Abstract
Vascular malformations are frequent in newborns, and they persist throughout life, which differentiates them from vascular tumors (eg, hemangiomas). Arteriovenous malformations are high-flow vascular malformations. They are considered nonmalignant but can expand and become a significant clinical risk when extensive. To characterize endothelial cells from arteriovenous malformations (AMEC), we cultured cells obtained from surgical specimens and studied their properties. After selection, the cells that grew out from explants had phenotypic and antigenic features (platelet endothelial cell adhesion molecule, von Willebrand factor) of human endothelial cells. Their spontaneous proliferation rate was higher (1.8 to 6.4 times) than that of human umbilical vein, arterial, or microvascular endothelial cells. The proliferation rate of AMEC was not sensitive to the inhibitory activity of various cytokines (interleukin-1β, tumor necrosis factor-, transforming growth factor-β, Interferon-γ). In basal conditions, intercellular adhesion molecule (ICAM-1) was detected at a higher level of expression (6- to 10-fold) on AMEC, but these cells failed to express E-selectin or the vascular cell adhesion molecule (VCAM-1) after cytokine stimulation. Expression of c-ets-1 proto-oncogene was shown by in situ hybridization. The low response to cytokines, the higher propensity to proliferate, and the ets-1 expression suggest that AMEC have a defective regulation of proliferation that may be due to a reduced apoptotic process.
ANGIOGENESIS and vasculogenesis, which are observed during embryogenesis and body growth, are essential for organogenesis and tissue nutrition.1 Frequently, angiogenic abnormalities are observed in newborns. They are classified according to their anatomical location and angiographic and developmental characteristics. In the classification proposed by Mulliken and Glowacki,2 hemangiomas are different from vascular malformations. Hemangiomas are involutive tumors, whereas vascular malformations are composed of dysplastic vessels. Arteriovenous malformations are high-flow vascular abnormalities, which usually exhibit a slow rate of expansion. Growth can be acutely accelerated, however, depending on factors such as puberty, pregnancy, and trauma. The tumor mass produced may alter the contiguous tissues. Such arteriovenous malformations represent a critical risk when located in the head and cervicocephalic region. They are not responsive to pharmacologic treatment, (eg, corticosteroid or interferon α). Attempts have been made to limit tumor expansion by endovascular embolization. After this treatment, surgery is often needed to remove either the remaining lesion or the necrotic tissue. Little information is available on the characteristics of the cells present in arteriovenous malformations. Our goal was to attempt to culture endothelial cells from arteriovenous malformations and to characterize these cells, according to the antigens expressed, their rate of proliferation, and their response to cytokines and other factors involved in growth control.
PATIENTS
This study was conducted in accordance with the principles of the Declaration of Helsinki and the 1975 Declaration of Tokyo. Surgical excisions were performed in 11 consecutive patients with arteriovenous malformations, and samples were collected (Fig1A). Cells were successfully cultured from the surgical biopsies of 6 patients. Because a limited number of cells grew in culture, a complete characterization could only be achieved in 4 cases. In the other 5 cases, the tissues were either necrotic after embolization, infected, or did not grow in in vitro conditions. The main clinical features of the patients are summarized in Table1. The hemostatic parameters were normal and there was no evidence of intravascular coagulation.
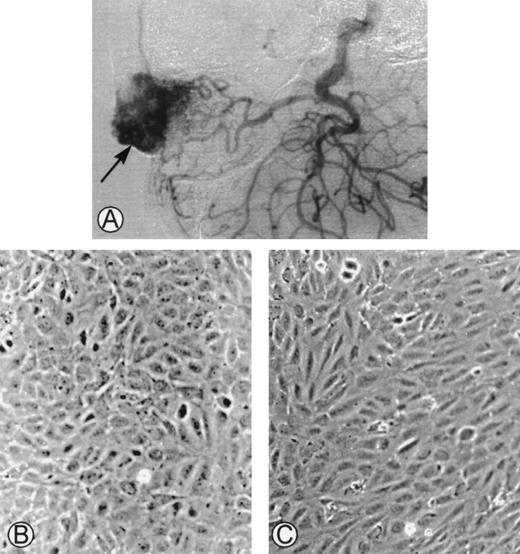
(A) Angiogram of one of the cephalic arteriovenous malformations. The vascular tumor was embolized using an endovascular technique and was operated on. The specimen was used as starting material to obtain endothelial cells derived from the malformation. (B) Phase-contrast microphotographs of confluent HUVEC or (C) confluent AMEC grown on gelatin-coated plastic dishes (original magnification ×100).
(A) Angiogram of one of the cephalic arteriovenous malformations. The vascular tumor was embolized using an endovascular technique and was operated on. The specimen was used as starting material to obtain endothelial cells derived from the malformation. (B) Phase-contrast microphotographs of confluent HUVEC or (C) confluent AMEC grown on gelatin-coated plastic dishes (original magnification ×100).
Clinical Characteristics of the Patients
| Patients . | 1 . | 2 . | 3 . | 4 . |
|---|---|---|---|---|
| Sex | F | M | M | F |
| Age (yrs) | 17 | 33 | 20 | 21 |
| Location | Superior lip | Cheek and lip | Nose | Ear |
| Size (mm) | 30 × 30 × 20 | 70 × 90 × 20 | 60 × 60 × 20 | 26 × 22 × 10 |
| Presence at birth | Yes | No | Yes | No |
| Cardiac failure | No | No | No | No |
| Patients . | 1 . | 2 . | 3 . | 4 . |
|---|---|---|---|---|
| Sex | F | M | M | F |
| Age (yrs) | 17 | 33 | 20 | 21 |
| Location | Superior lip | Cheek and lip | Nose | Ear |
| Size (mm) | 30 × 30 × 20 | 70 × 90 × 20 | 60 × 60 × 20 | 26 × 22 × 10 |
| Presence at birth | Yes | No | Yes | No |
| Cardiac failure | No | No | No | No |
MATERIALS AND METHODS
Materials
Cytokine Growth Factors and Other Reagents
Recombinant human (rh) tumor necrosis factor-α (TNF-α), rh interleukin-1β (IL-1β), and rh interferon-γ (IFN-γ) were obtained from Boehringer Mannheim (Mannheim, Germany), transforming growth factor-β (TGF-β) purified from human platelets was from Calbiochem (La Jolla, CA), and [methyl-3H]thymidine was from Amersham Life Science (Buckinghamshire, UK). Amphotericin B, gentamicin, L-glutamine, epidermal growth factor (EGF), and Hanks’ balanced salt solution (HBSS) were purchased from GIBCO Life Technologies (Grand Island, NY); hydrocortisone was from Sigma Chemical Co (St Louis, MO).
Culture Media and Sera
Medium M199 medium MCDB131, RPMI medium, and fetal calf serum (FCS) were purchased from GIBCO Life Technologies. Essential basal medium (EBM) and all supplements, EGF, hydrocortisone, gentamicin, amphotericin B, bovine brain extract (BBE), and fetal bovine serum (FBS), which were used for dermal-derived normal human microvascular endothelial cells (HMVEC-d), were from Clonetics-BioWhittaker (Walkersville, MD).
Antibodies
Specific anti–vascular cell adhesion molecule-1 (VCAM-1) and anti-intercellular adhesion molecule-1 (ICAM-1) antibodies were obtained from British Biotechnologies (Abingdon, UK); the antibody directed against E-selectin (H18/7) and the isotype-matched nonbinding E1A monoclonal antibody (MoAb) were a gift from MP Bevilacqua (La Jolla, CA). The 125I-labeled sheep antimouse IgG F(ab′)2 fragment was from Amersham; bis Benzimide (Hoechst 33258) was from Sigma.
The antihuman fibroblast antibody, directed against human prolyl-4-hydroxylase,3 and the antihuman von Willebrand Factor (vWF) antibody were purchased from Dako (Glostrup, Denmark), anti-α smooth muscle cell actin (α actin) was from Sigma, and anti-CD14 from Immunotech (Lumigny, France). Anti-platelet endothelial cell adhesion molecule (PECAM; CD31) was a gift from P. Newman (Blood Center, Milwaukee, WI). The fluorescein isothiocyanate-conjugated (FITC) goat antimouse or goat antirabbit antibodies were obtained from Nordic Immunology (Tilburg, The Netherlands).
Cell Culture Equipment
T25 culture flasks (25 cm2), 35-mm plastic dishes, and tissue culture trays (96 wells or 24 wells) were all from Costar (Cambridge, MA).
Methods
Cell Culture
Human umbilical vein endothelial cells (HUVEC).
HUVEC were grown as previously described.4 Cells were cultured in M199 medium supplemented with glutamine, HEPES buffer, amphotericin B, and gentamicin and containing human serum AB (15% vol/vol). Cells were tested after 1 to 3 passages.
Human arterial endothelial cells (HAEC).
HAEC were cultured from human umbilical cords according to a previously described technique.5 After digestion with collagenase at 37°C for 10 minutes, endothelial cells were centrifuged, and the cell pellet was resuspended in M199 medium supplemented with glutamine, HEPES buffer, amphotericin B, and gentamicin and containing human serum AB (15% vol/vol). Arterial endothelial cells were seeded on gelatin (0.2% wt/vol) precoated dishes at a concentration of 40,000 per square centimeter. The medium was changed 3 or 4 hours later (when the cells were attached) and every 2 days, until the cells reached confluence (6 or 7 days). Cells were tested after 1 to 3 passages.
Human microvascular endothelial cells (HMEC).
Because in prior experiments we observed unexpected results with HMEC, as reported by Xu et al,6 we tested in parallel 2 types of human microvascular endothelial cells from different origins and cultured them according to the techniques described by the manufacturer. The HMEC-1 cell line was obtained from the Center for Disease Control (Atlanta, GA) and passaged in medium MCDB131, which was supplemented with hydrocortisone (1 μg/mL), EGF (10 ng/mL), and 15% FCS.
HMVEC-d were obtained from Clonetics and cultured in medium EBM supplemented (per 100 mL) with EGF (1 ng), hydrocortisone (0.1 mg), BBE (1.2 mg), and FBS (5%). Both types of microvascular cells were tested after 1 to 4 passages.
Arteriovenous malformation-derived endothelial cells (AMEC).
During the surgical operation, a specimen of the arteriovenous malformation was dissected and put into a container filled with gentamicin and amphotericin diluted in HBSS. The surgical explants were cut into pieces of 1 mm2 and plated on gelatin (0.2%) precoated 35-mm plastic dishes; 0.8 mL of medium M199 with 20% FCS was then added. After 4 to 6 weeks of culture, the selection of endothelial cells growing out from explants was performed in several steps. First, cell colonies, which did not have the gross morphology of endothelial cells (under inverted phase contrast microscopy), were detached by scraping and removed from the dish by aspiration. The colonies that exhibited the characteristic cobblestone morphology of endothelial cells were allowed to proliferate in the dish for 1 week, selected as before, and then subcultured. Cells generated from this selection were called AMEC. AMEC were obtained from 4 different patients (no. 1 to 4). The flow cytometry analysis (FACS) was performed on cells after 2 or more passages. FACS analysis, the proliferation assay, and adhesion molecule expression measurements were performed on the same passage.
Monocytes, smooth muscle cells, and fibroblasts.
Monocytes were isolated from normal human blood. Smooth muscle cells (SMC) isolated from human uterine arteries, and fibroblasts derived from human skin were kindly provided by INSERM U.353 (Paris, France).
Flow Cytometry Analysis
After harvesting, cells in suspension were washed, incubated for 30 minutes with the antibodies, and then washed again. The binding of antibodies was shown by incubating the cells with FITC-conjugated antimouse or antirabbit polyclonal antibodies at a dilution giving a negative signal when tested with cells in absence of the first antibody. The immunofluorescence was quantified by FACS (FacScan; Becton Dickinson, Mountain View, CA). For each antibody, 5,000 events were recorded. The cell population was analyzed, and the results were expressed as a percentage of fluorescent cells and the mean fluorescence intensity in arbitrary units of fluorescence (AUF). The forward side scatter (FSC) versus side scatter (SSC) dot plot of HUVEC or AMEC are shown in Fig 2.
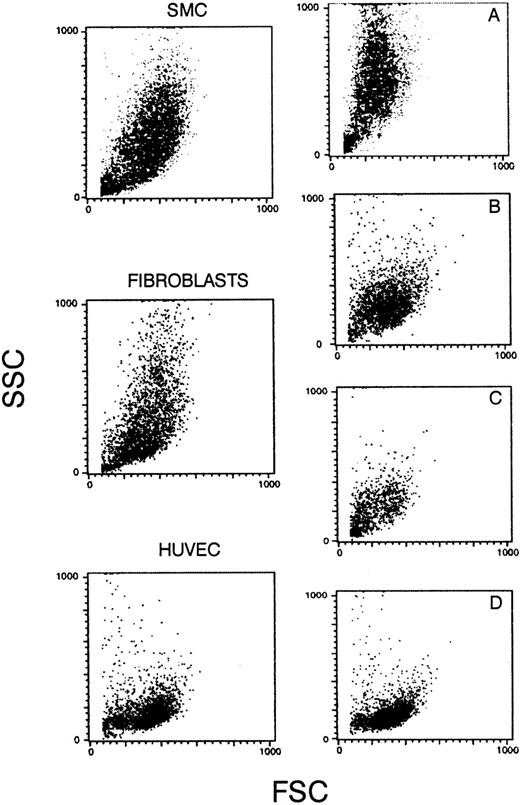
Forward and side scatters of SMCs, fibroblasts, and HUVECs. (A) A mixed population of SMCs (90%) and endothelial cells from AMEC (10%); (B) from fibroblasts (70%) and AMEC (30%); (C) from fibroblasts (40%) and AMEC (60%); and (D) from pure AMEC population (AMEC-1 or AMEC-2 after 2 or 3 passages).
Forward and side scatters of SMCs, fibroblasts, and HUVECs. (A) A mixed population of SMCs (90%) and endothelial cells from AMEC (10%); (B) from fibroblasts (70%) and AMEC (30%); (C) from fibroblasts (40%) and AMEC (60%); and (D) from pure AMEC population (AMEC-1 or AMEC-2 after 2 or 3 passages).
In Situ Hybridization With c-ets-1 Riboprobes
Immediately after biopsy, the material was fixed in paraformaldehyde (4%) for 24 hours at 4°C, dehydrated through graded alcohols, and embedded in paraffin. Five-micrometer–thick transverse sections were transferred to slides coated with 3-aminopropyl-triethoxysilane. In situ hybridization protocols with 35S-labeled c-ets-1 riboprobes were performed as described elsewhere.7 8 The slides were dipped in the NTB2 nuclear track emulsion and exposed for 10 to 15 days in sealed boxes with dessicator at 4°C. In all experiments, the antisense and the sense probes were hybridized on neighboring sections. The control c-ets-1 sense probe gave no signal. Under darkfield illumination, endothelial cells could be unambiguously identified by comparison with neighboring cells stained conventionally with hematoxylin and eosin.
Proliferation Assay
Cell proliferation was assayed in triplicate by incorporation of [methyl-3H]thymidine into acid-insoluble DNA of cultured endothelial cells (HUVEC, HAEC, HMEC, and AMEC) as previously described.9 Cells were seeded into 96-well plates, and 2 or 3 hours later (when the cells were adherent), the cultures were incubated for 24 hours with or without cytokines (20 U/mL IL-1β, 500 U/mL TNF-α, 250 U/mL IFN-γ, 2 ng/mL TGF-β) in medium M199 with 10% FCS. In previous work,9 we determined the cytokine concentration that produced 50% inhibition of HUVEC proliferation. The cells were then pulsed with [methyl-3H]thymidine for 16 hours and harvested using an automatic cell harvester (Skatron, Lier, Norway) on glass fiber filters. Incorporated radioactivity was counted in a beta counter (Beckman, Fullerton, CA). Percentage of inhibition of proliferation was determined for each cytokine by comparison of incorporation of radioactivity expressed in counts per minute in cytokine-treated endothelial cells and in untreated control cells.
Determination of Adhesion Molecules Expression
Confluent HUVEC, HMEC, or AMEC monolayers in microplates were stimulated with IL-1β (100 U/mL) or TNF-α (500 U/mL) for 4, 16, or 24 hours before the measurement of E-selectin (CD62E), VCAM-1 (CD106), and ICAM-1 (CD54) expression. After 2 washes, the monolayers were incubated for 1 hour on ice in RPMI medium plus 10% FCS containing specific antibodies (anti–E-selectin [H18/7], anti–VCAM-1, or anti–ICAM-1). The binding was shown with a second antibody,125I-labeled sheep antimouse IgG F(ab′)2fragment. The cells were washed, solubilized with sodium dodecyl sulfate (SDS) (0.1%)-NaOH (25 mmol/L) solution, and then collected. The radioactivity was measured in a gamma counter (Beckman, Fullerton, CA). The specific binding was calculated by subtracting the nonspecific radioactivity that was measured in wells labeled with the isotype-matched nonbinding E1A MoAb.10
Statistical Analysis
The results are expressed as mean ± standard deviation. Statistical analysis was performed by using Wilcoxon’s rank sum test for paired values for comparison of the effect of the different cytokines (IL-1β, TNF-α, IFN-γ, and TGF-β) on adhesion molecule expression on the cell surface of HUVEC, HMEC, and AMEC. Comparison of inhibition of proliferation of AMEC, HMEC, and HAEC to HUVEC by the different cytokines were performed by using one-way ANOVA followed by the parametric Dunnett’s test.
RESULTS
Morphological Aspect
The initial culture from surgical pieces of arteriovenous malformations (Fig 1A) contained heterogeneous populations. Only cultured cells that had the morphological characteristics of endothelial cells were kept. At confluence, HUVEC had a cobblestone aspect when cultured on gelatin-coated plastic dishes with a strict monolayer growth (Fig 1B). At confluence, AMEC cultured on gelatin-coated plastic dishes had an elongated form with an endothelial-like polygonal morphology. They were closely contiguous and grew as a monolayer (Fig 1C).
Flow Cytometry Analysis
Cultures in which mixed populations were present, ie, either SMCs and endothelial cells, or endothelial cells and fibroblasts, or a pure endothelial cell population (Fig 2D), demonstrated different scatter patterns, when analyzed for size and nuclearity.
The different cell types reacted differently with the different antibodies (Table 2). HMEC-1, HMVEC-d, and HUVEC were positively stained by anti-vWF and anti-PECAM. The mean fluorescence intensity (MFI) was similar with both antibodies: (MFI: 668,703 AUF) for HMVEC-d and slightly lower with HMEC-1 (MFI: 480,520 AUF).
Immunoreactivity of the Different Cell Types
| Cell Types . | Antibodies . | ||||
|---|---|---|---|---|---|
| Anti-vWF . | Anti-PECAM (CD31) . | Anti-α Actin . | Anti-Fibroblast . | Anti-CD14 . | |
| HUVEC | Pos | Pos | Neg | Neg | Neg |
| HMEC-1 | Pos | Pos | Neg | Neg | Neg |
| HMVEC-d | Pos | Pos | Neg | Neg | Neg |
| Fibro | Neg | Neg | Neg | Pos | Neg |
| SMC | Neg | Neg | Pos | Neg | Neg |
| Mono | Neg | Pos | Neg | Neg | Pos |
| Cell Types . | Antibodies . | ||||
|---|---|---|---|---|---|
| Anti-vWF . | Anti-PECAM (CD31) . | Anti-α Actin . | Anti-Fibroblast . | Anti-CD14 . | |
| HUVEC | Pos | Pos | Neg | Neg | Neg |
| HMEC-1 | Pos | Pos | Neg | Neg | Neg |
| HMVEC-d | Pos | Pos | Neg | Neg | Neg |
| Fibro | Neg | Neg | Neg | Pos | Neg |
| SMC | Neg | Neg | Pos | Neg | Neg |
| Mono | Neg | Pos | Neg | Neg | Pos |
Immunoreactivity of the different endothelial cells (HUVEC, HMEC-1, and HMVEC-d), fibroblasts (Fibro), smooth muscle cells (SMC), and blood monocytes (Mono) with anti-vWF, anti-PECAM (CD31), anti-SMC α actin, anti-fibroblast (anti–prolyl-4-hydroxylase), and anti-CD14 (monocyte marker). The MFIs recorded when cells were positively stained are given in the corresponding Result section.
Monocytes were labeled with anti-CD14 (MFI: 450 AUF) and anti-PECAM (MFI: 370 AUF). Fibroblasts reacted with anti–prolyl-4-hydroxylase (MFI: 453 AUF), and SMCs with anti-α actin (MFI: 560 AUF). None of the other cell types reacted with the antibodies directed against fibroblast or SMC components.
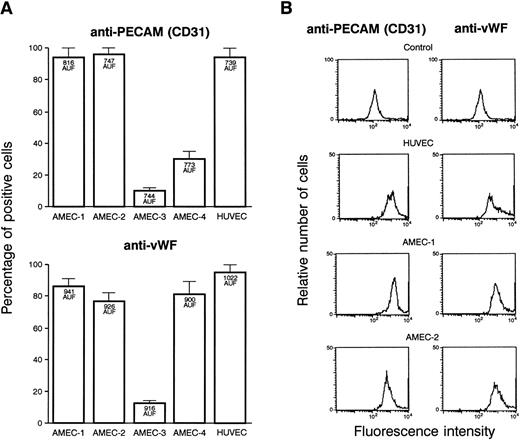
A sufficient amount of endothelial cells were obtained from 4 patients and were tested in the different assays. After endothelial cell selection and 2 passages, more than 90% of the cells from AMEC-1 and AMEC-2 reacted positively with anti-vWF and anti-PECAM antibodies with an MFI similar to that recorded with HUVEC (Fig3A). The results were different with AMEC-3. The cell cultures contained a mixed population, and a proportion of them were negative for endothelial cell markers while not being positive with anti-fibroblast or SMC. The results with AMEC-4 were discordant with anti-vWF and anti-PECAM antibodies. The flow cytometry profile of AMEC-1 (passage 2) or AMEC-2 (passage 3) in the presence of anti-CD31 and anti-vWF antibodies, demonstrated that the population is as homogeneous as HUVEC for these 2 markers of endothelial cells (Fig 3B). This experiment was performed 3 times and gave a similar profile at passage 2 and 3. They were also negative with the antibodies that are specific for the possible contaminating cells. Based on the results, we decided to use only AMEC-1 and AMEC-2 as representative of endothelial cells in the subsequent experiments.
(A) FACS of the different cells obtained from culture explants of 4 patients. The results are presented in percentages of cells that were fluorescent with anti-vWF and anti-PECAM antibodies. Bars denote standard deviation. The MFI, expressed in arbitrary units of fluorescence, is indicated inside the corresponding histograms. AMEC-1 and AMEC-2 (passages 2 and 3) were positive with anti-vWF, and the same percentage of cells was labeled with anti-CD31. In the further experiments, only AMEC-1 and AMEC-2 were considered as AMEC. (B) Flow cytometry profiles of HUVEC, AMEC-1, and AMEC-2 (passages 2 and 3) obtained with anti-CD31 and anti-vWF antibodies. The results showed that the AMEC were as homogeneous as the HUVEC with the 2 antibodies. The control corresponds to HUVEC or AMEC incubated with FITC conjugated antimouse or antirabbit Igs.
(A) FACS of the different cells obtained from culture explants of 4 patients. The results are presented in percentages of cells that were fluorescent with anti-vWF and anti-PECAM antibodies. Bars denote standard deviation. The MFI, expressed in arbitrary units of fluorescence, is indicated inside the corresponding histograms. AMEC-1 and AMEC-2 (passages 2 and 3) were positive with anti-vWF, and the same percentage of cells was labeled with anti-CD31. In the further experiments, only AMEC-1 and AMEC-2 were considered as AMEC. (B) Flow cytometry profiles of HUVEC, AMEC-1, and AMEC-2 (passages 2 and 3) obtained with anti-CD31 and anti-vWF antibodies. The results showed that the AMEC were as homogeneous as the HUVEC with the 2 antibodies. The control corresponds to HUVEC or AMEC incubated with FITC conjugated antimouse or antirabbit Igs.
Cell Proliferation Studies
Under our conditions, the spontaneous rates of proliferation of HUVEC, HAEC, and HMVEC-d were similar. The spontaneous rate of proliferation of HMEC-1 was higher, but the difference was not statistically significant (Fig 4A). [methyl-3H]thymidine incorporation in AMEC (passage 2 and 3) was higher than that of HUVEC or HAEC (P < .001); the proliferation of AMEC was higher than that of HMEC-1 and HMVEC-d (2- and 3-fold, respectively).
(A) Spontaneous proliferation of HUVEC, HAEC, HMEC-1, HMVEC-d, and AMEC. Proliferation was measured by using [methyl-3H]thymidine incorporation in different sets of experiments. Each experiment was performed in quintuplicate with cells obtained from 2 different cell cultures. Results are expressed as means and bars denote standard deviation. The results of spontaneous proliferation of AMEC are expressed as means of AMEC-1 and AMEC-2 (passages 2 and 3). (B through E) Histological studies show the staining of immature spindle AMEC by hematoxylin-eosin (B), and demonstration of c-ets-1 transcripts (light grains) by in situ hybridization (C, darkfield illumination of the section after fluorescent counterstaining of the nuclei with Hoechst 33258). (D) Negative results are obtained with the c-ets-1 sense riboprobe. (E) C-ets-1 expression within the stromal capillaries of an invasive ductal breast carcinoma was used as a positive control for active angiogenesis.8
(A) Spontaneous proliferation of HUVEC, HAEC, HMEC-1, HMVEC-d, and AMEC. Proliferation was measured by using [methyl-3H]thymidine incorporation in different sets of experiments. Each experiment was performed in quintuplicate with cells obtained from 2 different cell cultures. Results are expressed as means and bars denote standard deviation. The results of spontaneous proliferation of AMEC are expressed as means of AMEC-1 and AMEC-2 (passages 2 and 3). (B through E) Histological studies show the staining of immature spindle AMEC by hematoxylin-eosin (B), and demonstration of c-ets-1 transcripts (light grains) by in situ hybridization (C, darkfield illumination of the section after fluorescent counterstaining of the nuclei with Hoechst 33258). (D) Negative results are obtained with the c-ets-1 sense riboprobe. (E) C-ets-1 expression within the stromal capillaries of an invasive ductal breast carcinoma was used as a positive control for active angiogenesis.8
C-ets-1 in situ Hybridization
Morphologically, the arteriovenous malformations presented typical histological features. They consisted of mature and nonproliferating thick-walled blood vessels with features of arteries and veins or without specific characteristics. In addition, a growing capillary component was present, including immature endothelial spindle cells (Fig 4B). C-ets-1 mRNA was detected by in situ hybridization exclusively within capillaries and endothelial spindle cells (Fig 4B and C) in all 4 cases. In contrast, mature arterial or venous structures without active angiogenesis were negative. This was consistent with our findings in cultured arterial and capillary endothelial cells, which downregulate ets-1 transcription after confluence.8
Cytokine Stimulation
In proliferation assays, IL-1β (20 U/mL), TNF-α (500 U/mL), IFN-γ (250 U/mL), and TGF-β (2 ng/mL) inhibited [methyl-3H]thymidine incorporation in HUVEC (50%) (Fig5) and to a greater extent in HAEC (P < .05). HMEC-1 proliferation was inhibited (50%) by TNF-α, but less by IL-1β, IFN-γ, or TGF-β (30% [P < .01], 20% [P < .001], and 38%, respectively). IL-1β, TNF-α, IFN-γ, and TGF-β did not alter AMEC proliferation (passages 2 and 3).
Endothelial cell proliferation in presence of IL-1β (20 U/mL), TNF- (500 U/mL), IFN-γ (250 U/mL), or TGF-β (2 ng/mL). The effect of cytokines was tested by using [methyl-3H]thymidine incorporation into HUVEC, HAEC, HMEC-1, and AMEC and was expressed as a percentage of thymidine incorporation inhibition. For HUVEC, HAEC, and HMEC-1, the experiments were done in triplicate with cells obtained from 2 to 4 different cell cultures. For AMEC, the experiments were performed in triplicate with cells obtained after 2 and 3 passages. The results are expressed as means of AMEC-1 and AMEC-2.
Endothelial cell proliferation in presence of IL-1β (20 U/mL), TNF- (500 U/mL), IFN-γ (250 U/mL), or TGF-β (2 ng/mL). The effect of cytokines was tested by using [methyl-3H]thymidine incorporation into HUVEC, HAEC, HMEC-1, and AMEC and was expressed as a percentage of thymidine incorporation inhibition. For HUVEC, HAEC, and HMEC-1, the experiments were done in triplicate with cells obtained from 2 to 4 different cell cultures. For AMEC, the experiments were performed in triplicate with cells obtained after 2 and 3 passages. The results are expressed as means of AMEC-1 and AMEC-2.
Adhesion Molecule Expression
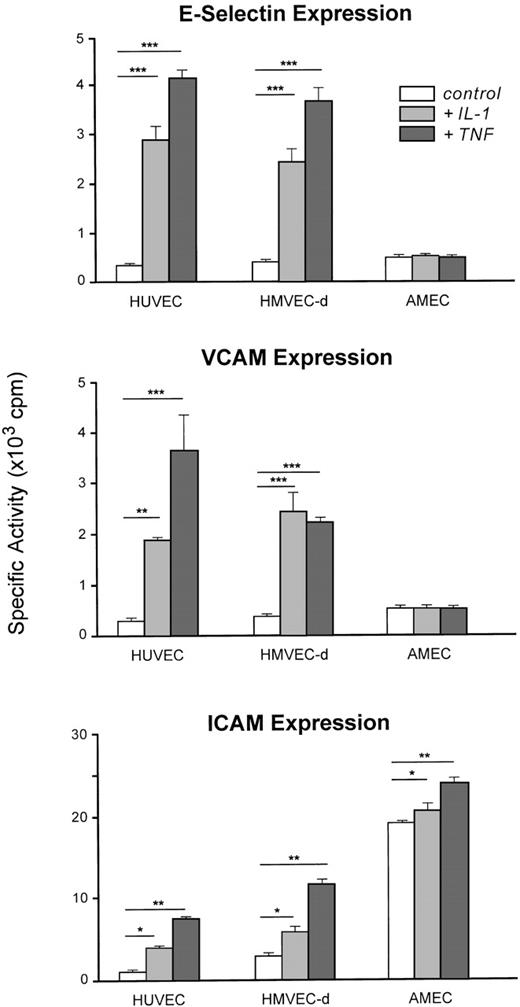
IL-1β or TNF-α induced E-selectin and VCAM-1 expression and enhanced ICAM-1 on the cell surface of HUVEC or HMVEC-d (Fig6).
Adhesion molecule (E-selectin, VCAM-1, ICAM-1) expression on HUVEC (n = 6), HMVEC-d (n = 6), and AMEC (n = 6) under basal conditions or induced by IL-1β (100 U/mL) and TNF- (500 U/mL). The results are expressed as means and bars denote standard deviation (*P < .05, **P < .01, ***P < .001). For AMEC, the results are expressed as means of AMEC-1 and AMEC-2 (passage 3).
Adhesion molecule (E-selectin, VCAM-1, ICAM-1) expression on HUVEC (n = 6), HMVEC-d (n = 6), and AMEC (n = 6) under basal conditions or induced by IL-1β (100 U/mL) and TNF- (500 U/mL). The results are expressed as means and bars denote standard deviation (*P < .05, **P < .01, ***P < .001). For AMEC, the results are expressed as means of AMEC-1 and AMEC-2 (passage 3).
In contrast to HUVEC or HMVEC-d, neither E-selectin nor VCAM-1 was detectable on AMEC (passage 3) after stimulation by IL-1β (100 U/mL) or TNF-α (500 U/mL). Constitutive ICAM-1 expression on AMEC was 6- to 15-fold higher than that observed with HMEC and HUVEC and was potentiated by IL-1β and TNF-α stimulation.
DISCUSSION
The endothelial cells cultured from explants of arteriovenous malformations may originate from the vascular malformation or, for a small proportion, from adjacent vessels. The characteristics of these cells, compared with endothelial cells from arterial or venous origin or from normal skin microvessels, are different, which is in favor of their origin from the arteriovenous malformation.
The endothelial cells derived from arteriovenous malformations, when obtained at a degree of homogeneity, had the same reactivity with antibodies specific for PECAM and vWF as normal endothelial cells.11 These results were in agreement with those of Takahashi et al12 obtained with 1 specimen of an arteriovenous malformation. The AMEC proliferation rate was higher than that observed with HUVEC, HAEC, and HMEC, which may correspond to a propensity of these cells to replicate more rapidly and could be consistent with the abnormal vessel development in vivo.
In vitro, AMEC growth was not inhibited by IL-1β, TNF-α, IFN-γ, and TGF-β. ICAM expression on AMEC, in the absence of exogenous stimulation, was expressed at a high level. E-selectin was not stimulated by IL-1β or TNF-α. AMEC have characteristics that are different from venular or arterial endothelial cells and HMVEC. Similar defective responses have been observed on endothelial cells in the vicinity of melanomas and carcinomas.13 The nonexpression of leukocyte adhesion molecules may represent a barrier to lymphocyte-monocyte infiltration of the tumors or of the angioma and constitute a resistance to the defense mechanism mediated by leukocytes.
The lack of response to cytokines was observed after several weeks of culture, a long period after a possible influence of the previous in vivo microenvironment, suggesting that a factor secreted by local tissue was not responsible for the down-response to cytokines, as previously reported.14-16 Because endothelial cells in arteriovenous malformations are subjected to special flow conditions, we expect that the stress responsive elements can be simulated by the shear stress forces.17 18 We speculate that some of the growth regulation alteration may be secondary to the abnormal flow conditions. However, the abnormal control of proliferation remained when the cells were in culture; furthermore, at variance with endothelial cells stimulated by physical forces, they did not express VCAM-1. The lack of inhibition of proliferation was not related to a lack of cell receptor for IL-1β or TNF-α, as indicated by the upregulation of ICAM-1 by these cytokines.
Expression of proto-oncogene ets-1 has been demonstrated in different cultured endothelial cells.8,19 Recently, ets-1 has been shown to regulate genes involved in the proliferation and apoptotic processes.20,21 A binding site for ETS family members has been identified in the Bcl-2 enhancer region of mature cells.22 23
Dexamethasone, which is known to induce programmed cell death (apoptosis) by an indirect pathway, consisting of unmasking or exposing DNA regions to endonucleases,24 and TGF-β, which regulates the endothelial growing by inducing apoptosis,25were not effective. For AMEC, the lack of response to dexamethasone in vivo and TGF-β in vitro suggests an alteration in the triggering of apoptosis.
The cells obtained from arteriovenous malformations, a particular fast-flow type of angiomas, have characteristics that distinguish them from normal endothelial cells and are compatible with an abnormal regulation of proliferation, which persists in culture and may be related to an intrinsic cell defect.
ACKNOWLEDGMENT
The authors are grateful to F. Vileyn (INTS) and LPH/Hôpital St Louis members (Paris) for their help in preparing this manuscript. Pr J. Levin is acknowledged for helpful reviewing of the manuscript (University of California School of Medicine, San Francisco, CA).
O.C. was a recipient of a fellowship from ADR Biologie vasculaire cellulaire, Paris, France and N.W. was a recipient of a grant from the Dr Mildred Scheel Foundation, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jean-Luc Wautier, MD, Laboratoire de Biologie Vasculaire et Cellulaire, Institut National de la Transfusion Sanguine, 6, rue Alexandre Cabanel, 75739 Paris Cedex 15, France; e-mail: wautier@ints.fr.

![Fig. 4. (A) Spontaneous proliferation of HUVEC, HAEC, HMEC-1, HMVEC-d, and AMEC. Proliferation was measured by using [methyl-3H]thymidine incorporation in different sets of experiments. Each experiment was performed in quintuplicate with cells obtained from 2 different cell cultures. Results are expressed as means and bars denote standard deviation. The results of spontaneous proliferation of AMEC are expressed as means of AMEC-1 and AMEC-2 (passages 2 and 3). (B through E) Histological studies show the staining of immature spindle AMEC by hematoxylin-eosin (B), and demonstration of c-ets-1 transcripts (light grains) by in situ hybridization (C, darkfield illumination of the section after fluorescent counterstaining of the nuclei with Hoechst 33258). (D) Negative results are obtained with the c-ets-1 sense riboprobe. (E) C-ets-1 expression within the stromal capillaries of an invasive ductal breast carcinoma was used as a positive control for active angiogenesis.8](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.2020/5/m_blod41823004w.jpeg?Expires=1769217698&Signature=s-FJCIdlEqGCPtE2jJnIxFYBvy3wySdR9ZLs9PFaiY4V7zLaXibl5qUh1~G35nLxhSXJJqz4bnkp31YdR3GkjFzFLF7UkO4DiK3CwXpdz1e-ymezGnfb8SNuhVoQNghSkm34hdLaIUrzSfxi80yjaxtnWaf7GUJKQKPxN~jklMIaUpUmqEKt-ZN4KThDkCofI1XDH6aPZ0dZMYvmajd6G5QcHqCD6onVSyCm-yX3sEosD9qj4aTPFP8F2FKXiwBvZPwMzK5e-Qqwz6p8flK8xJGHo8YGQuEccStcPjPmNyC71GTIcOIno1l0l787QL2Xtztoveihj5gd-IE64606cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Endothelial cell proliferation in presence of IL-1β (20 U/mL), TNF- (500 U/mL), IFN-γ (250 U/mL), or TGF-β (2 ng/mL). The effect of cytokines was tested by using [methyl-3H]thymidine incorporation into HUVEC, HAEC, HMEC-1, and AMEC and was expressed as a percentage of thymidine incorporation inhibition. For HUVEC, HAEC, and HMEC-1, the experiments were done in triplicate with cells obtained from 2 to 4 different cell cultures. For AMEC, the experiments were performed in triplicate with cells obtained after 2 and 3 passages. The results are expressed as means of AMEC-1 and AMEC-2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.2020/5/m_blod41823005x.jpeg?Expires=1769217698&Signature=ZMG7bT~Mabr5fkfUX0JwybY1yziHPMbrIg~HEeT3Q1PZ~GnQZ6oXq6koxz56fcm3u0Jg7bNIaGQF0gipCOY3ntta0neDPj8aTKZa-mD7vdt8nOYYYR~nd1AGSGJviUsKRgQ1wQQY05UGuJpxHCH3aNotULTTA0S7tbFNimBnTi~aPrVEQ19EU3APmC7xisaRec9ygJaZ4oF6EJPr3B2~gX0mp~PIbmsB2sxJ9bs2txeJvu5sMEaEjyK11ombM6PprJxvdQ3cFAX-SxwOiH0quUhY5A06w8Nkm~kT5~2EAJoF-z7VP2lMkqjWJhgaINqeCS96FJdbONRM-tUx4pnigA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal