Fas (CD95/Apo-1) mutations were previously reported as the genetic defect responsible for human lymphoproliferative syndrome associated with autoimmune manifestations (also known as autoimmune lymphoproliferative syndrome or Canale-Smith syndrome). We have identified 14 new heterozygous Fas mutations. Analysis of patients and families allow us to further dissect this syndrome with regards to the relationship between Fas mutations, inheritance pattern, and phenotype as observed on long-term follow-up. In vitro studies show that lymphocytes from all Fas mutant carriers exhibit a Fas-antibody–induced apoptosis defect. However, among the 8 inherited mutations, 4 of 4 Fas missense mutations were associated with high clinical penetrance, whereas 3 of 4 mutations leading to a truncated Fas product were associated with variable clinical penetrance. This suggests that a second defect, in another yet undefined factor involved in apoptosis and/or lymphoproliferation control, is necessary to induce full clinical expression of the disease. These results also indicate that the currently available antibody-mediated in vitro apoptosis assay does not necessarily reflect the in vivo ability of abnormal Fas molecules to trigger lymphocyte death. In addition, we found that lymphoproliferative manifestations resolved with age, whereas immunological disorders [ie, hypergammaglobulinemia and detection of TcR β(+) CD4(−) CD8(−) lymphocytes] persisted. This observation suggests that Fas-mediated apoptosis plays a more important role in lymphocyte homeostasis in early childhood than later on in life.
Fas IS A MOLECULE belonging to the tumor necrosis factor (TNF) receptor family.1 A subfamily has been defined as those expressing an intracytoplasmic domain called the death domain (DD).2 Several newly described molecules, such as Apo-3 (also called DR3, Tramp, Wsl-1, and Lard)3-7 and the Trail receptors,8 belong to this growing subfamily, in which Fas and TNFR are the prototypes. They are all able to trigger a death signal upon oligomerization. This signal is given through the interactions of their DD with DD-containing adapter molecules (such as FADD, TRADD, Rip, and RAIDD).9-14 These adapters are thought to interact with proteases of the caspase family through interactions of domains called death effector domains (DED) and caspase recruitment domains (CARD).15 In particular, formation of a death-inducing signaling complex (DISC) including Fas, FADD, and Flice/Caspase-8 seems to be a crucial step in the cascade activation of Caspases and delivering a death signal.9,16 17
The role of the Fas-FasL interaction in controlling lymphocyte homeostasis has been described in the lpr and gld mouse models.1 These mice, carrying genetic defects in the Fas18 and FasL19 genes, respectively, exhibit lymphoproliferation and severe autoimmune manifestations. Similar manifestations have also been observed in humans.20-22 The human disease appears to be more complex than the mouse disease models, because the lymphoproliferative syndrome and autoimmune manifestations (also called ALPS) are associated with mutations on both21,23,24 or only 1 Fas allele.21-23,25 Both homozygous Fas mutations described were null mutations as Fas expression could not be detected at the cell surface. In these cases, the phenotype is extremely severe.23,26 Heterozygous Fas mutations have been shown to be responsible for a less severe lymphoproliferative syndrome and variable autoimmune manifestations.26,27 Two forms of inheritance have been reported. Autosomal recessive inheritance has been described in patients in whom both Fas alleles are mutated, whereas heterozygous carriers are asymptomatic.21,23,24 In contrast, inheritance of lymphoproliferative syndrome with autoimmunity associated with heterozygous Fas mutations is less clear. In some pedigrees, a clear autosomal dominant inheritance pattern is found.22,25,27 In others, only some carriers present with clinical symptoms.25-28 We describe here the spectrum of clinical, immunological, and genetic features of 16 patients with lymphoproliferative syndrome and autoimmunity who are heterozygous for Fas mutations.
PATIENTS, MATERIALS, AND METHODS
Patients
Patients were assessed for lymphoproliferative syndrome associating splenomegaly and/or lymphadenopathy and autoimmune manifestations. Investigations consisted of determining serum Ig levels, screening for serum autoantibodies (to polymorphonuclear cells, platelets, erythrocytes, nuclear antigen, DNA, and phospholipids), peripheral blood lymphocyte subset phenotype determinations [including testing for TcR α/β (+) CD4(−) CD8(−) lymphocytes], and anti-CD95 monoclonal antibody (MoAb)-induced T-lymphocyte apoptosis assays. A defect in anti-CD95 MoAb-induced T-lymphocyte apoptosis was detected in 14 patients. Phenotypic and functional analysis performed on patient no. 4’s cells were previously reported.29 A total of 45 relatives were at risk, and 36 were screened using the apoptosis assay and analysis of Fas mutations. In addition to the 14 patients, heterozygous Fas mutations were found in 15 relatives, and clinical manifestations were found in 10. Informed consent from patients or families was obtained before performing the studies.
Antibodies
The following MoAbs were used in immunofluorescence studies: anti-CD3: Leu 4 (IgG2a; Becton Dickinson, San Diego, CA); anti–T-cell receptor (TcR) α/β: BMA031 (IgG1; Immunotech, Marseille, France); anti-CD4: Leu 3a (IgG1, Becton Dickinson); anti-CD8: Leu 2a (IgG1; Becton Dickinson); anti-CD19: J4 119 (IgG1; Immunotech); anti-CD95: Apo-1 (kindly provided by Dr K.N. Debatin30). Phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated MoAbs were used for fluorescence staining of whole blood samples, and PE-conjugated goat antimouse IgG (Caltag, San Francisco, CA) was used for the Apo-1 antibody on activated lymphocytes. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson).
Apoptosis Assay
Peripheral mononuclear cells were isolated from freshly drawn heparinized blood by means of Ficoll-hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. They were activated for 6 days with phytohemagglutinin (PHA) and interleukin-2 (IL-2; Genzyme; 20 IU/mL) and then cultured with Apo-1 MoAb (250 ng/mL) and a rat-antimouse IgG (Jackson Laboratories, Westgrove, PA; 10 μg/mL) for 24 hours. Apoptotic cells were quantified as described elsewhere.31 Briefly, cells were resuspended in a hypotonic solution containing 0.1% sodium citrate, 0.1% Triton X-100 (Sigma), and 50 μg/mL propidium iodide (Sigma). Red fluorescence was measured using a FACStar plus flow cytometer (Becton Dickinson). Apoptotic cells were counted as hypodiploid. The data are expressed as the percentage calculated as follows: (apoptosis observed in the patient)/(apoptosis observed in the control) × 100. The in vitro apoptosis observed in the controls and mutation negative relatives was always greater than 80%.
Detection of Fas Mutations
DNA samples were prepared from activated lymphocytes using standard methods.32 Single-strand conformation polymorphism (SSCP) was assayed as described elsewhere.22 Genomic DNA segments of 90 to 350 bp were amplified with following primers: Exon I F (5′ GGAACACACCCTGAGGCCAG 3′); Exon I R (5′ CCTCCACCCGGGCAGGGAAG 3′); Exon II F (5′ TAAAATTCTCTTCATGCTTT 3′); Exon II R (5′ CTGTAATCTCTGGATGTTTG 3′); Exon IIIa F (5′ TTGTCTGTCATCCCTCTATACTTCCC 3′); Exon IIIa R (5′ ATCACACAATCTACATCTTCTGCA 3′); Exon IIIb F (5′ TGCCAAGAAGGGAAGGAGTA 3′); Exon IIIb R (5′ ATTTGAGCTGATGAACCCTGTTCC 3′); Exon IV F (5′ TAACTAATAGTTTCCAAACT 3′); Exon IV R (5′ TGTTTTAATCTCTGAAAGAC 3′); Exon V F (5′ TTTGAATTTCTCCTGTATTT 3′); Exon V R (5′ GGGGAAAGGAGAATATAACC 3′); Exon VI F (5′ CATATAATATGCCAATGTTC 3′); Exon VI R (5′ AATCTGCAGTTTGAACAAAG 3′); Exon VII F (5′ CATGCATTCTACAAGGCTGAGACC3′); Exon VII R (5′ TTTTCTTTTCAAGGAAAGCTGATACC 3′); Exon VIII F (5′ ACTTCTTTCTGAATTAAGGA 3′); Exon VIII R (5′ GCAGGTAGAATTGTATGAGA 3′); Exon IXa F (5′ CTGAAGTACTATAAAGAGAAAT 3′); Exon IXa R (5′ CTTTCTGTTCTGCTGTGTCTTG 3′); Exon IXb F (5′ GAGATCAAGAATGACAATGT 3′); and Exon IXb R (5′ ACAGCCAGCTATTAAGAATC 3′).
Amplimers were labeled by incorporation of a-dCTP32 (cold dNTPs-a dCTP32 ratio of 1:10) and boiled single-stranded products were separated on MDE acrylamide gels (AT biochem, Malvern, PA) and shown by autoradiography.
For cDNA analysis, reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as previously described21 using Fas-specific primers. Bidirectional sequencing was performed directly on PCR products using the Dye terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Foster City, CA) and an ABI-377 automated sequencer. Sequences were analyzed with a Sequencing analysis program (Perkin Elmer). Evidence that both alleles were examined included the determination of the complete sequence of cDNAs as well as the demonstration of previously described polymorphisms.33 For individuals with homozygous allotypes or for sequences that were unreadable due to insertions or deletions, PCR products were cloned into pGEM T easy vector (Promega, Madison, WI). Subsequently, 6 to 10 clones were sequenced.
RESULTS
Fas Mutation Identification (Table 1)
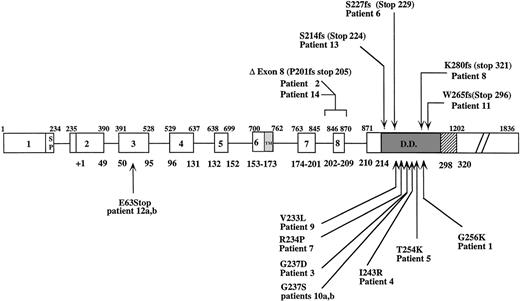
Fourteen different heterozygous Fas gene mutations were detected in this series of 14 families. Eleven mutations mapped to exon 9, 2 to intron 8 (donor splice site), and 1 to exon 3 (Fig 1). In 6 patients (no. 1, 2, 4, 5, 6, and 13) Fas gene mutations were found as de novo mutations. Patients no. 1, 4, and 5 had missense mutations in exon 9 leading to a Glu256→Lys, Ile243→Arg, and Thr254→Lys substitution, and in patient no. 6 a 4-bp duplication (at nucleotide 921 or 928) resulted in a premature stop codon at residue 229. In patient no. 13, a 2-bp insertion at nucleotide 885 led to a frame-shift and a premature stop codon at amino acid position 224. This mutation should result in a truncated Fas protein lacking the DD.
Mutations
| Patient No. . | Mutation . | Anti-CD95 MoAb- Induced Apoptosis (%) in Patient* . | Mutation-Positive Relative . | Anti-CD95 MoAb- Induced Apoptosis (%) in Relatives* . | Clinical Manifestations in Relatives . |
|---|---|---|---|---|---|
| 1 | E256K | 6 | De novo | ||
| 2 | Del Exon8(P201fs)† | 6 | De novo | ||
| 3 | G237D | 61 | Father | 30 | 1/1 |
| 4 | I243R | 4 | De novo | ||
| 5 | T254K | 40 | De novo | ||
| 6 | S227fs‡ | 13 | De novo | ||
| 7 | R234P | 12 | Mother | 8 | 1/11-153 |
| Maternal grandfather | 12 | ||||
| 8 | K280fs1-154 | 38 | Father | 49 | 0/1 |
| 9 | V233L | 4 | Mother | 12 | 2/2 |
| 10a | G237S | 23 | Father | 25 | 1/11-153 |
| Brother (10b) | 14 | ||||
| 11 | W265fs1-155 | 19 | Mother | 20 | 0/1 |
| 12a | E63Stop | 23 | Mother | 50 | 3/6 |
| Sister | 53 | ||||
| Cousin (12b) | 40 | ||||
| Cousin (12b’s brother) | 39 | ||||
| Maternal aunt | 35 | ||||
| Maternal grandfather | 45 | ||||
| 13 | S214fs# | 1 | De novo | ||
| 14 | Del Exon8(P201fs)† | 8 | Mother | 7 | 2/2 |
| Patient No. . | Mutation . | Anti-CD95 MoAb- Induced Apoptosis (%) in Patient* . | Mutation-Positive Relative . | Anti-CD95 MoAb- Induced Apoptosis (%) in Relatives* . | Clinical Manifestations in Relatives . |
|---|---|---|---|---|---|
| 1 | E256K | 6 | De novo | ||
| 2 | Del Exon8(P201fs)† | 6 | De novo | ||
| 3 | G237D | 61 | Father | 30 | 1/1 |
| 4 | I243R | 4 | De novo | ||
| 5 | T254K | 40 | De novo | ||
| 6 | S227fs‡ | 13 | De novo | ||
| 7 | R234P | 12 | Mother | 8 | 1/11-153 |
| Maternal grandfather | 12 | ||||
| 8 | K280fs1-154 | 38 | Father | 49 | 0/1 |
| 9 | V233L | 4 | Mother | 12 | 2/2 |
| 10a | G237S | 23 | Father | 25 | 1/11-153 |
| Brother (10b) | 14 | ||||
| 11 | W265fs1-155 | 19 | Mother | 20 | 0/1 |
| 12a | E63Stop | 23 | Mother | 50 | 3/6 |
| Sister | 53 | ||||
| Cousin (12b) | 40 | ||||
| Cousin (12b’s brother) | 39 | ||||
| Maternal aunt | 35 | ||||
| Maternal grandfather | 45 | ||||
| 13 | S214fs# | 1 | De novo | ||
| 14 | Del Exon8(P201fs)† | 8 | Mother | 7 | 2/2 |
The apoptosis observed in the controls are always greater than 80%.
Abbreviation: fs, frameshift.
The data are expressed as the percentage of the apoptosis observed in the control and calculated as follows: (apoptosis observed in the patient)/(apoptosis observed in the control) × 100.
Three missense amino acid codons before stop.
Two missense amino acid codons before stop.
One relative clinically not evaluable.
Forty-one missense amino acid codons before stop.
Ten missense amino acid codons before stop.
#Ten missense amino acid codons before stop.
Fas gene structure and mutations found in patients with lymphoproliferative syndrome and autoimmunity. Ins, insertion; ▵, deletion; Fs, frameshift; bp, basepair; SP, signal peptide; TM, transmembrane domain; DD, death domain. Upper and lower numbers delineate exon locations at nucleotide and amino acid residues, respectively, according to Itoh et al.44
Fas gene structure and mutations found in patients with lymphoproliferative syndrome and autoimmunity. Ins, insertion; ▵, deletion; Fs, frameshift; bp, basepair; SP, signal peptide; TM, transmembrane domain; DD, death domain. Upper and lower numbers delineate exon locations at nucleotide and amino acid residues, respectively, according to Itoh et al.44
PCR amplification of cDNA from patient no. 2 gave an abnormal 2-band profile (data not shown). Sequencing reactions using a sense primer showed a double profile at nucleotide 845 and those with a reverse primer at nucleotide 870. This suggests the presence of 2 products differing by the presence or absence of the exon 8. Sequencing of cloned RT-PCR products demonstrated that half of the clones were normal and that half lacked exon 8. To investigate this exon 8 deletion, genomic DNA from patient no. 2 and DNA from her parents and from 10 unrelated controls were checked by SSCP. An aberrant migration profile was detected in patient no. 2 only. Sequencing of this product showed a heterozygous substitution (T to C) at the second position of the 5′ splice site (splice donor) of intron 8. This mutation is probably responsible for the exon 8 skipping, because this type of splice donor mutation has been shown to affect mRNA splicing in several other human genetic diseases.34 35
In 8 cases, the mutations were inherited from 1 of the parents. In 4 cases (no. 3, 7, 9, and 10), these mutations were missense mutations (Fig 1). In 1 case (patient no. 8), a 1-bp deletion at nucleotide position 1082 resulted in a frameshift introducing an aberrant 41 amino acids and a stop codon at amino acid position 321. Sequencing RT-PCR products of exon 9 from patient no. 11 showed the presence of 2 different sequences at nucleotide 1126. Cloned RT-PCR products showed a wild-type and a mutated allele. The mutation was a 22-bp deletion (from 1136 to 1158) along with a duplication of the ctagtc hexamer at position 1149-1155. This mutation is probably the consequence of a deletion-insertion event resulting from slipped mispairing, possibly mediated by the 2 direct repeats (agaaaattc at 1117-1127 and 1157-1167) surrounding the affected region.36 Consequently, a stop codon led to a truncated protein 296 amino acids long.
Patients no. 12a and 12b carried a stop codon in exon 3 at Glu 63 encoding a normal Fas protein up to the central part of the first cysteine-rich domain (CRD1). The deduced protein has no transmembrane domain and should hence be unable to anchor onto the membrane. Additionally, this mutation could be observed on genomic DNA but was hardly detectable on cDNA (data not shown). This suggests that the corresponding RNA is probably unstable and that this mutation behaves as a null mutation. We observed reduced Fas expression and function at day 6, whereas the results of both tests were normal at day 11 (data not shown), suggesting that sufficient expression of only 1 allele would require an extended time of stimulation. In patient no. 14, a single basepair substitution in the intron 8 donor splice site was found. The invariable G of the GT dimer was replaced by an A. The consequence of this mutation was identical to that of patient no. 2 and resulted in skipping of exon 8 and a premature stop codon at position 205.
Clinical Features
Lymphoproliferative syndrome.
The patients’ clinical features are summarized in Table 2. Two relatives’ cases were also reported (patient no. 10’s brother and patient no. 12’s first cousin) as they were fully investigated. The first manifestations appeared before 2 years of age in half of the patients (range, 1 month to 7 years). A splenectomy was performed in 7 patients either because of discomfort (patients no. 8 and 9) or because of hypersplenism (patients no. 5, 7, 11, 12b, and 13). The lymphadenopathy was severe in 12 patients, consisting of multiple lymph nodes larger than 2 cm. No malignancy was observed, but this may relate to the young age and limited duration of medical follow-up of several patients.
Clinical Manifestations and Immunological Investigations
| Patient No. . | Sex/ Age (yrs) . | Age at Presentation (yrs) . | Lymph- adenopathy . | Spleno- megaly . | Splenec- tomy Age (yrs) . | Autoimmune Manifestations . | Serum Ig . | Lympho- cytes (/μL) . | TCR αβ/ CD4−CD8− (%) . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG . | IgA . | IgM . | |||||||||
| 1 | M/6 | 1.2 | +++ | +++ | − | Urticarial rash | ↗↗ | ↗↗ | N | 16,017 | 13 |
| 2 | F/15 | 1.5 | + | − | − | 0 | ↗↗ | N | ↘ | 1,800 | 6 |
| 3 | M/6 | 4 | − | +++ | − | 0 | ↗↗ | ↗ | ↗ | 1,600 | 6 |
| 4 | F/18 | 0.1 | ++ | ++ | − | Bilateral uveitis | ↗ | N | N | 1,600 | 23 |
| Iridocyclitis | |||||||||||
| Hemolytic anemia* | |||||||||||
| 5 | F/14 | 0.2 | ++ | ++† | + (2) | 0 | ↘ | ↘ | ↘ | 21,000 | 63 |
| 6 | F/15 | 4 | + | ++ | − | Anterior uveitis vasculitis | ↗↗ | ↗ | ↘ | 1,600 | 21 |
| Glomerulopathy | |||||||||||
| Thrombocytopenia* | |||||||||||
| 7 | M/12 | 3 | ++ | +++† | + (5) | Hemolytic anemia | ↗↗ | ↗↗ | N | 4,900 | 5 |
| 8 | M/24 | 6 | ++ | +++† | + (12) | Arthralgia | ↗↗ → N | ↗↗ → N | N | 2,728 | 3 |
| Urticarial rash | |||||||||||
| 9 | M/35 | 2 | ++ | +++† | + (16) | 0 | ↗↗ → N | ↗↗ | ↘ | 5,278 | 27 |
| 10a | M/11 | 3 | − | + | − | Skin vasculitis | ↗↗ | ↗↗ | ↘ | 2,420 | 10 |
| Guillain-Barre syndrome | |||||||||||
| 10b | M/12 | 4 | ++ | + | − | Skin vasculitis | ↗↗ | ↗ | ↘↘ | 1,540 | 5 |
| 11 | M/20 | 7 | +++ | +++ | + (11) | Hemolytic anemia* | ↗ | ↗ | N | 5,480 | 7 |
| Thrombocytopenia* | |||||||||||
| Neutropenia* | |||||||||||
| Arthritis | |||||||||||
| 12a | F/9 | neonatal | − | + | − | 0 | N | N | N | 1,200 | 3 |
| 12b | M/25 | 12 | + | ++† | + (14) | Thrombocytopenia* | ↗ | ↗↗ | ↘ | 5,000 | 1 |
| 13 | F/0.6 | neonatal | + | +++† | + (0.6) | 0 | ↗↗ | N | N | 19,650 | 65 |
| 14 | M/14 | neonatal | ++ | +++† | − | 0 | ↗↗ | ↗ | ↘ | 1,300 | 21 |
| Patient No. . | Sex/ Age (yrs) . | Age at Presentation (yrs) . | Lymph- adenopathy . | Spleno- megaly . | Splenec- tomy Age (yrs) . | Autoimmune Manifestations . | Serum Ig . | Lympho- cytes (/μL) . | TCR αβ/ CD4−CD8− (%) . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG . | IgA . | IgM . | |||||||||
| 1 | M/6 | 1.2 | +++ | +++ | − | Urticarial rash | ↗↗ | ↗↗ | N | 16,017 | 13 |
| 2 | F/15 | 1.5 | + | − | − | 0 | ↗↗ | N | ↘ | 1,800 | 6 |
| 3 | M/6 | 4 | − | +++ | − | 0 | ↗↗ | ↗ | ↗ | 1,600 | 6 |
| 4 | F/18 | 0.1 | ++ | ++ | − | Bilateral uveitis | ↗ | N | N | 1,600 | 23 |
| Iridocyclitis | |||||||||||
| Hemolytic anemia* | |||||||||||
| 5 | F/14 | 0.2 | ++ | ++† | + (2) | 0 | ↘ | ↘ | ↘ | 21,000 | 63 |
| 6 | F/15 | 4 | + | ++ | − | Anterior uveitis vasculitis | ↗↗ | ↗ | ↘ | 1,600 | 21 |
| Glomerulopathy | |||||||||||
| Thrombocytopenia* | |||||||||||
| 7 | M/12 | 3 | ++ | +++† | + (5) | Hemolytic anemia | ↗↗ | ↗↗ | N | 4,900 | 5 |
| 8 | M/24 | 6 | ++ | +++† | + (12) | Arthralgia | ↗↗ → N | ↗↗ → N | N | 2,728 | 3 |
| Urticarial rash | |||||||||||
| 9 | M/35 | 2 | ++ | +++† | + (16) | 0 | ↗↗ → N | ↗↗ | ↘ | 5,278 | 27 |
| 10a | M/11 | 3 | − | + | − | Skin vasculitis | ↗↗ | ↗↗ | ↘ | 2,420 | 10 |
| Guillain-Barre syndrome | |||||||||||
| 10b | M/12 | 4 | ++ | + | − | Skin vasculitis | ↗↗ | ↗ | ↘↘ | 1,540 | 5 |
| 11 | M/20 | 7 | +++ | +++ | + (11) | Hemolytic anemia* | ↗ | ↗ | N | 5,480 | 7 |
| Thrombocytopenia* | |||||||||||
| Neutropenia* | |||||||||||
| Arthritis | |||||||||||
| 12a | F/9 | neonatal | − | + | − | 0 | N | N | N | 1,200 | 3 |
| 12b | M/25 | 12 | + | ++† | + (14) | Thrombocytopenia* | ↗ | ↗↗ | ↘ | 5,000 | 1 |
| 13 | F/0.6 | neonatal | + | +++† | + (0.6) | 0 | ↗↗ | N | N | 19,650 | 65 |
| 14 | M/14 | neonatal | ++ | +++† | − | 0 | ↗↗ | ↗ | ↘ | 1,300 | 21 |
Lymphadenopathy: +, multiple small nodes <2 cm; ++, multiple nodes >2 cm <5 cm; +++, multiple and large nodes >5 cm. Splenomegaly: +, above umbilicus; ++, below umbilicus; +++, palpable in iliac fossa. Ig serum level: N, in normal range; ↗, >+2 SD <+4 SD; ↗↗, >+4 SD; ↘, <−2 SD.
All had autoantibodies.
Size before splenectomy.
Autoimmune manifestations.
Autoimmune manifestations were detected in 9 patients (Table 2). Urticarial rash (patients no. 1 and 8) and uveitis (patients no. 4 and 6) were each found in 2 patients, respectively. Glomerulonephritis, cutaneous vasculitis, Guillain Barre syndrome, and arthritis were found in 1 patient each. Three patients suffered severe autoimmune thrombopenia and 3 suffered autoimmune hemolytic anemia with the detection of corresponding autoantibodies. In patient no. 7, the hemolytic anemia was associated with a dyserythropoiesis that was sensitive to corticosteroid therapy. Seven patients presented no autoimmune manifestations.
Immunological studies.
Hypergammaglobulinemia was found in all but 1 patient. It was associated with hyper IgA in 10 cases. The serum IgM level was either normal or low in 7 patients. Panhypogammaglobulinemia was detected in 1 patient (no. 5). Blood lymphocyte counts were high in 3 cases (patients no. 1, 5, and 13). In all cases, a subset of TcR α/β CD4(−) CD8(−) T lymphocytes was detected in peripheral blood. The size of this population as a proportion of all TcR α/β T lymphocytes varied from one patient to another (between 1% and 63% of T cells).
Evolution of clinical manifestations with age (Table3).
Seven patients were observed for at least 10 years. For 3 of them (no. 2, 6, and 9), the medical follow-up was performed by the same physician. In 4 patients (no. 2, 6, 9, and 14), splenomegaly and/or lymphadenopathy were detected within the first 5 years of life. For the other 3, the lymphoproliferative syndrome was detected at 6 (patient no. 8), 8 (patient no. 11), and 12 years of age (patient no. 12b), respectively. In the 3 patients, who are now more than 20 years of age (patients no. 8, 9, and 12b), the lymphoproliferative syndrome has progressively regressed. Nevertheless, a single lymph node more than 5 cm in diameter was detected in patient no. 8 (when 24 years old), and in patient no. 9 (when 35 years old) an enlarged accessory spleen, detected 2 years ago, was surgically removed. In patient no. 12b, a splenomegaly was first noted at 12 years of age; lymphadenopathy appeared after splenectomy was performed, but was no longer detectable at 25 years of age. In addition, patient no. 14’s mother presented in childhood with splenomegaly and lymphadenopathy that had resolved by 26 years of age. In patient no. 2, although massive lymphadenopathy was observed before 5 years of age, this was no longer detectable by 15 years of age. In patient no. 6, no major change was observed during the follow-up. In patient no. 11, lymphadenopathy decreased with time and by 19 years only a few cervical nodes were detected. These observations suggest that the lymphoproliferative syndrome is more pronounced during childhood and tends to regress in adulthood. In contrast, the immunological features of the syndrome, ie, hypergammaglobulinemia and the presence of circulating CD4(−) CD8(−) T cells in the blood did not change with time (data not shown). Longitudinal study of autoimmune manifestations was more difficult, because only 9 patients developed such manifestations. Autoimmune phenomena in patient no. 6 were no longer detectable by 10 years of age, and in patient no. 11, who is now 20 years old, they disappeared by 16 years of age. Similarly, the last autoimmune manifestation occurred at 18 years of age in patient no. 8, who is now 24 years old.
Evolution of the Lymphoproliferative Syndrome
| Patient No. . | . | Age Range (yrs) . | Age at Last Follow-Up (yrs) . | ||||
|---|---|---|---|---|---|---|---|
| 0-5 . | 5-10 . | 10-15 . | 15-20 . | >20 . | |||
| 2 | Lymphadenopathy | +++ | ++ | + | 0 | 15 | |
| Splenomegaly | + | 0 | 0 | 0 | |||
| 6 | Lymphadenopathy | 0 | + | + | 13 | ||
| Splenomegaly | ++ | ++ | ++ | ||||
| 8 | Lymphadenopathy | NE | ++ | + | 0 | 1 (5 cm × 5 cm) | 24 |
| Splenomegaly | NE | +++ | 12 yrs3-150 | (axillar) | |||
| 9 | Lymphadenopathy | +++ | +++ | +++ | ++/+ | Accessory | 35 |
| Splenomegaly | +++ | +++ | +++ | 16 yrs3-150 | Spleen | ||
| 11 | Lymphadenopathy | NE | 0 | +++ | ++/+ | 19 | |
| Splenomegaly | NE | ++ | 10 yrs3-150 | ||||
| 12b | Lymphadenopathy | NE | NE | +++ | + | 0 | 25 |
| Splenomegaly | NE | NE | +14 yrs3-150 | ||||
| 14 | Lymphadenopathy | ++ | + | 0 | 15 | ||
| Splenomegaly | +++ | +++ | +++ | ||||
| Patient No. . | . | Age Range (yrs) . | Age at Last Follow-Up (yrs) . | ||||
|---|---|---|---|---|---|---|---|
| 0-5 . | 5-10 . | 10-15 . | 15-20 . | >20 . | |||
| 2 | Lymphadenopathy | +++ | ++ | + | 0 | 15 | |
| Splenomegaly | + | 0 | 0 | 0 | |||
| 6 | Lymphadenopathy | 0 | + | + | 13 | ||
| Splenomegaly | ++ | ++ | ++ | ||||
| 8 | Lymphadenopathy | NE | ++ | + | 0 | 1 (5 cm × 5 cm) | 24 |
| Splenomegaly | NE | +++ | 12 yrs3-150 | (axillar) | |||
| 9 | Lymphadenopathy | +++ | +++ | +++ | ++/+ | Accessory | 35 |
| Splenomegaly | +++ | +++ | +++ | 16 yrs3-150 | Spleen | ||
| 11 | Lymphadenopathy | NE | 0 | +++ | ++/+ | 19 | |
| Splenomegaly | NE | ++ | 10 yrs3-150 | ||||
| 12b | Lymphadenopathy | NE | NE | +++ | + | 0 | 25 |
| Splenomegaly | NE | NE | +14 yrs3-150 | ||||
| 14 | Lymphadenopathy | ++ | + | 0 | 15 | ||
| Splenomegaly | +++ | +++ | +++ | ||||
Lymphadenopathy: 0, absence; +, multiple nodes <2 cm; ++, multiple nodes >2 cm and <5 cm; +++, multiple nodes >5 cm. Splenomegaly: +, above the umbilicus; ++, below the umbilicus; +++, palpable in the iliac fossa.
Abbreviation: NE, not evaluated.
Age at splenectomy.
Family analysis.
Genetic analysis was performed in 14 families. De novo mutations were detected in 6 patients (Table 1). In these families, Fas-induced apoptosis was normal in both parents, consistent with the absence of Fas gene mutations. Neither clinical nor immunological features were detectable in the parents. Inherited mutations were found in 8 families (Table 1). Mutations were inherited from the mother in 5 cases and from the father in 3 cases. In 1 case (patient no. 7), the mutation was also detected in the maternal grandfather. In 5 families (patients no. 3, 7, 9, 10, and 14), all relatives who carried the mutated Fas gene presented with abnormal anti-Fas MoAb-mediated apoptosis (7/7) and clinical manifestations (7/7), including splenomegaly associated in some cases with lymphadenopathy. The severity of lymphoproliferative syndrome did not correlate with the number of TcR α/β CD4(−)CD8(−) T cells in blood (data not shown). In the 3 other families (families of patients no. 8, 11, and 12), relatives with Fas mutations had in vitro anti-Fas MoAb-induced apoptosis defects, but they did not develop clinical manifestations. The family of patient no. 12 is particularly intriguing, because both carriers, mothers of patients no. 12a and 12b (who are sisters), were healthy, whereas patient no. 12a’s brother died from aplastic anemia at the age of 2 years, and patient no. 12b’s brother presented with a mild lymphoproliferative syndrome (Table 1). In the 5 families (no. 3, 7, 9, 10, and 14) with full clinical penetrance, 4 mutations consisted of missense mutations (no. 3, 7, 9, and 10), whereas the 2 mutations detected in the 2 families (no. 8 and 11) with no clinical penetrance led to an altered product in the intracytoplasmic tail of the Fas molecule.
DISCUSSION
We have studied 16 patients (in 14 families) with lymphoproliferative syndrome and autoimmunity associated with defect in Fas-induced apoptosis. All of the 14 new Fas mutations are heterozygous. This finding is consistent with previous reports in which patients with lymphoproliferative syndrome and autoimmunity (also referred to as autoimmune lymphoproliferative syndrome [ALPS] and Canale-Smith syndrome) were shown to carry such mutations.21-23,25,28The dominance of this genetic defect is likely to be due to the fact that trimerization of the Fas molecule is necessary to form a DISC and to trigger the death signal.9 37 Therefore, a mutated Fas protein would have to be produced to exert a dominant negative effect and partially (or totally) inhibit the apoptotic signal.
Eleven mutations were found in exon 9 and should therefore affect the death domain structure without affecting membrane expression. The intron 8 donor splice site mutations lead to abnormal splicing (Exon 7-Exon 9 junction) and a premature stop codon at resulting amino acid codon 205. Although this mutant protein is most likely devoid of a death domain, it is presumably produced as observed in mutant cell lines carrying exon 8 deletions. Moreover, this mutant protein was shown to exert a full transdominant effect on Fas-mediated apoptosis of cell lines.38 In vitro Fas-induced apoptosis seems to correlate with the size of truncation in the DD. For instance, a stop codon at residue 205 (patients no. 2 and 14), 224 (patient no. 13), and 229 (patient no. 6) was associated with Fas-induced apoptosis of 6%, 8%, 1%, and 13% of control values, respectively, whereas Fas-mediated apoptosis from patient no. 8, in whom the Fas molecule is intact up to residue 280, is 38% of control. This is in keeping with previous studies in which a stop codon at residue 253 was associated with Fas-induced apoptosis of 25% of control.21,26 Results from patient no. 11 cannot be included, because the apoptosis test was not performed under the same conditions. All carriers (29/29) of these Fas mutations had defective in vitro apoptosis, showing a full penetrance as far as anti-Fas antibody-mediated apoptosis is concerned. In contrast, although 100% (9/9) of evaluated individuals with a missense mutation in the DD had clinical manifestations, 66% (4/6) of individuals carrying a premature stop (families no. 11 and 14) or an altered DD (family no. 8) had clinical manifestations. Two recently published Fas-mutation reports reinforce this observation.39,40 They show that 90% (40/44) individuals with missense mutation and 75% (21/28) individuals with a truncated intracytoplasmic domain had symptoms of lymphoproliferative syndrome. Two older reports in which 66% (2 of 3 in both) of individuals with truncated Fas DD had clinical symptoms also lead to the same conclusion.21 23
These findings paradoxically suggest that missense mutations in the Fas DD are more likely to result in a high clinical penetrance of the syndrome, whereas major mutations associated with the deletion or truncation of the DD may result in a lower clinical penetrance. This may indicate that the in vitro anti-Fas antibody-triggered T-lymphocyte apoptosis assay does not entirely reflect the possible in vivo consequences of Fas mutations. Indeed, Fas mutations with high and low clinical penetrance both lead to reduced MoAb-mediated apoptosis in vitro, the magnitude of which is not discriminative. One possible explanation for this may be that Fas molecules with a single amino acid substitution are more likely to be incorporated into Fas trimers and disrupt apoptosis signaling by failing to interact appropriately with FADD (both upon antibody cross-linking and upon binding of FasL trimers in vivo). Fas products with more important alterations in their structure (deletion or long stretch of missense amino acid codon) may complex less efficiently (as a consequence of a diminished stability), allowing trimers to be formed mainly by nonmutated Fas molecules and resulting in less consequences in vivo (the overall number of normal Fas trimers being sufficient to transmit death signals when binding FasL trimers in vivo). Indeed, whereas penetrance was 16 of 25 (2 of 4 in this study and 14 of 21 in the reports by Jackson et al40 and Vaishnaw et al39) for such mutations in nonindex cases, it was 36 of 40 (5 of 5 in this report and 31 of 35 elsewhere) for relatives carrying missense mutations. In contrast, oligomerization by anti-Fas antibodies might exacerbate the defect in part by clustering normal and abnormal Fas molecules together. This model predicts that Fas-mediated apoptosis will be less profoundly affected in vivo when Fas gene mutations lead to a truncated form of the molecule. Adapted apoptotic assays may make it possible to test this hypothesis. Clinical penetrance was found to be strikingly reduced for extracytoplasmic mutation, as exemplified here in family no. 12. This mutation is most likely a null mutation and therefore cannot act as a dominant negative. It is likely that the observed in vitro apoptosis defect was caused by a reduced Fas expression in a given time window of in vitro cell activation. Penetrance was 3 of 6 and is slightly higher than penetrance observed for similar mutations in other reports (0 of 539 and 2 of 1440).
Another, as yet undefined, function would have to be defective to account for the disease onset in families with lower clinical penetrance, as previously discussed.21,22 26 Fas ligand, proteins of the DISC (FADD, Caspase 8), or other factors involved in controlling cell death and/or proliferation could be involved and are currently under investigation.
Alternatively, the lymphoproliferative syndrome could be induced by the combination of a Fas mutation and other genes that predispose the individual to autoimmune disease. In patient no. 14’s family, the maternal grandmother and aunt, who do not carry the Fas mutation, were diagnosed with autoimmune thyroiditis at 35 and 47 years of age, respectively. This observation suggests that the combination of heterozygous Fas mutation and autoimmune-prone genes may have contributed to the onset of the syndrome in the proband and his mother. On the other hand, the early onset of disease in the proband correlated with cytomegalovirus (CMV) infection, raising the hypothesis that an acquired second factor could also precipitate the clinical manifestations (P.A., personal communication, October 1999).
Seven patients (no. 2, 3, 5, 9, 12a, 13, and 14) did not exhibit any autoimmune manifestations. Nine patients suffered autoimmune disorders. There was no correlation between the Fas-induced apoptosis rate (from 4% in patient no. 4 up to 38% in patient no. 8), the double-negative T-cell counts, the hypergammaglobulinemia, and the type of mutations. Autoimmune manifestations are thus inconstant and could not be related to any other features of the disease. This is reminiscent of the observations in mice in which severity of autoimmunity appears to correlate closely with the genetic background.1 41 Thus, autoimmunity appears to be intrinsic to each patient exhibiting such manifestations. The Fas mutation seems to be a susceptibility factor for autoimmune disease, as could be deficiencies of other molecules involved in lymphocyte homeostasis.
Among 7 patients observed over a period of 10 years, 3 patients (no. 8, 9, and 12b) are more than 20 years of age. One patient (no. 8) exhibited a single persisting large lymph node, whereas patient no. 9 developed enlargement of an accessory spleen 9 years after a first splenectomy. In patient no. 12b, all clinical manifestations disappeared. Strikingly, the immunological features persisted over time. Both double-negative T-cell count and Ig serum levels were unchanged. Although this finding is based on a small number of patients, it is consistent and has been recently reported in a large pedigree of Fas-deficient patients.28 This contrasts with what is observed in lpr mice, in which the double-negative T-cell count increases with age and life-span is shortened.42 However, these mice have a more severe phenotype, because homozygous Fas mutation is a condition associated with a complete functional defect. It is possible that, in patients with partial Fas-mediated apoptotic defects, alternative Fas-independent apoptotic pathways compensate for the deficiency. Also, secretion of immunosuppressive cytokines such as IL-1043 may control lymphoproliferation and autoimmunity. In keeping with this hypothesis is the observation of increased in vitro apoptosis of double-negative T cells (data not shown) and in vivo spontaneous apoptosis in blood and spleen from Fas-deficient patients.26 Alternatively, control of autoimmune clones in the periphery may be a more stringent phenomenon in early childhood, because the rate of T-cell production is higher than in late childhood or adulthood. In any case, the longitudinal study of a larger cohort of patients is required to further assess this intriguing observation.
Supported by grants from Institut National de la Santé et de la Recherche Médicale, Assistance Public-Hôpitaux de Paris (PHRC AOM 96127), Association Française contre les Myopathies, Ligue Nationale contre la Cancer, and PHRC European concerted action Biomed-2 983007.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Frédéric Rieux-Laucat, PhD, INSERM U429, Hôpital Necker-Enfants Malades, 149, rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: rieux@necker.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal