We have investigated the influence of alterations in plasma coagulation factor levels between 50% and 150% of their mean values for prothrombin, factor X, factor XI, factor IX, factor VII, factor VIII, factor V, protein C, protein S, antithrombin III (AT-III), and tissue factor pathway inhibitor (TFPI) as well as combinations of extremes, eg, 50% anticoagulants and 150% procoagulants or 50% procoagulants and 150% anticoagulants in a synthetic “plasma” system. The reaction systems were constructed in vitro using purified, natural, and recombinant proteins and synthetic phospholipid vesicles or platelets with the reactions initiated by recombinant tissue factor (TF)-factor VIIa complex (5 pmol/L). To investigate the influence of the protein C system, soluble thrombomodulin (Tm) was also added to the reaction mixture. For the most extreme situations in which the essential plasma procoagulants (prothrombin, and factors X, IX, V, and VIII) and the stoichiometric anticoagulants (AT-III and TFPI) were collectively and inversely altered by 50%, a 28-fold difference in the total available thrombin generated was observed. Variations of most of these proteins 50% above and below the “normal” range, with the remainder at 100%, had only modest influences on the peak and total levels of thrombin generated. The dominant factors influencing thrombin generation were prothrombin and AT-III. When these 2 components were held at 100% and all other plasma procoagulants were reduced to 50%, there was a 60% reduction in the available thrombin generated. No increase in the thrombin generated was observed when the 150% level of all plasma procoagulants other than prothrombin was evaluated. When only prothrombin was raised to 150%, and all other factors were maintained at 100%, the thrombin generated increased by 71% to 121%. When AT-III was at 50% and all other constituents were at 100%, thrombin production was increased by 104% to 196%. The additions of protein C and protein S over the 50% to 150% ranges with Tm at 0.1 nmol/L concentration had limited influence on thrombin generation. Individual variations in factors VII, XI, and X concentrations had little effect on the duration of the initiation phase, the peak thrombin level achieved, or the available thrombin generated. Paradoxically, increases in factor IX concentration to 150% led to lowered thrombin generation, while decreases to 50% led to enhanced thrombin generation, most likely a consequence of factor IX as a competitive substrate with factor X for factor VIIa-TF. Reductions in factor V or factor VIII concentration led to prolongations of the initiation phase, while the reduction of TFPI to 50% led to shortening of this phase. However, none of these alterations led to significant changes in the available thrombin generated. Based on these data, one might surmise that increases in prothrombin and reductions in AT-III, within the normal range, would be potential risk factors for thrombosis and that algorithms that combine normal factor levels may be required to develop predictive tests for thrombosis.
THROMBIN IS the essential enzyme product of the blood coagulation enzymatic cascade.1 In humans and a variety of natural and transgenic animal models, the regulation of the production of this enzyme is vital to the maintenance of the hemostatic balance.2-9 Genetic and acquired deficiencies that cause reductions in, or the absence of, thrombin generation lead to hemorrhagic syndromes.10-16 Defects in the regulatory stoichiometric and dynamic processes that downregulate thrombin generation are associated with thrombotic risk.17-24
At a minimum, the procoagulant process is mediated by an array of at least 6 plasma proteins (prothrombin, factor VII, factor IX, factor X, factor V, and factor VIII) and 1 tissue protein, tissue factor (TF). The anticoagulant process is governed by a minimum of 4 plasma proteins: antithrombin III (AT-III), protein C, protein S, and the tissue factor pathway inhibitor (TFPI), and by 1 membrane-bound protein contributed by vascular tissue, thrombomodulin (Tm).1 Thus, a minimum of 12 proteins are explicitly and centrally associated with the maintenance of blood fluidity and protection from vascular injury. In addition to these proteins, membrane-binding sites essential to both procoagulant and anticoagulant processes are provided by vascular cell injury either by trauma and inflammatory mediators and by platelets.25,26 The latter are essential contributors to the initiation and propagation of the process.27
Other proteins contributed by the so-called “contact pathway of coagulation,” while apparently not essential for the procoagulant response, may perform important supplemental roles in the coagulation process. These include high–molecular-weight kininogen, factor XII, prekallikrein, and factor XI. Potential additional anticoagulant roles are played by heparin cofactor II, α2-macroglobulin, and α1-antitrypsin.28 29
Following mechanical or inflammatory damage to the vascular wall, the procoagulant reaction is thought to begin by the binding of small amounts of preexistent 2-chain plasma factor VIIa to TF. The resulting membrane bound factor VIIa-TF enzyme complex activates the plasma zymogens factor X and factor IX by limited proteolysis. Factor Xa generation is further accelerated by the formation of the membrane-bound intrinsic factor Xase complex composed of factor IXa and factor VIIIa. Ultimately, the factor Xa formed by both enzyme complexes binds to membrane-bound factor Va to produce the prothrombinase complex, which converts prothrombin to thrombin.1 The composite of these procoagulant reactions leads to the biphasic generation of thrombin. During an initiation phase, picomolar amounts of enzymes are produced while the procofactors, factor V and factor VIII, are nearly quantitatively activated. Subsequently, during a propagation phase, the bulk of the thrombin is generated.30The initiation and propagation phases of the coagulation system are differentially regulated by the stoichiometric inhibitors AT-III and TFPI and by activated protein C produced dynamically by thrombin-Tm. TFPI, which has as its principle targets factor Xa, and the factor VIIa-TF-factor Xa product complex,31 serves principally to regulate the initiation phase of the reaction.32 AT-III, which reacts with all of the serine proteases produced in the coagulation system, is in significant molar excess to its target enzymes and serves principally to quench enzyme activity once formed.33 As a consequence, AT-III makes a larger contribution to ablating the propagation phase than in altering the initiation phase of the reaction.32 The combinations of AT-III and TFPI, and TFPI and the protein C system, act in synergy to produce threshold limits of thrombin generation, which are related to initiator concentration.32 34
From studies of “normal” human physiology, it is generally inferred that the range of procoagulant and anticoagulant species in the average blood sample ranges from 50% to 150% of some mean value, and the assessment of potential pathology based on clinical laboratory blood studies, in general, attributes values between 50% and 150% of any the coagulation factors as within the “normal” range.35 36
In previous studies, empirical and theoretical models of the coagulation have been provided.30,37-40 We have assessed the influence of alterations in the concentrations of the vascular components TF and Tm and the stoichiometric inhibitors TFPI and AT-III in regulating the procoagulant system when the levels of all other proteins are set at normal, 100% values.32,34 From those studies, it is clear that the process of the blood coagulation system, although made up of fairly conventionally functioning complex enzymes, behaves in such a manner that synergy between procoagulants and inhibitors produces threshholded responses with respect to stimuli, and the process of blood clotting functions effectively in a “yes/no” configuration in which the procoagulant initiating stimulus must be at a certain level to bring about the uncompromised generation of thrombin.32 34
A number of epidemiologic studies have shown that concentration variations of blood coagulation proteins within the 50% to 150% range, including prothrombin, AT-III, proteins C and S, factors VII, VIII, and IX, and fibrinogen, are associated with thrombotic risk.19,24 41-44 We therefore elected to evaluate the influence of alterations in the concentration of each procoagulant and anticoagulant (zymogens, procofactors, and inhibitors) species of the extrinsic pathway with respect to its influence on the generation of thrombin, at a given stimulus.
MATERIALS AND METHODS
Materials.
Phospholipid vesicles (PCPS) composed of 25% phosphatidylserine and 75% phosphatidylcholine were prepared as described.45Phospatidylserine, phosphatidylcholine, and EDTA were purchased from Sigma (St Louis, MO). Spectrozyme TH was purchased from American Diagnostica (Greenwich, CT). FPRck was obtained as a gift from Haematologic Technologies (Essex Junction, VT). The enzyme-linked immunosorbent assay (ELISA) thrombin-AT-III (TAT) kit (Enzygnost TAT) was purchased from Behring (Marborg, Germany).
Proteins.
Human coagulation factors VII, X, and IX, and prothrombin and protein C were isolated from fresh-frozen plasma using the general methods of Bajaj et al,46 and were purged of trace contaminants and traces of active enzymes as described.32Human factor V and AT-III were isolated from freshly frozen plasma.47,48 Human protein S was purified using Blue-Sepharose chromatography.49 Recombinant factor VIII and recombinant TF (residues 1-242) were provided as gifts from Drs Shu Len Liu and Roger Lundblad (Hyland Division, Baxter Healthcare, Duarte, CA). Recombinant human factor VIIa was purchased from NOVO Pharmaceuticals (Denmark). Recombinant full-length TFPI produced in Escherichia coli was provided as a gift from Dr K. Johnson (Chiron, Emeryville, CA). Recombinant soluble Tm (Solulin) was provided as a gift from Dr J. Morser (Berlex, Richmond, CA) and human plasma factor XI was a gift from Dr R. Jenny (Haematologic Technologies). Corn trypsin inhibitor was isolated from popcorn as described elsewhere.50 Washed platelets were prepared by the procedure of Mustard et al.51
Coagulation factor activation experiments.
Thrombin generation initiated by factor VIIa-TF in a reconstituted procoagulant model using mean plasma protein concentrations was studied as described previously.32,34 37 TF (0.5 nmol/L) was relipidated into 400 μmol/L PCPS by incubation in 20 mmol/L HEPES, 150 mmol/L NaCl, and 2 mmol/L CaCl2 pH 7.4 (HBS/Ca2+) for 30 minutes at 37°C. The relipidated TF was incubated with 10 pmol/L factor VIIa for 20 minutes to allow factor VIIa-TF complex formation. Factor V, factor VIII, and Tm (when desired) were added to the relipidated factor VIIa-TF complex, and thrombin generation was started by addition of an equal volume of a zymogen-inhibitor mixture containing prothrombin, factors X and IX, TFPI, AT-III, proteins C and S, and factors VII and XI (the last 4 when desired) prepared in HBS/Ca2+ and preheated at 37°C for 3 minutes. The final concentrations of the proteins in the reaction, chosen to represent mean plasma values (100%), are indicated in Table1. The Tm concentration was 0.1 nmol/L or 1 nmol/L, factor VIIa and TF concentrations were 5 pmol/L and 0.25 nmol/L, respectively. In experiments in which PCPS was substituted by washed platelets at 2 × 108/mL concentration, final concentrations of factor VIIa and TF were 100 pmol/L and 12.5 pmol/L, respectively. The concentrations of selected proteins in mixtures were varied from 50% to 150% of their mean plasma values. Following initiation of the reaction, at selected time points, 10-μL aliquots were withdrawn from the reaction mixture and quenched in 20 mmol/L EDTA in HBS (pH 7.4) containing 0.2 mmol/L Spectrozyme TH and assayed immediately for thrombin activity. The hydrolysis of the substrate was monitored by the change in absorbance at 405 nm using a Molecular Devices Vmax spectrophotometer (Molecular Devices, Menlo Park, CA). Thrombin generation was calculated from a standard curve prepared by serial dilutions of α-thrombin. The total active thrombin generated was evaluated by integrating the area under thrombin versus time curve.
Mean Plasma Concentrations of Coagulation Proteins and Inhibitors
| Protein . | Mean Plasma Concentration . | E1%280nm† . | Source . | |
|---|---|---|---|---|
| nmol/L . | μg/mL . | |||
| Prothrombin | 1,400 | 100 | 13.8 | Plasma |
| Factor X | 170 | 10 | 13.9 | Plasma |
| Factor IX | 90 | 5.1 | 13.2 | Plasma |
| Factor XI | 30 | 4.8 | 13.4 | Plasma |
| Factor V | 20 | 6.6 | 9.6 | Plasma |
| Factor VII | 10 | 0.5 | 13.9 | Plasma |
| Factor VIII* | 0.7 | 0.2 | — | Recombinant |
| Factor VIIa* | 0.1 | 0.005 | — | Recombinant |
| Tissue factor* | NA | NA | — | Recombinant |
| Antithrombin III | 3,400 | 200 | 6.2 | Plasma |
| Protein S | 300 | 21 | 9.5 | Plasma |
| Protein C | 60 | 3.7 | 14.5 | Plasma |
| TFPI* | 2.5 | 0.1 | — | Recombinant |
| Tm* | NA | NA | — | Recombinant |
| Protein . | Mean Plasma Concentration . | E1%280nm† . | Source . | |
|---|---|---|---|---|
| nmol/L . | μg/mL . | |||
| Prothrombin | 1,400 | 100 | 13.8 | Plasma |
| Factor X | 170 | 10 | 13.9 | Plasma |
| Factor IX | 90 | 5.1 | 13.2 | Plasma |
| Factor XI | 30 | 4.8 | 13.4 | Plasma |
| Factor V | 20 | 6.6 | 9.6 | Plasma |
| Factor VII | 10 | 0.5 | 13.9 | Plasma |
| Factor VIII* | 0.7 | 0.2 | — | Recombinant |
| Factor VIIa* | 0.1 | 0.005 | — | Recombinant |
| Tissue factor* | NA | NA | — | Recombinant |
| Antithrombin III | 3,400 | 200 | 6.2 | Plasma |
| Protein S | 300 | 21 | 9.5 | Plasma |
| Protein C | 60 | 3.7 | 14.5 | Plasma |
| TFPI* | 2.5 | 0.1 | — | Recombinant |
| Tm* | NA | NA | — | Recombinant |
Data for mean plasma concentrations from Kalafatis et al.75
Abbreviation: NA, not applicable (membrane protein).
Concentration provided by manufacturer.
Absorbance of 1% solution at 280 nm wavelength.
Coagulation in whole blood.
The protocol used is a modification of the protocol of Rand et al.52
Clotting in freshly drawn, nonanticoagulated whole blood was performed in 32 tubes (2 series per experiment, 16 tubes per series). All tubes were loaded with corn trypsin inhibitor (100 μg/mL blood), 12.5 pmol/L relipidated TF/mL blood (PCPS:TF = 2,000) in HBS with 2 mmol/L CaCl2 (all tubes in each series except phlebotomy control tube), 50 μg/mL blood of prothrombin or 100 μg/mL blood of AT-III (all tubes, experiment series only), and equivalent volume prothrombin/AT-III dilution buffer (HBS, pH 7.4, all tubes control series only). No more than 35 μL of reagents was loaded in each tube. The zero tube of each series was pretreated using 1 mL of 50 mmol/L EDTA and 10 μL of 10 mmol/L FPRck (diluted in 0.01 mol/L HCl). Normal donor blood was drawn by venipuncture, delivered into the reagent-loaded tubes, and the tubes periodically quenched with EDTA and FPRck. The clotting time was monitored visually by two observers. Tubes were centrifuged and the supernatants were aliquoted for further analyses. Commercial ELISAs for TAT were performed according to the manufacturer’s protocol. Thrombin generation analyses were accomplished using 10 μg/mL of a polyclonal burro α-human prethrombin-1 antibody as described elsewhere.52
RESULTS
Thrombin generation induced by the factor VIIa-TF complex in a mixture containing the essential coagulation proteins (factors X, IX, V, and VIII, and prothrombin) and the inhibitors TFPI and AT-III evolves after an initiation phase as a peak of thrombin activity, the formation of which is downregulated and quenched by the action of AT-III. Under these conditions, explosive thrombin generation becomes a threshold-limited event with respect to the initiating factor VIIa-TF concentration.32 Based on this observation, the factor VIIa-TF enzymatic complex was used at 5 pmol/L, a concentration that permits observation of thrombin generation even under the least favorable conditions of this study, ie, the extreme cases when procoagulants are present at 50% and anticoagulants at 150% of their mean plasma values. On the other hand, this concentration of initiator does not eliminate the responsiveness of the system to the variations of reactant concentrations at the most favorable thrombin generation conditions.
Procoagulants and stoichiometric inhibitors.
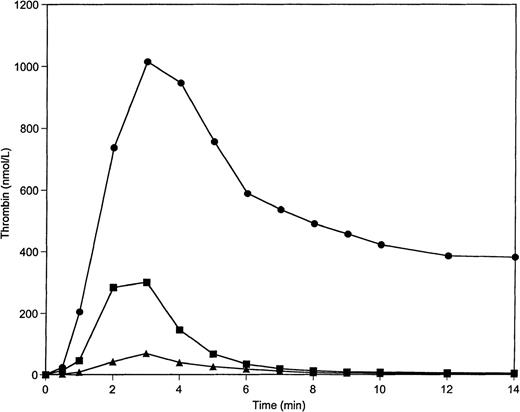
Figure 1 displays thrombin generation profiles, which result when the reaction is initiated by 5 pmol/L TF-factor VIIa complex on 200 μmol/L PCPS vesicles. Under conditions in which all procoagulant factors and stoichiometric inhibitors are at their mean plasma concentrations, thrombin generation occurs after an initiation phase and reaches a maximum concentration of approximately 300 nmol/L. The formation and inhibition rates are equivalent by 2.5 minutes; subsequently declining until the thrombin is largely inhibited by 10 minutes into the reaction. When AT-III and TFPI are present at 150% and the procoagulants are reduced to 50% of mean values, a severely depressed thrombin generation profile is observed. The area under the curve (total thrombin) under these conditions is reduced to approximately 25% of the normal profile. Conversely, a decrease in the concentration of the anticoagulants by 50% in combination with an increase of the concentration of all procoagulants to 150% results in an approximately 700% increase (from control) in total thrombin generation, which reaches a maximum concentration of 1 μmol/L. The observed stable level of thrombin at the latter time points of this profile is the result of the consumption of AT-III in this situation (1.7 μmol/L) and the quantitative conversion of prothrombin (2.1 μmol/L) to thrombin. The initial rate of thrombin generation is altered approximately 16-fold between extremes (8.9 nmol/L/s and 0.57 nmol/L/s, respectively), which results from accumulated 50% variations from the mean concentrations of coagulation factors and inhibitors.
Thrombin generatin on 200 μmol/L PCPS at varying procoagulant and anticoagulant concentrations in the absence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of all proteins at mean plasma concentrations (▪), in the presence of procoagulants (prothrombin, factors V, VIII, IX, and X) at 150% and anticoagulants (AT-III and TFPI) at 50% of their mean plasma concentrations (•), and in the presence of procoagulants at 50% and anticoagulants at 150% of their mean plasma concentrations (▴).
Thrombin generatin on 200 μmol/L PCPS at varying procoagulant and anticoagulant concentrations in the absence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of all proteins at mean plasma concentrations (▪), in the presence of procoagulants (prothrombin, factors V, VIII, IX, and X) at 150% and anticoagulants (AT-III and TFPI) at 50% of their mean plasma concentrations (•), and in the presence of procoagulants at 50% and anticoagulants at 150% of their mean plasma concentrations (▴).
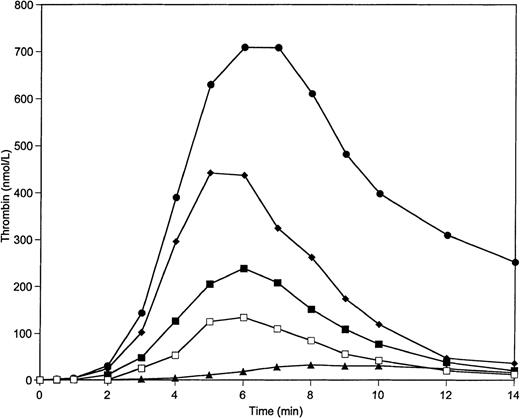
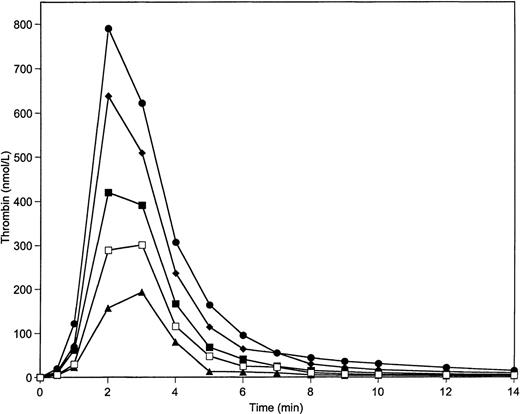
Figure 2 illustrates data for experiments in which only prothrombin and AT-III are varied over the range from 50% to 150% with factors X, V, VIII, and IX, and with TFPI maintained at 100% of mean plasma value. The control is 100% of all species. The contrast between 150% prothrombin and 50% AT-III, and 50% prothrombin and 150% AT-III, is most similar to the extremes represented in Fig 1 when all constituents were varied over this range. This suggests that prothrombin and AT-III are the principle agents responsible for the major excursions observed in Fig 1. This observation is supported by the data of Fig3, which demonstrates that variations of prothrombin concentration over the range from 50% to 150% with other proteins present (including AT-III) at 100% cause an approximately 4-fold increase in maximum thrombin concentration (from 200 nmol/L to 800 nmol/L) and in total thrombin (Fig 3; Table2). The variation of AT-III concentration with other proteins present at 100% leads to an approximately 50% decrease in the maximum thrombin concentration and in total thrombin generated (Table 2).
Thrombin generation on 200 μmol/L PCPS at varying prothrombin and AT-III concentrations in the absence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of all proteins at their mean plasma concentrations (▪), in the presence of prothrombin at 150% and AT-III at 50% of mean plasma concentrations (•), in the presence of prothrombin at 50% and AT-III at 150% of mean plasma concentrations (▴), in the presence of prothrombin at 125% and AT-III at 75% of mean plasma concentrations (⧫), and in the presence of prothrombin at 75% and AT-III at 125% of mean plasma concentrations (□). Factors V, VIII, IX, and X and TFPI were present at mean plasma concentrations in all experiments.
Thrombin generation on 200 μmol/L PCPS at varying prothrombin and AT-III concentrations in the absence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of all proteins at their mean plasma concentrations (▪), in the presence of prothrombin at 150% and AT-III at 50% of mean plasma concentrations (•), in the presence of prothrombin at 50% and AT-III at 150% of mean plasma concentrations (▴), in the presence of prothrombin at 125% and AT-III at 75% of mean plasma concentrations (⧫), and in the presence of prothrombin at 75% and AT-III at 125% of mean plasma concentrations (□). Factors V, VIII, IX, and X and TFPI were present at mean plasma concentrations in all experiments.
Prothrombin titration on 200 μmol/L PCPS in a coagulation protein activation experiment in the absence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of prothrombin at 150% (•), 125% (⧫), 100% (▪), 75% (□), and 50% (▴) of its mean plasma concentration. All other proteins were present at mean plasma concentrations.
Prothrombin titration on 200 μmol/L PCPS in a coagulation protein activation experiment in the absence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of prothrombin at 150% (•), 125% (⧫), 100% (▪), 75% (□), and 50% (▴) of its mean plasma concentration. All other proteins were present at mean plasma concentrations.
Total Thrombin, Maximum Thrombin Concentration, and Initiation Phase Duration (%) Dependence on Protein Concentration
| Protein . | Protein Concentration (% of normal) . | |||||
|---|---|---|---|---|---|---|
| 50 . | 150 . | |||||
| Total IIa* . | Max.IIa* . | Init.Phase* . | Total IIa* . | Max.IIa* . | Init.Phase* . | |
| Factor V† | 80 | 77 | 140 | 93 | 93 | 87 |
| Factor VIII† | 100 | 77 | 112 | 118 | 152 | 87 |
| Factor VII† | 106 | 132 | 100 | 98 | 112 | 100 |
| Factor IX† | 109 | 129 | 75 | 87 | 73 | 112 |
| Factor X† | 82 | 74 | 112 | 93 | 99 | 87 |
| Factor XI† | 104 | 80 | 100 | 100 | 101 | 100 |
| Factors V, VIII, IX, X† | 41 | 34 | 170 | 90 | 90 | 114 |
| Prothrombin | ||||||
| A. 200 μmol/L PCPS | ||||||
| No proteins C, S, and Tm | 45 | 50 | 100 | 184 | 195 | 100 |
| Proteins C, S, and Tm | 50 | 61 | 125 | 171 | 150 | 100 |
| B. 2 × 108/mL platelets | 41 | 41 | 140 | 221 | 193 | 100 |
| AT-III | ||||||
| A. 200 μmol/L PCPS | ||||||
| No proteins C, S, and Tm | 204 | 162 | 100 | 74 | 98 | 100 |
| Proteins C, S, and Tm | 211 | 136 | 80 | 70 | 71 | 112 |
| B. 2 × 108/mL platelets | 296 | 262 | 70 | 74 | 90 | 100 |
| TFPI† | 98 | 118 | 67 | 106 | 90 | 117 |
| Protein C‡ | 110 | 90 | 100 | 108 | 93 | 100 |
| Protein S‡ | 93 | 100 | 100 | 101 | 103 | 100 |
| Protein . | Protein Concentration (% of normal) . | |||||
|---|---|---|---|---|---|---|
| 50 . | 150 . | |||||
| Total IIa* . | Max.IIa* . | Init.Phase* . | Total IIa* . | Max.IIa* . | Init.Phase* . | |
| Factor V† | 80 | 77 | 140 | 93 | 93 | 87 |
| Factor VIII† | 100 | 77 | 112 | 118 | 152 | 87 |
| Factor VII† | 106 | 132 | 100 | 98 | 112 | 100 |
| Factor IX† | 109 | 129 | 75 | 87 | 73 | 112 |
| Factor X† | 82 | 74 | 112 | 93 | 99 | 87 |
| Factor XI† | 104 | 80 | 100 | 100 | 101 | 100 |
| Factors V, VIII, IX, X† | 41 | 34 | 170 | 90 | 90 | 114 |
| Prothrombin | ||||||
| A. 200 μmol/L PCPS | ||||||
| No proteins C, S, and Tm | 45 | 50 | 100 | 184 | 195 | 100 |
| Proteins C, S, and Tm | 50 | 61 | 125 | 171 | 150 | 100 |
| B. 2 × 108/mL platelets | 41 | 41 | 140 | 221 | 193 | 100 |
| AT-III | ||||||
| A. 200 μmol/L PCPS | ||||||
| No proteins C, S, and Tm | 204 | 162 | 100 | 74 | 98 | 100 |
| Proteins C, S, and Tm | 211 | 136 | 80 | 70 | 71 | 112 |
| B. 2 × 108/mL platelets | 296 | 262 | 70 | 74 | 90 | 100 |
| TFPI† | 98 | 118 | 67 | 106 | 90 | 117 |
| Protein C‡ | 110 | 90 | 100 | 108 | 93 | 100 |
| Protein S‡ | 93 | 100 | 100 | 101 | 103 | 100 |
Abbreviations: Max., maximum; Init., initial.
The value achieved in the presence of 100% protein concentration is 100%.
In the absence of protein C, protein S, and Tm.
In the presence of protein C, protein S, and 0.1 nmol/L Tm.
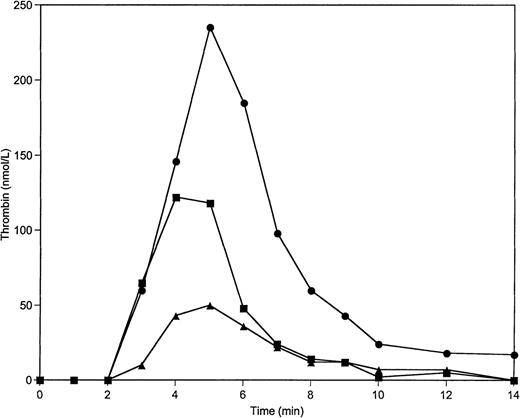
In a protein activation experiment in which washed platelets were substituted for PCPS vesicles, variations of prothrombin and AT-III concentrations over the 50% to 150% range led to results similar to experiments conducted on PCPS (Fig 4; Table 2).
Thrombin generation on 2 × 108/mL platelets at varying prothrombin concentrations in the absence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of prothrombin at 100% (▪), 150% (•), and 50% (▴) of its mean plasma concentration. All other proteins were present at mean plasma concentrations.
Thrombin generation on 2 × 108/mL platelets at varying prothrombin concentrations in the absence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of prothrombin at 100% (▪), 150% (•), and 50% (▴) of its mean plasma concentration. All other proteins were present at mean plasma concentrations.
Adding the dynamic anticoagulant system.
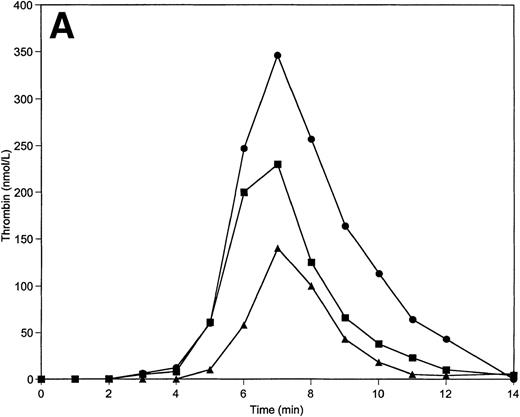
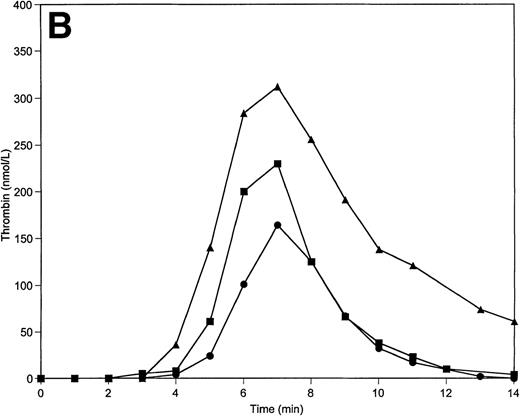
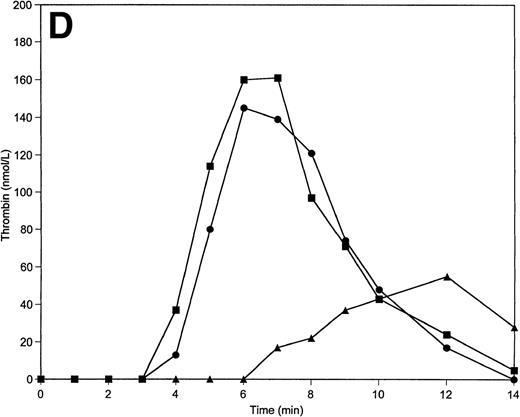
Tm is an essential thrombin cofactor required for protein C activation. Since the local concentration of Tm on endothelial cells may vary widely, an explicit knowledge of effective Tm concentration in vivo is not available. We chose to use soluble Tm at 0.1 nmol/L and 1 nmol/L concentrations based on previously published data that indicated these concentrations of Tm are able to support protein C activation in the reconstituted model.34 The inclusion of soluble Tm at these concentrations and protein C and protein S at their mean plasma concentrations into the reaction mixture does not significantly change the response of the system to variations of prothrombin and AT-III. The total thrombin and maximum levels of thrombin generated are similar to those observed in the absence of the protein C pathway. However, the duration of the initiation phase is affected by the variations of prothrombin and AT-III concentrations when protein C, protein S, and Tm are present (Table 2). Thus, a decrease in prothrombin concentration by 50% extends the initiation phase by 25% (Fig5A), whereas a 50% decrease in AT-III concentration leads to the shortening of this phase by 20% (Fig 5B).
Thrombin generation on 200 μmol/L PCPS at varying prothrombin (A) or AT-III (B) concentrations in the presence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of prothrombin or AT-III at 100% (▪), 150% (•), and 50% (▴) of their mean plasma concentration. All other proteins were present at mean plasma concentrations.
Thrombin generation on 200 μmol/L PCPS at varying prothrombin (A) or AT-III (B) concentrations in the presence of protein C pathway. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of prothrombin or AT-III at 100% (▪), 150% (•), and 50% (▴) of their mean plasma concentration. All other proteins were present at mean plasma concentrations.
Individual variations in the concentration of factors V, VIII, VII, IX, X, and XI, TFPI, and proteins C and S.
Individual variations in the concentration of these proteins over the range from 50% to 150% do not alter significantly the levels of total available thrombin generated or maximum thrombin concentration (Table2). However, the influence of these variations on the initiation phase is not identical for all proteins that were tested. This group of proteins can be divided into 2 subgroups: (1) those for which varying concentrations have an observable influence on this phase (factors V, VIII, and IX, and TFPI); and (2) those that do not alter the initiation phase (factors X, VII, and XI and proteins C and S).
Individual variations in the concentrations of factors V, VIII, and IX, and TFPI.
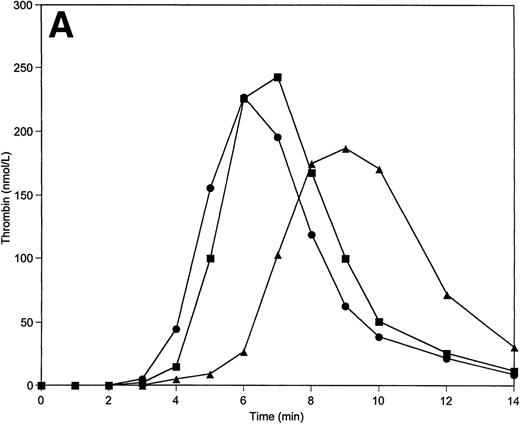
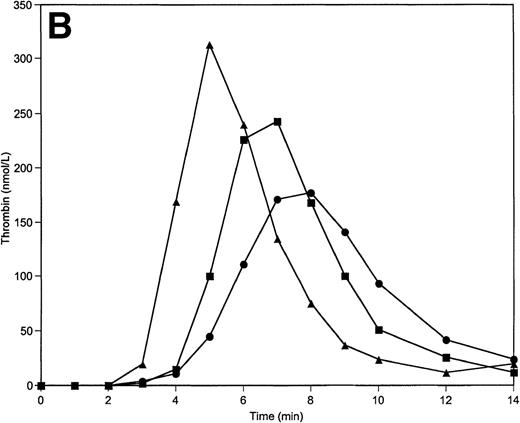
When factor V concentrations are varied over the 50% to 150% range, the initiation phase of the reaction is prolonged by more than 1 minute (from 4 minutes to 5.5 minutes) when factor V levels are reduced to 50% of the nominal average value (Fig 6A). The initiation phase is only slightly shortened when factor V is present at 150%. A similar observation is made when factor VIII levels are varied (Table 2); however, the initiation phase is influenced by factor VIII concentration to a lesser extent than for factor V. Prolongation of the initiation phase at 50% of factor VIII is similar to the shortening of this phase obtained at 150% of this protein (≈0.5 minutes). The reduction of TFPI from the mean plasma value to 50% results in a significant shortening (by ≈1 minute) of the initiation phase of the reaction (Table 2). However, the peak concentrations of thrombin generated and the total thrombin generated over the time course of the experiment are only moderately influenced by TFPI concentration changes within the “normal” range. Raising the TFPI concentration to 150% produced little change over 2.5 nmol/L TFPI.
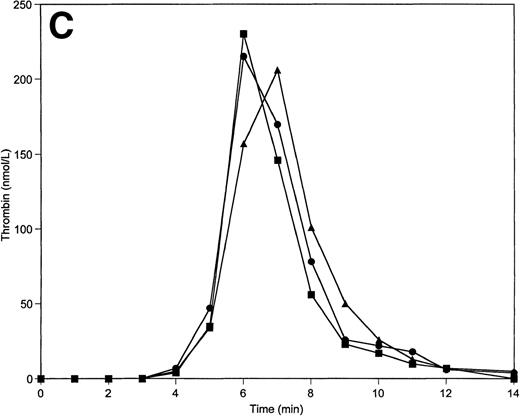
Thrombin generation on 200 μmol/L PCPS at varying factor V (A), factor IX (B), protein C (C), and factors V, VIII, IX, and X simultaneously (D) concentrations. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of either factor V or factor IX, or protein C, or factors V, VIII, IX, and X simultaneously at 100% (▪), 150% (•), and 50% (▴) of their mean plasma concentrations. All other proteins were present at mean plasma concentrations.
Thrombin generation on 200 μmol/L PCPS at varying factor V (A), factor IX (B), protein C (C), and factors V, VIII, IX, and X simultaneously (D) concentrations. Thrombin generation was initiated by 5 pmol/L factor VIIa-TF in the presence of either factor V or factor IX, or protein C, or factors V, VIII, IX, and X simultaneously at 100% (▪), 150% (•), and 50% (▴) of their mean plasma concentrations. All other proteins were present at mean plasma concentrations.
Paradoxically, variations in the procoagulant factor IX concentration led to a similar pattern of thrombin generation as variations in TFPI concentration. Thus, a shortening of the initiation phase is observed at 50% levels of factor IX (by ≈1 minute), while a prolongation (by ≈0.5 minute) of the initiation phase is observed when factor IX levels are increased to 150% of the nominal average value (Fig 6B; Table 2). The total thrombin generated is also inversely related to the factor IX concentration. At the lowest level of factor IX, the highest peak levels of thrombin are produced (310 nmol/L), while the highest level of factor IX gives the lowest peak thrombin level (175 nmol/L).
Individual variations in the concentrations of factors X, VII, and XI, and proteins C and S.
Variations in these protein levels from 50% to 150% produces only a slight (if any) effect on the duration of the initiation phase, the peak thrombin levels achieved, and the total available thrombin (Table2). A representative illustration is shown for protein C in Fig 6C.
As stated earlier, thrombin generation is not influenced by changes in factor VII or factor XI concentrations over the range from 50% to 150% (Table 2). The experimental conditions used did not permit a direct comparison of alterations in factor VII levels because an excess of TF was used to insure complete complexation of the factor VIIa added. Factor VIIa generated from factor VII forms a complex with excess TF, which increases the concentration of the initiator until all TF is bound to factor VIIa. The increased concentrations of the factor VIIa-TF complex caused by the addition of factor VII shorten the initiation phase of the reaction at all concentrations of factor VII tested (5 to 15 nmol/L) compared with experiments performed in the absence of this zymogen; however, variation in factor VII concentration from 50% to 150% does not influence this phase (data not shown).
The data presented thus far suggest that while individual variations in factors IX, X, V, and VIII have some influence on thrombin generation, the contributions of these components are relatively small when compared with the contributions of prothrombin and AT-III to the overall process. Their combined influence was tested by analysis of thrombin generation on 200 μmol/L PCPS in the absence of protein C pathway proteins with prothrombin and AT-III at 100% by varying all other procoagulants in the reaction system from 50% to 150%. The data presented in Fig 6D demonstrate that an increase in all the procoagulants, save prothrombin, had little influence on the thrombin generation profile. In contrast, a reduction by 50% of all these constituents delays the onset of the propagation phase by approximately 3 minutes, reduces peak thrombin levels by 66% and total thrombin by 60%.
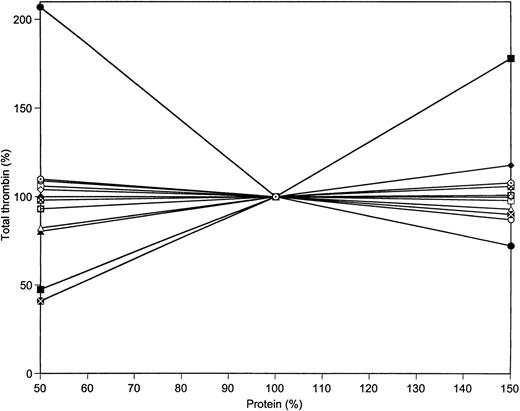
The collected data, in terms of total thrombin produced, for the variations of prothrombin, AT-III, factors X, IX, VIII, V, VII, and XI, as well as TFPI and proteins C and S in the reaction system are illustrated in Fig 7. The total thrombin data are extracted from the experimental curves of Figs 3 and 6, as well as from similar data not shown. The relative magnitudes of thrombin production change with variations of the 7 procoagulants and the 4 anticoagulants illustrate the dominance of prothrombin and AT-III to the overall generation of thrombin. Halving the level of AT-III leads to more than a doubling of the total thrombin generated, while a 50% increase in prothrombin concentration leads to a 75% increase in thrombin relative to that generated at nominal average prothrombin concentrations. In contrast, a reduction in prothrombin concentration to 50% of the mean level leads to a halving of the total thrombin available in the reaction system. Similar alterations of all other procoagulants lead to a 60% reduction relative to that obtained at the nominal plasma concentrations. In contrast to prothrombin, a 50% increase in all other procoagulants has virtually no effect on the total available thrombin. Individually, factor X and factor V at 50% levels each produces only a 20% reduction in total thrombin.
Total thrombin generated at varying protein concentrations. Data were taken from Figs 3 and 6 and from Table 2. The following proteins were varied in the range from 50% to 150% of their mean plasma value: prothrombin (▪), AT-III (•), factor V (▴), factor VIII (⧫), factor VII (□), factor IX (○), factor X (▵), factor XI (◊), factors V, VIII, IX, and X simultaneously ( ), TFPI (⊠), protein C (⊙), and protein S ().
), TFPI (⊠), protein C (⊙), and protein S ().
Total thrombin generated at varying protein concentrations. Data were taken from Figs 3 and 6 and from Table 2. The following proteins were varied in the range from 50% to 150% of their mean plasma value: prothrombin (▪), AT-III (•), factor V (▴), factor VIII (⧫), factor VII (□), factor IX (○), factor X (▵), factor XI (◊), factors V, VIII, IX, and X simultaneously ( ), TFPI (⊠), protein C (⊙), and protein S ().
), TFPI (⊠), protein C (⊙), and protein S ().
Additions of prothrombin or AT-III to whole blood.
An experimental model to study the TF pathway of coagulation in minimally altered whole blood was developed previously in our laboratory.52 In this model, clotting of fresh blood is initiated by TF under conditions in which contact activation is suppressed. This system allows for the testing of theories of blood coagulation under the conditions in which the native state of blood components is preserved. This model has been validated in analyses of blood from healthy persons,52 as well as from patients with hemophilia A and factor XI deficiency.50 Direct comparison of the synthetic plasma system and the whole blood models indicates that activation initiated by the same concentration of TF leads to similar thrombin generation profiles in both experiments (data not shown). This suggests that conclusions drawn from the reconstituted model data can be applied to whole blood as well. To test this hypothesis, we evaluated how thrombin generation is affected by additions of either prothrombin or AT-III to whole blood. The indicator of thrombin generation in this experiment is thrombin-AT-III complex (TAT) formation. The concentration of free thrombin was also evaluated by immunoblotting to quantitate thrombin B chain.
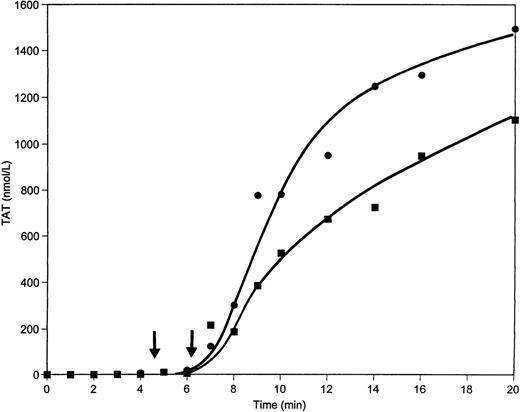
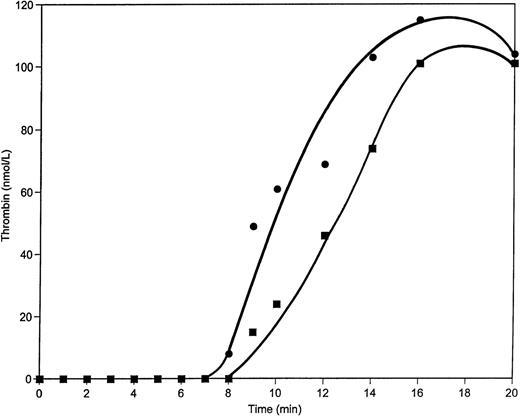
When a 50% supplement of prothrombin (50 μg/mL) is added to contact pathway suppressed whole blood and coagulation is initiated by 12.5 pmol/L relipidated TF, the clotting time is shortened by almost 1.5 minutes relative to the control (4:40 minutes v 6:06 minutes) and the maximum rate of TAT formation in this case is approximately 50% greater than that in the control (4.0 nmol/L/s v 2.7 nmol/L/s, respectively) (Fig 8). The maximum levels of TAT observed are also elevated when excess prothrombin is added to whole blood (1,500 nmol/L v 1,100 nmol/L in control after 20 minutes of activation). Detectable amounts of free thrombin are observed by approximately 1 minute earlier in the excess prothrombin experiment (Fig 9) than in the control and remain higher during all 20 minutes of the experiment.
TAT generation in whole blood when 50 μg/mL of prothrombin is added. Whole blood clotting was initiated by 12.5 pmol/L relipidated TF in unaltered blood (▪) or after the addition of 50 μg/mL of prothrombin (•). TAT concentrations were evaluated by ELISA. Clot times are indicated by arrows.
TAT generation in whole blood when 50 μg/mL of prothrombin is added. Whole blood clotting was initiated by 12.5 pmol/L relipidated TF in unaltered blood (▪) or after the addition of 50 μg/mL of prothrombin (•). TAT concentrations were evaluated by ELISA. Clot times are indicated by arrows.
Thrombin generation in whole blood when 50 μg/mL of prothrombin is added. Concentration of free thrombin evaluated from Western blots by densitometry in control (▪) and in excess prothrombin (•) experiments.
Thrombin generation in whole blood when 50 μg/mL of prothrombin is added. Concentration of free thrombin evaluated from Western blots by densitometry in control (▪) and in excess prothrombin (•) experiments.
The addition of 0.1 mg/mL AT-III (50% of mean plasma value) to whole blood increases the clotting time by 1.3 minutes and the initiation phase of TAT formation is slightly prolonged (data not shown). However, the maximum rate of this complex generation is only marginally altered by the addition of AT-III.
DISCUSSION
Numerous epidemiologic studies indicate that deficiencies in blood coagulation proteins cause serious bleeding problems, whereas the deficiencies in coagulation inhibitors and certain mutations in coagulation factors may lead to thrombotic disorders. From clinical studies it is assumed that the normal concentration range of proteins involved in blood coagulation and regulation of this process may vary in the average blood sample from 50% to 150% of their mean plasma values.35,36 However, a number of epidemiologic studies have shown that concentration variations of some of these proteins within the normal range are associated with thrombotic risk.19,24 41-44
Data presented in this study indicate that of all the procoagulants and stoichiometric inhibitors that influence thrombin generation, prothrombin and AT-III are the standout contributors to significant alterations. Varying these two proteins (in combination or individually) within their felt to be “normal” concentration range causes significant alterations in the amount of total thrombin available, as well as in its maximum levels and rates of generation. Any prothrombin concentration used in this study (0.7 to 2.1 μmol/L) is below saturation for the prothrombinase complex.53 The term “KM” for membrane-bound reactions is complex, involving not only the intrinsic association of enzyme and substrate, but also accumulation of substrate by the membrane.54 The value reported for this constant for prothrombin activation by the prothrombinase complex in the presence of 20 μmol/L PCPS is 1.1 μmol/L.53 Our data indicate that the KM for this process in the presence of 200 μmol/L PCPS is 1.0 μmol/L (manuscript in preparation). Thus, further increases in the substrate (prothrombin) concentration would be associated with an increased rate of thrombin generation. A reduced rate of AT-III inhibition55 would allow elevated levels of thrombin during the initiation phase, thus accelerating factor V and factor VIII activation and shortening this phase. Therefore, in the whole blood experiments, the clotting time, which occurs at the inception of the propagation phase, is significantly influenced by the addition of either prothrombin or AT-III. In both synthetic plasma and whole blood, a similar response of the initiation phase duration to the concentration of prothrombin or AT-III is observed. Increased levels of prothrombin produce significant increases in the thrombin generation. Recently, the in vivo influence of elevated prothrombin levels associated with prothrombin gene mutation G20210A has been studied intensively. Population studies suggest that increased prothrombin levels are associated with multiple thrombotic disorders,5,41,56-59 particularly venous thrombosis, although conclusions on this subject are still controversial.60 The importance of prothrombin levels for TF initiated coagulation-related processes in plasma of warfarin-treated rabbits was also well established.61
No substantial differences in total thrombin were observed when the concentrations of factors V, VIII, IX, and X were individually varied in the range used in this study. The duration of the initiation phase was also not very sensitive to increased levels (150%) of these proteins. Only when factor V was reduced to 50% of its mean plasma value, was the initiation phase prolonged by 40%, whereas in the presence of 50% factor IX, this phase was reduced by 25%. However, the combined reduction of these 4 proteins to 50% of their mean plasma values caused a substantial decrease in total thrombin and significantly prolonged the initiation phase. These conclusions are consistent with the observations that individuals with any of these proteins at concentrations exceeding 25% to 50% of their mean plasma values do not exhibit any bleeding problems.14,62 On the other hand, acquired or hereditary multiple coagulation factor deficiency may cause bleeding problems, sometimes severe or even lethal.63-66
The lack of response in thrombin generation to increased concentrations of all four coagulation factors (V, VIII, IX, and X) is most likely ascribed to a paradoxical effect of factor IX. This effect on the initiation phase and the total thrombin and peak thrombin concentration is probably a consequence of the competition of factor IX and factor X for the factor VIIa-TF catalyst. At a limited catalyst concentration and at a higher affinity of factor IX for the factor VIIa-TF complex,67 the presence of a competing substrate (factor IX) decreases the rate of factor X activation and, as a consequence, the initial prothrombinase concentration because factor Xa is a limiting component of this enzymatic complex during the initiation and propagation phases.30 A similar effect on thrombin generation was observed when another competitive substrate of factor X, factor VII at its mean plasma concentration, was introduced into the reconstituted model.68
Factor XI appears to perform an accessory function in the TF coagulation system by its activation to factor XIa by thrombin, which leads to enhancement of the formation of thrombin by the activation of factor IX to factor IXa. From whole blood studies, this feedback was shown to be important only in thrombin generation initiated at very low levels of TF.50 Therefore, it was predictable that under the present experimental conditions variations in factor XI concentration would have only a marginal effect on the reaction system. Similarly, no bleeding complications are observed in individuals who display factor XI concentrations greater than 10% of the mean plasma value.62
Plasma TFPI concentrations are nominally 2.5 nmol/L. However, the true physiologically effective concentration of TFPI is uncertain. This inhibitor is sequestered by platelets, bound by the endothelium and plasma lipoproteins, released by a variety of effectors, and major portions circulate in plasma as variably truncated forms.69,70 Thus, the biologically relevant concentration of TFPI may not be reflected by the mean plasma concentration. In addition, proteolytic processing of TFPI significantly alters it effectiveness as an inhibitor. These limitations are inescapable in an interpretation of in vitro concentration changes in TFPI. The results of a study, which evaluated TFPI levels in normal individuals and patients with a variety of diseases, indicate that variations of this inhibitor in a wide range are not associated with thrombotic or bleeding tendencies.69 Similarly, no significant impact on thrombin generation was observed in the reconstituted model of this study with normal factor V when TFPI concentration was varied from 1.25 nmol/L to 3.75 nmol/L. In the presence of mutant factor V, factor VLEIDEN, a 50% decrease in TFPI concentration led to a dramatic increase in thrombin levels.71
The absence of a significant response to varying concentrations of protein S and protein C was predictable on the basis of previous studies that concluded protein S is not able to inhibit thrombin generation in a reconstituted model when phospholipids are present at relatively high concentrations.72 It has also been established that variations of only protein C concentration in the “normal” range do not cause coagulation disorders.24,73 74
The data presented here clearly indicate than only prothrombin and AT-III when varied within a “normal” range are able to significantly influence the coagulation process. Other proteins involved in blood coagulation and regulation of coagulation are able only marginally (if at all) to alter this process.
ACKNOWLEDGMENT
We thank Neal J. Golden and Jason J. Penucci for their technical assistance with a few of the experiments. We thank Dr Shu Len Liu and Dr Roger Lundblad for providing us with recombinant factor VIII and recombinant TF; Dr Kirk Johnson for providing recombinant TFPI; Dr John Morser for providing recombinant soluble Tm; and Dr Richard Jenny for providing factor XI.
Supported by Program Project Grant No. HL 46703 from the National Institutes of Health (K.G.M.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenneth G. Mann, PhD, Department of Biochemistry, C401 Given Bldg, University of Vermont, Burlington, VT 05405-0068.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal