Abstract
Primary effusion lymphoma (PEL) is a distinct type of lymphoma associated with Kaposi’s sarcoma-associated herpesvirus (KSHV) infection. To determine the factors responsible for the unrestrained proliferation of PEL, we have studied the growth factor requirements of the PEL-derived BCBL-1 and BC-1 cell lines. Both cell lines were found to be autocrine growth factor dependent and to release human interleukin-6 (IL-6), viral IL-6 (vIL-6), and human IL-10 in the culture supernatant. To establish whether these cytokines contribute to autocrine growth, neutralizing antibodies against human IL-6, vIL-6, human IL-10, and soluble IL-10 receptor were used. These experiments showed that human IL-10 and, to a lesser degree, vIL-6 serve as autocrine growth factors for BCBL-1 and BC-1 cells. Thus, human IL-10 and vIL-6 are growth factors released and used by PEL cells for autonomous proliferation and may be critical to the development and progression of PEL.
BODY CAVITY-BASED/PRIMARY effusion lymphoma (PEL) is a rare and distinct type of non-Hodgkin’s B-cell lymphoma occurring in patients with acquired immunodeficiency syndrome (AIDS) and some human immunodeficiency virus (HIV)-negative individuals that is typically associated with Kaposi’s sarcoma-associated herpesvirus (KSHV; also known as herpes virus 8 [HHV-8]) infection.1-6 Characteristic features of this lymphoma include involvement of the pleural, pericardial, and abdominal cavities as lymphomatous effusions in the absence of a solid tumor mass, B-cell lineage of the lymphoma cells based on Ig gene rearrangement, and, in most cases, coinfection with Epstein-Barr virus (EBV).5,7,8The lymphoma cells display immunoblastic morphology, express the cell activation CD38 marker, and are generally monoclonal. They also generally fail to express B-cell–associated surface antigens, lack rearrangements of the c-myc gene, and do not display alterations of the bcl-2, ras, and p53 genes.3,8 9
The presence of KSHV in PEL has raised questions on the virus’ potential pathogenetic role. In addition to its association with PEL, KSHV is regularly found in all types of Kaposi’s sarcoma and is believed to infect asymptomatically a proportion of normal individuals. Unlike 2 other gammaherpesviruses, EBV and Herpesvirus Saimiri, KSHV has not been previously shown to immortalize or reproducibly infect normal cells in vitro.10,11 However, many spontaneous cell lines have been derived from PEL that grow autonomously in vitro.6 12-14 Reflecting the infectious status of the original lymphomas, some of these cell lines are coinfected with KSHV and EBV, whereas others are infected with KSHV alone.
Previous studies on EBV-infected B cell lines have shown that growth and survival of these cells is dependent on autocrine and/or paracrine growth factors.15,16 Human interleukin-6 (IL-6) and IL-10 are present in the culture supernatants of EBV-infected cells and play an important role in promoting the continuous proliferation of EBV-immortalized cells in vitro.16-18 Patients with posttransplant lymphoproliferative syndrome, an illness attributed to the unbridled expansion of B cells latently infected with EBV, often display abnormally high levels of circulating IL-6 and IL-10.19,20 In addition, IL-10 was reported to serve as an autocrine growth factor for AIDS-related B-cell lymphoma.21
Efforts aimed at identifying factors responsible for PEL autonomous growth in vitro have suggested a role for human IL-6 based on experiments with antisense oligonucleotides.22 However, antisense oligonucleotides containing 4 contiguous guanosine residues, such as the human IL-6 antisense oligonucleotide used in these experiments, can inhibit cell proliferation via a hybridization-independent mechanism.23 In addition, because PEL cell growth was not inhibited by neutralizing antibodies against human IL-6 or stimulated by IL-6 protein added to the cells, the role of human IL-6 as a growth or survival factor for PEL is uncertain.22 In particular, KSHV is known to code for viral IL-6 (vIL-6), a cytokine that shares 24.7% amino acid identity with human IL-6.24,25 Like human IL-6, vIL-6 can support the growth of the murine hybridoma B9 cell line and the human myeloma INA-6 cell line, albeit at concentrations approximately 1,000-fold greater than human IL-6.24
In this study, we have examined the potential contribution of vIL-6 and other growth factors to the growth and survival of PEL cells.
MATERIALS AND METHODS
Cell lines.
The PEL-derived BCBL-16,11 and BC-111 cell lines and the murine Ad5mE526 B-cell line were maintained in RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 2 mmol/L L-glutamine (Life Technologies, Grand Island, NY), 10% fetal bovine serum (BioWhittaker), and 5 μg/mL gentamicin (Life Technologies).
Reagents.
The human cytokines IL-1β, tumor necrosis factor α (TNFα), IL-4, transforming growth factor β (TGFβ), and IL-10 were purchased from R&D Systems, Inc (Minneapolis, MN); IL-6 was either a gift from Sandoz Pharmaceutical Co (Basel, Switzerland) or was purchased from R&D Systems, Inc. Purified recombinant vIL-6 (MBP-vIL-6) was obtained fromEscherichia coli-expressing a fusion protein of vIL-6 (amino acids 22-204) and a maltose binding protein (MBP), as described.27 Neutralizing rat monoclonal antibodies (MoAbs) against human IL-10 (antibody 19F1) and an isotype control rat MoAb (R35.95) were purchased from Pharmingen (San Diego, CA). A neutralizing rabbit antibody against vIL-6 was obtained by immunizations with recombinant purified vIL-6 (MBP-vIL-6; 1 mg/injection) and subsequent serum purification over protein G column (Amersham Pharmacia Biotech, Piscataway, NJ), following the manufacturer’s instructions. A neutralizing mouse MoAb against human IL-6 (6709.111) was purchased from R&D Systems, Inc. Soluble human IL-10 receptor was obtained from R&D Systems, Inc.
Cytokine measurements.
Conditioned media from the BCBL-1 and BC-1 cell lines were tested by enzyme-linked immunosorbent assay (ELISA) for the human cytokines human IL-1β, IL-6, leukemia inhibitory factor (LIF), and IL-10 using commercially available kits from R&D Systems, Inc. The sensitivity of detection for IL-1β, IL-6, and IL-10 ranged between 20 and 40 pg/mL. Viral IL-10 was measured as previously described28 using the rat anti–vIL-10 6B11 MoAb that recognizes viral but not human IL-10. The sensitivity of vIL-10 detection was estimated to be 20 to 25 pg/mL. IL-6 bioactivity was measured by the B9 cell proliferation assay, as described previously.29 The lower limit of sensitivity of the B9 assay was estimated to correspond to 15 pg/mL human IL-6. One unit of B9 activity, defined as the activity stimulating 1 half-maximal cell proliferation, corresponds to approximately 20 pg/mL human IL-6. Viral IL-6 was detected by Western blotting, using a rabbit antiserum raised against recombinant vIL-6.
Flow cytometric analysis.
Biotinylated human IL-10 (1.5 mg/mL), human IL-6 (1.5 mg/mL), vIL-6 (MBP-vIL-6, 1.5 mg/mL), MBP (1.5 mg/mL), or biotinylated control protein (soybean trypsin inhibitor, 1.5 mg/mL) was added to 1 × 105 IgG-treated cells and incubated for 60 minutes at 4°C. After incubation, streptavidin was added to the cells (10 μg/mL), and incubation was continued at 4°C for 30 minutes in the dark. After washing, the cells were analyzed by flow cytometric analysis (FACScan flow cytometer). Biotinylation of vIL-6 (MBP-vIL-6), human IL-6, and MBP was performed using EZ-Link Sulfo-NHS-LC-Biotinylation Kit (Pierce, Rockford, IL). Reagents for human IL-10 receptor detection were purchased from R&D Biosystems, Inc.
Preparation of autologous conditioned media.
BCBL-1 and BC-1 cells obtained 48 to 72 hours after subculture were washed free of serum (6 washes in RPMI 1640 medium), suspended at 2 × 106 cells/mL in culture medium (consisting of RMPI 1640 medium [BioWhittaker] supplemented with 0.25 mg/mL bovine serum albumin [Boehringer Mannheim, Indianapolis, IN], 2.5 mg/mL transferrin [Sigma Chemical Co, St Louis, MO], and 5 mg/mL gentamicin [Life Technologies]), and incubated for 48 hours in 25-cm2 culture flasks (Corning, Corning, NY). At the end of incubation, culture supernatants were harvested by centrifugation, filtered, and frozen at −30°C until use.
Western blot analysis.
Aliquots (0.1 to 1 mL) of conditioned media, prepared in serum-free conditions as described above and then subjected to deoxycholate trichloroacetic acid (DOC-TCA) precipitation, cell lysates from 1 × 105 cells, or purified proteins (vIL-6, human IL-6, human IL-1α, human TGFβ, and human TNFα) were solubilized in tricine sodium dodecyl sulfate (SDS) sample buffer (Novex, San Diego, CA), boiled, and run through 10% to 20% tricine gels (Novex). Protein was then transferred onto Immobilon-P membranes (Millipore Corp, Bedford, MA). Membranes were reacted with a rabbit anti–vIL-6 antiserum (1:1,000 dilution); bound antibody was detected with an affinity-purified, peroxidase-linked, donkey antirabbit IgG antibody (Amersham Pharmacia Biotech) and a chemiluminescence detection system (ECL kit; Amersham Pharmacia Biotech). For detection of human IL-6 (recombinant human IL-6, 100 ng), the membranes were reacted with a biotinylated, affinity-purified goat antihuman IL-6 antiserum (R&D Systems), followed by a streptavidin-horseradish-peroxidase–conjugated antigoat IgG antibody (Life Technology) and a chemiluminescence detection system (ECL kit).
Cell proliferation assays.
Exponentially growing BCBL-1 and BC-1 cells were first washed free of serum, suspended in RMPI 1640 medium (BioWhittaker) supplemented with 0.25 mg/mL bovine serum albumin (Boehringer Mannheim), 2.5 μg/mL transferrin (Sigma Chemical Co), and 5 μg/mL gentamicin (Life Technologies) and then incubated (5 to 10 × 103cells/well) alone or with additives in microtiter culture plates (Costar, Cambridge, MA) for 3 days. DNA synthesis was measured by3H thymidine deoxyribose uptake (0.5 mCi/well, 6.7 Ci/mmol; New England Nuclear, Boston, MA) during the last 18 to 20 hours of culture. The results of proliferation assays were expressed as the mean radioactivity (± standard deviation) of triplicate cultures.
Cytokine neutralization assays.
Exponentially growing BCBL-1 and BC-1 cells that had been washed free of serum were incubated (5 to 10 × 103 cells/well for 3 days) in microtiter culture wells (Costar) in RMPI 1640 medium (BioWhittaker) supplemented with 0.25 mg/mL bovine serum albumin (Boehringer Mannheim), 2.5 μg/mL transferrin (Sigma Chemical Co), and 5 μg/mL gentamicin (Life Technologies) alone or with the addition of autologous conditioned medium (prepared as described above) with or without the addition of purified neutralizing antibodies (1 to 10 μg/mL) against various cytokines or soluble human IL-10 receptor (0.1 to 0.2 μg/mL). The specificity of reactions was tested by addition of the appropriate cytokines (1 to 10 ng/mL) to the neutralized cultures. Cell proliferation was measured by 3H thymidine deoxyribose uptake, as described above.
RESULTS
Dependency of body cavity lymphoma cell lines on autocrine growth factors.
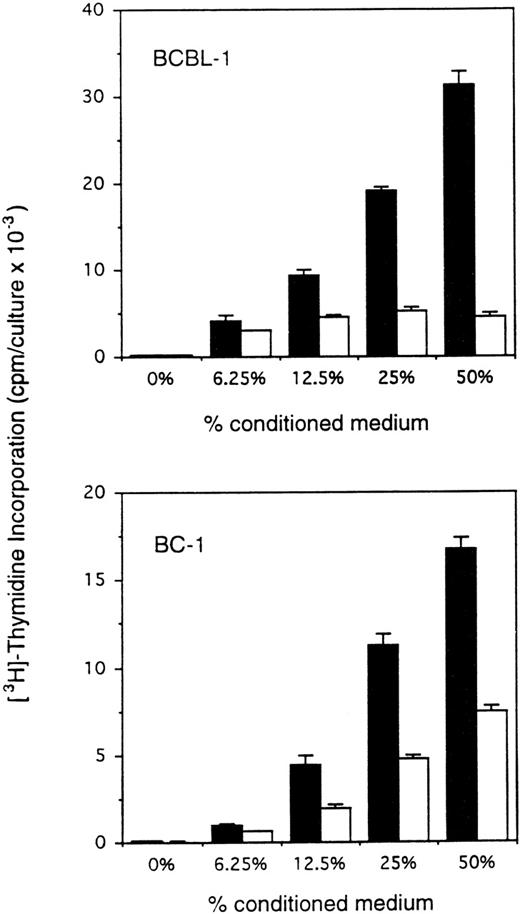
Body cavity-based/PEL-derived cell lines are either infected with HHV-8 alone or with HHV-8 plus EBV. We therefore selected the HHV-8–infected BCBL-111 and the HHV-8 plus EBV-infected BC-114cell lines. To test for autocrine growth factor dependency, PEL cells were cultured under conditions that minimize the contribution of autocrine growth factors, including low cell densities (2.2 to 10 × 104 cells/mL) and restricted culture medium (RPMI 1640 culture medium supplemented with 0.25 mg/mL bovine serum albumin and 2.5 μg/mL transferrin). Conditioned medium was prepared by incubation of PEL cell lines and a control murine B-cell line (Ad5mE5) at 2 × 106 cells/mL in RPMI 1640 culture medium supplemented with 0.25 mg/mL bovine serum albumin and 2.5 μg/mL transferrin for 2 days. When incubated in medium alone (RPMI 1640 medium supplemented with 0.25 mg/mL bovine serum albumin and 2.5 μg/mL transferrin) for 3 days at low cell densities, BCBL-1 and BC-1 cells showed minimal spontaneous proliferation (Fig 1). However, when incubated with autologous conditioned medium under otherwise identical conditions, BCBL-1 and BC-1 cells showed a dose-dependent increase in proliferation (Fig 1). Conditioned medium from a control murine B-cell line prepared under identical conditions had variable stimulatory effects on BCBL-1 and BC-1 cells (Fig 1). These experiments demonstrate that BCBL-1 and BC-1 cells are dependent on autocrine growth factors for sustained growth.
Enhanced proliferation of BCBL-1 and BC-1 cell lines in response to autologous conditioned medium. The cell lines BCBL-1 and BC-1 in exponential growth phase (5 × 103 and 10 × 103/0.2 mL flat-bottom microwell, respectively) were cultured for 3 days in RPMI 1640 medium supplemented with either fresh medium alone or medium that had previously been conditioned for 24 hours by either the autologous (BCBL-1 or BC-1; ▪) or control (murine AdmEm5; □) cells seeded at 2 × 106 cells/mL in tissue culture flasks. 3H thymidine was added during the final 18 hours of culture. The results represent the mean radioactivity (±SD) of triplicate cultures. Shown is a representative experiment of 9 performed.
Enhanced proliferation of BCBL-1 and BC-1 cell lines in response to autologous conditioned medium. The cell lines BCBL-1 and BC-1 in exponential growth phase (5 × 103 and 10 × 103/0.2 mL flat-bottom microwell, respectively) were cultured for 3 days in RPMI 1640 medium supplemented with either fresh medium alone or medium that had previously been conditioned for 24 hours by either the autologous (BCBL-1 or BC-1; ▪) or control (murine AdmEm5; □) cells seeded at 2 × 106 cells/mL in tissue culture flasks. 3H thymidine was added during the final 18 hours of culture. The results represent the mean radioactivity (±SD) of triplicate cultures. Shown is a representative experiment of 9 performed.
Expression of human IL-10, human IL-6, and vIL-6 by PEL cells.
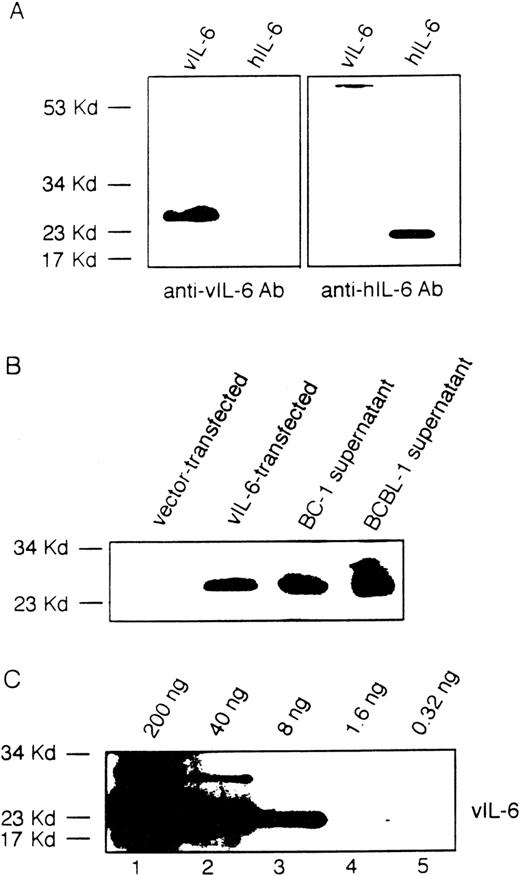
To identify potential compounds responsible for autocrine growth factor activity, we first measured by ELISA the levels of selected cytokines in the conditioned media from BCBL-1 and BC-1 cell lines. In representative determinations (Table 1), human IL-10 was detected at the concentration of 20.8 ng/mL in BCBL-1 and at 3.3 ng/mL in BC-1 conditioned media; and human IL-6 was detected at 116.0 pg/mL in BCBL-1 and at 12.7 ng/mL in BC-1 conditioned media. However, vIL-10 (assay sensitivity, 10 × 10−3U/mL; corresponding to ∼20 to 25 pg/mL), IL-1β (assay sensitivity, ∼35 pg/mL), and LIF (assay sensitivity, ∼50 pg/mL) were not detectable in BCBL-1 and in BC-1 conditioned media. Because of a lack of suitable reagents, vIL-6 cannot currently be measured by ELISA. Instead, we used a rabbit antiserum raised against recombinant vIL-6 that recognizes vIL-6 but not human IL-6 (Fig 2A), IL-1α, IL-10, TGFβ, or TNFα (not shown) in immunoblotting. Using this method, vIL-6 was detected in the culture supernatants of BCBL-1 and BC-1 cells (Fig 2B). Using various concentrations of recombinant purified vIL-6 in Western blotting (Fig 2C), we estimated the content of vIL-6 in culture supernatants of BC-1 and BCBL-1 cell lines to range between 5 and 20 ng/mL. Consistent with these results, BCBL-1 and BC-1 culture supernatants contained IL-6 bioactivity (42 and >100 IL-6 units/mL, respectively), as measured by the B9 cell proliferation assay that can detect both cellular and vIL-6. These results demonstrate that conditioned media from BCBL-1 and BC-1 cells contain human IL-10, human IL-6, and vIL-6.
Detection of Selected Cytokines in BCBL-1 and BC-1 Culture Supernatants
| Cytokine . | Cytokine Concentration (pg/mL) . | |
|---|---|---|
| BCBL-1 . | BC-1 . | |
| Human IL-10 | 20,800 | 3,300 |
| Human IL-6 | 116 | 12,700 |
| Human IL-1β | <20 | <20 |
| Human LIF | <30 | <30 |
| Viral IL-10 | <25 | <25 |
| Cytokine . | Cytokine Concentration (pg/mL) . | |
|---|---|---|
| BCBL-1 . | BC-1 . | |
| Human IL-10 | 20,800 | 3,300 |
| Human IL-6 | 116 | 12,700 |
| Human IL-1β | <20 | <20 |
| Human LIF | <30 | <30 |
| Viral IL-10 | <25 | <25 |
Conditioned media from the BCBL-1 and BC-1 cell lines was tested by ELISA for the presence of selected cytokines by ELISA.
Viral IL-6 in the culture supernatants of BCBL-1 and BC-1 cells detected by immunoblotting with a specific rabbit antiserum. (A) A rabbit antiserum to vIL-6 was used in immunoblotting of viral and human IL-6 protein. (B) Conditioned medium from the BCBL-1 and BC-1 cell lines (1 mL) was TCA-precipitated and then analyzed by immunoblotting with a rabbit antiserum to vIL-6. Cell lysates from NIH3T3 cells (1 × 103 cells) transfected with a control vector or with the vIL-6 gene were used as controls. (C) Detection of purified recombinant vIL-6 (0.32 to 200 ng) in immunoblotting using a rabbit antiserum to vIL-6.
Viral IL-6 in the culture supernatants of BCBL-1 and BC-1 cells detected by immunoblotting with a specific rabbit antiserum. (A) A rabbit antiserum to vIL-6 was used in immunoblotting of viral and human IL-6 protein. (B) Conditioned medium from the BCBL-1 and BC-1 cell lines (1 mL) was TCA-precipitated and then analyzed by immunoblotting with a rabbit antiserum to vIL-6. Cell lysates from NIH3T3 cells (1 × 103 cells) transfected with a control vector or with the vIL-6 gene were used as controls. (C) Detection of purified recombinant vIL-6 (0.32 to 200 ng) in immunoblotting using a rabbit antiserum to vIL-6.
Effect of IL-10, IL-6, and vIL-6 neutralizing antibodies on PEL cell growth.
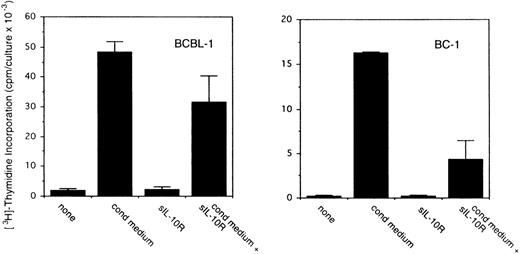
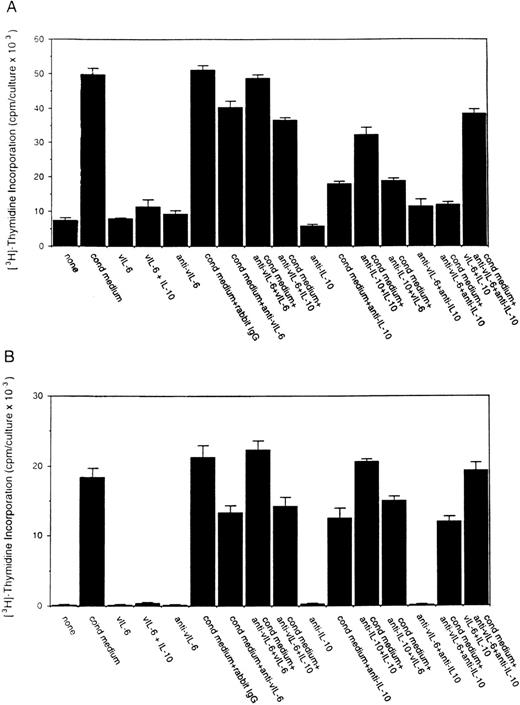
To assess the potential role for cytokines as autocrine growth factors, neutralizing antibodies specific for human IL-10, IL-6, and vIL-6 or control antibodies were added to PEL cell cultures, and their effects on cell proliferation were measured. Neutralizing antibodies against human IL-10 (19F1 MoAb, 5 μg/mL) reduced autocrine growth factor-induced proliferation of BCBL-1 cells by approximately 88% and of BC-1 cells by 70% (Fig 3). An isotype-matched control rat MoAb (R35-95) had a minimal effect (Fig 3). Addition of IL-10 (25 ng/mL) to these cultures reduced the level of antibody neutralization by 33% to 60%. Titration experiments showed that, at the concentration of 2.5 μg/mL, the antihuman IL-10 antibody neutralized 42% and 51% of the BCBL-1 and BC-1 autocrine growth factor activity, respectively, whereas at the concentration of 1.25 μg/mL, the antihuman IL-10 antibody had minimal neutralizing effect with either cell line. However, IL-10 (25 ng/mL) added alone to BCBL-1 and BC-1 cultured under conditions that minimize the contribution of autocrine growth factors stimulated little cell proliferation (Fig 3). Because these results suggested that IL-10 is a critical component of the autocrine growth factor activity in BCBL-1 and BC-1 cells, we tested the effects of soluble IL-10 receptor. The addition of soluble IL-10 receptor (100 ng/mL) to BCBL-1 and BC-1 cells substantially reduced cell proliferation in the presence of autologous conditioned medium (Fig 4). Thus, soluble IL-10 receptor and antibodies against human IL-10, reagents that specifically bind and neutralize IL-10, reduced autocrine growth factor-dependent PEL proliferation, substantiating a role for IL-10 in PEL cell growth.
Effects of a neutralizing antibody to human IL-10 on the proliferation of BCBL-1 and BC-1 cells. Cells (5 × 103cells/microwell) from the BCBL-1 and BC-1 cell lines were cultured for 3 days with or without the addition of 25% autologous conditioned medium either alone or in the presence of a neutralizing MoAb (19F1; 5 μg/mL) to human IL-10 or an isotype-matched control MoAb (R35-95; 5 μg/mL). Sets of cultures were supplemented with recombinant human IL-10 (25 ng/mL). 3H thymidine was added during the final 20 hours of culture. The results represent the mean (±SD) radioactivity of triplicate cultures. Shown is a representative experiment of 4 performed.
Effects of a neutralizing antibody to human IL-10 on the proliferation of BCBL-1 and BC-1 cells. Cells (5 × 103cells/microwell) from the BCBL-1 and BC-1 cell lines were cultured for 3 days with or without the addition of 25% autologous conditioned medium either alone or in the presence of a neutralizing MoAb (19F1; 5 μg/mL) to human IL-10 or an isotype-matched control MoAb (R35-95; 5 μg/mL). Sets of cultures were supplemented with recombinant human IL-10 (25 ng/mL). 3H thymidine was added during the final 20 hours of culture. The results represent the mean (±SD) radioactivity of triplicate cultures. Shown is a representative experiment of 4 performed.
Effects of soluble IL-10 receptor on the proliferation of BCBL-1 and BC-1 cells. Cells (5 × 103 cells/microwell) from the BCBL-1 and BC-1 cell lines were cultured for 3 days with or without the addition of 25% autologous conditioned medium with medium alone or in medium supplemented with soluble IL-10 receptor (100 ng/mL). 3H thymidine was added during the final 20 hours of culture. The results represent the mean (±SD) radioactivity of triplicate cultures. Shown is a representative experiment of 5 performed.
Effects of soluble IL-10 receptor on the proliferation of BCBL-1 and BC-1 cells. Cells (5 × 103 cells/microwell) from the BCBL-1 and BC-1 cell lines were cultured for 3 days with or without the addition of 25% autologous conditioned medium with medium alone or in medium supplemented with soluble IL-10 receptor (100 ng/mL). 3H thymidine was added during the final 20 hours of culture. The results represent the mean (±SD) radioactivity of triplicate cultures. Shown is a representative experiment of 5 performed.
To test for a potential contribution of human IL-6 to PEL autocrine cell growth, we selected a MoAb against human IL-6 that can neutralize human, but not viral, IL-6 bioactivity detected by the B9 assay and can recognize human but not viral IL-6 in immunoblotting (not shown). This antihuman IL-6 antibody consistently failed to inhibit BCBL-1 and BC-1 cell proliferation induced by autologous conditioned media (not shown). Also, human recombinant purified IL-6 (100 pg/mL to 1 mg/mL) consistently failed to stimulate the proliferation of BCBL-1 and BC-1 cells grown under restricted culture conditions (not shown). Similarly, the addition of the cytokines TGFβ, IL-4, and TNFα (used at concentrations ranging between 1 and 100 ng/mL) consistently failed to stimulate the proliferation of BCBL-1 and BC-1 cells grown under restricted culture conditions (not shown). Thus, human IL-6, which is detected in the culture supernatant of BCBL-1 and BC-1 cells, appears not to function as an autocrine growth factor for these cells.
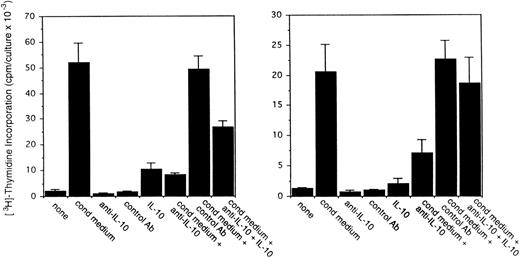
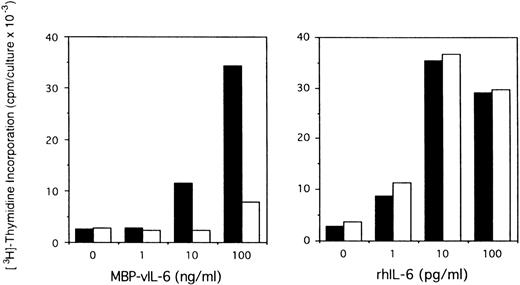
To assess the potential role of vIL-6 to autocrine growth factor activity, we tested the effects of a purified rabbit antiserum against vIL-6. This reagent can specifically neutralize vIL-6–induced B9 cell proliferation, but has minimal neutralizing effect on human IL-6–induced B9 cell proliferation (Fig5). When tested for its ability to neutralize PEL autocrine growth factor activity (Fig 6), anti–vIL-6 antibody (5 μg/mL) reduced autocrine growth factor activity by 23% in BCBL-1 cells and by 28% in BC-1 cells. A dose-response study showed that, at the reduced concentration of 1 μg/mL, this anti–vIL-6 antibody neutralized 0% and 4% of BCBL-1 and BC-1 autocrine growth factor activity, respectively. A control rabbit IgG preparation did not reduce the autocrine growth factor activity in BCBL-1 and BC-1 cells (Fig 6). The specificity of the neutralizing effect was documented by the addition of recombinant purified vIL-6, which reversed the neutralizing effect of the anti–vIL-6 antibody, whereas human IL-10 did not. We compared the neutralizing effect of anti–vIL-6 and antihuman IL-10 antibodies used at the same concentration (5 μg/mL). In parallel cultures (Fig 6), antihuman IL-10 antibodies were more effective at neutralizing autocrine growth factor activity than anti–vIL-6 antibodies (75% v 23% in BCBL-1 cells; and 32%v 28% in BC-1 cells). When added together in culture, antihuman IL-10 and anti–vIL-6 antibodies neutralized 89% and 35% of the autocrine growth factor activity in BCBL-1 and BC-1 cells, respectively (Fig 6). The addition of recombinant purified vIL-6 and human IL-10 to these cultures reversed the combined neutralizing effect of the anti–vIL-6 and antihuman IL-10 antibodies (Fig 6). However, when added together in the absence of conditioned medium, IL-10 and vIL-6 displayed little growth stimulation of BCBL-1 and BC-1 cells (Fig6). These experiments document that human IL-10 and vIL-6 contribute to the autocrine growth factor activity in the BCBL-1 and BC-1 cell lines and suggest that other growth factors yet to be defined are important contributors to autocrine growth in these cells.
Effects of anti–vIL-6 antibody on B9 cell proliferation induced by viral and human IL-6. Exponentially growing B9 cells (2 × 103 cells/microwell) were cultured in medium alone or medium supplemented with vIL-6 (MBP-vIL-6; 1 to 100 ng/mL) or human IL-6 (1 to 100 pg/mL), with (□) or without (▪) anti–vIL-6 antibody (10 μg/mL). 3H thymidine was added during the final 6 hours of culture. The results represent the mean radioactivity of triplicate cultures; SDs were within 5% of the mean.
Effects of anti–vIL-6 antibody on B9 cell proliferation induced by viral and human IL-6. Exponentially growing B9 cells (2 × 103 cells/microwell) were cultured in medium alone or medium supplemented with vIL-6 (MBP-vIL-6; 1 to 100 ng/mL) or human IL-6 (1 to 100 pg/mL), with (□) or without (▪) anti–vIL-6 antibody (10 μg/mL). 3H thymidine was added during the final 6 hours of culture. The results represent the mean radioactivity of triplicate cultures; SDs were within 5% of the mean.
Effects of a neutralizing antibody to vIL-6 on the proliferation of BCBL-1 and BC-1 cells. Cells (5 × 103cells/microwell) from the BCBL-1 and BC-1 cell lines were cultured for 3 days with or without the addition of 25% autologous conditioned medium either alone or in the presence of a rabbit neutralizing antibody (5 μg/mL) against vIL-6, control rabbit IgG (5 μg/mL), a MoAb against human IL-10 (19F1; 5 μg/mL), or anti–vIL-6 plus antihuman IL-10 antibodies (each at 5 μg/mL). Selected cultures were supplemented with recombinant purified vIL-6 (MBP-vIL-6; 50 ng/mL) or human IL-10 (25 ng/mL). 3H thymidine was added during the final 20 hours of culture. The results represent the mean (±SD) radioactivity of triplicate cultures.
Effects of a neutralizing antibody to vIL-6 on the proliferation of BCBL-1 and BC-1 cells. Cells (5 × 103cells/microwell) from the BCBL-1 and BC-1 cell lines were cultured for 3 days with or without the addition of 25% autologous conditioned medium either alone or in the presence of a rabbit neutralizing antibody (5 μg/mL) against vIL-6, control rabbit IgG (5 μg/mL), a MoAb against human IL-10 (19F1; 5 μg/mL), or anti–vIL-6 plus antihuman IL-10 antibodies (each at 5 μg/mL). Selected cultures were supplemented with recombinant purified vIL-6 (MBP-vIL-6; 50 ng/mL) or human IL-10 (25 ng/mL). 3H thymidine was added during the final 20 hours of culture. The results represent the mean (±SD) radioactivity of triplicate cultures.
IL-10 and vIL-6 receptor expression by BCBL-1 and BC-1 cells.
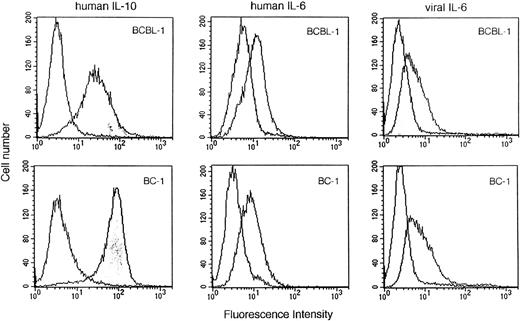
To define further the roles of IL-10 and vIL-6 as growth factors for PEL cells, we evaluated the expression of the IL-10, vIL-6, and human IL-6 receptors using biotinylated IL-10, vIL-6, or human IL-6 and flow-cytometric analysis. BCBL-1 and BC-1 cells exhibited significant binding of IL-10, vIL-6, and human IL-6 (Fig 7). The specificity of cytokine binding was confirmed by use of a control biotinylated protein, soybean trypsin inhibitor, whereas the specificity of vIL-6 (MBP-vIL-6) binding was confirmed by use of control biotinylated MBP (Fig 7). These results are consistent with BCBL-1 and BC-1 cell expression of human IL-10, human IL-6, and vIL-6 receptors.
Human IL-10, human IL-6, and vIL-6 binding to BCBL-1 and BC-1 cells. Biotinylated human IL-10 (1.5 μg/mL), human IL-6 (1.5 μg/mL), soybean trypsin inhibitor (1.5 μg/mL), vIL-6 (MBP-vIL-6; 1.5 μg/mL), or MBP (1.5 μg/mL) was incubated with BCBL-1 and BC-1 cells. Cell-bound protein was shown by the addition of avidin-fluorescein (10 μg/mL). After washing, surface fluorescence was evaluated by FACScan analysis. Unshaded histograms reflect binding from control biotinylated control proteins (soybean trypsin inhibitor or MBP).
Human IL-10, human IL-6, and vIL-6 binding to BCBL-1 and BC-1 cells. Biotinylated human IL-10 (1.5 μg/mL), human IL-6 (1.5 μg/mL), soybean trypsin inhibitor (1.5 μg/mL), vIL-6 (MBP-vIL-6; 1.5 μg/mL), or MBP (1.5 μg/mL) was incubated with BCBL-1 and BC-1 cells. Cell-bound protein was shown by the addition of avidin-fluorescein (10 μg/mL). After washing, surface fluorescence was evaluated by FACScan analysis. Unshaded histograms reflect binding from control biotinylated control proteins (soybean trypsin inhibitor or MBP).
DISCUSSION
In this study, we show that KSHV-infected spontaneous cell lines derived from PEL are dependent on autocrine growth factors for continuous proliferation in vitro and demonstrate that human IL-10 and, to a lesser degree, vIL-6 account for some of the autocrine growth factor activity. When cultured at low cell densities in serum-free medium, the BCBL-1 and BC-1 cell lines showed little spontaneous proliferation. However, the addition of autologous supernatant prepared in serum-free medium at optimal cell densities induced a high rate of cell proliferation. Human IL-10, human IL-6, and vIL-6, but not other cytokines, were detected in BCBL-1 and BC-1 culture supernatants. Neutralizing antibodies to human IL-10 and to vIL-6 added individually to BCBL-1 and BC-1 cell cultures reduced significantly cell proliferation induced by autologous conditioned media. When added together, these neutralizing antibodies removed a substantial amount of the autocrine growth factor activity in BCBL-1 and BC-1 culture supernatants. The neutralizing effect was specific, because human IL-10 and vIL-6 proteins reversed the growth-neutralizing effect of the respective antibodies. However, when added together to BCBL-1 and BC-1 cells grown at low cell densities in serum-free medium, human IL-10 and vIL-6 failed to stimulate cell growth. Neutralizing antibodies to human IL-6, oncostatin M, LIF, ciliary neurotropic factor (CNTF), and IL-11 had minimal effect on the growth of BCBL-1 or BC-1 cells (not shown). Thus, we show that 2 PEL-derived cell lines are dependent, in part, on the cellular cytokine IL-10 and, to a lesser extent, the viral cytokine IL-6 for autonomous proliferation in vitro. Other growth factors yet to be defined may contribute to the autocrine growth of PEL cells.
The cell lines used in the current studies are representative of PEL that is either infected with KSHV alone or is coinfected with EBV and KSHV.23 Previous studies have demonstrated that EBV-infected cell lines derived from the spontaneous outgrowth of either normal B cells from EBV-seropositive individuals or posttransplant lymphoproliferative disease tissues are largely dependent on autocrine growth factors, particularly IL-6 and IL-10, for continuous proliferation.16-18 By contrast, vIL-10, a product of the EBV gene BCRF-1, and a variety of other cytokines and chemokines are not active as a growth-stimulatory factor for these cell lines.30 LMP-1, an EBV latency protein that is regularly expressed in EBV-immortalized cell lines, is known to mediate the activation of NF-kB transcription factor and, by this mechanism, stimulate expression of IL-10, IL-6, and other cellular genes that contain kB elements.31-33 Previous studies have documented that LMP-1 is not expressed in the PEL-derived cell line BC-1 that is coinfected with KHSV and EBV, suggesting that EBV-infected PEL may display a limited expression of EBV latency genes similar to certain EBV-infected Burkitt cells.34,35 Thus, IL-6 and IL-10 expression in BC-1 cells may not be attributable to EBV infection and expression of the LMP-1 protein. Rather, because the pattern of autocrine cytokine requirement is quite similar in BCBL-1 and BC-1 cell lines, which differ with respect to their EBV status while both being KSHV-infected, KSHV could play an important and unifying role in the regulation of autonomous cell growth in these cell lines. KSHV open reading frame K2 encodes vIL-6; thus, the virus plays a very direct role in the production of this cytokine.24,25 36 By contrast, the mechanism by which human IL-10 is expressed in PEL cell lines is currently undefined.
In addition to being expressed in PEL and the cell lines derived from PEL, vIL-6 is detected in some forms of KSHV-positive multicentric Castleman’s disease, whereas it is generally not expressed in KSHV-positive Kaposi’s sarcoma tissues.24,37 Because vIL-6 exhibits 24.7% amino acid identity to human IL-6, it was suggested that KSHV may have captured this cellular gene during evolution for its own advantage.24,25 36 The results presented here demonstrate that vIL-6 contributes some of the autocrine growth factor activity in PEL cell lines. By promoting the expansion of KSHV-infected cells, vIL-6 may help KSHV persist and spread in the human host.
It is worth noting that, whereas both viral and human IL-6 are detected in PEL culture supernatants, our results suggest that human IL-6 and vIL-6 differ with respect to growth stimulation of PEL cells. The reasons for this difference, particularly the failure of human IL-6 to promote PEL cell growth, are currently not clear. We know that the human IL-6 present in the culture supernatant of PEL cell lines is biologically active, because it promotes the growth of the IL-6–dependent indicator B9 cells. We also know that the failure of human IL-6 to stimulate PEL cell growth is not due to insufficient cytokine amounts, because even microgram quantities of human IL-6 added to culture were inactive. Previous studies with the Hep-G2 cell line, a human hepatoma cell line that expresses cellular IL-6 receptors, demonstrated potential differences in IL-6 receptor use by human and vIL-6.38 Other studies using the human myeloma cell line INA-6 provided evidence that the IL-6 receptor α chain as well as GP130 are involved in viral as well as human IL-6 signaling, albeit with different affinities, perhaps due to amino acid differences at human IL-6 positions Phe74 and Arg182.25,39 In addition, Western blot analysis has demonstrated that the IL-6 receptor α chain is expressed in several PEL cell lines.22 We showed here that PEL cells bind human IL-6. Thus, current information on IL-6 receptor structure and signaling does not explain the biological differences of human and vIL-6 noted here, and additional studies will be needed.
Human IL-10, a cytokine normally produced by activated T lymphocytes, monocytes, B lymphocytes, and other cells, can promote the growth of primary B lymphocytes that are costimulated by anti-IgM or anti-CD40 antibodies and can profoundly inhibit T-cell immunity.40 In conjunction with IL-4, IL-10 promoted the expansion and increased substantially the survival of primary B lymphocytes in vitro.41-43 When added to B lymphocytes in conjunction with EBV, IL-10 potentiated virus-induced cell proliferation.44,45 IL-10 is expressed by PEL cells in vitro and in vivo.6 10 The results presented here suggest that, in addition to other previously reported stimulatory effects on B lymphocytes, IL-10 may cooperate with KSHV to potentiate virus-induced proliferation of cells of B-cell lineage.
Currently, there are no effective treatments available for PEL, and patients usually succumb of their illness within months from diagnosis. The observation that PEL cells are dependent on IL-10 and vIL-6 for expansion, suggests that rational treatments could be designed to impair production or cell responsiveness to these cytokines.
ACKNOWLEDGMENT
The authors thank Sandy Pike, Lei Yao, Barry Cherney, Fonda Newcomb, and Andy Lewis.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Giovanna Tosato, MD, Division of Hematologic Products, Center for Biologics Evaluation and Research, Bldg 29A, Room 2D16, 8800 Rockville Pike, Bethesda, MD 20892.
K.D.J. and Y.A. contributed equally to this report.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal