We have characterized dendritic cell precursors (pre-DC) in the human thymus. These CD1a−CD3−CD4+CD8−cells express high levels of interleukin-3R (IL-3R) on the membrane and are able to develop into mature DC upon culture with IL-3 and CD40 ligation. The DC precursors are predominantly located in the thymic medulla. Interestingly, the pre-DC express pT mRNA, which is also present in CD1a+CD3−CD4+ CD8−pre-T cells. Yet, the pre-DC lack expression of recombination activating gene-1 mRNA and fail to develop into T cells in appropriate assays. The thymic pre-DC are very similar to the recently characterized pre-DC found in the T cell areas of the tonsil, and it is suggested that these pre-DC populations are of lymphoid origin.
DENDRITIC CELLS (DC) ARE highly specialized antigen-presenting cells present in peripheral lymphoid and nonlymphoid organs. These cells play an essential role in the initiation of immune responses.1,2 DC are also present in the thymus, where they serve to eliminate potentially autoreactive cells from the T-cell repertoire.3 It has become clear that there are distinct subsets of DC in the mouse with different ontogenetic origins. While many DC in the periphery are of myeloid origin, a second subset, which includes thymic DC, appears to be related to lymphocytes.4-6 The notion that thymic DC are related to lymphocytes is based on studies of Ardavin et al,4 which demonstrated that DC in the mouse thymus are derived from an intrathymic precursor with the capacity to develop into natural killer (NK) and T cells, but not into myeloid cells. Recently, a more mature precursor population in the mouse thymus was shown to have T and DC differentiation capacity.7 Others and we have shown that the human thymus contains precursors able to develop into T, NK, and DC,8,9 but not into monocytes,10 suggesting that also in man thymic DC may be of lymphoid origin. Therefore, analysis of thymic DC may provide insight into the characteristics of human DC.
So far our knowledge about mature thymic DC and their immediate precursors in man is limited. Sotzic et al11 have described a CD4+ population in the human thymus, which is able to differentiate into DC after overnight culture. These cells expressed relatively high levels of major histocompatability complex (MHC) class II antigens, but lacked CD1a, which is expressed on Langerhans cells in the skin. It is unclear whether this population are committed DC precursors, represent already matured DC, or is a mixture of mature DC and their immediate precursors. Here we have analyzed the CD1a−CD3−CD4+CD8−population in the human thymus in more detail with emphasis on features that these cells might share with T-cell precursors. We found that these cells mature to DC upon coculture with interleukin-3 (IL-3) and CD40 ligation. The thymic DC precursors share characteristics with pre-T cells, including expression of pre-T cell receptor alpha chain (pTα) mRNA, strongly suggesting their relationship with T cells. However, these DC precursors are committed to the DC lineage, as they are unable to develop into T and NK cells in appropriate assays. Interestingly, the phenotype and precursor activities of these cells are very similar to that of a committed DC precursor in the tonsil, which was previously referred to as plasmacytoid T cell.12
MATERIALS AND METHODS
Isolation of DC precursors from tonsil and thymus.
Thymocyte tissues were obtained from children ranging from 3 weeks to 3 years of age undergoing median sternotomy and corrective cardiovascular surgery. Tonsils had been removed from children for therapeutic purpose. Suspensions were made by mincing tissues and pressing them through a stainless steel mesh. Large aggregates were removed and the cells were washed once before separating subpopulations. Thymocytes were kept at 4°C overnight followed by centrifugation over a ficoll gradient. The ficoll interphase cells were collected and depleted for CD8+ cells by staining with RPA-T8 (kindly provided by G. Aversa, DNAX, Palo Alto, CA) and subsequent negative depletion using Dynal magnetic beads (Dynal, Oslo, Norway). Remaining thymocytes were stained with anti-CD3 fluorescein isothiocyanate (FITC), anti-CD8 FITC (which recognizes a different epitope on CD8 than RPA-T8 used for depletion), anti-CD1a phycoerythrin (PE) and anti-CD4 Tricolor monoclonal antibodies (MoAbs) and the CD1a−CD3−CD4+CD8−DC precursors and the CD1a+CD3−CD4+CD8−immature single positive (CD4 ISP) thymocytes were isolated by sorting on a FACStar plus (Becton and Dickinson, San Jose, CA). In some experiments CD1a−CD4+CD3+mature thymocytes were sorted after staining of total thymocytes with CD3 FITC, CD1a PE and CD4 Tricolor.
Single cell suspensions of tonsillar cells were labeled with anti-CD3, anti-CD8 (RPA-T8), anti-CD14, anti-CD19 (CLB, CD14 and CD19, respectively, gifts from Dr R. van Lier, CLB, Amsterdam, The Netherlands) and anti-CD56 (L185, from J.H. Philips, DNAX, Palo Alto, CA) and labeled cells were subsequently removed by depletion with magnetic beads. Remaining cells were stained with goat antimouse F(ab)2-FITC (Zymed, San Francisco, CA), CD11c PE and CD4 Tricolor and the CD11c−CD4+CD3−CD14−CD19−CD56−cells were sorted using a FACStar plus.
Antibodies.
FITC-labeled antibodies specific for CD3, CD5, CD7, CD8, CD10, CD14, CD19, CD33, CD40, CD45RA, CD56, and CD69 were purchased from Becton Dickinson. Anti-CD1a FITC was obtained from Serotec (Kidlington, Oxford, UK), anti-CD33 FITC from Immunotech (Marseille, France), anti-CD86 FITC from Instruchemie (Hilversum, The Netherlands), and anti-CD40 FITC from Pharmingen (San Diego, CA). PE-labeled antibodies against CD2, CD4, CD13, CD20, CD80, and HLA-DR were purchased from Becton and Dickinson. Anti-CD1a PE, anti-CD54 PE, anti-CD83 PE, and anti-T-cell receptor (TCR)γδ PE were purchased from Coulter/Immunotech (Luminy, France), anti-CD11c PE from Biosource International (Nivelle, Belgium), and anti-IL-3Rα PE from Pharmingen. Anti-CD4tricolor was obtained from DAKO A/S (Glostrup, Denmark) and anti-TCRαβ Tricolor from Immunotech. The nοnconjugated antibody against CD1a was purchased from Becton and Dickinson. Anti-CD45RA (GI-15) was a gift of Bristol-Meyers Squibb (Seattle, WA) and anti-CD86 (IT2.2) was kindly provided by M. Azuma (Juntendo University, Tokyo, Japan).
Immunohistochemical analysis.
Single staining was performed as described previously using a standard immunoperoxidase method.13 Briefly, cryostat fragments of thymic tissue were cut in 4-μm sections, air-dried overnight, and fixed in acetone for 10 minutes at room temperature. The slides were first incubated with 10% (vol/vol) normal rabbit serum (CLB), then with optimal dilutions of primary MoAb (in phosphate-buffered saline [PBS] containing 1% (wt/vol) bovine serum albumin [BSA] [PBS/BSA]) for 30 minutes at room temperature, followed by incubation with biotinylated goat antimouse IgG (DAKO A/S). After incubation with streptavidin/biotin-conjugated peroxidase complex (ABC-protocol, DAKO), the bound peroxidase was developed with 3-amino-9-ethylcarbazole (Sigma Chemical Co, St Louis, MO), 0.4 mg/mL in 0.1 mol/L sodium acetate buffer, pH 5.0. Between incubation steps, the sections were extensively rinsed in PBS/BSA. The sections were counterstained with hematoxylin and mounted. Within each staining procedure, negative control antibodies were included.
For double-staining, acetone-fixed cryostat sections were incubated subsequently with primary MoAb, biotinylated rabbit antimouse IgG and streptavidin/biotin-conjugated peroxidase complex. After incubation with normal mouse serum (CLB), sections were stained with either PE-labeled or FITC-labeled secondary mouse MoAb. PE staining was visualized by incubation with rabbit anti-PE (Biogenesis, Poole, England, UK) followed by goat antirabbit-complexed to alkaline phosphatase (Immunotech) and FITC staining with sheep anti-FITC–complexed to alkaline phosphatase (Boehringer Mannheim, Almere, The Netherlands). Color development for alkaline phosphatase and peroxidase was performed by incubations with naphtol AS-MX phosphate (0.4 mg/mL) plus Fast blue BB base (0.6 mg/mL, Sigma, in 0.1 mol/L Tris-HCl, pH 8.5) and for peroxidase by incubation with and 3-amino-9-ethylcarbazole (0.4 mg/mL in 0.1 mol/L sodium acetate buffer, pH 5.0).
For triple-staining, acetone fixed cryostat sections were incubated subsequently with primary mouse antihuman MoAb, biotinylated rabbit antimouse IgG, Cy5-conjugated streptavidin (Jackson Immunoresearch Laboratories, Inc, Palo Alto, CA), normal mouse serum, secondary PE-labeled MoAb, rabbit anti-PE (Biogenesis), Cy3-conjugated goat antirabbit (Jackson Immunoresearch Laboratories), normal mouse serum and FITC-labeled tertiary MoAb. For each fluorochrome label, negative control antibodies were included.
Confocal laser scanning microscope analysis.
Confocal fluorescence images were obtained on a Leica TCS NT (Leica Microsystems, Heidelberg, Germany) confocal system, equipped with an Ar/Kr laser. Images were taken using a 40x 1.25 NA objective. Possible cross-talk between FITC, Cy3, and Cy5, which could give rise to false-positive colocalization of different signals, was avoided by careful selection of the imaging conditions. The standard FITC/Cy3/Cy5 filter combination and Kalman averaging was used. Color photomicrographs were taken from electronic overlays. Processing of images for presentation was performed on a PC using the software packages Photoshop (Adobe Systems Inc, Mountain View, CA) and Freelance Graphics (Lotus Development Corp, Cambridge, MA).
Generation of DC in IL-3– and CD40L-supported cultures of thymic DC precursors.
CD1a−CD3−CD4+CD8−thymocytes were cultured in a 96-well flat bottomed plate in the presence of 10 ng/mL IL-3 with or without 104 CD40L transfected mouse fibroblasts (kindly provided by J. Banchereau, Schering Plough, Dardilly, France) preirradiated with 104rad. Culture medium consisted of Yssel’s medium14 with 5% normal human serum.
Staining of freshly isolated and IL-3–cultured thymic DC precursors on slides.
Cytocentrifuge preparations of freshly isolated CD1a−CD3−CD4+CD8−thymocytes were fixed with acetone, incubated in rabbit antihuman HLA-DR antibody (1/500 serum dilution, obtained from J. Neefjes, Netherlands Cancer Institute, Amsterdam, The Netherlands) for 30 minutes at room temperature, washed in PBS/BSA, followed by incubation in Cy3-conjugated goat antirabbit antibody for 30 minutes at room temperature. Clusters of cells that were cultured in the presence of IL-3 for 5 days were resuspended in medium and layered on 3-amino-propyltriethoxysilane–coated glass slides. After 3 minutes at 37°C, adhering cells were fixed in Cellfix solution (1/10 diluted solution of buffered 10% formalin/1% sodium azide) (Becton Dickinson) for 30 minutes at room temperature. Upon subsequent incubations in PBS/BSA for 5 minutes and in PBS/0.25% saponin for 30 minutes, cells were stained for HLA-DR and confocal fluorescence images were obtained as described above.
Hybrid human/mouse fetal thymic organ cultures.
The in vitro development of human T cells was studied using the hybrid human/mouse fetal thymic organ culture (FTOC).9 Fetal thymuses were dissected from embryos of recombination activating gene-1 (RAG-1)–deficient mice on day 15 to 16 of gestation and precultured for 5 days in the presence of 1.35 mmol/L 2-deoxyguanosine (Sigma) to remove endogenous thymocytes. Next, the thymic lobes were cocultured for 2 days in hanging drops in wells of a Terasaki plate with human progenitor cells and transferred to nucleopore filters, which were layered over gelfoam rafts in 6-well plates (Costar, Badhoevedorp, Netherlands). The lobes populated with human cells were cultured for the indicated number of days in Yssel’s medium supplemented with 2% normal human serum and 5% fetal calf serum. To analyze differentiation of human cells, the mouse thymuses were dispersed into single cell suspensions and stained with MoAbs specific for human cell surface antigens.
Reverse transcriptase-polymerase chain reaction (RT-PCR) assays.
RNA was isolated from fluorescence-activated cell sorting (FACS)-sorted or cultured postnatal thymocytes and tonsil cells using TRIzol reagent (GIBCO, Paisley, Scotland) according to the manufacturer’s instructions and reverse transcribed using a poly-dT15oligonucleotide (Promega, Madison, WI) and 400 U of Moloney murine leukemia virus (M-MuLV) reverse transcriptase (GIBCO) at 42°C for 1 hour. PCR assays were performed in 50 μL reaction volumes using 1 μL of cDNA template, 2 mmol/L MgCl2, 0.25 mmol/L of each dNTP, 1 μmol/L of each primer and 3 U Taq-polymerase (GIBCO) in 1× buffer (10 mmol/L TRIS-HCl, pH 8.5, 50 mmol/L KCl). Reaction conditions were a 5-minute denaturation step at 94°C followed by 30 cycles of 1 minute at 94°C, 1 minute at 65°C, and 2 minutes at 72°C. PCR products were separated on 1.2% agarose gels, stained with ethydium bromide, and analyzed by videodensitrometry using the Eagle Eye still video system and Eagle Sight Software (Stratagene, La Jolla, CA).
The hydroxyphosphoribosyl transferase (HRPT), RAG-1, and pTα primers that were used are:
HPRT sense: 5′-TATGGACAGGACTGAACGTCTTGC-3′, HPRT antisense: 5′-GACACAAACATGATTCAAATCCCTGA-3′, RAG-1 sense: 5′-GAACACACTTTGCCTTCTCTTTGG-3′, RAG-1 antisense: 5′-CGCTTTGCCTCTTGCTTTCTCGTT-3′, pTα sense: 5′-GTCCAGCCCTACCCACAGGTGT-3′, pTα antisense: 5′-CGGGAATTCGACGTCCCTGGCTGTAGAAGCCTCTC-3′.
Semiquantitative RT-PCR (semi-Q RT-PCR).
To compare the amount of pTα mRNAs expressed in different samples, expression was related to the level of expression of the housekeeping enzyme HPRT. Standard curves for HPRT and pTα were established by simultaneous amplification of serial dilutions of cDNA prepared from RNA isolated from total thymus. Total thymocytes contain a high concentration of the target sequence being amplified to ensure that quantitative values fell within the linear range of the standard curve. PCR was performed in a total volume of 50 μL consisting of 1 μmol/L of each primer set, 200 μmol/L each dNTP (Pharmacia Biotech, Uppsala, Sweden), 2.5 mmol/L MgCl2, 1x PCR buffer, 1 U Taq DNA polymerase (GIBCO-BRL, Gaithersburg, MD), and 10 μL of the sample cDNA. Samples were covered with 50 μL paraffin oil and heated to 94°C for 5 minutes followed by amplification for 30 cycles of 1 minute 94°C, 1 minute 65°C (HPRT, RAG-1), or 1 minute 59°C (pTα), and 2 minutes 72°C. After the last cycle, a final extension step at 72°C for 10 minutes was performed. A total of 10 μL of each PCR reaction was dotblotted in duplicate onto a nylon filter (Hybond N+, Amersham International plc, Slough, UK). Filters were prehybridized at 60°C for at least 1 hour (6x SSC, 0.5% sodium dodecyl sulfate [SDS], 5x Denhardt’s, 100 mg herring sperm DNA/L) and hybridized overnight with an oligoprobe specifically recognizing the HPRT or pTα PCR product internal to the PCR primers. The sequences of the probes were:
pTα probe: 5′-CTGCCTTCTGAGGAGCTGGCAT-3′. HPRT probe: 5′-GTCCCCTGTTGACTGGTCATTACAAT-3′.
Oligoprobes were 32-P endlabeled according to the manufacturer’s recommendations (Boehringer Mannheim). To remove any nonspecifically bound probe, the filters were washed with excess amount of 2x SSC; 0.1% SDS at 55°C for 30 minutes. Measurement of the cpm/dot values was performed with a phosphoimager (Fujix Bas 2000, Fuji). Standard curves were calculated using the Softmax program (Molecular Dynamics, Sunnyvale, CA), in which the first point of the curve, containing the highest amount of cDNA, was set arbitrarily to 1,000 U. Using the same Softmax program, also the values of the samples were calculated in units. Finally the ratios of (pTα)/(HPRT) was calculated to compare the expression of the above mRNA in the different samples.
NK cell assays.
Thymocytes were cultured in 96-well U bottom plates in Yssel’s medium supplemented with 5% normal human serum in the presence of 25 U/mL Flt-3 ligand (Flt-3L from Peprotech, Rocky Hill, NJ), 10 ng/mL IL-7 (Peprotech,) and 10 ng/mL IL-15 (Peprotech) or in the presence of 10 ng/mL IL-7, 20 ng/mL stem cell factor (SCF, Amgen, Thousand Oaks, CA) and 50 U/mL IL-2 (EuroCetus, Amsterdam, The Netherlands).
RESULTS
Identification of a thymic CD3−CD4+CD8−population, which is similar to tonsillar plasmacytoid T cells.
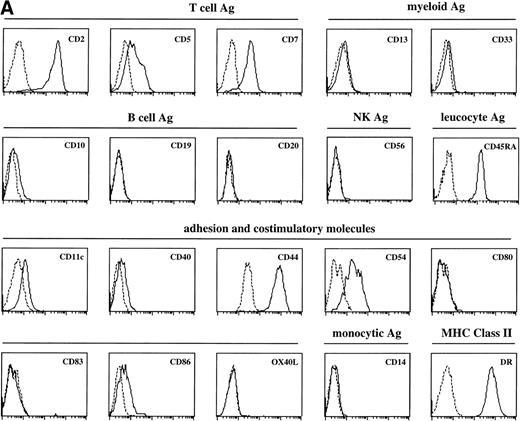
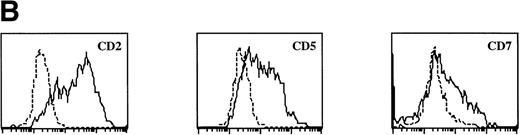
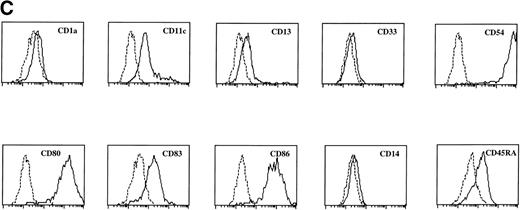
Sotzic et al11 have described a CD4+ thymic population that lacks CD1a. Upon overnight culture, these cells acquired DC characteristics. It was unclear whether these cells represent mature DC or DC precursors. In this study, we isolated CD1a−CD3−CD4+CD8−cells from the thymus and examined their cell surface phenotype extensively by flow cytometry. As illustrated in Fig 1A, these cells lack expression of markers for NK (CD56), B cells (CD10, CD19, and CD20), and monocytes (CD14), which is consistent with previous observations.11Further analysis showed that CD1a−CD3−CD4+CD8−thymocytes express CD44, CD45RA, HLA-DR, and CD54, but not CD13, CD33, CD80, and CD86 (Fig 1A). This phenotype is similar to that of a committed DC precursor in the tonsil, previously referred to as plasmacytoid T cells.12 However, differences were observed, as in contrast to plasmacytoid T cell, all thymic CD1a−CD3− CD4+CD8−cells expressed the T/NK cell markers CD2 and CD7 and most of these cells expressed CD5 (Fig 1A). These markers are also expressed at specific stages of NK development. To exclude that expression of these markers was due to passive absorption of CD2, CD5, and CD7 shed from thymocytes, we reanalyzed expression of CD2, CD5, and CD7 after a 5-day culture in IL-3. Under these conditions, no contaminating thymocytes survive. Figure 1B shows that cultured cells still express significant levels of CD2, CD5, and CD7, although the level of expression of CD7 is much lower than on freshly isolated cells.
FACS analysis of thymic DC precursors. (A) Thymocytes sorted on basis of a CD1a−CD3−CD4+CD8−phenotype were analyzed for the expression of a number of lymphoid, myeloid, costimulatory, and adhesion markers. (B) Expression of CD2, CD5, and CD7 on CD1a−CD3−CD4+CD8−cells cultured for 5 days in IL-3.
FACS analysis of thymic DC precursors. (A) Thymocytes sorted on basis of a CD1a−CD3−CD4+CD8−phenotype were analyzed for the expression of a number of lymphoid, myeloid, costimulatory, and adhesion markers. (B) Expression of CD2, CD5, and CD7 on CD1a−CD3−CD4+CD8−cells cultured for 5 days in IL-3.
CD1a−CD3−CD4+CD8−thymocytes develop to mature dendritic cells upon stimulation with IL-3 together with CD40L.
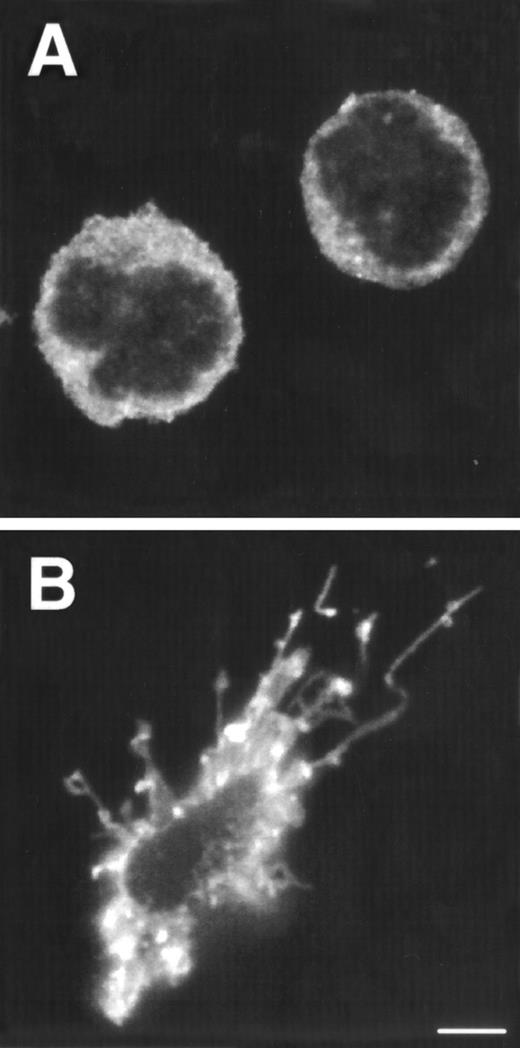
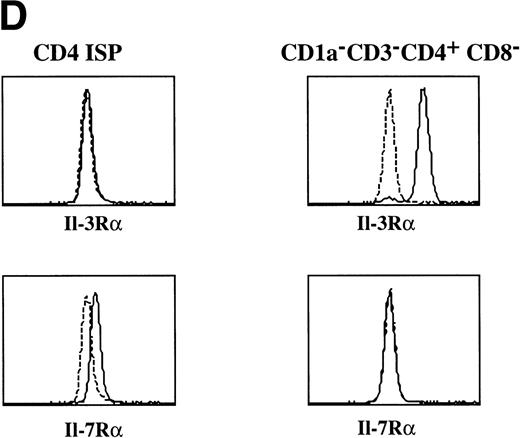
Tonsillar DC precursors can differentiate into DC when cultured in the presence of IL-3 and CD40L expressing fibroblasts.12 Given the similarity of the CD1a−CD3−CD4+CD8−thymocytes with the DC precursors found in the tonsil, we examined the response of the thymic CD1a−CD3−CD4+CD8−thymocytes to the same culture conditions. After a few hours, these cultures contained large cell clusters. IL-3 together with CD40L induced the strongest activation of DC cells. In the first 5 days, the cells activated with IL-3 and CD40L did not expand significantly, but in the period of 5 to 10 days, the cells expanded 2-fold to 3-fold. IL-3 alone was sufficient for generation of DC, but was less efficient than in combination with CD40L. After stimulation with IL-3, the DC precursor cells with rounded cell morphology (Fig 2A) developed into a mature DC with a typical veiled dendritic like morphology, exhibiting enhanced expression of HLA-DR (Fig 2B). Furthermore, stimulation of CD1a−CD3−CD4+CD8−thymocytes with IL-3 and CD40L resulted in the upregulation of expression of the costimulatory molecules CD80 and CD86 and the DC-specific marker CD83 (Fig 2C). The DC obtained after culture with IL-3 and CD40L were potent activators of proliferation of allogeneic T cells in mixed cell cultures (data not shown). These data demonstrate that the thymic CD1a−CD3−CD4+CD8−cells have DC precursor activities similar to those of the previously described committed DC precursors in the tonsil. Consistent with the responsiveness of the thymic DC precursors to IL-3, these cells express IL-3Rα (Fig 2D). In contrast, these cells fail to express the IL-7Rα chain and were unable to respond to IL-7. As a comparison, we studied the expression of both molecules on CD1a+CD3−CD4+CD8−immature single positive (CD4 ISP) thymocytes. As indicated, this latter population did not express IL-3Rα, but displayed low levels of IL-7Rα (Fig 2D). CD4 ISP cells fail to respond to IL-3 and died within 2 days of culture, consistent with the lack of IL-3Rα on these cells.
Morphology and phenotype of thymic DC precursors freshly isolated and after in vitro stimulation with IL-3. For confocal laser scanning microscope analysis, CD1a−CD3−CD4+CD8−thymocytes were sorted and either directly cytocentrifuge preparations were made or cells were cultured for 5 days in the presence of IL-3, resuspended and layered on precoated glass slides. Both fresh and cultured cells were stained for HLA-DR. For FACS analysis, CD1a−CD3−CD4+CD8−thymocytes were cultured for 5 days in the presence of IL-3 and CD40L+ mouse fibroblasts. (A) Freshly isolated DC precursors show a rounded morphology and weak staining for HLA-DR. (B) Activated DCs upon culture in IL-3 acquire a dendritic morphology and more intense staining for HLA-DR (bar, 5 μm). (C) IL-3– and CD40L-activated DCs display a phenotype specific for mature DC. (D) The expression of IL-3R and IL-7R on thymic DC precursors compared with CD4 ISP pre-T cells. Solid lines represent staining with specific antibodies as compared with staining with isotype-matched controls indicated with dotted lines.
Morphology and phenotype of thymic DC precursors freshly isolated and after in vitro stimulation with IL-3. For confocal laser scanning microscope analysis, CD1a−CD3−CD4+CD8−thymocytes were sorted and either directly cytocentrifuge preparations were made or cells were cultured for 5 days in the presence of IL-3, resuspended and layered on precoated glass slides. Both fresh and cultured cells were stained for HLA-DR. For FACS analysis, CD1a−CD3−CD4+CD8−thymocytes were cultured for 5 days in the presence of IL-3 and CD40L+ mouse fibroblasts. (A) Freshly isolated DC precursors show a rounded morphology and weak staining for HLA-DR. (B) Activated DCs upon culture in IL-3 acquire a dendritic morphology and more intense staining for HLA-DR (bar, 5 μm). (C) IL-3– and CD40L-activated DCs display a phenotype specific for mature DC. (D) The expression of IL-3R and IL-7R on thymic DC precursors compared with CD4 ISP pre-T cells. Solid lines represent staining with specific antibodies as compared with staining with isotype-matched controls indicated with dotted lines.
CD1a−CD3−CD4+CD8−thymic DC precursors are predominantly located in the medulla.
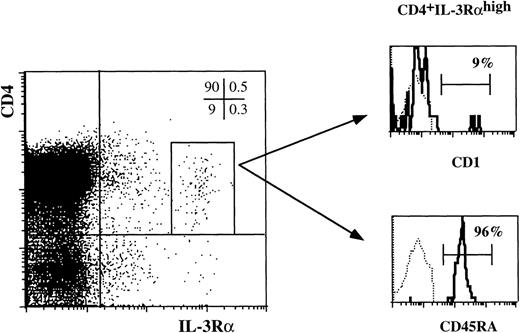
As shown in Fig 2D, thymic DC precursors express high levels of IL-3Rα. To explore whether this feature is unique for these DC, we analyzed IL-3Rαhigh cells in a sample of total unseparated thymocytes. Total thymocytes were stained with antibodies against CD4 and IL-3Rα and either CD1a or CD45RA. Electronically gated CD4+ IL-3Rαhigh cells were analyzed for CD1a and CD45RA expression. As shown in Fig3, the great majority of the CD4+ IL-3Rαhighexpressed CD45RA and lacked CD1a strongly suggesting that the majority of cells that express high levels of IL-3Rα are identical to the precursors characterized in the preceding experiments (Figs 1 and 2). In addition, very small populations of CD4−IL-3Rαhigh and CD1a+CD4+IL-3Rαhigh were detectable. It is possible that these cells represent intermediate stages of DC development in the thymus. Unfortunately this point could not be unambiguously checked. Because of the small sizes of these populations, we were unable to purify them sufficiently to evaluate their DC precursor activities.
The majority of thymocytes with a high level of IL-3R are thymic DC precursors. Total thymocytes were stained for three-color FACS analysis with anti-IL–3RPE, anti-CD4 Tricolor and either anti-CD1 FITC or anti-CD45RA FITC. The histograms show the staining of CD1a and CD45RA of CD4+ IL-3Rhigh cells as gated in the dot plot (0.1% of total thymocytes).
The majority of thymocytes with a high level of IL-3R are thymic DC precursors. Total thymocytes were stained for three-color FACS analysis with anti-IL–3RPE, anti-CD4 Tricolor and either anti-CD1 FITC or anti-CD45RA FITC. The histograms show the staining of CD1a and CD45RA of CD4+ IL-3Rhigh cells as gated in the dot plot (0.1% of total thymocytes).
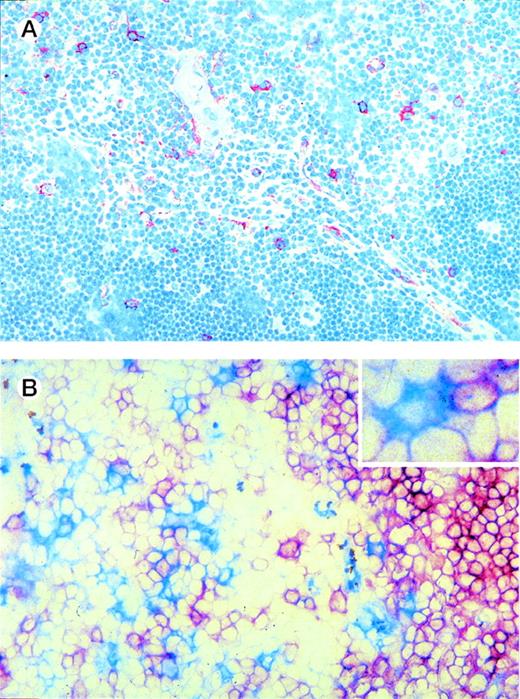
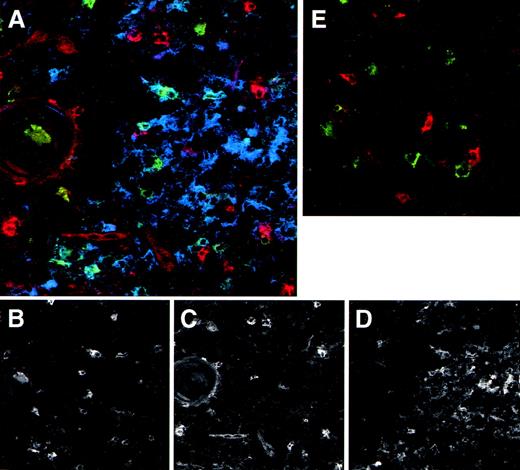
We made use of the elevated expression of IL-3Rα by the thymic DC precursors to study their localization in the thymus using immunohistochemistry on tissue sections. Figure 4A shows that IL-3Rαhigh cells are predominantly located in the medulla, whereas a few cells are also present in the corticomedullary border and the cortex. In line with the phenotype observed for freshly isolated DC precursors, the majority of IL-3Rαhighthymocytes in the medulla coexpressed CD45RA (not shown) and lacked CD1a− (Fig 4B). Many thymic pre-DC were in close contact with CD1a+ thymocytes (Fig 4B, inset). Three-color immunofluorescence analyses of tissue sections furthermore demonstrated that the medullary IL-3Rαhigh thymocytes showed virtually no costaining with the costimulatory molecule CD86 (Fig 5A). In addition, there was no distinct costaining of IL-3Rαhigh cells with CD83, which is a marker specific for mature DC (Fig 5A and E). However, the medulla does contain a population of CD83+ cells, which lack significant IL-3Rα expression. Many of these mature DC coexpress CD86 (Fig 5A).
Thymic localization of DC precursors. (A) Thymic tissue sections were incubated with anti-IL3R MoAb and counterstained with hematoxylin. IL-3Rhigh cells (red), which comprise DC precursors, are predominantly located in the medulla and a few are scattered through the cortex. (B) Thymic tissue sections were double-stained for IL-3R and CD1a. IL-3Rhigh cells (blue) are often found adjacent to CD1a+ thymocytes (red) (see inset). Original magnification (A) ×200, (B) ×400.
Thymic localization of DC precursors. (A) Thymic tissue sections were incubated with anti-IL3R MoAb and counterstained with hematoxylin. IL-3Rhigh cells (red), which comprise DC precursors, are predominantly located in the medulla and a few are scattered through the cortex. (B) Thymic tissue sections were double-stained for IL-3R and CD1a. IL-3Rhigh cells (blue) are often found adjacent to CD1a+ thymocytes (red) (see inset). Original magnification (A) ×200, (B) ×400.
The medulla contains both DC precursors and mature DC. Thymic tissue sections were triple-stained with anti-IL–3R (red), anti-CD83 (green), and anti-CD86 (blue). Patterns of the individual colors, which combined are shown in (A), are depicted separately in (B) (anti-CD83), (C) (anti-IL-3R ), and (D) (anti-CD86). As a comparison in (E), a control staining of anti-IL–3R (red), anti-CD83 (green), and control IgG (blue) is included. The round structure on the left side represents a Hassall’s corpuscle, which indicates the thymic medulla. Note in (A) the red stained pre-DC versus the blue/green stained mature DC. Original magnification ×400.
The medulla contains both DC precursors and mature DC. Thymic tissue sections were triple-stained with anti-IL–3R (red), anti-CD83 (green), and anti-CD86 (blue). Patterns of the individual colors, which combined are shown in (A), are depicted separately in (B) (anti-CD83), (C) (anti-IL-3R ), and (D) (anti-CD86). As a comparison in (E), a control staining of anti-IL–3R (red), anti-CD83 (green), and control IgG (blue) is included. The round structure on the left side represents a Hassall’s corpuscle, which indicates the thymic medulla. Note in (A) the red stained pre-DC versus the blue/green stained mature DC. Original magnification ×400.
CD1a−CD3−CD4+CD8−thymic DC precursors express high levels of mRNA for the pre-TCRα chain.
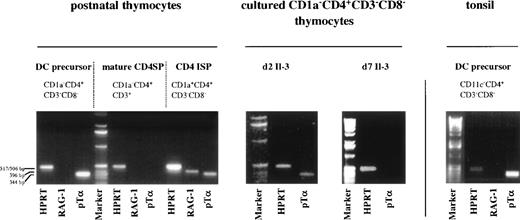
The phenotype of thymic DC precursors does not only resemble that of DC precursors in the tonsil, but also that of a population of cells present in adult peripheral blood, which has been shown to contain committed T-cell precursors.15 This latter CD1a−CD3−CD4+CD8−CD14−population of medium sized peripheral blood mononuclear cell (PBMC) has been shown to give rise to mature CD4+ SP TCRαβ+ T cells in a thymus-independent fashion. TCRβ DJ, but no VDJ rearrangements, were detected in these cells.15 Furthermore these cells were found to express mRNA for pTα and RAG-1. Although not shown, the peripheral blood CD1a−CD3−CD4+CD8−CD14−population contains also cells that express high levels of IL-3Rα, able to differentiate into mature DC in the presence of IL-3 and CD40L. The high level of expression of pTα mRNA in CD1a−CD3−CD4+CD8−CD14−PBMC15 prompted us to examine pTα mRNA levels in thymic DC precursors. As controls, we included CD1a−CD3+CD4+ mature thymocytes that should lack pTα16 and CD1a+CD3−CD4+CD8−ISP pre-T cells17-19 expected to express pTα mRNA.16 Figure 6 demonstrates that not only committed pre-T cells express pTα mRNA, but also the thymic DC precursors. The ratio of the pTα and HPRT signal intensities in the DC precursors is even higher than in the CD4+ pre-T cells, suggesting that the detected pTα mRNA in the pre-DC is not due to contamination with CD4 ISP pre-T cells. We also detected high levels of pTα mRNA in cells that were isolated from CD8-depleted thymocytes on the basis of expression of CD4, IL-3Rαhigh and CD45RA. These pTα mRNA levels were higher that those of CD45RA−CD4+IL-3Rα−cells obtained from the same sort (results not shown). Although the level of expression was decreased, pTα mRNA was still observed in the thymic DC after 2 days of culture with IL-3. This observation confirms the notion that pTα expression in these cells is not due to contamination with CD4 ISP pre-T cells, as these latter cells are unable to survive for 2 days with IL-3 (Fig 6). After 7 days of culture, the mature DC did not express pTα mRNA anymore, suggesting that concomitant with acquisition of a mature phenotype, pTα is downregulated. As expected, the CD1a−CD3+CD4+ SP mature thymocytes lacked pTα mRNA. The CD4 ISP thymocytes displayed RAG-1 mRNA expression, in contrast to the mature CD4 SP thymocytes and the thymic DC precursors. To investigate whether pTα mRNA expression is specific for thymic DC precursors, we examined pTα mRNA expression in lineage markers negative CD11c−CD3−CD4+ DC precursors from the tonsil. Figure 6 shows that pTα mRNA is present in these DC precursors as well.
Thymic and tonsillar DC precursors express mRNA for pT. Thymic DC precursors, CD4 ISP, mature CD4 SP thymocytes, and tonsillar DC precursors were analyzed for expression of mRNA for HPRT, pT, and RAG-1. pT mRNA expression was also determined for thymic DC precursors cultured for 2 and 7 days with IL-3.
Thymic and tonsillar DC precursors express mRNA for pT. Thymic DC precursors, CD4 ISP, mature CD4 SP thymocytes, and tonsillar DC precursors were analyzed for expression of mRNA for HPRT, pT, and RAG-1. pT mRNA expression was also determined for thymic DC precursors cultured for 2 and 7 days with IL-3.
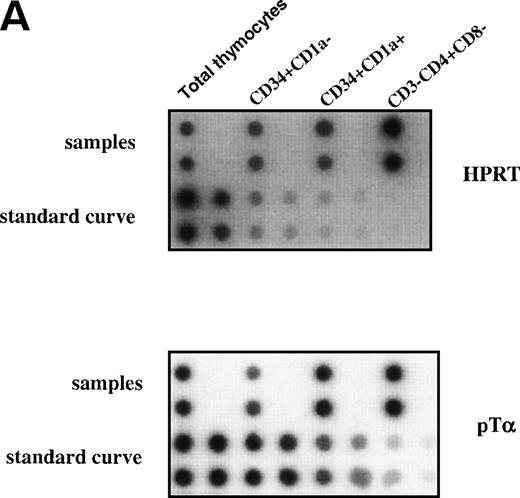
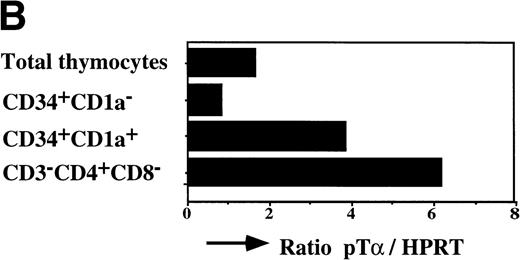
The earliest precursors in the thymus express CD34 and lack CD1a. This population contains T, NK, and DC precursor activities.8,9Upon upregulation of CD1a, the DC precursor activity is lost and the NK precursor activity is strongly diminished.20 The CD34+CD1a+ cells differentiate into CD4 ISP pre-T cells. To obtain insight into the levels of pTα in different subsets of early precursors in the thymus, we performed a semiquantitative PCR with CD34+CD1a−, CD34+CD1a+, and CD3−CD4+CD8− cells from the same thymic sample. These latter cells were sorted on the basis of CD4 expression and absence of CD3 and CD8 and therefore include pre-T cells and committed pre-DC. The pre-DC constitute 10% of the sorted CD3-CD4+CD8− population. As was found previously for fetal thymic CD34+CD1a−cells,21 the postnatal CD34+CD1a− cells express low levels of pTα mRNA (Fig 7). Around 10-fold higher levels of pTα mRNA were observed in CD34+CD1a+ cells, which increased a further 1.5-fold in CD3-CD4+CD8− cells (Fig 7). It is feasible that the CD4+ pre-DC are responsible for this increase. These results shown in Figs 6 and 7 indicate that pTα is present in uncommitted thymic precursors, but at levels lower than those of the committed IL-3Rα+ DC precursors.
Expression levels pre-T mRNAs in subsets of thymic precursors. Total RNA was isolated from sorted CD34+CD1a−, CD34+CD1a+, and CD4 ISP human thymic subpopulations (> 99% pure upon reanalysis) and analyzed for the levels of pT mRNA expression by semiquantitative RT-PCR as described in Materials and Methods (A). mRNA levels were compared with HPRT expression and calculated in arbitrary units. Standard curves were established by simultaneous amplification of 2-fold dilutions of cDNA prepared from RNA isolated from total thymus. PCR product of standard curves and samples were dot-blotted in duplicate onto nylon filters and hybridized with end-labeled oligo nucleotides shown on the right-hand site of the figure. (B) Measurement of the cpm/dot values was performed with a phosphoimager. Standard curves were calculated in which the first point of the curve, containing the highest amount of cDNA, was set arbitrarily to 1,000 U. Values of the samples were calculated in units. The ratios of (pT)/(HPRT) were determined and visualized in the bar graph.
Expression levels pre-T mRNAs in subsets of thymic precursors. Total RNA was isolated from sorted CD34+CD1a−, CD34+CD1a+, and CD4 ISP human thymic subpopulations (> 99% pure upon reanalysis) and analyzed for the levels of pT mRNA expression by semiquantitative RT-PCR as described in Materials and Methods (A). mRNA levels were compared with HPRT expression and calculated in arbitrary units. Standard curves were established by simultaneous amplification of 2-fold dilutions of cDNA prepared from RNA isolated from total thymus. PCR product of standard curves and samples were dot-blotted in duplicate onto nylon filters and hybridized with end-labeled oligo nucleotides shown on the right-hand site of the figure. (B) Measurement of the cpm/dot values was performed with a phosphoimager. Standard curves were calculated in which the first point of the curve, containing the highest amount of cDNA, was set arbitrarily to 1,000 U. Values of the samples were calculated in units. The ratios of (pT)/(HPRT) were determined and visualized in the bar graph.
CD1a−CD3−CD4+CD8−thymocytes are unable to develop into T cells or NK cells.
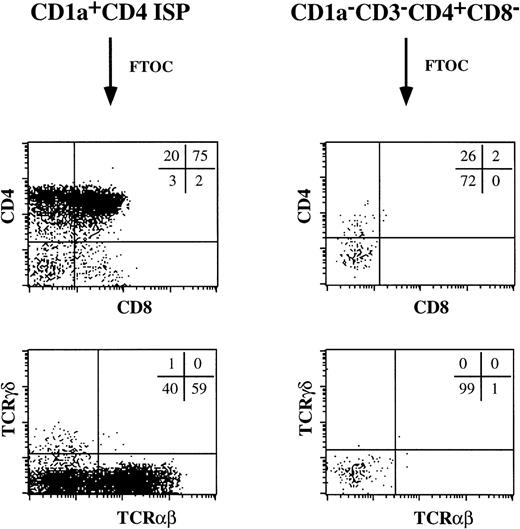
The cell surface expression of CD2, CD5, and CD7 and the presence of expression of pTα mRNA in the thymic CD1a−CD3−CD4+CD8−pre-DC population raised the possibility that these cells may have T-cell precursor activity. Therefore, we studied the potential of these cells to develop to T cells in fetal thymic organ culture. As a positive control, we included the CD4 ISP thymocytes. As expected, these CD4 ISP thymocytes developed through a DP stage into mature TCRαβ+ T cells (Fig 8). This population gave, in addition, rise to a small percentage of TCRγδ+ T cells. Very few cells could be recovered from the FTOC with the pre-DC. Those cells did not express CD8, TCRαβ, or TCRγδ, while a proportion still expressed CD4. Presumably these CD3−CD4+CD8− cells are surviving pre-DC. These data indicate that CD1a−CD3−CD4+CD8−thymocytes were unable to generate T cells in an FTOC.
CD1a−CD3−CD4+CD8−thymocytes do not contain T-cell precursors. The ability of CD1a−CD3−CD4+CD8−thymocytes to give rise to T cells was tested in a human/mouse FTOC. As a comparison, the developmental capacity of CD1a+CD4+ ISP thymocytes was examined.
CD1a−CD3−CD4+CD8−thymocytes do not contain T-cell precursors. The ability of CD1a−CD3−CD4+CD8−thymocytes to give rise to T cells was tested in a human/mouse FTOC. As a comparison, the developmental capacity of CD1a+CD4+ ISP thymocytes was examined.
Because the CD1a−CD3−CD4+CD8−CD14−peripheral blood population has been shown to generate T cells when directly stimulated with phytohemagglutinin (PHA), IL-2, IL-7, and allogeneic feeder cells,15 this same stimulation regimen was applied to the CD1a−CD4+CD3−CD8−thymocytes. Also under these conditions, no T-cell development was observed (data not shown). Taken together, these results therefore suggest that the CD1a−CD3−CD4+CD8−thymocytes do not contain T-cell precursors. To investigate NK precursor activity, we cultured the thymic CD1a−CD3−CD4+CD8−cells in either a mixture of IL-2, IL-7, and SCF or a mixture of IL-7, IL-15, and Flt-3L.22 While both mixtures simulated differentiation of NK cells from CD34+ thymocytes, they failed to induce NK cell development from the thymic CD1a−CD3−CD4+CD8−cells (results not shown).
DISCUSSION
Here we have characterized CD4+ thymic precursors committed to the DC lineage. Cells within this population lack CD1a, express high levels of CD45RA and the IL-3Rα chain, and are predominantly located in the thymic medulla. They develop into mature DC in vitro upon stimulation with IL-3 or IL-3 and CD40, but are unable to develop into T and NK cells in an FTOC or in mixtures of either IL-7, IL-15, and Flt-3L or IL-2, IL-7, and SCF. These committed DC precursors share many characteristics with pre-T cells. Both cell populations express CD2, CD5, and CD7. These antigens could be detected on DC precursors cultured in IL-3, indicating that expression of CD2, CD5, and CD7 was not due to absorption from T-lineage cells within the thymus. More strikingly, we detected pTα mRNA in the committed DC precursors. This was unexpected because pTα is considered to be specific for the T-cell lineage. Because we determined expression of pTα mRNA at a population level, we cannot completely rule out that pTα is expressed in cells within the population that are not committed DC precursors. However, we observed that high levels of pTα were also present in cells that were sorted on the basis of IL-3Rα expression, that CD1a−CD4+ pre-DC contain more pTα mRNA than CD1a+CD4+ pre-T cells, and that pTα mRNA could still be detected in DC precursors cultured for 2 days in IL-3. These observations together support the interpretation that the committed DC precursors do express pTα mRNA. The IL-3Rα+ DC precursors show also similarities with NK cells, as both cell types express CD2, CD5, and CD7. Whether committed NK precursors express pTα mRNA is not known, but it is interesting to note that pTα mRNA was detected in T/NK precursor cells from murine fetal blood.23
The similarity in phenotype of pre-T cells, pre-NK cells, and pre-DC strongly suggest a common origin of these cells. The observation that pTα transcripts, detected in both committed pre-T and pre-DC, are also expressed in CD34+CD1a− cells that have T, DC, and NK precursor activities8,9 is consistent with this notion. Such a developmental relationship of T cells and DC has been inferred from the seminal studies of Shortman et al,4 who demonstrated that mouse intrathymic DC and T cells are derived from a common CD4lowCD44+CD25− thymic precursor. This latter population can also produce B cells and NK cells24 and therefore may contain common lymphoid precursors (CLP).25 Because the mouse thymic DC originate from cells, which lack myeloid precursor activity, they were called lymphoid DC to distinguish them from DC derived through the myeloid lineage. Further studies in the mouse have made clear that lymphoid-derived CD8α+ DC are also present in the spleen and the circulation.26,27 The peripheral lymphoid dendritic cells may well arise from a CLP population that has been found in bone marrow and which may be the source of cells that colonize the thymus.25 In humans, there is also evidence for a lymphoid origin of a subset of DC. Galy et al28 have identified a CLP in the bone marrow, which can generate T, B, and NK and also DC, but not myeloid cells. We have found an almost identical precursor in the human thymus. Like the CLP, these cells express CD10, CD34 and CD45RA lack CD1a and can, next to T cells, develop into NK or DC dependent on the cytokines added to these cells.9 It is possible that the thymic committed pre-DC, identified here, are the progeny of the CD34+CD1a−CD10+CD45RA+thymic precursors and therefore may represent human lymphoid DC. It is, however, important to note that this hypothesis has yet to be directly verified. Not only the developmental origin of the IL-3Rα+ DC precursors has yet to be determined, but also their final destination. Our data indicate that, apart from committed pre-DC, the medulla contains mature CD83+CD86+DC, which lack IL-3Rα. Whether the IL-3Rα+ pre-DC give rise to these medullary mature DC in vivo is unknown. A detailed comparison between the mature CD83+, the IL-3Rα+ pre-DC, and their progeny is necessary to resolve this point.
The finding that DC precursors (plasmacytoid T cells) with similar features as thymic DC precursors are present in the tonsil12 indicates that this cell type is not confined to the thymus. Both cell populations express high levels of CD45RA, IL-3Rα, and pTα mRNA, but lack CD1a and express only very low levels of CD11c. Moreover, these cells respond to IL-3 and the combination of IL-3 and CD40L in an identical way. There appears to be a similar population in peripheral blood. Recently we identified a CD3−CD4+CD8−CD14−population in the peripheral blood of adults.15 Cells in this population expressed high levels of pTα mRNA. However, in contrast to the pre-DC in the thymus, they also expressed RAG-1 mRNA and demonstrated T-cell precursor activity. Confirming results of other groups, we have also found that CD3−CD4+CD8−CD14−peripheral blood cells have DC precursor activities (L. Bruno, P. Res, and H. Spits, unpublished findings). In addition, we observed that this population contains cells with a high level of IL-3Rα and, although this was not directly tested, it is likely that these IL-3Rα+ cells also express pTα mRNA. It is clear that the DC precursor found in the thymus is not unique for this organ. As discussed above, we speculate that the thymic IL-3Rα+ DC precursors originate from an intrathymic CD34+ progenitor, although this has yet to be directly proven. An interesting question is whether the pTα+ DC precursors found in peripheral blood and tonsil are derived from the common lymphoid/DC precursors present in bone marrow or from the CD34+ thymocytes. In the latter case, pTα mRNA expression in lymphoid DC could be a thymus-induced event.
ACKNOWLEDGMENT
The authors thank Lauran Oomen and Ilja van de Pavert for help with confocal microscopy analysis and Trees Dellemijn and Natascha Verra for preparation and antibody labeling of tissue sections. Bianca Blom is acknowledged for her help with the semiquantitative PCR.
Supported by Grant No. NKI 95-960 from the Dutch Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests Hergen Spits, MD, Division of Immunology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: hergen@nki.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal