and IL-3 action by a single agent.
We show here the development of the first simultaneous antagonist of IL-5, GM-CSF, and IL-3 in the form of monoclonal antibody (MoAb) BION-1 directed against the major cytokine binding region of βc. BION-1 blocked the production and activation of human eosinophils stimulated by IL-5, GM-CSF, or IL-3 by inhibiting the high-affinity binding of all 3 cytokines to eosinophils and preventing receptor heterodimerization and βc phosphorylation. The results demonstrate the feasibility of blocking the 3 eosinophilopoietic cytokines with a single compound and support the notion of simultaneously inhibiting more than 1 cytokine by targetting the shared signaling subunit in their receptors.
MATERIALS AND METHODS
Domain 4 cDNA.
Domain 4 of βc was expressed with an N-terminal Flag-epitope in the form of an activated βc mutant, ΔQP.29 An extracellular deletion removes domains 1 to 3 in this construct, but the transmembrane and cytoplasmic regions are retained. This was cloned into the eukaryotic expression vector pcDNA3 (Invitrogen, San Diego, CA).
Cytokines, cell lines, and primary cells.
Recombinant human IL-3 and GM-CSF were produced in Escherichia coli as described.22,30 Recombinant human IL-5 was purified from E coli by Bresagen (Adelaide, South Australia). Tumor necrosis factor α (TNFα) was a gift from Dr J. Gamble (Hanson Centre for Cancer Research, Adelaide, Australia). COS cells were transfected with receptor cDNA as described previously.26CHO βc and CHO ΔQP cells stably expressing either full-length βc or ΔQP, respectively, were generated by electroporation.22 TF1.8 cells were a gift from Dr J. Tavernier (University of Gent, Gent, Belgium). MO7e cells, a human megakaryoblastic cell line, were from Dr P. Crozier (Auckland, New Zealand). Human eosinophils were purified from the peripheral blood of normal or slightly eosinophilic volunteers via sedimentation through dextran and centrifugation through a discontinuous density gradient of hypertonic metrizamide, as previously described,31 and were greater than 95% pure. Human neutrophils and monocytes were purified from peripheral blood as described previously,2 32 with greater than 95% purity.
Generation of anti-βc MoAbs.
BALB/c mice were immunized intraperitonally with 1 × 107 COS cells transfected with βc or ΔQP expression constructs. The immunizations were repeated 4 times at 2-week intervals. Four weeks after the final immunization, a mouse was boosted with 2 × 106 COS transfectants intravenously. Three days later, splenocytes were harvested and fused with NS-1 myeloma cells as previously described.33 Hybridoma supernatants were screened on CHO βc or CHO ΔQP cells by flow cytometry, with untransfected CHO cells as a control. All antibodies were from single hybridoma clones as selected by a limiting dilution method. Using this procedure, several anti-βcMoAbs were generated; 8B8, 8E4,34 and 1C135 are nonfunctional MoAbs raised against full-length βc and have been used as control anti-βc MoAbs throughout these studies. MoAbs were purified from ascites fluid or hybridoma supernatant by a protein A sepharose column. The isotypes of MoAbs were tested with a Mouse MoAb Isotyping Kit (Boehringer Mannheim, Mannheim, Germany). Fab fragments were generated using a Fab Preparation kit (Pierce, Rockford, IL) following the supplied protocol.
Immunofluorescence.
Freshly purified neutrophils, eosinophils, monocytes, or CHO and COS cell transfectants (5 × 105) were incubated with 50 μL of hybridoma supernatant or 0.25 μg of purified MoAb for 45 to 60 minutes at 4°C. Cells were washed twice and then incubated with fluorescein isothiocyanate (FITC)-conjugated rabbit antimouse Ig (Silenus, Hawthorn, Victoria, Australia) for another 30 to 45 minutes. Cell were then washed and fixed before analyzing their fluorescence intensity on an EPICS-Profile II Flow Cytometer (Coulter Electronics, Hialeah, FL).
Ligand binding assay.
IL-3 and GM-CSF were radio-iodinated using the iodine monochloride method.36125I–IL-5 was purchased from Dupont NEN (North Sydney, New South Wales, Australia). MoAb BION-1 was radio-iodinated using the chloramine T method.37 Binding assays were performed as previously described.38 Briefly, 1 to 2 × 106 cells were preincubated with either BION-1 whole IgG, Fab fragments, or control MoAbs or with a range of concentrations of IL-3 or TNFα for 1 hour at 4°C. Radio-labeled ligand was then added and incubated for a further 2 hours before the cells were separated from free label by centrifugation through fetal calf serum (FCS). Counts associated with the resulting cell pellets were determined by counting on a γ-counter (Cobra Auto Gamma; Packard Instruments Co, Meridien, CT). Nonspecific binding was determined for each ligand by determining binding in the presence of a 200-fold excess of unlabeled ligand.
βc mutants and MoAb epitope mapping.
Alanine substitutions in the B-C and F-G loops of domain 4 of the βc have been described previously.26,27 The cDNAs for wild-type βc and each of the βcmutants in the B-C and F-G loops were introduced into COS cells by electroporation,26 and binding assays were performed on the transfected cells 48 hours posttransfection. The anti-βcMoAb, BION-1, and 1C1 were labeled using the chloramine T method,37 and binding assays were performed essentially as described previously.27
TF-1.8 cell proliferation assay.
TF-1.8 cells were grown in the presence of 2 ng/mL of GM-CSF. The cells were starved for 24 hours before setting up proliferation assays as described previously.33 From dose-response curves, the half-maximal proliferation dosage of IL-5 (0.3 ng/mL), GM-CSF (0.03 ng/mL), IL-3 (0.1 ng/mL), or erythropoietin (EPO; 5 ng/mL) was chosen to perform proliferation experiments in the presence of a range of concentrations of MoAbs. The 3H-Thymidine incorporation of each sample was determined by liquid scintillation.
Colony assay.
Ethics approval was obtained to collect bone marrow cells from healthy adult donors. The mononuclear cells were isolated after dextran sedimentation and density gradient centrifugation. The cells (50,000/mL) were cultured in semisolid methylcellulose medium for 14 days in humidified conditions supplied with 5% CO2. Colonies of more than 40 cells were scored after staining as previously described.39
Eosinophil survival assays.
The maximal dose of IL-5 required to support eosinophil survival after 36 hours was determined. Eosinophils were then cultured with 1 nmol/L of IL-5, GM-CSF, or IL-3 plus anti-βc MoAbs for 36 hours. The viability of eosinophils was quantitated by propidium iodide staining and flow cytometry analysis as described.40
CD69 expression.
Eosinophils were treated with a range of concentrations of cytokines for 3 hours either in the presence or absence of anti-βcMoAbs. CD69 expression was measured by immunofluorescence using an anti-CD69 monoclonal MoAb coupled to phycoerythrin (PE; Becton Dickinson Immunocytochemistry Systems, San Jose, CA).
Coimmunoprecipitation of α and β chains and the βc phosphorylation assays.
MO7e cells were surface labeled with 125I using the lactoperoxidase method, as described previously.41 The labeled cells were preincubated with MoAbs BION-1, control anti-βc MoAb 1C1 (0.5 mg/mL), or medium alone for 1 minutes before being stimulated or not with IL-3 (6 nmol/L) for 5 minutes. Cells were lysed in lysis buffer consisting of 137 mmol/L NaCl, 10 mmol/L Tris-HCl (pH 7.4), 10% glycerol, and 1% Nonindet P-40 with protease and phosphatase inhibitors (10 μg/mL leupeptin, 2 mmol/L phenylmethlysulphonyl fluoride, 10 μg/mL aprotonin,and 2 mmol/L sodium vanadate) for 30 minutes at 4°C, followed by centrifugation of the lysate at 10,000g for 15 minutes to remove cellular debris. The lysate was precleared with mouse-Ig–coupled Sepharose beads for 18 hours at 4°C and incubated with anti–IL-3Rα, anti-βc MoAb beads for 2 hours at 4°C. The beads were washed 6 times with lysis buffer and immunoprecipited proteins were separated on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The gel was transferred onto nitrocellulose and the immunoprecipited proteins were detected by a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The blot was then probed with an antiphosphotyrosine MoAb, 3-365-10 (Boehringer Mannheim, Frankfurt, Germany), as described previously.35
RESULTS
Development of MoAb BION-1.
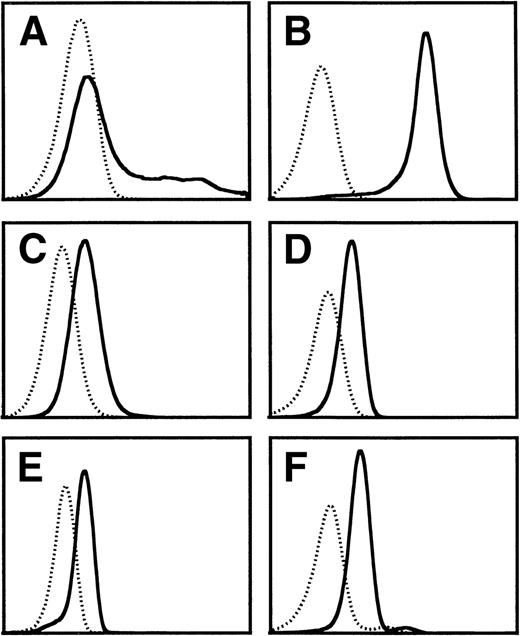
We have previously shown by site-directed mutagenesis that domain 4 of βc and, more specifically, the putative B-C and F-G loops are involved in high-affinity binding and receptor activation by IL-5, GM-CSF, and IL-3.26-28 To ascertain the feasibility of targetting this region of βc for developing molecules that can simultaneously block IL-5, GM-CSF, and IL-3 stimulation of eosinophils, we have attempted to raise functional MoAbs against this domain. However, previous attempts using cells expressing the full-length βc as the immunogen failed to produce any MoAb to domain 4 of βc, and immunization with chemically synthesized peptides encompassing either the predicted B-C and F-G loops or the whole domain 4 led to MoAb that recognized the immunogen but failed to bind to native βc. We have now succeeded in obtaining a blocking MoAb by immunizing mice with COS cells overexpressing a cDNA encoding only the extracellular domain 4 of βc (ΔQP). This MoAb, termed BION-1, immunoprecipitated full-length βc as well as ΔQP from transfected COS cells and recognized native βc in purified eosinophils, neutrophils, and monocytes as judged by flow cytometry (Fig 1).
Flow cytometry analysis of the staining of MoAb BION-1 (solid line) and an isotype-matched IgG1 control MoAb (dotted line) to (A) COS cells transiently transfected with βc, (B) CHO cells constitutively expressing βc, (C) TF-1.8 cells, (D) neutrophils, (E) eosinophils, and (F) monocytes.
Flow cytometry analysis of the staining of MoAb BION-1 (solid line) and an isotype-matched IgG1 control MoAb (dotted line) to (A) COS cells transiently transfected with βc, (B) CHO cells constitutively expressing βc, (C) TF-1.8 cells, (D) neutrophils, (E) eosinophils, and (F) monocytes.
BION-1 inhibits the high-affinity binding of IL-5, GM-CSF, and IL-3 to human eosinophils.
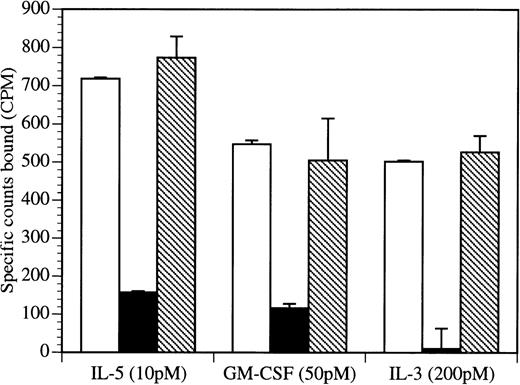
Given that domain 4 of βc is crucial for the high-affinity binding of IL-5, GM-CSF, and IL-3, we examined whether BION-1 was able to affect this binding. Initially, we found that BION-1, either as a whole IgG or as a Fab fragment, inhibited, in a dose-dependent manner, the binding of 125I–IL-5,125I–GM-CSF, and 125I–IL-3 to the human erythroleukemic cell line TF-1.8 (data not shown). Significantly, BION-1 blocked the binding of IL-5, GM-CSF, and IL-3 to primary purified human eosinophils (Fig 2). In contrast, other anti-βc MoAb or IgG1 MoAb controls failed to do so. For each cytokine, we used the lowest practicable concentration of radioligand, thereby allowing binding to high-affinity sites with minimal binding to low-affinity sites.
BION-1 blocks high-affinity binding of IL-5, GM-CSF, and IL-3 to human eosinophils. Human eosinophils (1.8 × 106) were preincubated either alone (□) or in the presence of 1 μmol/L BION-1 MoAb (▪) or an isotype IgG1-matched control antibody (▧) before the addition of radiolabeled cytokine. Nonspecific binding was in each case less than 1% of total counts added. Each point is the mean of duplicate determinations and error bars represent 1 standard deviation.
BION-1 blocks high-affinity binding of IL-5, GM-CSF, and IL-3 to human eosinophils. Human eosinophils (1.8 × 106) were preincubated either alone (□) or in the presence of 1 μmol/L BION-1 MoAb (▪) or an isotype IgG1-matched control antibody (▧) before the addition of radiolabeled cytokine. Nonspecific binding was in each case less than 1% of total counts added. Each point is the mean of duplicate determinations and error bars represent 1 standard deviation.
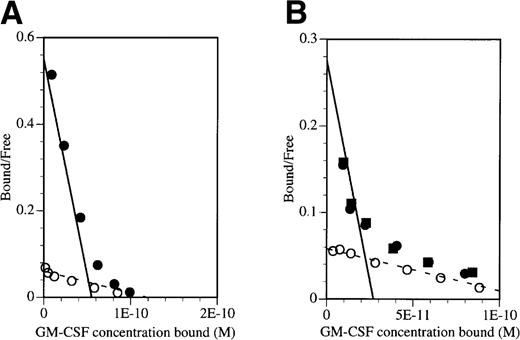
To demonstrate that the inhibitory effect of BION-1 was solely due to the blocking of high-affinity receptors, saturation binding studies were performed on TF-1 cells and peripheral blood mononuclear cells (PBMNC) in the presence or absence of BION-1 (Fig 3). Binding was performed with GM-CSF over a wide range of concentrations, ie, 10 pmol/L to 10 nmol/L. Scatchard transformation of the binding data obtained shows that BION-1 but not anti-βc antibody 8E4 completely blocked GM-CSF binding to high-affinity binding sites but not to low-affinity sites (Fig 3). These results, together with those in Fig 2, suggest that BION-1 recognizes the same binding site on βc used by IL-5, GM-CSF, and IL-3 or binds to an epitope in close proximity to it.
BION-1 blocks high-affinity binding of GM-CSF to TF-1 and PBMNC. Scatchard transformation of saturation binding studies performed on (A) TF-1 cells or (B) PBMNC preincubated either alone (•) or in the presence of 1 μmol/L BION-1 MoAb (○) or 1 μmol/L of a control anti-βc MoAb, 8E4 (▪) before the addition of radiolabeled GM-CSF. Binding assays were performed with125I-labeled GM-CSF over a concentration range of 10 pmol/L to 10 nmol/L. The dashed line indicates the best fit for BION-1 noninhibitable binding and the solid line indicates the high-affinity GM-CSF binding in the absence of BION-1.
BION-1 blocks high-affinity binding of GM-CSF to TF-1 and PBMNC. Scatchard transformation of saturation binding studies performed on (A) TF-1 cells or (B) PBMNC preincubated either alone (•) or in the presence of 1 μmol/L BION-1 MoAb (○) or 1 μmol/L of a control anti-βc MoAb, 8E4 (▪) before the addition of radiolabeled GM-CSF. Binding assays were performed with125I-labeled GM-CSF over a concentration range of 10 pmol/L to 10 nmol/L. The dashed line indicates the best fit for BION-1 noninhibitable binding and the solid line indicates the high-affinity GM-CSF binding in the absence of BION-1.
Epitope mapping of BION-1.
In an attempt to define the region in βc recognized by BION-1, we used several mutants of βc and examined whether substitutions of individual aminoacids in the predicted B-C loop or F-G loop impaired BION-1 binding. To perform these experiments, we transfected COS cells with wild-type and mutant βc and measured the affinity of 125I–BION-1 and125I-1C1 (as binding control). The results showed that βc mutants carrying either substitutions M363A/R364A, E366A, or R418A were not recognized by BION-1 (Table 1), indicating that these residues form part of the BION-1 epitope.
Epitope Mapping of BION-1
| . | kd (mol/L) . | |
|---|---|---|
| BION-1 . | 1C1 . | |
| Wt βc | 5.00 × 10−9 | 2.72 × 10−9 |
| B-C loop mutants | ||
| M363A/R364A | NB | 3.82 × 10−9 |
| Y365A | 6.94 × 10−8 | 1.55 × 10−9 |
| E366A | NB | 2.43 × 10−9 |
| H367A | 2.70 × 10−8 | 2.80 × 10−9 |
| I368A | 2.17 × 10−8 | 2.43 × 10−9 |
| D369A/H370A | 3.24 × 10−8 | 3.64 × 10−9 |
| F-G loop mutants | ||
| R418A | NB | 1.83 × 10−9 |
| T419A | 3.22 × 10−8 | 3.21 × 10−9 |
| G420A | 5.30 × 10−8 | 1.54 × 10−9 |
| Y421A | 3.89 × 10−8 | 2.31 × 10−9 |
| . | kd (mol/L) . | |
|---|---|---|
| BION-1 . | 1C1 . | |
| Wt βc | 5.00 × 10−9 | 2.72 × 10−9 |
| B-C loop mutants | ||
| M363A/R364A | NB | 3.82 × 10−9 |
| Y365A | 6.94 × 10−8 | 1.55 × 10−9 |
| E366A | NB | 2.43 × 10−9 |
| H367A | 2.70 × 10−8 | 2.80 × 10−9 |
| I368A | 2.17 × 10−8 | 2.43 × 10−9 |
| D369A/H370A | 3.24 × 10−8 | 3.64 × 10−9 |
| F-G loop mutants | ||
| R418A | NB | 1.83 × 10−9 |
| T419A | 3.22 × 10−8 | 3.21 × 10−9 |
| G420A | 5.30 × 10−8 | 1.54 × 10−9 |
| Y421A | 3.89 × 10−8 | 2.31 × 10−9 |
Abbreviation: NB, no detectable binding.
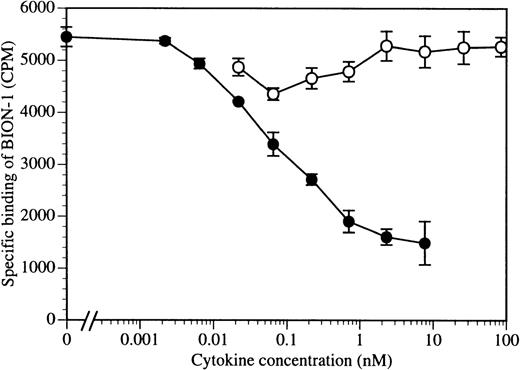
To address this issue further, we performed a reciprocal experiment in which increasing concentrations of IL-3 were used to compete for125I–BION-1 binding. As can be seen in Fig 4, the addition of IL-3 to TF1.8 cells expressing endogenous IL-3 receptor α and βc chains prevented, in a dose-dependent manner, the binding of125I–BION-1 to βc. In contrast, TNFα did not prevent the binding of 125I–BION-1 to βc. These results emphasize the close proximity of the epitopes on βc that the cytokines and BION-1 bind.
The binding of BION-1 to TF1.8 cells is inhibited by IL-3 but not by TNF. TF1.8 cells (2 × 106) were incubated with a range of concentrations of IL-3 (•) or TNF (○) in the presence of 125I-labeled MoAb BION-1 (1 nmol/L). Each point is the mean of duplicate determinations and error bars represent 1 standard deviation.
The binding of BION-1 to TF1.8 cells is inhibited by IL-3 but not by TNF. TF1.8 cells (2 × 106) were incubated with a range of concentrations of IL-3 (•) or TNF (○) in the presence of 125I-labeled MoAb BION-1 (1 nmol/L). Each point is the mean of duplicate determinations and error bars represent 1 standard deviation.
BION-1 specifically inhibits eosinophil colony formation and activation induced by IL-5, GM-CSF, and IL-3.
To ascertain whether the inhibition of IL-5, GM-CSF, and IL-3 binding by BION-1 was translated into inhibition of IL-5, GM-CSF, and IL-3 stimulation, we initially used the factor-dependent TF-1.8 cell line that proliferates in response to either IL-5, GM-CSF, IL-3, or EPO. We found that BION-1 inhibited the stimulation of TF-1.8 cell proliferation by IL-5, GM-CSF, and IL-3 in a dose-dependent manner, with an ED50 of approximately 0.2 to 2 mmol/L, whereas a control anti-βc MoAb , 8E4, had little or no effect (Table 2). The Fab fragment of BION-1 behaved similarly with virtually identical ED50 values to the BION-1 antibody (data not shown). Additionally, the stimulating ability of EPO was not inhibited by BION-1, showing the specificity of BION-1 for the IL-5/GM-CSF/IL-3 receptors system (data not shown).
Inhibition of IL-5–, GM-CSF–, and IL-3–Induced TF 1.8 Cell Proliferation by BION-1
| . | IL-5 . | GM-CSF . | IL-3 . |
|---|---|---|---|
| BION-1 MoAb (nmol/L) | |||
| 20 | 0.3 ± 1.9 | 7.9 ± 1.7 | 23.3 ± 1.6 |
| 65 | 11.8 ± 6.8 | 12.5 ± 2.3 | 58.9 ± 5.1 |
| 200 | 36.2 ± 3.7 | 21.7 ± 0.1 | 31.8 ± 1.4 |
| 650 | 76.5 ± 1.3 | 63.4 ± 0.7 | 8.2 ± 0.7 |
| 2,000 | 99.4 ± 0.2 | 96.1 ± 0.9 | 100 ± 0.3 |
| 8E4 MoAb (nmol/L) | |||
| 20 | 0 ± 1.0 | 7.1 ± 3.5 | 12.1 ± 3.5 |
| 65 | 0 ± 6.5 | 0 ± 3.7 | 11.7 ± 3.7 |
| 200 | 11.4 ± 3.3 | 0 ± 3.3 | 15.8 ± 3.4 |
| 650 | 13.1 ± 4.0 | 4.5 ± 1.0 | 11.5 ± 0.8 |
| 2,000 | 8.5 ± 3.2 | 7.0 ± 2.4 | 17.2 ± 1.8 |
| . | IL-5 . | GM-CSF . | IL-3 . |
|---|---|---|---|
| BION-1 MoAb (nmol/L) | |||
| 20 | 0.3 ± 1.9 | 7.9 ± 1.7 | 23.3 ± 1.6 |
| 65 | 11.8 ± 6.8 | 12.5 ± 2.3 | 58.9 ± 5.1 |
| 200 | 36.2 ± 3.7 | 21.7 ± 0.1 | 31.8 ± 1.4 |
| 650 | 76.5 ± 1.3 | 63.4 ± 0.7 | 8.2 ± 0.7 |
| 2,000 | 99.4 ± 0.2 | 96.1 ± 0.9 | 100 ± 0.3 |
| 8E4 MoAb (nmol/L) | |||
| 20 | 0 ± 1.0 | 7.1 ± 3.5 | 12.1 ± 3.5 |
| 65 | 0 ± 6.5 | 0 ± 3.7 | 11.7 ± 3.7 |
| 200 | 11.4 ± 3.3 | 0 ± 3.3 | 15.8 ± 3.4 |
| 650 | 13.1 ± 4.0 | 4.5 ± 1.0 | 11.5 ± 0.8 |
| 2,000 | 8.5 ± 3.2 | 7.0 ± 2.4 | 17.2 ± 1.8 |
Values are the percentage of maximal inhibition of triplicate determinations ± SEM. One hundred percent inhibition represents values obtained in the absence of factors or antibody, and 0% inhibition represents the amount of proliferation obtained with 0.3 ng/mL IL-5, 0.03 ng/mL GM-CSF, and 0.1 ng/mL IL-3 in the absence of antibody.
Because eosinophils are believed to be the major effector cells in asthma and they respond to IL-5, GM-CSF, and IL-3, we examined BION-1 for its ability to block eosinophil production and survival in response to these 3 cytokines. We found that BION-1, but not another anti-βc MoAb, 8E4, inhibited the ability of IL-5, GM-CSF, and IL-3 to stimulate the formation of eosinophil colonies from human bone marrow cells (Table 3). In addition, BION-1 inhibited the prosurvival activity of IL-5, GM-CSF, and IL-3 on purified peripheral blood human eosinophils (Fig 5). Because these cytokines are essential for maintaining eosinophil viability, blocking of βc by MoAb BION-1 promoted eosinophil cell death to levels similar to those observed in the absence of cytokines (Fig 5).
Inhibition of IL-5–, GM-CSF–, and IL-3–Mediated Eosinophil Colony Formation by BION-1
| . | Medium . | MoAb 8E4 (100 μmol/L) . | MoAb BION-1 (μmol/L) . | |||
|---|---|---|---|---|---|---|
| 0.1 . | 1 . | 10 . | 100 . | |||
| IL-5 (1 nmol/L) | 13 ± 4 | 12 ± 3 | 13 ± 4 | 8 ± 2 | 2 ± 2 | 2 ± 0 |
| GM-CSF (1 nmol/L) | 9 ± 4 | 21 ± 4 | 20 ± 4 | 13 ± 4 | 2 ± 2 | 0 ± 0 |
| IL-3 (1 nmol/L) | 4 ± 2 | 9 ± 1 | 8 ± 2 | 4 ± 1 | 1 ± 1 | 0 ± 1 |
| None | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2 ± 0 | 0 ± 0 |
| . | Medium . | MoAb 8E4 (100 μmol/L) . | MoAb BION-1 (μmol/L) . | |||
|---|---|---|---|---|---|---|
| 0.1 . | 1 . | 10 . | 100 . | |||
| IL-5 (1 nmol/L) | 13 ± 4 | 12 ± 3 | 13 ± 4 | 8 ± 2 | 2 ± 2 | 2 ± 0 |
| GM-CSF (1 nmol/L) | 9 ± 4 | 21 ± 4 | 20 ± 4 | 13 ± 4 | 2 ± 2 | 0 ± 0 |
| IL-3 (1 nmol/L) | 4 ± 2 | 9 ± 1 | 8 ± 2 | 4 ± 1 | 1 ± 1 | 0 ± 1 |
| None | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2 ± 0 | 0 ± 0 |
Values are the number of day-14 eosinophil colonies per 50,000 seeded bone marrow cells. Values shown are the mean from triplicate determination ± SEM.
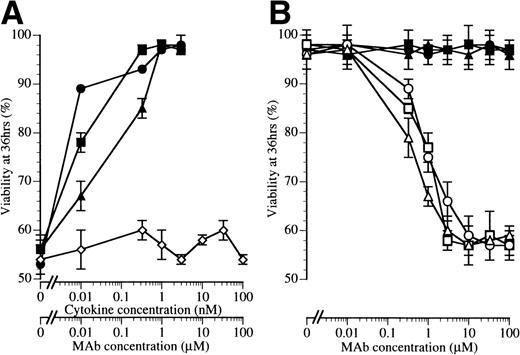
BION-1 blocks IL-5–, GM-CSF–, and IL-3–induced survival of eosinophils. (A) The percentage of viability of eosinophils after 36 hours in the presence of a range of concentrations of IL-5 (▪), GM-CSF (•), IL-3 (▴), or MoAb BION-1 (◊). (B) The percentage of viability of eosinophils after 36 hours in the presence of IL-5, GM-CSF, and IL-3 (1 nmol/L) and different concentrations of MoAb BION-1 (open symbols) and a control anti-βc MoAb, 8E4 (solid symbols). Each point is the mean of triplicate determinations and error bars represent 1 standard deviation.
BION-1 blocks IL-5–, GM-CSF–, and IL-3–induced survival of eosinophils. (A) The percentage of viability of eosinophils after 36 hours in the presence of a range of concentrations of IL-5 (▪), GM-CSF (•), IL-3 (▴), or MoAb BION-1 (◊). (B) The percentage of viability of eosinophils after 36 hours in the presence of IL-5, GM-CSF, and IL-3 (1 nmol/L) and different concentrations of MoAb BION-1 (open symbols) and a control anti-βc MoAb, 8E4 (solid symbols). Each point is the mean of triplicate determinations and error bars represent 1 standard deviation.
Eosinophil functional activity can be activated by IL-5, GM-CSF, and IL-3 as well as by TNFα, with the latter using p55 and p75 of the TNFα receptor but not βc. A sign of eosinophil activation is the upregulation of the CD69 surface antigen, a marker of eosinophil activation in asthma42 and in vitro (Fig 6A). We found that BION-1 inhibited the upregulation of eosinophil CD69 induced by IL-5, GM-CSF, and IL-3 (Fig 6B), whereas another anti-βc MoAb failed to do so. The blocking effect of BION-1 was found to be specific for the IL-5, GM-CSF, and IL-3 receptors, because the stimulating activity of TNFα was not inhibited (Fig 6B).
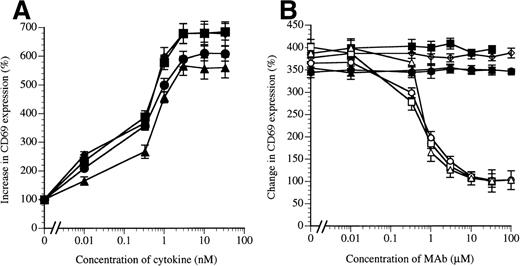
MoAb BION-1 inhibits IL-5–, GM-CSF–, and IL-3–stimulated but not TNF-stimulated CD69 upregulation on human eosinophils. (A) CD69 upregulation in the presence of different concentrations of IL-5 (▪), GM-CSF (•), IL-3 (▴), and TNF (⧫). (B) CD69 upregulation stimulated by 0.3 nmol/L of IL-5, GM-CSF, IL-3, or TNF in the presence of different concentrations of MoAb BION-1 (open symbols) or control anti-βc MoAb, 8E4 (solid symbols). Results are expressed as the percentage increase or change in CD69 expression relative to unstimulated cells. Each point is the mean value of 3 replicates and error bars represent 1 standard deviation.
MoAb BION-1 inhibits IL-5–, GM-CSF–, and IL-3–stimulated but not TNF-stimulated CD69 upregulation on human eosinophils. (A) CD69 upregulation in the presence of different concentrations of IL-5 (▪), GM-CSF (•), IL-3 (▴), and TNF (⧫). (B) CD69 upregulation stimulated by 0.3 nmol/L of IL-5, GM-CSF, IL-3, or TNF in the presence of different concentrations of MoAb BION-1 (open symbols) or control anti-βc MoAb, 8E4 (solid symbols). Results are expressed as the percentage increase or change in CD69 expression relative to unstimulated cells. Each point is the mean value of 3 replicates and error bars represent 1 standard deviation.
BION-1 specifically inhibits IL-3 receptor dimerization and activation.
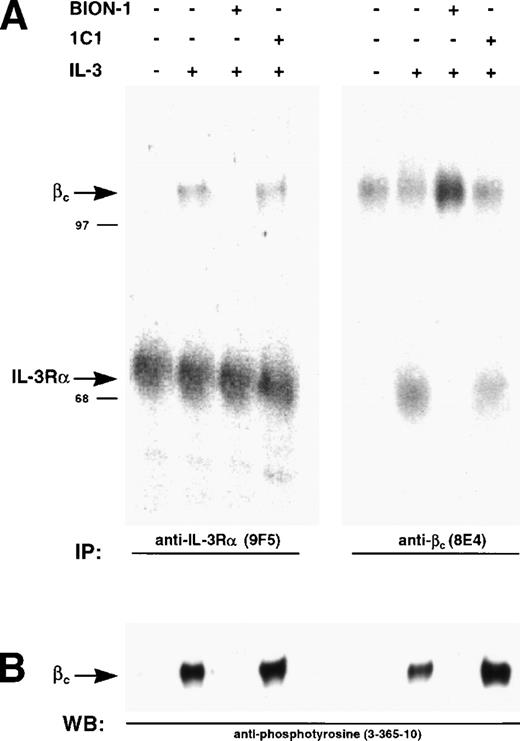
We have previously shown that IL-3, GM-CSF, and IL-5 induce dimerization of the respective α chains with βc, a phenomenon that leads to receptor activation as measured by phosphorylation of βc on tyrosine residues.35 43 We used this system to show that preincubation of Mo7e cells with BION-1 blocks receptor dimerization and tyrosine phosphorylation of βc, whereas anti-βc MoAb 1C1 was unable to prevent receptor dimerization and activation (Fig 7). This result suggests that, although IL-5, GM-CSF, and IL-3 direct binding to βc is not detectable, a direct interaction is obligatory for receptor dimerization and subsequent cellular activation.
Inhibition of IL-3–induced and β chain dimerization and phosphorylation by MoAb BION-1. (A) Immunoprecipitations using anti–IL-3R MoAb, 9F5, or anti-βc MoAb, 8E4, from 125I-surface-labeled MO7e cells preincubated with MoAbs BION-1, control anti-βc MoAb 1C1, or medium alone (−), before being stimulated (+) or not (−) with IL-3. The radiolabeled proteins were spearated by SDS-PAGE and visualized by PhosphorImaging. The position and molecular weight of marker proteins are shown. (B) Immunoprecipitated proteins were probed using antiphosphotyrosine MoAb 3-365-10.
Inhibition of IL-3–induced and β chain dimerization and phosphorylation by MoAb BION-1. (A) Immunoprecipitations using anti–IL-3R MoAb, 9F5, or anti-βc MoAb, 8E4, from 125I-surface-labeled MO7e cells preincubated with MoAbs BION-1, control anti-βc MoAb 1C1, or medium alone (−), before being stimulated (+) or not (−) with IL-3. The radiolabeled proteins were spearated by SDS-PAGE and visualized by PhosphorImaging. The position and molecular weight of marker proteins are shown. (B) Immunoprecipitated proteins were probed using antiphosphotyrosine MoAb 3-365-10.
DISCUSSION
We show here that targetting the common β subunit of the human IL-5, IL-3, and GM-CSF receptors allows for the simultaneous blocking of the binding and stimulating activities of all 3 eosinophilopoietic cytokines. The MoAb BION-1 and its Fab fragment thus represent initial reagents with which to inhibit eosinophil production, survival, and activation in vitro and in vivo. Furthermore, the ability of BION-1 to directly bind to the cytokine-interacting region of βcoffers a new approach to identifying small molecule antagonists of βc.
IL-5, GM-CSF, and IL-3 are the only cytokines so far discovered that stimulate the production of eosinophils from the bone marrow. In addition, these cytokines stimulate the adhesion capacity of eosinophils, their cytotoxic activity, and their survival. Because of these properties, IL-5, GM-CSF, and IL-3 have been implicated in chronic inflammatory conditions mediated by eosinophils, of which allergic inflammation such as asthma is one of the best studied. In bronchial asthma, eosinophils have long been recognized to play a central role, as judged by their accumulation in the bronchoalveolar lavage fluid and the presence in the sputum of asthmatics of eosinophil cationic proteins believed to contribute to local tissue damage.7 Of the different eosinophil regulators, IL-5 has been a major focus of attention, largely due to its specificity for the eosinophil lineage. Elevated IL-5 mRNA and protein levels have been observed in biopsies of bronchial mucosa in asthma.8,9Furthermore, recruitment of eosinophils is associated with elevated IL-5 in the airways of asthma patients.10 Given the involvement of IL-5 in allergic inflammation, new therapeutic approaches have involved the use of anti–IL-5 antibodies20,21 and the development of specific IL-5 antagonists.23 24
BION-1 may offer an alternative approach by blocking IL-5 through its interaction with βc, with the added advantage of also blocking GM-CSF and IL-3. It is becoming clear that not only IL-5, but also other eosinophilopoietic (GM-CSF and IL-3)11,14,15,44-46 as well as noneosinophilopoietic cytokines (IL-4)47-49 are elevated in asthma. Furthermore, the production of IL-5, GM-CSF, and IL-3 by CD4 cells is believed to prolong eosinophil survival in vivo.12,13 In the case of GM-CSF, for example, transgene expression allows the development of allergic airway inflammation.50,51 The extent to which these cytokines contribute to the lung inflammation is not clear. Their concomitant production may accentuate local eosinophilia or, alternatively, induce temporal and site-specific phases of eosinophil recruitment and activation.18 In either case, the simultaneous antagonism of the 3 eosinophilopoietic cytokines may more profoundly downregulate eosinophil-dependent inflammation.
The use of BION-1 or similar molecules that can simultaneously inhibit IL-5, GM-CSF, and IL-3 would be advantageous not only in asthma, but also in other eosinophil-dependent inflammatory diseases such as chronic hyperplastic sinusitis52 and nasal polyposis,16 in which all 3 cytokines have been found to be elevated. Furthermore, BION-1 and functionally analogous molecules may also be antagonists of choice in certain leukemias that produce and respond to GM-CSF and IL-3. A novel model of chronic myeloid leukemia has recently implicated bcr/abl in the production of excess GM-CSF and IL-3 and the development of disease in mice.53 The degree of βc inhibition required would have to be carefully ascertained in each case, because the complete absence of βc can lead, at least in mice, to lung disease.54
BION-1 was able to greatly decrease eosinophil colony formation by IL-5, GM-CSF, and IL-3. This illustrates the importance of βc for eosinophil production and is consistent with βc gene knockout experiments showing a major reduction in eosinophil numbers in the peripheral blood and bone marrow of these mice.54 In addition, BION-1 profoundly inhibited eosinophil viability down to levels observed in the absence of cytokines, a property similar to that seen by some glucocorticoids such as fluticasone 17-propionate.55 This ability of BION-1 to inhibit IL-5–, IL-3–, and GM-CSF–mediated eosinophil viability suggests its use as an adjuvant of steroid therapy, its use to reduce the dosage of concomitant steroid therapy, and its use in steroid-resistant asthma.
The membrane proximal domain 4 of βc was chosen as the immunogen to generate BION-1, because this domain contains the major sites supporting cytokine high-affinity binding and receptor activation.26-28 We and others have previously shown that the B-C and F-G loops in domain 4 of βc support high-affinity binding to IL-5, IL-3, and GM-CSF and are important for receptor activation. Consistent with this and with the inhibition of BION-1 and IL-3 for each other’s binding, we found that residues M363 R364, E366 in the predicted B-C loop, and R418 in the predicted F-G loop form part of the BION-1 epitope. Interestingly, these residues do not appear to be directly involved in mediating high-affinity binding; however, they may be close in the 3-dimensional structure to Y365, H367, I368, and Y421, the mutation of which has a profound effect on cytokine binding and activation.26-28 Thus, these data suggest a more complex cytokine binding site than that found by mutagenesis and identify new target amino acids in βc for the development of novel antagonists. Furthermore, these data, together with the ability of BION-1 to prevent IL-3–mediated dimerization of the IL-3 receptor α chain and βc, support the concept that receptor dimerization and subsquent activation require ligand contacting both receptor subunits. However, we cannot rule out that, given the size of BION-1 as a Fab molecule of 50,000 MW, inhibition of receptor dimerization is the result of steric hindrance.
The development of BION-1 may have 2 immediate uses. Firstly, it may be used as a therapeutic in its own right after humanization and affinity maturation to improve its ED50. The use of MoAbs in clinical medicine is rapidly gaining momentum to the point that they now encompass 27% of biotechnology therapeutics in development and several anticytokine antibodies such as anti-TNFα56 are already showing their efficacy in clinical trials. In relation to IL-5, animal models show that anti–IL-5 antibodies are proving to be efficacious in asthma.31 Secondly, BION-1 may be used as the basis for a novel screening assay for antagonists of βc. The observation that IL-3 could reciprocally inhibit the binding of 125I-labeled BION-1 to βc (Fig4) suggests that compounds with the same specificity may be obtained. The major advance lies in the fact that, unlike IL-5, GM-CSF, and IL-3, which require prior binding to each α subunit before interacting with βc, BION-1 can directly bind to βc, thereby facilitating a cell-free solid-phase assay on which natural or engineered products may be screened for inhibition of binding of labeled BION-1 to immobilized βc or domain 4 of βc. The approach described here may also be extended to other receptors systems, such as the common subunit of the IL-4 and IL-13, which mediates the allergen-induced asthma triggered by IL-4 and IL-13.57 58
Supported by the NH&MRC of Australia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to A.F. Lopez, MD, PhD, The Hanson Centre for Cancer Research, IMVS, Frome Road, Adelaide, SA 5000, Australia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal