We have compared adeno-associated virus (AAV)-based and retrovirus-based vectors for their ability to transduce primary T lymphocytes in vitro and then tracked the persistence of these genetically marked lymphocytes in vivo, using the rhesus monkey model. To avoid the complication of immune rejection of lymphocytes transduced with xenogeneic genes in tracking studies primarily designed to investigate transduction efficiency and in vivo kinetics, the vectors were designed without expressed genes. All vectors contained identically mutated β-galactosidase gene (β-gal) and neomycin resistance gene (neo) DNA sequences separated by different length polylinkers, allowing simple differentiation by polymerase chain reaction (PCR). Each of 2 aliquots of peripheral blood lymphocytes from 4 rhesus monkeys were transduced with either AAV or retroviral vectors. The in vitro transduction efficiency (mean vector copy number/cell) after the ex vivo culture was estimated by PCR at 0.015 to 3.0 for AAV, varying depending on the multiplicity of infection (MOI) used for transduction, and 0.13 to 0.19 for the retroviral transductions. Seven days after transduction, Southern blot analysis of AAV-transduced lymphocytes showed double-stranded and head-to-tail concatemer forms but failed to show integration of the AAV vector. AAV and retroviral aliquots were reinfused concurrently into each animal. Although the retrovirally marked lymphocytes could be detected for much longer after infusion, AAV transduction resulted in higher short-term in vivo marking efficiency compared with retroviral vectors, suggesting possible clinical applications of AAV vectors in lymphocyte gene therapy when long-term vector persistence is not required or desired.
LYMPHOCYTES HAVE several features that are attractive as targets for gene therapy; ease of ex vivo manipulation, acceptable transduction efficiencies with retroviral vectors, and long-term in vivo life span. Therefore, many investigators have been studying lymphocytes as potential targets for gene-therapy applications, including correction of severe combined immunodeficiency syndromes, targeted anti-cancer/anti–human immunodeficiency virus (HIV) therapy, or as factories for soluble factors.1,2 However, human in vivo trials with retroviral vectors have resulted in very low expression levels from retrovirally introduced genes in circulating lymphocytes, precluding clinical efficacy for most applications.3 Interpretation of results has been complicated by the stimluation of immune responses against xenogeneic genes included in the vectors, such as the bacterial neomycin resistance gene (neo) or the herpes thymidine kinase gene.4,5 Lymphocytes expressing at high levels may have been immunologically selected against in vivo, or shut down of expression from proviral control elements may have occurred.6
Adeno-associated viruses (AAVs) are small, nonenveloped, single-stranded DNA viruses of the Parvoviridaefamily.7 Much current interest in AAVs stems from their potential use as vectors for gene therapy. To date, 5 primate AAVs have been distinguished serologically by their antigenically distinct capsid proteins, but AAV-2 has been predominantly used for the vector platform.8 9 The AAV-2 viral genome is composed of 4,679 nucleotides, flanked at both ends by 145 nucleotides of palindromic inverted terminal repeats (ITRs).
Several characteristics of AAV-based vectors suggest that they might be useful for gene transfer to T lymphocytes. AAVs infect a wide variety of cell types, including lymphocytes,10 and infection of humans or other animals with AAVs has not been associated with any disease or pathogenicity. AAV vectors will transduce both dividing and nondividing cells in culture.11 High-level, long-term expression in myocytes, neurons, and hepatocytes in vivo has been achieved, even without chromosomal integration, implying expression from a stable episomal form of AAV vectors.12-14Replication-defective AAV vectors have been shown to integrate, but inefficiently and randomly,15,16 although wild-type AAV integrates more efficiently into a specific locus on human chromosome 19, a process dependent on intact rep gene function.17 18
Although successful AAV vector transduction of human T lymphocytes accompanied by transgene expression has been documented in vitro,19-21 in vivo analysis of AAV-transduced lymphocytes has not been performed. Using the rhesus macaque, we have compared the in vivo fate of autologous lymphocytes transduced with either AAV or standard retroviral vectors. Prior studies of rhesus and human T-lymphocyte retroviral transductions have been reported and clinical trials using these cells completed; thus, retroviral vectors are an appropriate standard for comparison.22,23 In these initial studies, vectors that do not produce xenogeneic gene products were used to avoid complicating the analysis of the persistence of transduced cells by active immune rejection.4 5
MATERIALS AND METHODS
Plasmid construction.
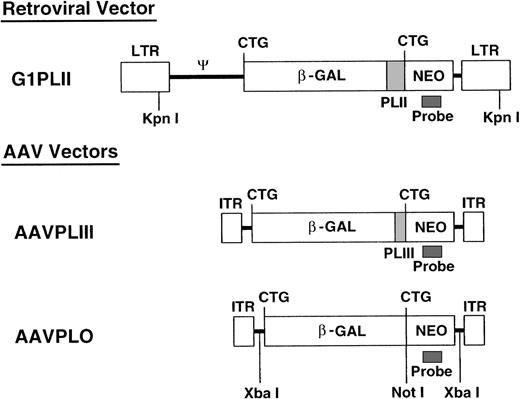
We constructed 2 AAV-2 and 1 retroviral nonexpression vector (Fig 1). The first ATGs of the β-gal sequences in pCMVβ (Clontech, Palo Alto, CA) and theneo sequences in pG1Na (Genetic Therapy Inc, Gaithersburg, MD) were mutated into CTGs by standard polymerase chain reaction (PCR) mutagenesis. The mutated neo gene cassette from pG1Na was introduced into the 3′ end of the mutated β-gal gene in pCMVβ (pCMVβneo). Polylinkers (PLII and PLIII) derived from pSE420 (Invitrogen, San Diego, CA) were introduced between the mutated β-gal and neo genes in pCMVβneo. The β-gal-polylinker-neo fragments were then subcloned into pBluescript (Stratagene, La Jolla, CA) and XbaI linkers were generated at the both ends of the β-gal-polylinker-neo fragments. The resulting β-gal-polylinker-neo cassettes (4.3 kb) were inserted into XbaI sites of pSub201 (a gift of Dr Jude Samulski, University of North Carolina, Chapel Hill, NC9) (pAAVPLO and pAAVPLIII) and into the SnaBI and HindIII sites of pG1 (Genetic Therapy Inc) (pG1PLII).
Vector construction. All 3 nonexpression vectors contain identical β-gal and neo sequences separated by different-length polylinkers. KpnI releases the retroviral sequence at the length of 6.0 kb. XbaI cuts the AAV vector sequence twice and NotI cuts once. These restriction enzymes were used for Southern blotting as shown in Figs 4 and 5.
Vector construction. All 3 nonexpression vectors contain identical β-gal and neo sequences separated by different-length polylinkers. KpnI releases the retroviral sequence at the length of 6.0 kb. XbaI cuts the AAV vector sequence twice and NotI cuts once. These restriction enzymes were used for Southern blotting as shown in Figs 4 and 5.
Vector production and purification.
The nonexpression AAV vectors, AAVPLIII and AAVPLO, were produced in an adenovirus-free system and CsCl-purified as previously reported.19 The titers of AAV vector stocks used in these studies were estimated to be 1012 to 1013genome copies/mL by Southern blot.
The nonexpression retroviral vector G1PLII producer cell line was generated by first transfecting the retroviral plasmid into BOSC23 ecotropic packaging cells using calcium phosphate precipitation, and harvesting supernatants 48 to 60 hours later.24 These supernatants were then used to infect PA317 amphotropic packaging cells using a standard protocol.25Stable producer clones were then isolated by limiting dilution. Fresh supernatants from 80% to 90% confluent producer clones were collected and used for rhesus lymphocyte transductions. The titers of these G1PLII supernatants were 5 × 105/mL according to viral RNA slot blot and Southern blotting of target HeLa cells.26
Lymphocyte transduction and infusions.
Four rhesus monkeys were enrolled in the study, and all animals were housed and handled according to guidelines set forth by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (Bethesda, MD, Department of Health and Human Services [DHHS] Publication No. NIH 85-23) and the protocol was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute. Fifty milliliters of heparinized peripheral blood was separated on a density gradient (LSM; ICN Biomedical, Aurora, OH) to obtain mononuclear cells. These cells were incubated in AIM-V medium (Life Technology, Gaithersburg, MD) in the presence of 500 IU/mL recombinant human interleukin-2 (IL-2) (R&D, Minneapolis, MN) and FN18, a monoclonal antibody to rhesus CD3 (Biosource International, Camarillo, CA) for 3 days. The cells were then divided into 2 equal aliquots. One sample was transduced with AAVPLO or III once on day 3 by adding the purified AAV vector to culture medium in the presence of 500 IU/mL human IL-2, and was incubated for another 7 days. Lymphocytes from rhesus 94E068 were transduced with 2,000 viral genomic copies (gc) of AAVPLO per cell, those from RQ1307 with 20,000 gc AAVPLO per cell, those from RQ1303 with 20,000 gc AAVPLIII per cell, and those from RQ854 with 200,000 gc AAVPLO per cell. The other aliquot was transduced with the retroviral vector G1PLII 3 times over 7 days according to the published method.22 Briefly, cells were first incubated in phosphate-free media for 6 hours, and then resuspended in the retroviral G1PLII supernatant at a multiplicity of infection (MOI) of 1 to 5, along with 4 μg/mL protamine sulfate (Sigma, St Louis, MO) and 500 IU/mL human IL-2 , followed by centrifugation at 1,000g at 32°C for 1 hour and incubation at 32°C overnight. This optimized transduction was performed twice, and a standard transduction was performed once, without phosphate depletion, centrifugation, or low-temperature transduction. Finally, both aliquots were reinfused simultaneously into each monkey after a total of 10 days of culture and transduction.
PCR analysis.
DNA was isolated from peripheral blood mononuclear cells using the QIAamp Blood Kits (Qiagen, Valencia, CA). Five hundred nanograms of DNA was used in each reaction. The standards consisted of log dilutions of DNA extracted from the G1PLII-producer clone (which contains 3 copies of the vector proviral sequence per cell, documented by Southern blot) into control normal rhesus peripheral blood mononuclear cell (PBMNC) genomic DNA. The negative control consisted of DNA extracted from pretransduced rhesus PBMNCs. PCR primers were used that span the polylinker in the vectors, allowing differentiation of the AAV and retroviral vector sequences. Overall reactions were optimized to yield linear amplifications in the range of the intensity of the positive controls. The outer primer set was 5′-CGT CAG TAT CGG CGG AAT TAC AGC-3′ and 5′-CAG TCA TAG CCG AAT AGC CTC T-3′. The inner primer set was 5′-CGC TAC CAT TAC CAG TTG GTC-3′ and 5′-AGA ACC TGC GTG CAA TCC ATC-3′. Amplification conditions were 95°C for 1 minute, 54°C for 1 minute, and 72°C for 2 minutes with 20 cycles both for both outer and inner PCR. The outer PCR products were purified by QIAquick PCR purification kits (Qiagen). The inner PCR was performed in the presence of 12.5 μCi/mL [α-32P]dCTP. The expected sizes of the final PCR products were 508 bp, 438 bp, and 355 bp for G1PLII, AAVPLIII, and AAVPLO, respectively.
PCR for β-gal and β-actin was also performed. The primer set for β-gal was 5′-CTA CAC CAA CGT AAC CTA TCC C-3′ and 5′-TTC TCC GGC GCG TAA AAA TGC G-3′. The primer set for β-actin was 5′-CAT TGT GAT GGA CTC CGG AGA CGG-3′ and CAT CTC CTG CTC GAA GTC TAG AGC-3′. Amplification conditions were 95°C for 1 minute, 54°C for 1 minute, and 72°C for 2 minutes in the presence of 12.5 μCi/mL [α-32P]dCTP with 30 cycles for β-gal and 26 cycles for β-actin. The expected sizes of the PCR products were 249 bp for β-gal and 234 bp for β-actin. The final PCR products were separated on 8% polyacrylamide gels. The quantification of the band intensity was performed using a Phosphorimager ImageQuant (Molecular Dynamics, Sunnyvale, CA).
Southern blotting.
Ten micrograms of DNA was digested with either KpnI,XbaI, or NotI restriction enzymes, separated on a 0.8% agarose gel, and transferred onto Hybond-N+ (Amersham, Cleveland, OH). The radiolabeled DNA probe was a neo gene-specific sequence generated by PCR. The forward primer was 5′-TCC ATC ATG GCT GAT GCA ATG CGG C-3′ and reverse primer was 5′-GAT AGA AGG CGA TGC GCT GCG AAT CG-3′. Radiolabeling of the probes was done using an oligolabeling kit (Phamacia, Piscataway, NJ).
RESULTS
Vector design.
We constructed 2 AAV vectors and 1 retroviral vector (Fig 1), with mutations of ATGs in neo and β-gal sequences to prevent translation (“nonexpression” vectors). All 3 vectors contain identical β-gal and neo sequences, but with different-length polylinkers (PLII and PLIII) inserted between the 2 genes to allow identification of the vectors by PCR and direct comparison of the levels of vector-containing cells in vivo. We confirmed that significant levels of functional gene products were not translated by showing a lack of X-gal staining and G418 resistance, as well as the absence of full-length or truncated proteins on immunoblots in 3T3 cells transduced with the nonexpression vectors (Y.H., manuscript in preparation).27
Ex vivo expansion and transduction.
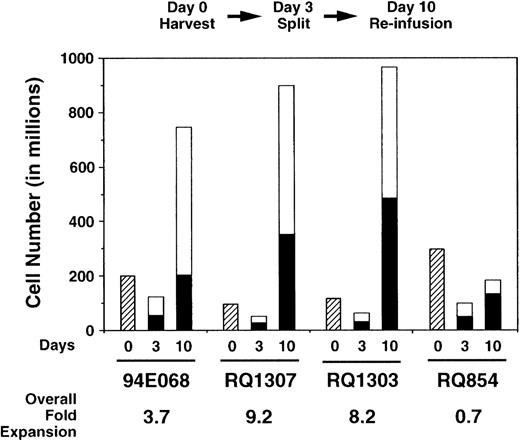
Using these nonexpression vectors, we compared transduction of autologous rhesus monkey lymphocytes with AAV and retroviral vectors. Mononuclear cells were obtained from 50 mL peripheral blood and incubated with IL-2 and anti-CD3 antibody for 3 days. The cells were then divided into 2 equal aliquots; 1 aliquot was transduced once with the AAV vector, while the other aliquot was transduced with the retroviral vector (3 times). Several ratios of AAV vector particles to target cells (MOI) were used (Table 1). Expansion during the ex vivo culture period is shown in Fig 2 and was almost equivalent for the aliquots transduced with AAV or retrovirus. No toxic effects of either vector were observed during the ex vivo culture period. By fluorescence-activated cell-surface analysis, over 98% of cells were T cells expressing CD2 at the end of expansion (10 days after culture initiation; data not shown).
Ex Vivo Culture and Transduction of Rhesus Lymphocytes
| Rhesus Monkeys . | AAV (MOI*) . | Cell Numbers (in millions) . | Expansion (fold increase over starting no.) . | In Vitro Transduction Efficiency (mean copy no. per cell) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AAV . | RV . | |||||||||
| Day 0 (harvest) . | Day 3 (split) . | Day 10 (infusion) . | AAV . | RV . | Total . | PCR . | PCR . | Southern . | ||
| 94E068 | AAVPLO (2,000) | 200 | 120 | 740 | 5.4 | 2.0 | 3.7 | 0.015 | .13 | .14 |
| RQ1307 | AAVPLO (20,000) | 97 | 46 | 890 | 11.3 | 7.0 | 9.2 | .17 | .19 | .22 |
| RQ1303 | AAVPLIII (20,000) | 117 | 58 | 960 | 8.0 | 8.4 | 8.2 | .80 | .14 | .17 |
| RQ854 | AAVLPO (200,000) | 299 | 96 | 206 | 0.41 | 0.97 | 0.7 | 3.0 | .15 | .16 |
| Mean | NA | 178 | 80 | 699 | 4.5 | 3.3 | 3.9 | NA | .15 | .17 |
| Rhesus Monkeys . | AAV (MOI*) . | Cell Numbers (in millions) . | Expansion (fold increase over starting no.) . | In Vitro Transduction Efficiency (mean copy no. per cell) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AAV . | RV . | |||||||||
| Day 0 (harvest) . | Day 3 (split) . | Day 10 (infusion) . | AAV . | RV . | Total . | PCR . | PCR . | Southern . | ||
| 94E068 | AAVPLO (2,000) | 200 | 120 | 740 | 5.4 | 2.0 | 3.7 | 0.015 | .13 | .14 |
| RQ1307 | AAVPLO (20,000) | 97 | 46 | 890 | 11.3 | 7.0 | 9.2 | .17 | .19 | .22 |
| RQ1303 | AAVPLIII (20,000) | 117 | 58 | 960 | 8.0 | 8.4 | 8.2 | .80 | .14 | .17 |
| RQ854 | AAVLPO (200,000) | 299 | 96 | 206 | 0.41 | 0.97 | 0.7 | 3.0 | .15 | .16 |
| Mean | NA | 178 | 80 | 699 | 4.5 | 3.3 | 3.9 | NA | .15 | .17 |
Abbreviations: RV, retrovirus; NA, not available.
MOI indicates viral genomic copy number per lymphocyte.
Ex vivo expansion of rhesus lymphocytes. The numbers of lymphocytes decreased for the first 3 days. Thereafter, the cell numbers increased and the overall expansion is shown. The expansion was equivalent for both AAV- (□) and retrovirus-transduced (▪) aliquots.
Ex vivo expansion of rhesus lymphocytes. The numbers of lymphocytes decreased for the first 3 days. Thereafter, the cell numbers increased and the overall expansion is shown. The expansion was equivalent for both AAV- (□) and retrovirus-transduced (▪) aliquots.
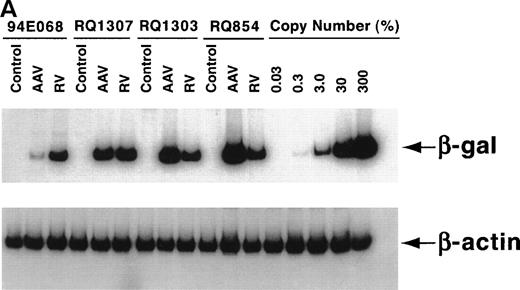
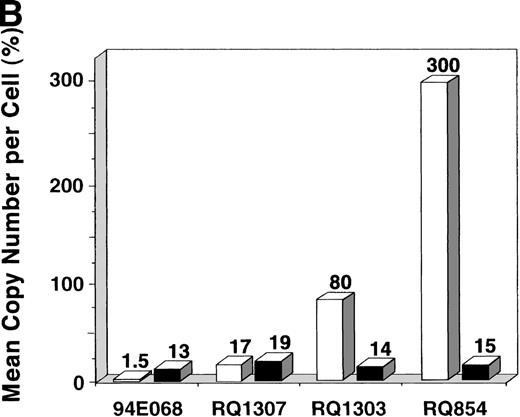
After the 10-day ex vivo culture and transduction period, PCR for the vector (β-gal) sequence and internal β-actincontrol sequence was performed to quantify the in vitro transduction efficiency (Fig 3A). When 200,000 AAV genomic copies per lymphocyte were used, very high-level transduction (on average 3 copies per lymphocyte in RQ854) was seen on analysis at day 10, just before reinfusion. When 20,000 copies per lymphocyte were used, copy numbers of 0.17 (RQ1307) and 0.80 (RQ1303) per lymphocyte resulted. When 2,000 copies per lymphocyte were used (94E068), only 0.015 copies per lymphocyte resulted (Fig 3B and Table 1). The transduction efficiencies for the retroviral vector were estimated by PCR to be 0.13 to 0.19, similar to levels previously reported using this retroviral transduction methodology.22
In vitro transduction efficiencies. (A) Semi-quantitative PCR for the β-gal and actin sequences was performed at the end of the ex vivo culture period. The β-gal is the vector sequence and the actin is the control sequence. (B) The graph summarizes the in vitro transduction efficiencies shown as a mean copy number per cell, calculated from the PCR results. The MOI used was different among monkeys. 94E068 had 2,000, RQ1307 and RQ1303 had 20,000, and RQ864 had 200,000 AAV genomic copies per lymphocyte. (□), AAV; (▪), retrovirus.
In vitro transduction efficiencies. (A) Semi-quantitative PCR for the β-gal and actin sequences was performed at the end of the ex vivo culture period. The β-gal is the vector sequence and the actin is the control sequence. (B) The graph summarizes the in vitro transduction efficiencies shown as a mean copy number per cell, calculated from the PCR results. The MOI used was different among monkeys. 94E068 had 2,000, RQ1307 and RQ1303 had 20,000, and RQ864 had 200,000 AAV genomic copies per lymphocyte. (□), AAV; (▪), retrovirus.
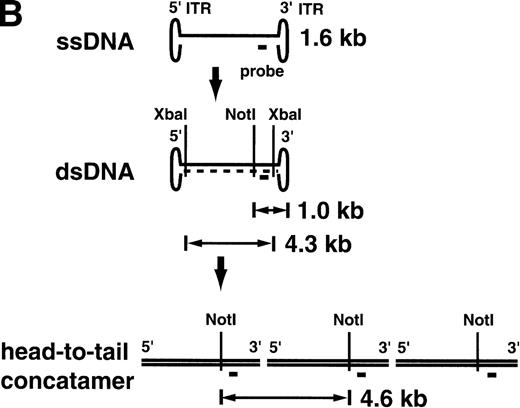
Southern blotting was also performed after the ex vivo culture on retrovirally transduced lymphocytes (Fig4). All 4 monkeys had the proviral sequence in their lymphocyte genome. A copy number of 0.14 to 0.22 integrated provirus per lymphocyte was estimated by Southern blotting, consistent with the PCR results (Table1). The Southern blot analysis of the AAV-transduced lymphocytes on day 10 just before reinfusion gave very different results (Fig 5A). In AAV-transduced aliquots from animals RQ1303 and RQ854, with relatively high-level transduction as estimated by PCR (0.80 and 3.0 copy number per lymphocyte, respectively), single-strand DNA (ssDNA) 1.6-kb bands were observed. These represent the expected size of the single-stranded AAV vector itself. In RQ854, with the highest in vitro transduction efficiency, besides the 1.6-kb ssDNA band, a 4.3-kb band was observed afterXbaI digestion (cutting at both ends of the vector genome), suggesting that some of the vector DNA has become double-stranded and could be released as viral double-stranded DNA (dsDNA). When single-cutter NotI was used, a 1.0 kb-band was observed, consistent with the size from the NotI site to the 3′-end of the dsDNA vector. Furthermore, one more band of 4.6 kb was observed in RQ854 when NotI was used. This band can be explained if a portion of the viral dsDNA has become a head-to-tail concatemer (Fig5B). Therefore, this Southern blot is consistent with the recent hypothesis that AAV vector ssDNA first becomes double-stranded and then forms a stable head-to-tail concatemer episome array before initiating transcription.13 The Southern blots did not show any evidence of integration of the vector into the genome by day 10 of culture (7 days after AAV transduction); however, there is no convenient assay to rule out the possibility of integration, at least at a low level. At this point, the cells were reinfused into the monkeys (see below). It is possible that conversion to the double-stranded, concatamerized form continued in vivo, but the low level of modified cells prevented Southern blot analysis of postinfusion blood samples.
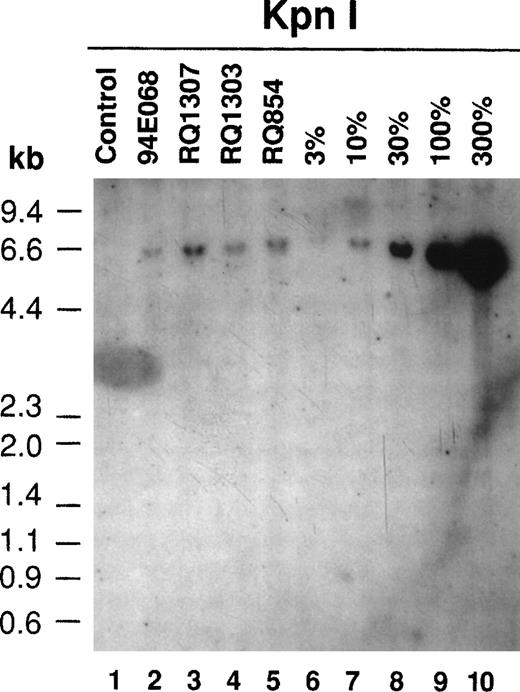
Southern blotting of the retrovirally transduced lymphocytes after the ex vivo culture (day 10). Ten micrograms of DNA from transduced lymphocytes was digested with KpnI, which released the proviral sequence at the length of 6.0 kb (see Fig 1), separated on an agarose gel, and transferred to a nylon membrane followed by hybridization with the neo DNA probe. The proviral sequence in the transduced lymphocyte genome was detected in all 4 monkeys. The positive controls (lanes 6 through 10) were made from dilutions of the G1PLII-retroviral producer DNA into control rhesus lymphocyte DNA.
Southern blotting of the retrovirally transduced lymphocytes after the ex vivo culture (day 10). Ten micrograms of DNA from transduced lymphocytes was digested with KpnI, which released the proviral sequence at the length of 6.0 kb (see Fig 1), separated on an agarose gel, and transferred to a nylon membrane followed by hybridization with the neo DNA probe. The proviral sequence in the transduced lymphocyte genome was detected in all 4 monkeys. The positive controls (lanes 6 through 10) were made from dilutions of the G1PLII-retroviral producer DNA into control rhesus lymphocyte DNA.
Southern blot analysis for the AAV vector-transduced rhesus lymphocytes with XbaI and NotI (A). Ten micrograms of DNA from transduced lymphocytes was digested withXbaI or NotI, separated on agarose gels, and transferred to nylon membranes followed by hybridization with theneo DNA probe. In RQ854, besides the single-strand DNA (ssDNA) band around 1.6 kb, the double-strand DNA (dsDNA) bands were detected, which were released by digestion with XbaI (4.3 kb, lane 5) and with NotI (1.0 kb, lane 10). Another band of 4.6 kb withNotI (lane 10) is consistent with a head-to-tail concatamer form of the vector. (B) The hypothesis of AAV vector conversion to a head-to-tail concatemer. This Southern blot is consistent with the hypothesis.
Southern blot analysis for the AAV vector-transduced rhesus lymphocytes with XbaI and NotI (A). Ten micrograms of DNA from transduced lymphocytes was digested withXbaI or NotI, separated on agarose gels, and transferred to nylon membranes followed by hybridization with theneo DNA probe. In RQ854, besides the single-strand DNA (ssDNA) band around 1.6 kb, the double-strand DNA (dsDNA) bands were detected, which were released by digestion with XbaI (4.3 kb, lane 5) and with NotI (1.0 kb, lane 10). Another band of 4.6 kb withNotI (lane 10) is consistent with a head-to-tail concatamer form of the vector. (B) The hypothesis of AAV vector conversion to a head-to-tail concatemer. This Southern blot is consistent with the hypothesis.
In vivo tracking of transduced lymphocytes.
We reinfused transduced cells into each monkey and followed the in vivo persistence of marked cells by PCR (Fig 6). In animal 94E068, receiving cells transduced with low MOI AAV, the AAV signal disappeared 2 weeks after infusion, whereas the retroviral signal was still detectable at week 56. In RQ1307 and RQ1303, receiving the intermediate MOI AAV, in vivo marking was equivalently shown for AAV and retroviral vectors for the first 3 days. However, the AAV signals decreased more rapidly and disappeared at weeks 8 and 4, respectively, while the retroviral signals persisted. In RQ854 receiving cells transduced with the highest MOI AAV, an in vivo AAV signal was shown at copy number levels of up to 0.03, several-fold higher than retroviral marking. However, the intensity of the AAV marking decreased rapidly, although it was still detectable at weeks 5 and 6.
In vivo persistence of transduced cells as detected by PCR. Mononuclear cells from rhesus peripheral blood were obtained at indicated time points. DNA was extracted and subjected to semi-quantitative PCR spanning the polylinkers (see Fig 1). The longer PCR products are derived from the retroviral vector and the shorter ones are derived from the AAV vectors. The copy number control was made from log dilution of the G1PLII-retroviral producer clone into the control rhesus lymphocyte DNA.
In vivo persistence of transduced cells as detected by PCR. Mononuclear cells from rhesus peripheral blood were obtained at indicated time points. DNA was extracted and subjected to semi-quantitative PCR spanning the polylinkers (see Fig 1). The longer PCR products are derived from the retroviral vector and the shorter ones are derived from the AAV vectors. The copy number control was made from log dilution of the G1PLII-retroviral producer clone into the control rhesus lymphocyte DNA.
DISCUSSION
In this study we have shown that both AAV- and retrovirus-based vectors can be used to transduce primary T lymphocytes, using the rhesus monkey model. Transduction with retrovirus vectors was performed using the optimized transduction methods of phosphate depletion, centrifugation, and low-temperature transduction,22 but similar optimizations have not been performed for AAV transduction and, hence, in this study AAV vectors were added on a single occasion to the culture media, at the same time as the first retroviral transduction. Despite only a single transduction procedure, we have shown that in vitro transduction efficiency of lymphocytes using AAV vectors was greater than retroviral vectors when high-titer AAV was used: a viral preparation concentrated to allow a ratio of 200,000 genome copies per target cell achieved, on average, 3.0 AAV genomic copies per transduced cell.
It should be noted that this MOI calculation was based on gc of AAV vector. Using expression-competent vectors, it is known that only a small percentage of the overall recombinant AAV particle population (1 in 102-4 particles, depending on the vector preparation) are functional. High-level transduction and sustained gene expression has required very high recombinant AAV gc per target cell, compared with retroviral vectors, where the use of greater than 10 infectious particles per target cell has not resulted in increased transduction efficiencies.28 This requirement for very high numbers of concentrated AAV vectors might be a drawback for clinical applications, because generation of these stocks on a large scale is technically challenging, although improved approaches are being developed.29-31
It is possible that receptor expression on lymphocytes could be limiting for AAV transduction. Recently it has been proposed that heparan sulfate proteoglycan may act as a cellular receptor for AAV-2,32 with the fibroblast growth factor receptor, FGFR1, or αVβ5 intergrin acting as putative coreceptors.33,34These molecules are expressed on the cell surface of lymphocytes,35,36 but the expression levels are low and this factor may account for the high MOI requirement for AAV transduction of lymphocytes. Alternatively, our own studies have shown that these molecules are not required for successful transduction of cell lines, suggesting that other molecules may act as receptors42 Regardless, manipulations to increase AAV-receptor interactions, as has been accomplished via phosphate depletion for amphotropic retroviral receptors, may help overcome this possible limitation. Nondividing cells such as neurons, myocytes, and hepatocytes may be efficiently transduced with AAV vectors, as opposed to retroviral vectors.12 13 In our study, 2 animals received cells transduced at the same AAV dose (20,000 gc/cell) but had different patterns of ex vivo lymphocyte growth. Cells from 1 animal grew poorly, but transduced at a higher relative level than more rapidly proliferating cells from the other animal. Likewise, the animal that had cells transduced at the very high AAV MOI and had the highest transduction efficiency (3 copies per transduced cell) had very poor ex vivo growth of the target lymphocytes. More efficient transduction may be possible by either shortening the ex vivo culture period (potentially also retaining a greater repertory or improved functionality of infused T cells) or by adding the AAV vectors on the first day without prior stimulation.
We have confirmed transgene expression from AAV vectors in both human and rhesus lymphocytes using an AAV vector expressing the green fluorescent protein gene (data not shown) Similarly, several other researchers have reported high expression in vitro of AAV-transduced genes in lymphocytes.19-21 However, in the current study, as shown in the Southern blots (Fig 5), most of the vector DNA still remains as single-strand form at the time of reinfusion into the animals 7 days after transduction, and only a small fraction of the vector DNA formed the head-to-tail concatemers. Conversion from a single-stranded form of AAV vectors to a double-stranded form is considered to be rate-limiting among steps required for transgene expression.13,37,38 Radiation and some cytotoxic drugs may accelerate conversion to expressing forms and, thus, improve AAV transgene expression.37 38 However, these manipulations are not appropriate for clinical transductions of primary lymphocytes. The fact that on the day of reinfusion concatemers were observed suggests that, in vivo, the conversion process continued, and that expressed gene products could increase their levels in vivo during the 2-8-week period AAV-marked cells persisted in the circulation. It is also possible that integration occurred, although the disappearance of the AAV vector signal by PCR analysis after 5 to 7 weeks suggests that integration was not common.
As shown in Fig 6, in vivo retroviral marking persisted longer than AAV marking, detectable for 1 year after a single infusion to date in several animals. The lack of xenogeneic expressed and translated sequences may have contributed to this long persistence. In fact, in separate experiments, rhesus lymphocytes transduced with the nonexpression retroviral vector persisted stably in vivo (more than 1 year), whereas lymphocytes transduced with the neo-expressing retroviral vector disappeared or decreased to minimal levels after 5 to 10 weeks.27 This long survival of lymphocytes transduced with the nonexpressed sequence is relevant to the prospects for lymphocyte gene therapy, and emphasizes the need to avoid inclusion of xenogeneic or allogeneic expressed sequences in vectors. There have been several reports documenting rejection of retrovirally transduced lymphocytes expressing xenogeneic genes, even in immunosuppressed HIV-positive patients or allogeneic transplantation recipients.4 39
The Southern blots for AAV-transduced lymphocytes showed no clear evidence for integration, although at only 7 days posttransduction this slow conversion and integration process may have not been completed. It may have continued in vivo after reinfusion, although AAV-transduced lymphocytes could not be detected in vivo beyond 6 to 8 weeks. These observations suggest that the integration frequency of AAV vectors in primary lymphocyte chromosomes is low. However, AAV transduction resulted in higher short-term in vivo marking as compared with retroviral transduction. The disappearance of the AAV-containing cells could have resulted from episomal loss during cell division. Alternatively, a preexisisting anti-AAV2 immune response against residual AAV proteins still present in the transduced lymphocytes could have contributed to rapid clearance. This seems unlikely, given the prolonged culture period after exposure to replication-incompetent vector. Western blotting for anti-AAV2 antibodies has not shown any evidence for anti-AAV2 humoral immunity in these animals before or after infusion of transduced lymphocytes (K.E.B., unpublished data, April 1999). Our results suggest possible clinical applications of AAV vectors in lymphocyte gene therapy when long-term vector persistence is not required or desired, for instance in targeted cancer gene therapy.1,40 41
D.H. was supported by grants from the Swiss National Science Foundation, the Ciba-Geigy Jubilaeumsstiftung, and from Cancer Research Switzerland KFS 500-8-1997.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Cynthia E. Dunbar, MD, Bldg 10, Room 7C103, 9000 Rockville Pike, Bethesda, MD 20892; e-mail:dunbarc@gwgate.nhlbi.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal