Eosinophils, which are prominent cells in asthmatic inflammation, undergo apoptosis and are recognized and engulfed by phagocytic macrophages in vitro. We have examined the ability of human small airway epithelial cells (SAEC) to recognize and ingest apoptotic human eosinophils. Cultured SAEC ingested apoptotic eosinophils but not freshly isolated eosinophils or opsonized erythrocytes. The ability of SAEC to ingest apoptotic eosinophils was enhanced by interleukin-1 (IL-1) or tumor necrosis factor (TNF) in a time- and concentration-dependent fashion. IL-1 was found to be more potent than TNF and each was optimal at 10−10 mol/L, with a significant (P < .05) effect observed at 1 hour postcytokine incubation that was maximal at 5 hours. IL-1 stimulation not only increased the number of SAEC engulfing apoptotic eosinophils, but also enhanced their capacity for ingestion. The amino sugars glucosamine, n-acetyl glucosamine, and galactosamine significantly inhibited uptake of apoptotic eosinophils by both resting and IL-1–stimulated SAEC, in contrast to the parent sugars glucose, galactose, mannose, and fucose. Incubation of apoptotic eosinophils with the tetrapeptide RGDS, but not RGES, significantly inhibited their uptake by both resting and IL-1–stimulated SAEC, as did monoclonal antibody against vβ3 and CD36. Thus, SAEC recognize apoptotic eosinophils via lectin- and integrin-dependent mechanisms. These data demonstrate a novel function for human bronchial epithelial cells that might represent an important mechanism in the resolution of eosinophil-induced asthmatic inflammation.
EOSINOPHILS ARE NOW recognized as major effector cells in the inflammatory process underlying much of the pathogenesis of asthma and other allergic diseases because of their ability to release potent mediators, including cytotoxic cationic proteins, lipid mediators, cytokines, and oxidative metabolites.1-3 Although many of the complex mechanisms involved in their accumulation in the asthmatic lung have been dissected,4,5 the factors responsible for their clearance are less well understood. Apoptosis or programmed cell death (PCD) is an ordered and fundamental biological process designed to dispose safely of surplus, aged, or damaged cells.6,7 Apoptotic cells are phagocytosed whole or as discrete fragments bound by an intact membrane, thus ensuring their disposal without inducing inflammation. Interest in eosinophil PCD has burgeoned,8due in no small part to the fact that elucidation of the mechanisms responsible for the induction and control of apoptosis might enable the development of therapies designed to induce eosinophil PCD, thereby allowing their rapid and safe removal by phagocytes, thus preventing their accumulation and limiting their toxic potential.
Eosinophils exhibit the classical changes in morphology associated with PCD, including cytoplasmic condensation and the internucleosomal cleavage of DNA by endogenous endonucleases. Apoptotic eosinophils are recognized and ingested as intact cells by autologous macrophages, often before any manifest signs of the morphological changes associated with apoptosis.9 Signals that have been shown to induce apoptosis in human eosinophils include withdrawal of viability-enhancing cytokines,10 monoclonal antibody (MoAb)-dependent ligation of Fas11,12 or CD69,13 and treatment with glucocorticoids,14interleukin-4 (IL-4),15 or transforming growth factor β (TGFβ).16 Furthermore, the level of intracellular protein tyrosine phosphorylation also appears to be an important factor in determining whether an eosinophil will survive or undergo PCD.17 Stern et al18 have demonstrated that monocyte-derived human macrophages recognize and ingest apoptotic eosinophils via integrin- and sugar/lectin-dependent mechanisms, a process similar to that described for the neutrophil. Other cells that are not professional phagocytes, such as fibroblasts,19smooth muscle cells,20 or dendritic cells,21have also been shown to have the ability to recognize and ingest apoptotic cells.
The airway epithelium is primarily made up of ciliated, nonciliated, and basal cells. Epithelial cell damage resulting in cilial dysfunction and loss is a major feature of asthma pathogenesis and is thought to be an important contributor to the development of airway hyperresponsiveness. Eosinophil-derived mediators, particularly granule-associated basic proteins such as major basic protein (MBP), have been heavily implicated in this process.22 The accepted view in asthma pathology, therefore, is that the epithelial cell is very much the victim of the eosinophil. Examination of induced sputum has provided evidence that the treatment of exacerbations of asthma with steroids results in the resolution of eosinophilic inflammation by inducing apoptosis in lung eosinophils that were subsequently recognized and phagocytosed by alveolar macrophages.23 The hypothesis addressed in this study therefore is that other resident cells in the asthmatic lung also recognize and phagocytose apoptotic eosinophils and that these might include bronchial epithelial cells. Thus, we have examined the ability of resting and cytokine-stimulated human small airway epithelial cells to recognize and engulf apoptotic eosinophils and investigated which recognition pathways are important in that process.
MATERIALS AND METHODS
Reagents and MoAbs.
Human recombinant human IL-1α (rhIL-1α) and recombinant human tumor necrosis factor α (rhTNFα) were purchased from R&D Systems (Oxon, UK). The tetrapeptides RGDS and RGES, monosaccharides, OPD, and bovine serum albumin (BSA) were supplied by Sigma Ltd (Poole, Dorset, UK). The specific αvβ3 MoAb 23C6,24 the CD36 MoAb SMO,25 anticytokeratin peptide 19, anti–intracellular adhesion molecule-1 (ICAM-1) and isotype-matched negative controls were all purchased from Serotec (Oxon, UK). The anti-MBP MoAb BMK-1326 was a kind gift from Prof Redwan Moqbel (University of Alberta, Edmonton, Alberta, Canada). The polyclonal antihuman erthrocyte membrane, anti-CD9-phyroerythrin (PE), anti-CD44, and the anti-CD11b MoAb were from Dako Ltd (High Wycombe, Bucks, UK). The latter MoAb has been previously shown to block CD11b-dependent eosinophil adhesion to cultured human microvascular endothelial cells.27
Small airway epithelial cells (SAEC) culture.
Small airway human bronchial epithelial cells were purchased as frozen primary cultures from Clonetics Ltd (Walkersville, MD). They were cultured in the supplier’s SAEC basal medium supplemented with gentamicin, amphotericin-B, bovine pituitary extract, hydrocortisone, human epithelial cell growth factor, epinephrine, transferrin, insulin, retinoic acid, triiodothyronine, and bovine serum albumin. Although human SAEC can be maintained in culture for 5 to 6 passages using the supplier’s serum-free medium,28 all experiments presented here were performed with SAEC that had been passaged a maximum of 3 times to ensure no loss of phenotype. These studies used SAEC from 5 different donors and their identity as epithelial cells was guaranteed by the supplier. However, we further confirmed the epithelial identity of SAEC that had undergone 2 or 3 passages by their cobblestone morphology and uniform positive immunostaining with MoAb to the epithelial marker cytokeratin peptide 19, CD9, CD44, and ICAM-1. Furthermore, the supplemented basal epithelial medium used for SAEC culture also inhibits fibroblast growth and fibroblasts do not express CD9. Moreover, we observed no positive immunostaining with MoAb to von Willebrand factor or smooth muscle α actin (both from Novocastra Laboratories Ltd, Newcastle-upon-Tyne, UK), ruling out any contamination of our SAEC cultures by these cell types (data not shown). Cultures were split at 80% to 90% confluence by trypsin digestion and subcultured in the same SAEC medium.
Eosinophil isolation and apoptosis induction.
Blood (100 mL) was obtained from normal donors or individuals with a history of mild allergic disease with an eosinophilia not greater than 0.5 × 106 eosinophils/mL who were not taking any medication at the time of venesection and who gave informed consent. Eosinophils were purified under sterile conditions using our modification of the immunomagnetic-dependent method of Hansel et al,29 which has been described in detail elsewhere.30 Briefly, after removal of red blood cells using dextran sedimentation, the leukocytes were centrifuged on Percoll gradients (Pharmacia Ltd, Beds, UK) at 700g for 20 minutes at 4°C. Contaminating red blood cells in the granulocyte pellet were removed by hypotonic shock with ice-cold, sterile, endotoxin-free, distilled water. Granulocytes were incubated on ice with micromagnetic beads coated with anti-CD16 for 40 minutes before passage through the magnetic-activated separation column (Miltenyi Ltd, Bisley, UK). Using this method, eosinophils with a purity of at least 99% were obtained with greater than 98% viability as assessed by trypan blue exclusion. No more than 3% of the freshly isolated eosinophil preparations displayed any morphological evidence of apoptosis. Flow cytometric analysis performed as previously described31 demonstrated that freshly isolated eosinophils did not bind annexin V-fluorescien isothiocyanate (FITC) and excluded propidium iodide.
Purified eosinophils were washed in RPMI 1640 supplemented with 10% fetal calf serum, antibiotics, and L-glutamine and resuspended in the same medium at a concentration of 1 × 106 cells/mL. Cells were cultured in 1-mL aliquots for 48 hours in a humidified atmosphere with 5% CO2 in flat-bottom 12-well plates (Life Technologies, Paisley, UK) that had been previously coated with BSA (1 mg/mL). After aging, eosinophils were assessed for apoptosis by morphological assessment and binding of annexin V-FITC.30All apoptotic cells used in the interaction/uptake experiments were greater than 70% apoptotic and more than 90% viable as judged by trypan blue exclusion.
Interaction assay.
This was a modification of a previously established assay.32 Before use, secondary passage SAEC were trypsinized and seeded into 24-well plates (Life Technologies) at a concentration of 5 × 105 cells/well and rested for 1 to 2 days before use. At this stage, cells were 80% to 90% confluent in each well as a whole, but were 95% to 100% confluent in the center of the well. After a change of medium, SAEC were stimulated with various concentrations of IL-1α or TNFα for 48, 24, 5, 2, or 1 hour. Apoptotic eosinophils were washed and added to resting or stimulated SAEC at a final concentration of 1 × 106/well and allowed to interact for 60 minutes at 37°C, which was found to be the optimum time for interaction and phagocytosis (Table1), although we did observe evidence that by 60 minutes some ingested eosinophils had started to undergo digestion within the SAEC. Noningested eosinophils were removed by 3 vigorous washes of the SAEC monolayer with phosphate-buffered saline (PBS) supplemented with 0.02 mol/L EDTA using a 1-mL Gilson pipette, and the cells were fixed with 2% gluteraldehyde in PBS. Ingested eosinophils were stained for peroxidase by incubation with o-phenylenediamine dihydrochloride (OPD) dissolved in PBS with hydrogen peroxide. Aged eosinophils could be clearly seen as brown cells within the SAEC. SAEC alone treated with OPD showed no positive peroxidase staining. All experiments were performed in duplicate and at least 200 SAEC were counted using an inverted microscope, and the proportion that had ingested 1 or more eosinophils was expressed as a percentage. In some experiments, the number of ingested apoptotic eosinophils within each resting or cytokine-stimulated SAEC were counted. To confirm that aged eosinophils were indeed ingested by SAEC and not merely adherent to them as a result of nonspecific stickiness, trypsin was added to washed monolayers after the interaction assay, but before the cells were fixed or stained. Cytospins were prepared from the epithelial cell suspensions and the cells fixed as before in gluteraldehyde/PBS and stained with OPD.
Determination of Optimal Timepoint for Uptake of Apoptotic Eosinophils by Unstimulated Monolayers of SAEC (n = 4, ±SEM)
| Time (min) . | % Engulfment by SAEC . |
|---|---|
| 15 | 4 ± 0.9 |
| 30 | 17 ± 2.1 |
| 45 | 21 ± 5.6 |
| 60 | 25 ± 2.6 |
| Time (min) . | % Engulfment by SAEC . |
|---|---|
| 15 | 4 ± 0.9 |
| 30 | 17 ± 2.1 |
| 45 | 21 ± 5.6 |
| 60 | 25 ± 2.6 |
In addition, SAEC were also grown in chamber slides and the following experiments were performed. SAEC that had ingested apoptotic eosinophils were permeabilized and stained with MoAb BMK-13, which is specific for the eosinophil granule protein MBP.26 Ingested aged eosinophils were visualized by the addition of antimouse IgG-FITC before viewing with a fluorescent microscope. Apoptotic eosinophils were also stained with a CD9 MoAb conjugated to PE (CD9-PE) before incubation with SAEC that had been stained with anti-CD44 MoAb and antimouse IgG-FITC before the interaction assay. The 2 cell types were then visualized with a fluorescent microscope. Interaction experiments were also performed with human erythrocytes opsonised with an antierythrocyte membrane polyclonal IgG antibody as previously described.33
Inhibition assays.
For these experiments, resting or cytokine-stimulated SAEC were preincubated for 30 minutes at 37°C with MoAb to αvβ3, CD36, isotype-matched controls at a final concentration of 10 μg/mL. Both untreated and treated SAEC were washed before use. We also assessed the tetrapeptides RGDS or RGES (final concentration, 2 mmol/L) and monosaccharides (final concentration, 25 mmol/L) for their effects on the uptake of apoptotic eosinophils by resting or IL-1α–stimulated SAEC. Aged eosinophils were preincubated for 30 minutes at 37°C with the tetrapeptides or sugars before their use in the interaction assay. We did not observe any increase in the uptake of trypan blue by treated aged eosinophils compared with untreated cells (data not shown). Aged eosinophils were also treated with a known adhesion blocking MoAb to CD11b to control for the possibility that adhesion via this receptor was responsible for apoptotic eosinophil interaction with SAEC.
Measurement of expression of αvβ3 and CD36 by SAEC.
The expression of the integrins αvβ3 and CD36 by resting and IL-1α–stimulated or TNFα-stimulated SAEC were measured using a specific enzyme-linked immunosorbent assay (ELISA) performed as previously described.29 Briefly, SAEC were seeded into 96-well flat-bottomed plates and grown to 90% confluence before stimulation with IL-1α or TNFα (final concentration, 10−10 mol/L for 5 or 20 hours). The resting or cytokine-stimulated SAEC were washed, fixed in 2% gluteraldehyde, and blocked with 0.5% BSA. Specific and control MoAb (10 μg/mL) were added in triplicate and incubated overnight at 4°C. After several washes in PBS, the SAEC were treated with rabbit antimouse IgG conjugated to horseradish peroxidase (HRP) for 30 minutes at room temperature (RT), washed in PBS, and treated with an HRP-conjugated goat antirabbit IgG. After development with OPD, the plate was read on an automated ELISA plate reader at an absorbance of 490 nm.
Statistical analysis.
All data are presented as the mean ± SEM and where n is given this represents the number of experiments each performed in duplicate. Statistical analysis was by the unpaired 2-tailed Student’st-test, where a P value of less than .05 was considered significant.
RESULTS
Resting and cytokine-stimulated SAEC ingest apoptotic eosinophils.
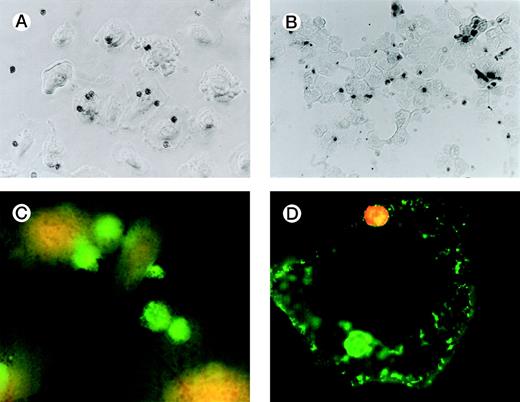
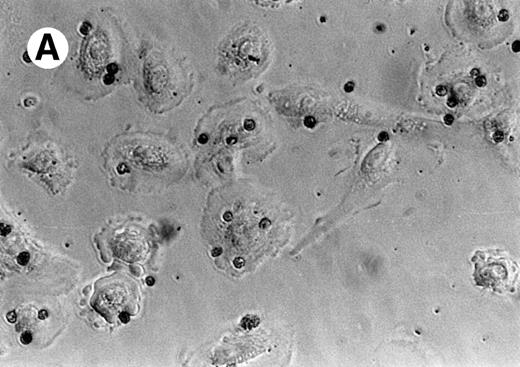
Figure 1A is a photomicrograph of a representative experiment that clearly demonstrates uptake of apoptotic eosinophils by unstimulated SAEC. Several SAEC can be seen to have ingested apoptotic eosinophils visualized as dark staining peroxidase-positive cells. Noningested or adherent aged eosinophils were removed by vigorous washing with PBS/EDTA, as described above. We confirmed that eosinophils had been ingested by addition of trypsin to washed SAEC after their interaction with aged eosinophils. Cytospins of the cell suspension were prepared, fixed, and stained with OPD. Figure1B clearly shows apoptotic peroxidase-positive eosinophils inside SAEC; note the absence of eosinophils on other areas of the slide. We further confirmed our observation by permeabilizing SAEC that had ingested apoptotic eosinophils and adding a MoAb specific for MBP. Stained eosinophils were visualized by the addition of antimouse IgG-FITC before viewing the cells with a fluorescent microscope. Figure 1C clearly demonstrates the presence of bright green-staining apoptotic eosinophils inside SAEC. Figure 1D shows an SAEC stained with CD44 MoAb followed by antimouse IgG-FITC before the addition of apoptotic eosinophils stained with CD9-PE before the interaction assay. In this example, 2 apoptotic eosinophils can be seen. The PE-positive eosinophil is interacting with the SAEC membrane but is not yet completely engulfed and, therefore, shows red fluorescence. The other is within a phagosome within the SAEC and is thus surrounded by a CD44+ membrane and, therefore, exhibits green fluorescence. We observed no evidence of either interaction with or the ingestion of freshly prepared nonapoptotic eosinophils or of human erythrocytes opsonized with IgG by either resting or cytokine-stimulated SAEC (data not shown). Any adhesive interactions between the freshly prepared eosinophils and the resting or cytokine-stimulated SAEC would have been disrupted by a combination of the 3 stringent washes together with the use of 0.02 mol/L EDTA in the washing buffer. Finally, our preliminary experiments determined that the optimal time-course for the engulfment of apoptotic eosinophils by SAEC was 60 minutes (Table 1).
Representative photomicrographs (×400) showing apoptotic eosinophil engulfment by unstimulated (A) SAEC. Eosinophils can be seen inside SAEC as peroxidase-positive dark staining cells. Cytospins were also prepared from SAEC that had ingested aged eosinophils and were removed from their wells by trypsin digestion. Under these conditions (B), OPD-stained apoptotic eosinophils can be seen inside the SAEC, with no eosinophils present on the portions of the slide from which epithelial cells were absent. (C) Representative fluorescent micrograph (×1,000) showing SAEC permeabilized before ingested eosinophils were stained with the anti-MBP MoAb BMK-13, followed by antimouse IgG-FITC. Bright green staining ingested eosinophils can be clearly seen inside the SAEC. (D) Apoptotic eosinophils were also stained with a CD9 MoAb conjugated to PE (CD9-PE) and allowed to interact with SAEC that had been stained with anti-CD44 MoAb and antimouse IgG-FITC. Both cell types were immunostained before the interaction assay (30 minutes) and visualized with a fluorescent microscope. The epithelial cell displays CD44+ green fluorescence. Two apoptotic eosinophils can be seen, both of which were stained with CD9-PE before the interaction assay. The PE-positive eosinophil (top) is interacting with the SAEC membrane but is not yet completely engulfed and therefore shows red fluorescence. The other (bottom) is within a phagosome inside the SAEC and is thus surrounded by a CD44+ membrane and therefore exhibits green fluorescence.
Representative photomicrographs (×400) showing apoptotic eosinophil engulfment by unstimulated (A) SAEC. Eosinophils can be seen inside SAEC as peroxidase-positive dark staining cells. Cytospins were also prepared from SAEC that had ingested aged eosinophils and were removed from their wells by trypsin digestion. Under these conditions (B), OPD-stained apoptotic eosinophils can be seen inside the SAEC, with no eosinophils present on the portions of the slide from which epithelial cells were absent. (C) Representative fluorescent micrograph (×1,000) showing SAEC permeabilized before ingested eosinophils were stained with the anti-MBP MoAb BMK-13, followed by antimouse IgG-FITC. Bright green staining ingested eosinophils can be clearly seen inside the SAEC. (D) Apoptotic eosinophils were also stained with a CD9 MoAb conjugated to PE (CD9-PE) and allowed to interact with SAEC that had been stained with anti-CD44 MoAb and antimouse IgG-FITC. Both cell types were immunostained before the interaction assay (30 minutes) and visualized with a fluorescent microscope. The epithelial cell displays CD44+ green fluorescence. Two apoptotic eosinophils can be seen, both of which were stained with CD9-PE before the interaction assay. The PE-positive eosinophil (top) is interacting with the SAEC membrane but is not yet completely engulfed and therefore shows red fluorescence. The other (bottom) is within a phagosome inside the SAEC and is thus surrounded by a CD44+ membrane and therefore exhibits green fluorescence.
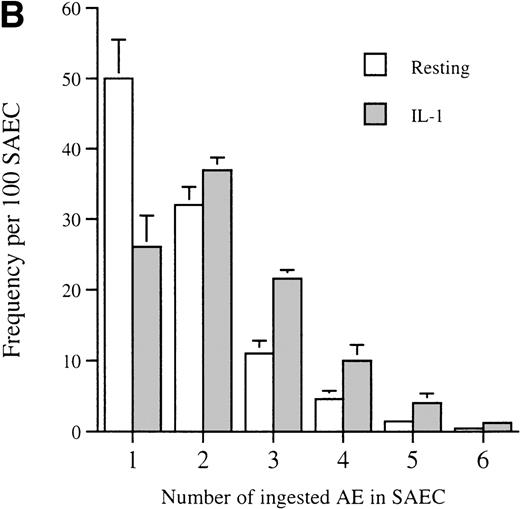
Having made the initial observation that SAEC are indeed capable of ingesting apoptotic eosinophils, the effect on this process of stimulation of SAEC with the proinflammatory cytokine IL-1α was examined. Stimulation with IL-1α resulted in a marked enhancement of the ability of SAEC to ingest apoptotic eosinophils (Fig2A). The proportion of SAEC that ingested eosinophils was enhanced by IL-1α stimulation, as was the ability of individual SAEC to ingest multiples of aged cells (Fig 2B).
(A) Representative experiment demonstrating that IL-1 stimulation (10−10 mol/L) increased the percentage of SAEC that ingested apoptotic eosinophils; (B) together with their capacity to ingest multiples of aged eosinophils. Each bar represents the mean ± SEM of 4 separate experiments in which the number of ingested aged eosinophils inside 200 resting or cytokine-stimulated SAEC were counted.
(A) Representative experiment demonstrating that IL-1 stimulation (10−10 mol/L) increased the percentage of SAEC that ingested apoptotic eosinophils; (B) together with their capacity to ingest multiples of aged eosinophils. Each bar represents the mean ± SEM of 4 separate experiments in which the number of ingested aged eosinophils inside 200 resting or cytokine-stimulated SAEC were counted.
Dose-response and kinetics of stimulation of SAEC with IL-1α or TNFα.
We next assessed the effects on uptake of aged eosinophils by SAEC after stimulation with a dose-range of IL-1α or TNFα. Figure3A shows the effect on the uptake of apoptotic eosinophils by resting SAEC after prestimulation with increasing concentrations of IL-1α or TNFα. The maximal increase in the number of SAEC ingesting apoptotic eosinophils was seen with IL-1α at a concentration of 10−10 mol/L, and this represented an approximate doubling of the numbers of stimulated SAEC capable of ingesting apoptotic eosinophils. Kinetic studies showed that a significant (P < .05) increase in uptake of apoptotic eosinophils by SAEC was observed at 1 hour post–IL-1α incubation, an effect that essentially had plateaued at 5 hours (Fig 3B). TNFα stimulation of SAEC resulted in a more modest, but statistically significant (P < .05), increase in the ability of SAEC to ingest apoptotic eosinophils (Fig 3A). Again, the optimal cytokine concentration was 10−10 mol/L, with the effect plateauing at 2 to 5 hours poststimulation (Fig 3B). In all subsequent interaction experiments that used cytokine-stimulated SAEC, IL-1α was used at a concentration of 10−10 mol/L for 24 hours.
(A) The effect of increasing concentrations of (□) IL-1 and (⧫) TNF on the uptake of aged human eosinophils by SAEC. Each point represents the mean ± SEM of at least 6 experiments. (B) A time course of the effect of stimulation of SAEC with IL-1 and TNF (10−10 mol/L final concentration in each case) on the engulfment of apoptotic eosinophils. In each case, each point represents the mean ± SEM of at least 4 experiments (*P < .05, **P < .001, ***P < .0005).
(A) The effect of increasing concentrations of (□) IL-1 and (⧫) TNF on the uptake of aged human eosinophils by SAEC. Each point represents the mean ± SEM of at least 6 experiments. (B) A time course of the effect of stimulation of SAEC with IL-1 and TNF (10−10 mol/L final concentration in each case) on the engulfment of apoptotic eosinophils. In each case, each point represents the mean ± SEM of at least 4 experiments (*P < .05, **P < .001, ***P < .0005).
The receptors involved in apoptotic eosinophil recognition and engulfment by SAEC.
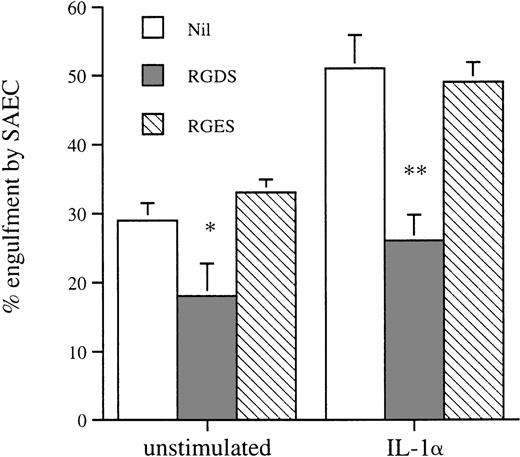
We assessed the effect of amino sugars to confirm that phagocytosis of aged eosinophils by resting and cytokine-stimulated SAEC was a specific receptor-mediated process as described in other phagocytic cells, including macrophages.18 Preincubation of apoptotic eosinophils with the amino sugars glucosamine, n-acetyl glucosamine, and galactosamine significantly inhibited their uptake by both resting and IL-1α–stimulated SAEC. In contrast, the parent sugars glucose, galactose, mannose, and fucose had no measurable inhibitory effect (Fig4). Incubation of apoptotic eosinophils with the tetrapeptide RGDS significantly inhibited their uptake by both resting and IL-1α–stimulated SAEC, whereas the control tetrapeptide RGES had no measurable effect on ingestion of apoptotic eosinophils (Fig 5). These data provided evidence that ingestion of apoptotic eosinophils by resting or IL-1α–stimulated SAEC is dependent on an RGD-dependent signal and that a membrane receptor molecule of the integrin family is involved. The effects of MoAb against αvβ3 and CD36 were therefore assessed, because these have been shown to be important in macrophage recognition of apoptotic eosinophils18 or neutrophils.34 35 Both MoAbs, but not an isotype-matched control or a known adhesion blocking MoAb against CD11b, significantly inhibited ingestion of apoptotic eosinophils by both resting and IL-1α–stimulated SAEC (Fig6). No additive effect was observed either when both MoAbs were used in combination or when either αvβ3 or CD36 MoAb was used in combination with the amino sugars glucosamine, n-acetyl glucosamine, and galactosamine by either resting or IL-1α–treated SAEC (data not shown).
Effect of the pretreatment of apoptotic eosinophils with sugar solutions on their uptake by (□) resting or (▩) IL-1–stimulated SAEC (10−10 mol/L for 20 hours). Washed aged eosinophils were resuspended in Hank’s balanced salt solution (HBSS) containing the sugars indicated at a final concentration of 25 mmol/L before use in the interaction assay. Each point represents the mean ± SEM of 4 experiments (*P < .05, **P < .001).
Effect of the pretreatment of apoptotic eosinophils with sugar solutions on their uptake by (□) resting or (▩) IL-1–stimulated SAEC (10−10 mol/L for 20 hours). Washed aged eosinophils were resuspended in Hank’s balanced salt solution (HBSS) containing the sugars indicated at a final concentration of 25 mmol/L before use in the interaction assay. Each point represents the mean ± SEM of 4 experiments (*P < .05, **P < .001).
The effect of the tetrapeptides RGDS and RGES on the recognition and engulfment of apoptotic eosinophils by resting or IL-1–stimulated SAEC (10−10 mol/L for 20 hours). Washed apoptotic eosinophils were resuspended in HBSS containing the tetrapeptides to give a final concentration of 2 mmol/L before use in the interaction assay. Each point represents the mean ± SEM of 5 experiments (*P < .05, **P < .001).
The effect of the tetrapeptides RGDS and RGES on the recognition and engulfment of apoptotic eosinophils by resting or IL-1–stimulated SAEC (10−10 mol/L for 20 hours). Washed apoptotic eosinophils were resuspended in HBSS containing the tetrapeptides to give a final concentration of 2 mmol/L before use in the interaction assay. Each point represents the mean ± SEM of 5 experiments (*P < .05, **P < .001).
The effect of preincubation of resting or IL-1–stimulated SAEC (10−10 mol/L for 20 hours) with isotype-matched (□) control or MoAb to the () VNR, (▧) CD36, or (▩) CD11b before their interaction with washed apoptotic eosinophils. All MoAbs were used at a final concentration of 50 μg/well. Each point represents the mean ± SEM of 4 experiments (*P < .05, **P < .001).
The effect of preincubation of resting or IL-1–stimulated SAEC (10−10 mol/L for 20 hours) with isotype-matched (□) control or MoAb to the () VNR, (▧) CD36, or (▩) CD11b before their interaction with washed apoptotic eosinophils. All MoAbs were used at a final concentration of 50 μg/well. Each point represents the mean ± SEM of 4 experiments (*P < .05, **P < .001).
Expression of αvβ3 and CD36 by resting or cytokine-stimulated SAEC.
To assess whether the observed increase in the capacity of cytokine-stimulated SAEC to ingest aged eosinophils was due to increased expression of αvβ3 or CD36, we performed specific ELISAs on resting and IL-1α–stimulated or TNFα-stimulated SAEC derived from 3 different donors. We observed modest but consistent expression of αvβ3 by resting SAEC comparable to that of ICAM-1, whereas expression of CD36 was somewhat higher. Incubation of the SAEC with either IL-1α or TNFα for 5 hours did not result in a significant increase in the expression of αvβ3 or CD36, although expression of ICAM-1 was enhanced to a modest degree (Table2). Near identical results were obtained when the incubation time with either IL-1α or TNFα was extended to 24 hours (data not shown).
ELISA Demonstrating Expression of vβ3, CD36, or ICAM-1 by Resting and IL-1–Stimulated or TNF-Stimulated SAEC (10−10 mol/L Final Concentration for 5 Hours)
| . | Isotype Control . | αvβ3 . | CD36 . | ICAM-1 . |
|---|---|---|---|---|
| Resting SAEC | 0.07 ± 0.02 | 0.16 ± 0.02* | 0.26 ± 0.05* | 0.16 ± 0.02* |
| SAEC + IL-1α | 0.07 ± 0.02 | 0.16 ± 0.02* | 0.29 ± 0.06* | 0.21 ± 0.01† |
| SAEC + TNFα | 0.08 ± 0.02 | 0.15 ± 0.005* | 0.24 ± 0.04* | 0.20 ± 0.006† |
| . | Isotype Control . | αvβ3 . | CD36 . | ICAM-1 . |
|---|---|---|---|---|
| Resting SAEC | 0.07 ± 0.02 | 0.16 ± 0.02* | 0.26 ± 0.05* | 0.16 ± 0.02* |
| SAEC + IL-1α | 0.07 ± 0.02 | 0.16 ± 0.02* | 0.29 ± 0.06* | 0.21 ± 0.01† |
| SAEC + TNFα | 0.08 ± 0.02 | 0.15 ± 0.005* | 0.24 ± 0.04* | 0.20 ± 0.006† |
Data (±SEM) are from 3 separate experiments that used SAEC from different donors, each performed in triplicate.
P < .01.
P < .005.
DISCUSSION
In the present study, we demonstrate for the first time that human SAEC recognize and ingest apoptotic eosinophils via specific recognition mechanisms and that this process is upregulated by the proinflammatory cytokine IL-1α and, to a more modest degree, TNFα. We have used several different approaches to confirm that apoptotic eosinophils were engulfed by SAEC and that internalized eosinophils are within phagosomes (Fig 1D). This might represent an additional pathway by which intact apoptotic tissue eosinophils are safely removed from the tissues surrounding the airways, thus preventing in situ leakage of their ubiquitous, granule-derived, highly cytotoxic proteins. It can be envisaged that failure to remove apoptotic eosinophils might result in secondary necrosis and subsequent leakage of their potent proinflammatory mediators, thereby making a major contribution to asthma pathogenesis. Thus, induction of apoptosis in eosinophils and their subsequent removal by macrophages and resident cells such as epithelial cells represents a potentially important therapeutic strategy in asthma treatment. Moreover, our observation that stimulation of SAEC with IL-1α or TNFα enhances their phagocytic capacity for apoptotic eosinophils is interesting in the light of the fact that these same cytokines are thought to promote eosinophil accumulation through upregulation of endothelial adhesion receptors, most notably vascular cell adhesion molecule-1 (VCAM-1), which interacts with eosinophil VLA-4.36 However, because inflammation is normally a beneficial and self-limiting response, it would make sense that the cytokines involved in eosinophil accumulation would also prepare resident cells to remove apoptotic eosinophils, a process analogous to that described for clearance of apoptotic neutrophils by cytokine-stimulated monocyte-derived macrophages.37
The mechanisms by which apoptotic cells are recognized appears to vary according to the cell type responsible for their engulfment. To date, at least 4 recognition mechanisms for apoptotic cells have been described: (1) an uncharacterized lectin-dependent interaction38; (2) a complicated charge-sensitive process involving the CD36/vitronectin receptor complex (αvβ3) on the macrophage surface interacting with unknown moieties on the apoptotic neutrophil surface via a thrombospondin bridge34,35; (3) a stereo-specific recognition of phosphatidylserine that is expressed on the surface of the apoptotic cell after the loss of membrane asymmetry39,40; and (4) macrophage scavenger receptors.41,42 In the present study, we observed that, in some respects, SAEC recognition of apoptotic eosinophils is similar to that of the human monocyte-derived macrophage reported by Stern et al.18 We found that the amino sugars glucosamine, n-acetyl glucosamine, and galactosamine significantly inhibited uptake of aged human eosinophils by both resting and IL-1α–stimulated SAEC, as did RGD-containing tetrapeptides. MoAbs to both αvβ3 and CD36 inhibited the phagocytic process. However, data from a sensitive ELISA indicated that the increased capacity of SAEC to ingest aged eosinophils after stimulation with either IL-1α or TNFα did not appear to be a consequence of increased expression of either αvβ3 or CD36, both of which had low but consistent constitutive expression by SAEC. These data suggest that factors other than a simple increase in the expression of αvβ3 or CD36 are involved, such as a conformational change in these receptors leading to increased affinity for their ligand. Expression of αvβ3 or CD36 has not been reported in studies that have examined the expression of integrins by the bronchial epithelium.43 The possibility that expression of αvβ3 or CD36 by SAEC is an artefact of their culture in vitro cannot therefore be excluded, although uniform positive immunostaining with MoAb to cytokeratin peptide 19, CD9, CD44, and ICAM-1 confirmed the epithelial identity of the SAEC. Moreover, bronchial airway epithelial cell expression of integrins is complex and, in particular, appears dependent on a number of diverse factors, including exposure to pollutants such as ozone, malignancy, and their stage of development or repair.44-46 Thus, other receptors, including αvβ3 or CD36, might also be expressed by the bronchial epithelium under certain circumstances. Our data suggest that both resting and cytokine-activated SAEC recognize apoptotic eosinophils via VnR-dependent and sugar-lectin–dependent mechanisms. However, it remains to be determined whether other receptors shown to be involved in both professional and nonprofessional phagocyte recognition and engulfment of apoptotic cells, including CD14,47,48CD68,49 or the ABC1 transporter system,50 might also be involved in the recognition of apoptotic eosinophils by SAEC.
Macrophages are thought to be one of the most important and efficient cells in the recognition and engulfment of apoptotic cells. These professional phagocytes have the ability to ingest multiples of apoptotic cells that, combined with rapid digestion of their apoptotic meal, probably explains much of their efficiency.51 A number of studies have demonstrated that several nonprofessional phagocytic cell types are also capable of recognizing and ingesting apoptotic cells.19-21 Moreover, a recent report has confirmed that dendritic cells recognize and ingest apoptotic cells via the scavenger receptor CD36 and also use a receptor not expressed by macrophages, namely αvβ5,52 whereas human liver Kupffer cells phagocytose apoptotic lymphocytes via lectin-dependent recognition of increased expression of n-acetylgalactosamine, D-galactose, and mannose residues.53 Hall et al19 have shown that human fibroblasts, including those derived from the lung, recognize and ingest apoptotic neutrophils and that this involves the participation of the vitronectin receptor and a mannose-/fucose-specific lectin. However, although SAEC also use the vitronectin receptor in recognition of apoptotic eosinophils, we did not observe a role for the mannose-/fucose-specific lectin, because neither sugar had any inhibitory effect on recognition or engulfment. Moreover, these workers did not observe any uptake of apoptotic neutrophils by human epidermal keratinocytes or mammary epithelial cells. These and the observations presented here suggest that epithelial cells at different organ sites vary in their ability to recognize and engulf apoptotic cells. The reasons for such differences are unclear. We also observed variation in the ability of SAEC to ingest more than 1 apoptotic eosinophil. This was particularly marked after IL-1α stimulation, with the majority of SAEC ingesting 2 or 3 aged eosinophils, whereas most unstimulated SAEC had only 1 or 2 ingested cells. The most likely explanation for this observation is the nature of the conditions required for the culture of SAEC. All of the experiments presented in this study were performed with SAEC that were 80% to 90% confluent. Culturing SAEC to 100% confluence is not possible, because this results in contact-dependent cell morbidity and loss. Thus, a minority of the SAEC in the monolayers used in these studies were at different stages of cell division and/or maturity that might explain in part the variability in their ability to ingest apoptotic eosinophils.
The present study has focused on clearance of apoptotic eosinophils by human small airway epithelial cells given the potential relevance of this process to asthma pathology. We therefore did not examine the ability of SAEC to ingest apoptotic neutrophils and neither did we examine the engulfment ability of large airway epithelial cells. Apoptosis is associated with the swift recognition of intact cells by macrophages or resident cells followed by their engulfment and degradation with the result that detection in vivo can be difficult. This fact is the most likely explanation for the reason that, to date, there have been no reports of the presence of apoptotic eosinophils inside epithelial cells in, for example, bronchial biopsies from patients with mild asthma.
Apoptotic cells are recognized and cleared by macrophages or nonprofessional phagocytes by a specific recognition process. Crucially, not only does the rapid phagocytosis of apoptotic cells prevent local tissue injury or inflammation, but it also suppresses activation of the macrophages’ usual proinflammatory secretory response.54 Similarly, engulfment of apoptotic eosinophils exerts profound effects on the functional ability of the ingesting macrophage, inducing an anti-inflammatory cytokine and mediator secretory profile, ie, TGFβ and prostaglandin E2. In contrast, ingestion of necrotic eosinophils induces a proinflammatory cytokine and mediator profile, ie, release of thromboxane B2 and granulocyte-macrophage colony-stimulating factor (GM-CSF).18 A number of studies have demonstrated that airway epithelial cells are also potent sources of proinflammatory substances, including cytokines and chemokines.55 The question as to whether ingestion of apoptotic eosinophils by SAEC modulates their ability to release chemokines and/or cytokines will be the subject of a future report.
In summary, we have demonstrated that human SAEC are capable of recognizing and ingesting apoptotic eosinophils and that this process can be enhanced by stimulation of SAEC with the proinflammatory cytokines IL-1α and, to a lesser extent, TNFα. The membrane receptors involved in SAEC recognition of apoptotic eosinophils appear similar to those reported for human monocyte-derived macrophages. These observations demonstrate that, together with macrophages and other resident cells, human SAEC might be active participants in the removal of apoptotic eosinophils and therefore play an important role in the resolution of eosinophilic inflammation in the asthmatic lung.
ACKNOWLEDGMENT
The authors thank Prof Andy Rees for his valuable comments on the manuscript and Sharon Gordon for providing some of the eosinophil preparations.
Supported by the Wellcome Trust (044988/2/95/2).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Garry M. Walsh, PhD, Department of Medicine & Therapeutics, IMS Building, University of Aberdeen, Foresterhill, Aberdeen AB25 2ZD, UK; e-mail: g.m.walsh@abdn.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal