Major histocompatibility complex (MHC) molecules play an important role in antigen presentation for induction of tumor as well as cellular and humoral immunities. Recent studies using anti-MHC antibodies demonstrated that antibodies specific for HLA class I molecules induced cellular activation and a type of apoptosis that may be distinct from Fas-dependent or TNFR (tumor necrosis factor- receptor)-dependent processes. We purified a previously untested apoptosis-inducing factor from HL-60 human leukemic cell-conditioned media to homogeneity and sequenced it. It was identified as β2-microglobulin (β2m), which has been previously known as thymotaxin and is a part of the HLA class I antigen complex. β2m acts on both T-leukemic cells and myeloid leukemic cells to induce apoptosis, which then activates caspase 1 and 3. Cross-linking studies showed that biotinilated β2m recognized an epitope distinct from those recognized by the anti-HLA class I antibody, as reported previously. We demonstrated that β2m plays a previously unrecognized and important role in regulating the elimination of tumor cells, which occurs as a result of the action of β2m as an apoptosis-inducing factor.
MAJOR HISTOCOMPATIBILITY antigen (MHC) molecules, including HLA class I, are heterodimers made of a 45-kD α chain with 3 extracellular domains noncovalently associated with the invariant 12-kD β2-microglobulin (β2m). They play an important role in regulating tumor immunity as well as cellular and humoral immunities.1,2 They function in the presentation of proteasome-generated antigens to CD8+ T cells.3
In addition to their role in antigen presentation, HLA class I molecules expressed on T cells may also be involved in the signal transduction.4-9 Intracellular signal transduction after HLA class I ligation includes protein tyrosine phosphorylation and an increase in intracellular free calcium.9-12
In addition, several studies have shown that HLA class I ligation on the surface of T cells may result in growth arrest, anergy, and, eventually, apoptosis induction.13-15 The expression of HLA class I antigens has been observed in some tumor cells. Chemoprevention agents, such as all-trans retinoic acid and retinamide, induce upregulation of HLA class I antigens and other factors (reactive oxygen radicals).16,17 Upregulation of HLA class I antigens explains how the enhancement of tumor immunity may occur. On the other hand, some kinds of viral infections, including adenovirus, cytomegalovirus, and human immunodeficiency virus (HIV), induce downregulation of HLA class I antigen expression and may be a factor in the immunodeficient state after infection with these viruses.18-20
In the immune system, apoptosis induced by surface molecules, such as Fas (CD95)21 and tumor necrosis factor receptor (TNFR), is the main mechanism for the homeostasis of T and B cells22,23 and for the lysis of target cells by cytotoxic T lymphocytes (CTL) and natural killer (NK) cells.21-23 In human T cells, apoptosis can be triggered not only by several membrane receptor families, including Fas, TNFR, and CD30, but also by CD2, CD45, CTLA4, and HLA class I molecules, especially in activated T cells.21-31 Recent reports indicate that cross-linking monoclonal antibodies (MoAbs), which recognize the β2m or the α3 domain of the heavy chain, can induce T-cell apoptosis, and this pathway may be distinct from Fas- or TNFR-induced apoptosis.32 Two antibodies against an epitope of the α1 domain of the HLA class I heavy chain may also induce apoptosis of activated T cells. These reactions do not involve Fas/Fas-ligand interaction.33,34 However, identification of specific ligands for the MHC class I complex has not yet been achieved. An interaction between human β2m35,36 and MHC I heavy chains has been studied by mutational analysis of the heavy chains.37
β2m, a chemotactic protein previously known as thymotaxin, plays an important role in T-cell precursor colonization in the thymus.38,39 We purified an apoptosis-inducing factor from a conditioned media of HL-60 cells stimulated by phorbol 12,13-dibutyrate (PDBu).40 41 One of the purified proteins has been identified as human β2m. A serum concentration of β2m induced apoptosis in both myeloid and lymphoid leukemia cell lines. We demonstrate a previously unknown function of β2m as an apoptosis-inducing factor in lymphoid cells.
MATERIALS AND METHODS
Cell lines and reagents.
The human cell lines used in this study were nonlymphocytic cell lines (K562 [chronic myelocytic], HL-60 and NB-4 [acute promyelocytic], THP-1 [acute monocytic], U937 [myelomonocytic], and KG-1 [acute erythrocytic]), B-lymphocytic cell lines (SKW, BALL, and Daudi), and T-lymphocytic cell lines (Jurkat, TALL, and CCRF-CEM). All of these cell lines were cultured in GIT media (Wako Co, Tokyo, Japan), which does not contain proteins such as β2m or cytokines, as demonstrated in our previous studies.40 41 Human β2m was purified from human urine to homogeneity (its sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] analysis showed a single band) and was used in the early experiments (data not shown). Endotoxin was not detectable by Limulus assay (data not shown). Recombinant human β2m consisting of 99 amino acids (Oriental Yeast Co, LTD, Shiga, Japan) was used for the same purposes as urine-derived β2m. Reducing SDS-PAGE analysis of the recombinant product showed homogeneity with a single band. The concentrations of β2m in supernatants of PDBu (Sigma, St Louis, MO) -treated HL-60 cells and in several maintained cell lines were measured by enzyme-linked immunosorbent assay (ELISA). In addition, anti-Fas MoAb (IgM, clone CH-11; MBL, Nagoya, Japan), recombinant human TNF-α (Calbiochem, La Jolla, CA), and endothelial interleukin-8 (IL-8; R&D System, Minneapolis, MN) were used as apoptosis-inducing factors in control studies.
Purification and sequencing of β2m as an apoptosis-inducing factor (AIF).
The HL60 cells were resuspended at 2 × 105 cells/mL in HamF12/Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Rockville, MD), which contains 10% GIT medium and 50 nmol/L PDBu and were incubated at 37°C for 3 days as in our previous studies.40,41 After 3 days, the supernatant was collected and precipitated in ammonium sulfate. The preparation was then purified further in a process using cation exchange fast protein liquid chromatography (FPLC) and gel filtration high performance liquid chromatography (HPLC). Additionally, the pool of active fraction eluted from the gel filtration HPLC column was added to reverse-phase HPLC. When the concentration of acetonitrile reached approximately 40%, the highest peak of AIF activity was eluted and added to the reverse-phase HPLC. The N-terminal sequence of the protein was determined by Edman degradation on an automated Applied Biosystems 473A sequencer (Applied Biosystems, Foster City, CA), as in our previous study.40 41
MTT and TUNEL assays for the detection of apoptosis.
Each of the human cell lines was seeded at 1 × 105cells/mL in HamF12/DMEM with 10% GIT medium, and β2m was added to the media at 10 μg/mL. After 2 days, the capacity to reduce 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) was determined. After adding 10 μL of MTT solution (5 mg/mL MTT in phosphate-buffered saline [PBS]), the preparation was incubated at 37°C for 3 hours. Cells with MTT formazan were dissolved in a solution of 2-propanol and 0.04 mol/L of HCl, and color absorbance was measured at 595 nm by a microplate reader (Bio-Rad, Hercules, CA). Additionally, residual cells were incubated with a digoxigenin-UTP terminal deoxynucleotide transferase mixture. Subsequently, they were stained with a peroxidase-conjugated antibody for digoxigenin by using an in situ Apoptosis Detection Kit (Apop Tag Plus; Oncor, Gaithersburg, MD); they were then counter-stained with 1% methyl green in sodium acetate (pH 4.0) and finally mounted. Specimens were examined and photographed with a microscope as in our previous study.40 41 Statistical analysis was performed using a Student’s t-test.
MoAbs.
The mouse anti-β2m MoAbs (IgG, clone NK1, NK2, and NK3) were derived from hybridoma cells and purified in a PBS together with ammonium sulfate. K562 cells and CCRF-CEM cells, at a concentration of 1 × 105 cells/mL were exposed in 1 mL of HamF12/DMEM to a 10% GIT medium for 48 hours at 37°C after preincubation of 100 μg of anti-β2m MoAb with 10 μg of β2m for 15 minutes at room temperature (RT). Inhibition of the β2m-induced apoptosis in these cells were detected using the MTT assays and TUNEL method as described above. The anti-Fas MoAb (IgG2b, clone SM1/23; Chemicon Int Inc, Temecula, CA) and anti-TNFR(p55) MoAb (IgG; AUSTRAL Biologicals, San Ramon, CA) at a concentration of 1 μg/mL were added to show whether β2m-induced apoptosis was inhibited. In addition, the anti–IL-8 MoAb (IgG1, clone #6217.11; R&D Systems), anti–IL-8 receptor A (CDW128), MoAb (IgG2b, clone 5A12; Pharmingen, San Diego, CA), and anti–IL-8 receptor B (CXCR2) MoAb (IgG1, clone 6C6; Pharmingen) were added at a concentration of 5μg/mL to show whether β2m-induced apoptosis was inhibited. Ten micrograms per milliliter each of TU149 MoAb (IgG2a, anti-HLA-B, C, and some A; Caltag, Burlingame, CA), YTH862 MoAb (IgG2b, anti-HLA-A, B, and C; Serotec, Oxford, UK), and W6/32 MoAb (IgG2a, anti-HLA-A, B, and C; Serotec) were added at 10 μg/mL to study the correlation with apoptosis induced by HLA-class I. The mouse anti-RecA MoAb (IgG2b, clone ARM414; MBL) was used as a negative control. These anti-HLA class I MoAbs and anti-RecA MoAb were used after dialysis against PBS.
Caspase inhibitors.
The IL-1β–converting enzyme (ICE)-like protease inhibitors Ac-Tyr-Val-Ala-Asp-aldehyde (Ac-YVAD-CHO) and Z-Val-Ala-Asp(OMe)-fluoromethylketone (Z-VAD-FMK) as well as the CPP32/Apopain-like protease inhibitor Ac-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-CHO) were purchased from Calbiochem-Novabiochem Co (San Diego, CA). These peptides were dissolved separately in dimethylsulfoxide (DMSO; Wako Co, Tokyo, Japan) and 10 or 100 μmol/L of each peptide was added to the cell suspensions with final concentrations of 0.1% or 0.3% DMSO as performed in our previous study.42
Fluorescence-activated cell sorter (FACS) analysis.
Fluorescein isothiocyanate (FITC)-conjugated mouse IgG1 (DAKO, Glostrup, Denmark) was used as a negative control. FITC-conjugated anti-HLA class I MoAb (clone W6/32; Serotec) was added to the solution to detect the presence of HLA class I on the cell surface membrane. β2m was biotinilated with an ECL protein biotinylation module (Amersham, Little Chalfont, Buckinghamshire, UK). Avidin R-PE (Caltag) was added. Cells from various cell lines were centrifuged at 2,000 rpm for 1 minute and washed once in PBS. The cells were exposed to the biotinilated-β2m (2μg) for 30 minutes, put on ice, washed twice in PBS, and exposed to avidin R-PE- and FITC-conjugated mouse anti-HLA class I MoAb simultaneously for 30 minutes on ice. The cells were analyzed using the FACScan (Nippon Becton Dickinson Co, Tokyo, Japan) as performed in our previous study.43 Each data point was derived from an analysis of 1 × 104 cells.
Protein cross-linking.
The results of our binding study are shown briefly. Biotinilated β2m was incubated with 5 × 106 K562 cells or CCRF-CEM cells for 30 minutes at 4°C. After resuspension in 450 μL PBS, cells were washed twice. After removal of PBS, 1 μL of 120 μg/mL biotinilated β2m was added. As a cold competitor, 100× β2m was added. Ten microliters of 1 mmol/L d-biotin (Sigma) was added and incubated at 4°C for 1 hour with occasional shaking. As for the manufacturer’s protocol, 1 × 106 cells of K562 cells were suspended in a total volume of 100 μL PBS and solubilized with 2.0% (vol/vol) Nonidet P 40.44 45 After removing insoluble material by centrifugation, 0.5 mL of the detergent extract was mixed with 5 μL disuccinimidyl suberate (DSS; Pierce, Rockford, IL) solution (20 mmol/L in DMSO; final concentration, 1 mmol/L) and allowed to react for 30 minutes at 14°C. The reaction was stopped by adding 2 μL of 1 mol/L Tris-HCl, pH 7.5.
SDS-PAGE and immunoblotting.
After further centrifugation to remove precipitates from this sample, the supernatant was loaded onto an SDS-PAGE and immunoblotted. Protein samples were submitted to SDS-PAGE analysis on a 4% to 20% gradient gel under standard conditions using a mini-Protean II system (Bio-Rad), followed by silver staining. For immunoblotting, the proteins were transferred to polyvinylidene difluoride (Bio-Rad) membrane filters and were blocked in 2% (wt/vol) bovine serum albumin in washing buffer Tween 20 in PBS.
RESULTS
β2m was detected as a type of AIF.
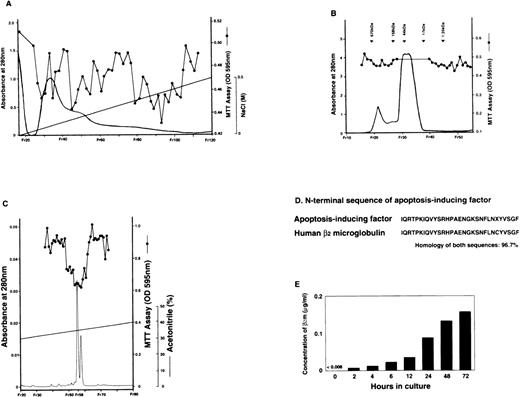
HL-60 cells at a concentration of 2 × 105 cells/mL were cultured with 50 nmol/L of PDBu at 37°C for 3 days and differentiated into the monocyte/macrophage lineage. We investigated whether apoptosis was induced in K562 cells when the supernatant from PDBu-treated HL-60 cells was added to a culture media of K562 cells. PDBu itself did not induce apoptosis in K562 cells (data not shown), and we did not detect any AIF activity in conditioned media from unstimulated HL-60 cells. To detect whether proteins were the AIFs, the protein in the supernatant was precipitated from a solution of 95% ammonium sulfate. The preparation was then purified further in a process using cation exchange (FPLC; Fig1A) and gel filtration (HPLC; Fig 1B). Additionally, the pool of active fractions eluted from the gel filtration column was applied to reverse-phase HPLC. The purified AIF activity was eluted when the concentration of acetonitrile reached approximately 40% in reverse-phase HPLC (Fig 1C). We obtained specific activity of 1 × 105 U/mg of protein (1 U AIF inhibited 50% of maximum reduction, as indicated by MTT reducing activity) and with a final purification of 270-fold, calculated from the first supernatant concentration.40 The overall yield was 0.05%.
The apoptosis-inducing factor was purified from the supernatant of PDBu-treated HL60 cells and recognized as human β2m. The supernatant of PDBu-treated HL60 cells collected as in Materials and Methods was purified in a process using (A) the cation exchange FPLC (SP Sepharose HP; Pharmacia, NJ) and (B) the gel filtration HPLC (TSKgel G3000SW; Tosoh, Tokyo, Japan). The pool of active fraction eluted from gel filtration HPLC was applied to (C) the reverse-phase HPLC. The column (μBondasphere; Waters, MA) was eluted through a 90-minute linear time gradient from 28% to 40% acetonitrile. The flow rate was maintained at 2 mL/min. (D) The N-terminal sequence of the fraction with AIF activity. It is closely homologous with the N-terminal sequence of human β2m. X shows an undetermined amino acid sequence. (E) The concentration of β2m in the supernatant of PDBu-treated HL60 cells gradually increased over time for 72 hours.
The apoptosis-inducing factor was purified from the supernatant of PDBu-treated HL60 cells and recognized as human β2m. The supernatant of PDBu-treated HL60 cells collected as in Materials and Methods was purified in a process using (A) the cation exchange FPLC (SP Sepharose HP; Pharmacia, NJ) and (B) the gel filtration HPLC (TSKgel G3000SW; Tosoh, Tokyo, Japan). The pool of active fraction eluted from gel filtration HPLC was applied to (C) the reverse-phase HPLC. The column (μBondasphere; Waters, MA) was eluted through a 90-minute linear time gradient from 28% to 40% acetonitrile. The flow rate was maintained at 2 mL/min. (D) The N-terminal sequence of the fraction with AIF activity. It is closely homologous with the N-terminal sequence of human β2m. X shows an undetermined amino acid sequence. (E) The concentration of β2m in the supernatant of PDBu-treated HL60 cells gradually increased over time for 72 hours.
We sequenced the protein that showed a single peak with AIF activity. From an analysis of the N-terminal sequence of the AIF, we found that it was strikingly (97%) homologous to the N-terminal sequence of human β2m (Fig 1D). In addition, using ELISA, the concentrations of β2m in the supernatants from the PDBu-treated HL-60 were measured and found to increase in relation to time (Fig 1E). We did not detect β2m in unstimulated HL-60 cell-conditioned media (data not shown). The expression of β2m mRNA in PDBu-treated HL-60 cells correlated with the concentration of β2m in the supernatant (data not shown). Therefore, β2m was recognized as a candidate AIF.
β2m induces apoptosis in K562 cells in a dose-dependent manner, with this apoptosis increasing in relation to time.
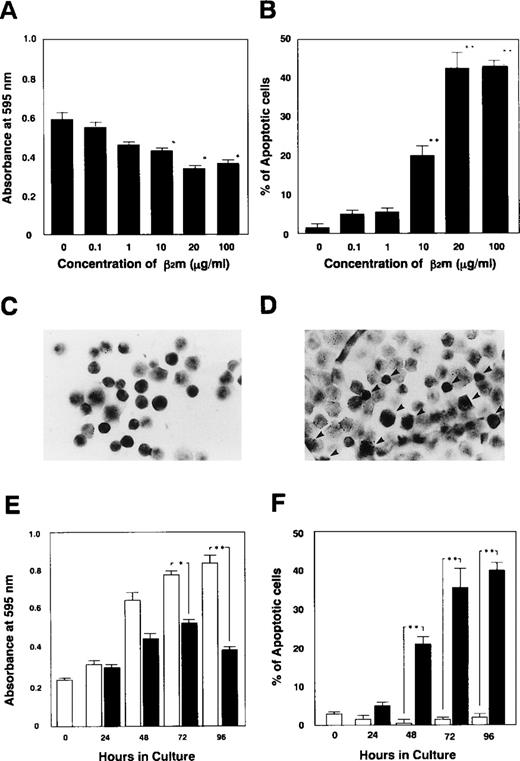
We initially examined whether β2m would induce apoptosis in K562 cells. The proliferation of K562 cells was suppressed in a dose-dependent manner in the presence of β2m at concentrations of more than 10 μg/mL, as detected by the MTT assay (Fig 2A). Simultaneously, we detected the induction of apoptosis in K562 cells with the TUNEL assay (Fig 2C and D), and we found that β2m induced apoptosis in these cells in a dose-dependent manner (Fig 2B). In the presence of β2m for 48 hours, the fraction of apoptotic K562 cells increased from 21.0% ± 2.2% (at 10 μg/mL) to 42.7% ± 4.5% (at 20 μg/mL) and to 43.3% ± 1.7% (at 100 μg/mL). Time course studies showed both inhibition of proliferation and induction of apoptosis most markedly between 48 and 96 hours (Fig 2E and F).
Inhibition of cell growth and induction of apoptosis in K562 cells by human β2m. (A) The proliferation of K562 cells (1 × 105 cells/mL) was suppressed in the presence of more than 10 μg/mL of β2m for 48 hours, and (B) apoptosis was induced. (C and D) The percentage of apoptotic cells was determined microscopically by counting 200 cells on in situ-stained slides. (C) Control K562 cells cultured without β2m or (D) with 10 μg/mL of β2m at 37°C for 48 hours. Apoptotic cells were detected using in situ staining with Apop Tag PLUS (Oncor), which gives a dark contrast to the insoluble precipitate, indicative of genomic fragmentation, as described. Arrows indicate apoptotic cells (original magnification × 200). (E) The proliferation of K562 cells was suppressed, and after more than 48 hours of incubation with 10 μg/mL of β2m apoptosis was induced (F). Results are expressed as the mean ± SE of 3 independent experiments. Standard deviations are shown by horizontal bars.P values of less than .05 are shown as (*) and less than .01 as (**).
Inhibition of cell growth and induction of apoptosis in K562 cells by human β2m. (A) The proliferation of K562 cells (1 × 105 cells/mL) was suppressed in the presence of more than 10 μg/mL of β2m for 48 hours, and (B) apoptosis was induced. (C and D) The percentage of apoptotic cells was determined microscopically by counting 200 cells on in situ-stained slides. (C) Control K562 cells cultured without β2m or (D) with 10 μg/mL of β2m at 37°C for 48 hours. Apoptotic cells were detected using in situ staining with Apop Tag PLUS (Oncor), which gives a dark contrast to the insoluble precipitate, indicative of genomic fragmentation, as described. Arrows indicate apoptotic cells (original magnification × 200). (E) The proliferation of K562 cells was suppressed, and after more than 48 hours of incubation with 10 μg/mL of β2m apoptosis was induced (F). Results are expressed as the mean ± SE of 3 independent experiments. Standard deviations are shown by horizontal bars.P values of less than .05 are shown as (*) and less than .01 as (**).
β2m induces apoptosis in human leukemic and lymphoma cell lines.
Based on the detection of β2m-induced apoptosis in K562 cells, various human leukemic and lymphoma cell lines were cultured at 1 × 105/mL in the presence of 10 μg/mL of β2m for 48 hours at 37°C. Proliferation and percentage of apoptotic cells were studied. We found that β2m induced apoptosis in more than 10% of U937, BALL, and CCRF-CEM cells (Fig 3A and B) as well as K562 cells (Fig 3C). Moreover, apoptosis both in K562 cells and in CCRF-CEM cells was induced by recombinant human β2m (Fig3D and E). FasL/Fas interaction is known to strongly and rapidly initiate apoptosis in human leukemic cell lines such as Jurkat cells. β2m does so more slowly, eg, over a few days instead of a few hours. K562 cells and CCRF-CEM cells were especially useful in this study of the mechanism of β2m-induced apoptosis, because apoptosis is not regularly induced in their natural in vitro growth patterns.
β2m induces apoptosis in other human leukemic and lymphoma cell lines. (A) Control CCRF-CEM cells were cultured without or (B) with 10 μg/mL β2m at 37°C for 48 hours. Apoptotic cells were detected using in situ staining with Apop Tag PLUS (original magnification × 200). (C) Induction of apoptosis by β2m in various cell lines. Nonlymphocytic cell lines (HL-60, NB4, THP-1, U937, and KG-1), B-lymphocytic cell lines (SKW, BALL, and Daudi), and T-lymphocytic cell lines (Jurkat, TALL, and CCRF-CEM) cells (1 × 105 cells/mL) were cultured with 10 μg/mL of β2m at 37°C for each or 48 hours, and TUNEL assays were performed. In the controls of U937 cells and BALL cells, apoptosis was induced in more than 5% of the cells. Therefore, they did not prove useful ([□] control; [▪] additive of β2m). (D and E) Apoptosis in K562 cells (D) and in CCRF-CEM cells (E) was induced by recombinant human β2m in the same way as with urine-derived human β2m.
β2m induces apoptosis in other human leukemic and lymphoma cell lines. (A) Control CCRF-CEM cells were cultured without or (B) with 10 μg/mL β2m at 37°C for 48 hours. Apoptotic cells were detected using in situ staining with Apop Tag PLUS (original magnification × 200). (C) Induction of apoptosis by β2m in various cell lines. Nonlymphocytic cell lines (HL-60, NB4, THP-1, U937, and KG-1), B-lymphocytic cell lines (SKW, BALL, and Daudi), and T-lymphocytic cell lines (Jurkat, TALL, and CCRF-CEM) cells (1 × 105 cells/mL) were cultured with 10 μg/mL of β2m at 37°C for each or 48 hours, and TUNEL assays were performed. In the controls of U937 cells and BALL cells, apoptosis was induced in more than 5% of the cells. Therefore, they did not prove useful ([□] control; [▪] additive of β2m). (D and E) Apoptosis in K562 cells (D) and in CCRF-CEM cells (E) was induced by recombinant human β2m in the same way as with urine-derived human β2m.
Anti-β2m MoAb inhibits the β2m-induced apoptosis.
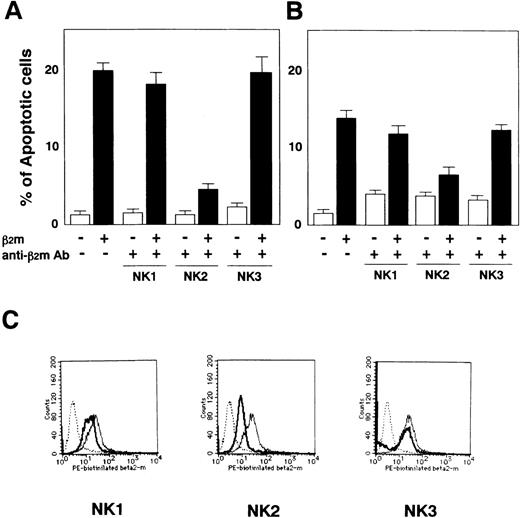
The mouse antihuman β2m MoAbs (clone NK1, NK2, and NK3) were derived from hybridoma cells and purified in PBS together with ammonium sulfate (Kubota et al, manuscript in preparation). K562 cells were grown in HamF12/DMEM to a 10% GIT medium for 48 hours at 37°C after preincubation of 100 μg of anti-β2m MoAb with 10 μg of β2m for 15 minutes at RT. The anti-β2m MoAb (clone NK2) completely blocked both the suppression of cell proliferation (data not shown) and the induction of apoptosis in K562 cells (Fig 4A) and coordinately blocked both the suppression of cell proliferation (data not shown) and the induction of apoptosis in CCRF-CEM cells (Fig 4B). The anti-β2m MoAb (NK2) inhibited any interaction with either biotinilated β2m or the cell surface of CCRF-CEM cells (Fig 4C). This result suggests that β2m induces apoptosis through an interaction with a receptor on the cell’s surface.
Inhibition of induction of apoptosis in K562 cells and in CCRF-CEM cells by anti-β2m MoAb. (A) β2m-induced apoptosis in K562 cells was suppressed by the addition of 100 μg anti-β2m MoAb derived from hybridoma clone NK2. Anti-β2m MoAbs (clone NK1 and NK3) were the negative controls at the same concentration. (B) β2m-induced apoptosis in CCRF-CEM cells was suppressed by preincubation with anti-β2m MoAb (clone NK2). Apoptotic cells were assayed by TUNEL. Data are expressed as the mean ± SE of 3 independent experiments. (C) The effects of anti-β2m MoAbs for the interaction of biotinilated β2m and the cell surface on CCRF-CEM cells were analyzed using FACS (dotted line, control; thin line, no additive of anti-β2m MoAb; thick line, additive of anti-β2m MoAb).
Inhibition of induction of apoptosis in K562 cells and in CCRF-CEM cells by anti-β2m MoAb. (A) β2m-induced apoptosis in K562 cells was suppressed by the addition of 100 μg anti-β2m MoAb derived from hybridoma clone NK2. Anti-β2m MoAbs (clone NK1 and NK3) were the negative controls at the same concentration. (B) β2m-induced apoptosis in CCRF-CEM cells was suppressed by preincubation with anti-β2m MoAb (clone NK2). Apoptotic cells were assayed by TUNEL. Data are expressed as the mean ± SE of 3 independent experiments. (C) The effects of anti-β2m MoAbs for the interaction of biotinilated β2m and the cell surface on CCRF-CEM cells were analyzed using FACS (dotted line, control; thin line, no additive of anti-β2m MoAb; thick line, additive of anti-β2m MoAb).
β2m-induced apoptosis is mediated by neither the FasL/Fas nor the TNF-α/TNFR systems.
To investigate whether β2m-induced apoptosis is mediated by the FasL/Fas or TNF-α/TNFR systems, we examined the effect of an anti-Fas neutralizing MoAb and an anti-TNFR neutralizing MoAb each at a concentration of 1 μg/mL. Blocking Fas/FasL interaction by an antagonistic anti-Fas MoAb did not interfere with β2m-induced apoptosis in either K562 or CCRF-CEM cells (Fig 5A and B). Similarly, blocking TNF-α/TNFR interaction also did not interfere in either cell line (Fig 5A and B). We propose that β2m-induced apoptosis is dependent on neither the FasL/Fas system nor the TNF-α/TNFR system.
In K562 cells, apoptosis is induced, which is distinct from the interaction of FasL/Fas, TNF-/TNFR, and IL-8/IL-8R. (A and B) After 2 hours of preincubation with 1 μg of anti-Fas MoAb (IgG2b, clone SM1/23) or 1 μg of anti-TNFR(p55) MoAb (IgG), K562 cells (A) and CCRF-CEM cells (B) at a concentration of 1 × 105cells/mL were exposed to 10 μg/mL β2m or at 37°C for 48 hours. β2m-induced apoptosis in both cell lines were prevented by neither anti-Fas MoAb nor anti-TNFR MoAb. (C and D) The preincubations with each 5 μg/mL of anti–IL-8 MoAb and anti–IL-8 receptor antibodies (receptor A [RA] and receptor B [RB]) did not inhibit the induction of apoptosis in K562 cells (C) and in CCRF-CEM cells (D) as described above. In addition, 10 ng/mL of anti-Fas MoAb, 10 ng/mL of recombinant human TNF-, and 20 ng/mL of endothelial IL-8 (data not shown) were used as apoptosis-inducing factors in control studies. Apoptotic cells were assayed by TUNEL. Data are expressed as the mean ± SE of 3 independent experiments.
In K562 cells, apoptosis is induced, which is distinct from the interaction of FasL/Fas, TNF-/TNFR, and IL-8/IL-8R. (A and B) After 2 hours of preincubation with 1 μg of anti-Fas MoAb (IgG2b, clone SM1/23) or 1 μg of anti-TNFR(p55) MoAb (IgG), K562 cells (A) and CCRF-CEM cells (B) at a concentration of 1 × 105cells/mL were exposed to 10 μg/mL β2m or at 37°C for 48 hours. β2m-induced apoptosis in both cell lines were prevented by neither anti-Fas MoAb nor anti-TNFR MoAb. (C and D) The preincubations with each 5 μg/mL of anti–IL-8 MoAb and anti–IL-8 receptor antibodies (receptor A [RA] and receptor B [RB]) did not inhibit the induction of apoptosis in K562 cells (C) and in CCRF-CEM cells (D) as described above. In addition, 10 ng/mL of anti-Fas MoAb, 10 ng/mL of recombinant human TNF-, and 20 ng/mL of endothelial IL-8 (data not shown) were used as apoptosis-inducing factors in control studies. Apoptotic cells were assayed by TUNEL. Data are expressed as the mean ± SE of 3 independent experiments.
β2m-induced apoptosis is independent of IL-8–induced apoptosis.
We previously reported that endothelial IL-8 is purified as an AIF from PDBu-conditioned media of HL-60 cells and induces apoptosis in human leukemic cell lines.40 41 To confirm that β2m induces apoptosis in human leukemic cell lines distinct from the pathway of IL-8–induced apoptosis, we examined the effect of an anti–IL-8 neutralizing MoAb and 2 anti–IL-8 receptors neutralizing MoAbs, each at a concentration of 5 μg/mL. These 3 antibodies could not inhibit β2m-induced apoptosis in either K562 (Fig 5C) or CCRF-CEM cells (Fig 5D). In addition, IL-8 was not detected in the purified human urine-derived β2m by ELISA (data not shown).
β2m induces apoptosis through the caspase cascade.
Recent studies have proposed that various types of apoptosis, such as Fas-mediated apoptosis, depend on the activation of caspases. To investigate whether a caspase cascade participates in β2m-induced apoptosis, we studied the inhibition of apoptosis through pretreatment with caspase inhibitors. Induction of apoptosis in K562 cells was blocked by Ac-DEVD-CHO, Ac-YVAD-CHO, and z-VAD-fmk 3 hours before adding the β2m (Fig 6A). The reduction of β2m-induced apoptosis in K562 cells was efficient in the presence of each of the 3 caspase inhibitors at concentrations of 10 μmol/L (data not shown). Ac-YVAD-CHO significantly inhibited apoptosis at 100 μmol/L, achieving more than 90% inhibition. Simultaneously, preincubation of CCRF-CEM cells with each of the 3 kinds of caspase inhibitors inhibited β2m-induced apoptosis (Fig 6B). The reduction of apoptosis in CCRF-CEM cells from our control was insufficient with concentrations of 10 μmol/L of each inhibitor (data not shown), but effective with 100 μmol/L of each. Z-VAD-fmk almost completely inhibited apoptosis at 100 μmol/L. We concluded that β2m-induced apoptosis depends on the activation of both ICE-like protease and CPP32-like protease.
The effect of Ac-DEVD-CHO (CPP32/Apopain-like protease inhibitor), Ac-YVAD-CHO (ICE-like protease inhibitor), and Z-VAD-FMK (pancaspase inhibitor) on induction of apoptosis by β2m. (A) K562 cells were preincubated for 3 hours in the presence of 3 caspase inhibitors separately, each at 100 μmol/L. After that, these cells were exposed at 1 × 105 cells/mL to 10 μg/mL of β2m at 37°C for 48 hours. (B) CCRF-CEM cells were similarly preincubated with the 3 caspase inhibitors separately and exposed to β2m. Control cells were cultured with the addition of 0.3% DMSO. Apoptotic cells were assayed by TUNEL. Data are expressed as the mean ± SE of 3 independent experiments.
The effect of Ac-DEVD-CHO (CPP32/Apopain-like protease inhibitor), Ac-YVAD-CHO (ICE-like protease inhibitor), and Z-VAD-FMK (pancaspase inhibitor) on induction of apoptosis by β2m. (A) K562 cells were preincubated for 3 hours in the presence of 3 caspase inhibitors separately, each at 100 μmol/L. After that, these cells were exposed at 1 × 105 cells/mL to 10 μg/mL of β2m at 37°C for 48 hours. (B) CCRF-CEM cells were similarly preincubated with the 3 caspase inhibitors separately and exposed to β2m. Control cells were cultured with the addition of 0.3% DMSO. Apoptotic cells were assayed by TUNEL. Data are expressed as the mean ± SE of 3 independent experiments.
Chemical cross-linking of β2m.
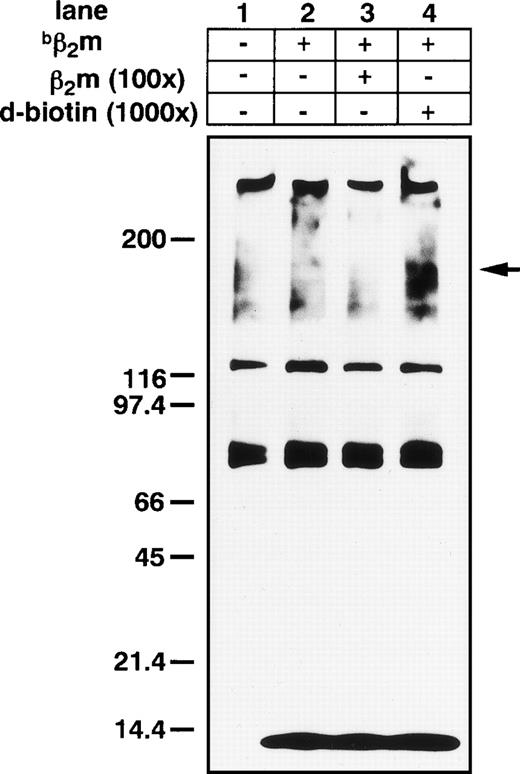
Addition of the amine-reactive, cross-linker, DSS, to Triton-X extracts of K562 cell membranes resulted in the formation of high molecular broad bands (its average complex showed a molecular weight [MW] of 150 kD) that were specifically inhibited by the addition of 100× unlabeled human purified β2m (Fig 7). Using anti-β2m MoAb, Western blot showed an approximately 150-kD β2m-binding protein complex (data not shown).
DSS cross-linking of β2m on the K562 leukemia cell line. Broad high molecular mass complexes of 150 kD (β2m and its binding protein complex) were generated by cross-linking. Several nonspecific bands were also stained; however, a band of appropriately 150 kD was specifically suppressed by the addition of 100× unlabeled β2m in lane 3.bβ2m, biotinilated β2m.
DSS cross-linking of β2m on the K562 leukemia cell line. Broad high molecular mass complexes of 150 kD (β2m and its binding protein complex) were generated by cross-linking. Several nonspecific bands were also stained; however, a band of appropriately 150 kD was specifically suppressed by the addition of 100× unlabeled β2m in lane 3.bβ2m, biotinilated β2m.
DISCUSSION
MHC class I antigens, or HLA antigens, play an important role in tumor immunity. Loss of HLA molecules in B-cell lymphomas is associated with an aggressive clinical effect, such as lung cancer.16,17,46In melanoma, whose tumor cells often lack HLA class I antigen expression, distinct mutations in the β2m gene have been identified.47 Antibodies against MHC class I antigen complexes induced apoptosis in activated T cells and T-cell leukemia cell lines. However, it is still not clear which epitope is responsible for apoptosis and which ligand binds to the responsible molecule on HLA class I antigens. We observed that human β2m itself induced apoptosis in various kinds of leukemic cells and β2m-enhanced antibody induced MHC complex-apoptosis in the T-cell leukemia cell line CCRF-CEM. We used K562 cells as target cells because they showed low background in MTT and TUNEL assay. Although K562 cells have no MHC class I antigen complex (data not shown),48,49 β2m induced apoptosis even in these cells. We strongly suggest that human β2m uses an apoptosis pathway in both T cells and myeloid leukemia cells distinct from the MHC class I antigen complex-induced apoptosis and Fas- or TNFR-mediated apoptosis pathways. Our report is the first evidence demonstrating the existence of human β2m-induced apoptosis. Neutralizing antibodies against β2m resulted in β2m-induced apoptosis being completely blocked. So far, we have not determined the epitopes that might be involved in cell death signaling. Our cross-linking study suggested the presence of a MW 150-kD complex including presumably a binding protein and β2m. Altered expressions of those genes that control apoptosis may lead to autoimmunity, malignant cell growth, neurodegenerative diseases, prolonged survival of cells during latent viral infections, and enhanced cell death during acquired immunodeficiency syndrome (AIDS). For example, Fas-ligand and Fas antigen, which control apoptosis and may lead to autoimmunity, are known to be mutations of lpr and gld, respectively, in mice.21,50-52 Christianson et al53reported that the phenotype in β2m null mice was predisposed to develop chronic lupus erythematosus and to have defective antibody responses. Thus, in addition to the role oflpr and gld in autoimmunity, gene knock-out technology showed the importance of the β2m gene in this disease. Obviously, a defective mechanism in apoptosis leads to autoimmunity, and β2m may be involved. As for the relationship between FasL and Fas antigen, the binding protein for β2m will be important. β2m-deficient and TAP-1–deficient mice show self-tolerance of NK cells due to low expression levels of MHC class I protein.54
TCR antigen-induced cell death occurs from the late G1phase of the cell cycle studies55 and is dependent on pRb (retinoblastoma).55 In the future, we will investigate whether pRb or cdk-2 (cyclin-dependent kinase) activation is also involved in our system.
Compared with the action of the Fas- or TNFR-dependent pathway, the dose and time frame required for β2m-induced apoptosis is large. β2m actions require a specific physiological concentration measured in micrograms (which corresponds to serum levels) to induce apoptosis.
In our system, β2m’s binding protein in the apoptosis pathway or its signaling pathway remains to be clarified. Ceramide, which is known as a second messenger, is produced by caspase-dependent apoptosis after activation by Fas or HLA class I antigen.56In Fas- and HLA class I-mediated peripheral T-cell apoptosis, caspase-dependent ceramide production, which acts downstream of the mitochondria, is important.32 Like the HLA class I-mediated pathway, our system also involves at least 2 different caspases. Table 1 shows the characterization of β2m-induced apoptosis and various types of HLA class I-induced apoptosis. The presence of antibody for HLA class I antigen induced more apoptosis in CCRF-CEM cells by β2m shown in TUNEL assay (data not shown). A recent report suggests that Fas-dependent apoptosis is dependent on ceramide.57
Characterizations of β2m-Induced Apoptosis and HLA Class I-Induced Apoptosis
| No.* . | MoAbs or Reagents . | Identical Epitope† . | Cells or Cell Lines . | Induction of Apoptosis . | Fas- Dependent . | Caspase(s)- Dependent . | Ceramide- Dependent . |
|---|---|---|---|---|---|---|---|
| (1) | Exogenous β2m | ND | K562, U937; CCRF-CEM | (+) | (−) | (+) | ND |
| (2) | Mouse MoAb90 Rat YTH862 | α1 domain | Activated peripheral B and T cells | (+) | (−) | (+) | (+) |
| (3) | Mouse CR11-351 Mouse RG1 | α2 domain | Jurkat E11‡ | (+) | (−) | (−) | ND |
| (4) | Mouse 5H7 | α3 domain | Peripheral T cells Jurkat BevD, JY and DBS-5211-153 14181-154 | (+) | (−) | (−) | (−) |
| (5) | Rabbit antihuman β2m | β2m (HLA class I) | Jurkat | (+) | (−) | (−) | ND |
| (6) | Mouse W6/32 | α2 + α3 domain | K562, CCRF-CEM | (−) | |||
| (7) | Mouse TU1491-155 | ND | K562, CCRF-CEM | (−) |
| No.* . | MoAbs or Reagents . | Identical Epitope† . | Cells or Cell Lines . | Induction of Apoptosis . | Fas- Dependent . | Caspase(s)- Dependent . | Ceramide- Dependent . |
|---|---|---|---|---|---|---|---|
| (1) | Exogenous β2m | ND | K562, U937; CCRF-CEM | (+) | (−) | (+) | ND |
| (2) | Mouse MoAb90 Rat YTH862 | α1 domain | Activated peripheral B and T cells | (+) | (−) | (+) | (+) |
| (3) | Mouse CR11-351 Mouse RG1 | α2 domain | Jurkat E11‡ | (+) | (−) | (−) | ND |
| (4) | Mouse 5H7 | α3 domain | Peripheral T cells Jurkat BevD, JY and DBS-5211-153 14181-154 | (+) | (−) | (−) | (−) |
| (5) | Rabbit antihuman β2m | β2m (HLA class I) | Jurkat | (+) | (−) | (−) | ND |
| (6) | Mouse W6/32 | α2 + α3 domain | K562, CCRF-CEM | (−) | |||
| (7) | Mouse TU1491-155 | ND | K562, CCRF-CEM | (−) |
Abbreviation: ND, not determined.
References: (1), (6), and (7), our study; (2) references 32, 33, 35, and 55; (3) reference 57; (4), references 5 and 39; and (5) references 58 and 59.
HLA class I heavy chain.
HLA-A2–expressing Jurkat cells.
EBV-transformed human lymphoblastoid cell lines.
B-cell line derived from a patient with Niemann-Pick disease.
Mouse monoclonal F(ab′)2 fragment.
Using specific MoAbs against HLA class I, α2 domain, which binds to the TCR, the results of several studies suggest that β2m induces Fas-independent cell death.58 This pathway does not involve ICE-like proteases or CPP32-like proteases.
The cell surface regulator Toso has been discovered to be a regulator of Fas-induced apoptosis in T cells and blocks the Fas pathway.59 In β2m-induced apoptosis, the molecule that blocks the β2m pathway has not yet been identified. A recent study using primary embryonal fibroblasts from transgenic mice expressing murine H-2 and a swine class I transgene, transformed with the highly oncogenic Ad12, showed abnormal turnover of β2m and suggests the existence of a novel β2m-binding molecule that sequesters β2m.60 One or more specific regulators or inhibitors for β2m-induced apoptosis remain to be discovered. However, the signaling pathway is addressed in our system to the extent that β2m-induced apoptosis is a distinct pathway from the Fas-dependent or MHC class I-dependent and the TNFR-dependent pathways of apoptosis.
ACKNOWLEDGMENT
The authors thank S. Kurokawa, K. Sato, and H. Ishikawa for technical assistance and we appreciate Dr Y. Miura.
Supported by a grant-in-aid from the Ministry of Education, Science and Culture of Japan; Research on Advanced Medical Technology, from the Ministry of Health and Welfare; the Japanese Foundation for Multidisciplinary Treatment of Cancer; and Jichi Medical School Young Investigator Award.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kiyohiko Hatake, MD, PhD, Associate Professor, Department of Hematology, Jichi Medical School, 3311-1 Yakushiji, Minamikawachi-machi, Kawachi-gun, Tochigi 329-0498, Japan; e-mail:kiyohiko@jichi.ac.jp.

![Fig. 3. β2m induces apoptosis in other human leukemic and lymphoma cell lines. (A) Control CCRF-CEM cells were cultured without or (B) with 10 μg/mL β2m at 37°C for 48 hours. Apoptotic cells were detected using in situ staining with Apop Tag PLUS (original magnification × 200). (C) Induction of apoptosis by β2m in various cell lines. Nonlymphocytic cell lines (HL-60, NB4, THP-1, U937, and KG-1), B-lymphocytic cell lines (SKW, BALL, and Daudi), and T-lymphocytic cell lines (Jurkat, TALL, and CCRF-CEM) cells (1 × 105 cells/mL) were cultured with 10 μg/mL of β2m at 37°C for each or 48 hours, and TUNEL assays were performed. In the controls of U937 cells and BALL cells, apoptosis was induced in more than 5% of the cells. Therefore, they did not prove useful ([□] control; [▪] additive of β2m). (D and E) Apoptosis in K562 cells (D) and in CCRF-CEM cells (E) was induced by recombinant human β2m in the same way as with urine-derived human β2m.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2744.420k34_2744_2753/7/m_blod42034003w.jpeg?Expires=1767941080&Signature=KejICmn6KaVpsyIo97xMzd7Zminj536De8gV7ID8QtpnvIQcoI6VW4YGbtAxkIcy-b~a2hq~jrQojNZciD3emXjoaYgsRox2LYAXLt7Z08GGIVC-G8Q4YtXRjbJ1B4XdQTKirh5SeYdedHCdNbupO~HQtKDqaeotW65AUwD1KpA7XDmv~Oad16PO913-d5eKEvIFvCXI8Rr6lZo8aFbZNXyll39BBRRFCBxn7xg36JnBiMbFbH1jYJA87-Zp~yMvPBXjATOeUNhgLadlohSoFVPPpqsN2QByGEIK3XAPEGJJPjohTl7IGZnYXIS~VM3h9vJwX7bJKr0-v3ie4chLZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. In K562 cells, apoptosis is induced, which is distinct from the interaction of FasL/Fas, TNF-/TNFR, and IL-8/IL-8R. (A and B) After 2 hours of preincubation with 1 μg of anti-Fas MoAb (IgG2b, clone SM1/23) or 1 μg of anti-TNFR(p55) MoAb (IgG), K562 cells (A) and CCRF-CEM cells (B) at a concentration of 1 × 105cells/mL were exposed to 10 μg/mL β2m or at 37°C for 48 hours. β2m-induced apoptosis in both cell lines were prevented by neither anti-Fas MoAb nor anti-TNFR MoAb. (C and D) The preincubations with each 5 μg/mL of anti–IL-8 MoAb and anti–IL-8 receptor antibodies (receptor A [RA] and receptor B [RB]) did not inhibit the induction of apoptosis in K562 cells (C) and in CCRF-CEM cells (D) as described above. In addition, 10 ng/mL of anti-Fas MoAb, 10 ng/mL of recombinant human TNF-, and 20 ng/mL of endothelial IL-8 (data not shown) were used as apoptosis-inducing factors in control studies. Apoptotic cells were assayed by TUNEL. Data are expressed as the mean ± SE of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2744.420k34_2744_2753/7/m_blod42034005x.jpeg?Expires=1767941080&Signature=SnhnOeUHmMaUCE7PGXjqTTclo-hEU12hGtZVSy3X1GQg62K79W5-NfvK4fcmbUouZsxPAyUhQFKsevK8mWyGyBDMLFvtGwkcN3KKNrmdhc6EFpONiJY8j6W70PmwDEsnMKe5Z4UmAG72PsuP51Jsk3w-9dYVhgHKGNabZUd7GTe2ywf1cj2jmEaIN8v72MDyT5rHRFVrowMAMmV9U4hQEWX9lRnbufaQAMwe33~T2-WYbi7iYulLZyeLwY6MB3lffMj7V~h1dghy6Jvr02pyBwfxPBERCbUrCP8-Ne1luwnlz-A7Z7pz2qshsFA4XDV8C4cpbHQhcZGIyfd8tKzHrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal