We retrospectively analyzed the B-cell function and leukocyte chimerism of 22 patients with severe combined immunodeficiency with B cells (B+ SCID) who survived more than 2 years after bone marrow transplantation (BMT) to determine the possible consequences of BMT procedures, leukocyte chimerism, and SCID molecular deficit on B-cell function outcome. Circulating T cells were of donor origin in all patients. In recipients of HLA-identical BMT (n = 5), monocytes were of host origin in 5 and B cells were of host origin in 4 and of mixed origin in 1. In recipients of HLA haploidentical T-cell–depleted BMT (n = 17), B cells and monocytes were of host origin in 14 and of donor origin in 3. Engraftment of B cells was found to be associated with normal B-cell function. In contrast, 10 of 18 patients with host B cells still require Ig substitution. Conditioning regimen (ie, 8 mg/kg busulfan and 200 mg/kg cyclophosphamide) was shown neither to promote B-cell and monocyte engraftment nor to affect B-cell function. Eight patients with B cells of host origin had normal B-cell function. Evidence for functional host B cells was further provided in 3 informative cases by Ig allotype determination and by the detection, in 5 studied cases, of host CD27+ memory B cells as in age-matched controls. These results strongly suggest that, in some transplanted patients, host B cells can cooperate with donor T cells to fully mature in Ig-producing cells.
SEVERE COMBINED immunodeficiencies (SCID) is a heterogeneous group of inherited disorders characterized by a severe impairment of both cellular and humoral immunity that leads to death during infancy in the absence of treatment.1-4 The most common SCID phenotype results from a selective block in T-cell and, usually, natural killer (NK) cell differentiation while there is a normal B-cell differentiation. It is thereafter called B+SCID. It is caused by mutation of either the γc5,6 or the JAK-3 genes.7,8 Recently, IL7Rα gene mutations have been shown to cause a SCID with a T− B+NK+ phenotype.9 The molecular basis of a small subset (5%) of B+ SCID cases so far remains unknown.1 Bone marrow transplantation (BMT) has been shown to be the treatment of choice for patients with SCID. Various studies have reported excellent results for HLA-identical BMT, with full restoration of T- and B-cell function in most patients.10-14 HLA-identical related donors are not available for most patients; therefore, HLA-nonidentical BMT have been performed since 1981, when it became feasible to deplete marrow specimen from T cells.15-21 However, the prospects of survival with long-term immune reconstitution are poorer, because HLA-nonidentical BMT led to 52% to 78% survival rates in different series.14,20-23 It has been recently demonstrated that SCID phenotype has an influence on the outcome of HLA-nonidentical T-cell–depleted BMT.24 In the latter study, the survival rate was 60% for B+ SCID patients, whereas it was only 35% for patients with SCID characterized by an absence of both T and B cells (B− SCID). Immune functions develop more rapidly and are more often of higher quality both for T-cell and B-cell immunity in transplanted B+ SCID than B−SCID patients.25 However, despite normal T-cell immunity development, normal humoral immune function is observed in only 50% to 60% of B+ SCID patients several years after HLA haploidentical T-cell–depleted BMT.14,25-28 In contrast, full T- and B-cell functions do develop in most recipients of histocompatible BMT.14,21,23 27
It has been suggested that the inconstant development of antibody responses after HLA haploidentical T-cell–depleted BMT in B+ SCID patients is a consequence of defective B-cell engraftment,20,23,26,27,29-31 thus reflecting an intrinsic B-cell deficiency. Involvement of γc and JAK-3 in interleukin-4 (IL-4) signaling supports this hypothesis.32 33 We therefore retrospectively studied the B-cell immune function of 22 B+ SCID patients who survived more than 2 years after HLA-identical or HLA-mismatched T-cell–depleted BMT to determine the effects of the underlying molecular deficiency together with BMT procedure factors and leukocyte chimerism on B-cell function outcome.
PATIENTS AND METHODS
Patients
Patients with SCID with B cells (B+ SCID) alive at least 2 years after BMT were enrolled in this study. BMTs were performed between 1976 and 1995 at the Hopital Necker-Enfants Malades (Paris, France). Twenty-two patients fulfilled the inclusion criteria. Median age at diagnosis was 5 months (range, 1 to 7 months). Fourteen patients had γc deficiency, 4 had JAK-3 deficiency, and 4 had a yet unknown molecular deficiency as defined by normal γc gene sequence and protein detection as well as of JAK-3 protein detection and normal phosphorylation after stimulation with IL-2 of Epstein-Barr virus (EBV)-transformed B cells.34
BMT characteristics.
Median age at BMT was 6.5 months (range, 1 to 93 months). Two patients required a second transplantation at 29 and 93 months of age, respectively, because of poor T-cell development. Data were analyzed from the last BMT in these 2 patients. The median length of follow-up was 5 years (range, 2 to 23 years). The end point for analysis was December 31, 1998.
Three patients received a BMT from an HLA-identical sibling and 2 received a BMT from a phenotypically HLA-identical aunt (n = 1) or mother (n = 1). These patients did not receive a conditioning regimen (CR). The marrow inoculum was not T-cell–depleted. No prophylaxis against graft-versus-host disease (GVHD) was used except for the patient who received a marrow transplant from his phenotypically HLA-identical mother. He received a 60-day course of intravenous cyclosporin A.
Seventeen patients underwent related HLA-nonidentical T-cell–depleted BMT. All the donors were parents. They were 2 HLA antigens (4 cases) or full haplotype (13 cases) mismatched. Eight of the 17 did not receive a CR, because they had a severe infection at the time of BMT. The CR administered to the other 9 patients consisted of busulfan (8 mg/kg total dose) and cyclophosphamide (200 mg/kg total dose). Twelve patients were also treated intravenously with a monoclonal anti-LFA1 antibody to prevent graft rejection.35 All marrow transplants were T-cell–depleted to prevent GVHD. E-rosetting was used to achieve depletion in 8 patients transplanted between 1983 and 1990,18 Campath 1-M Ab plus human complement was used in 6 patients transplanted between 1990 and 1994,36 and monoclonal anti-CD2 and anti-CD7 antibodies with complement lysis was used in 3 patients transplanted in 1994 and 1995. Post-BMT GVHD prophylaxis was administered to patients who received bone marrow depleted by E-rosetting (60-day course of cyclosporin A).18All patients were placed in a protective environment (sterile isolator) and received prophylactic antimicrobial medication to eliminate the intestinal microflora and intravenous immune globulin (IVIG) therapy weekly for 3 months after BMT and then every 3 weeks for at least 3 months. Acute and chronic GVHD was assessed in all patients according to standard criteria.37
Methods
Blood samples were collected and analyzed in 1998, which was 2 to 23 years after BMT.
B-cell function.
We studied B-cell function by determining serum concentrations of IgG, IgG isotypes, IgA, IgM, IgE, and serum antibodies to polioviruses, tetanus, and diphtheria toxoids in all patients except for those undergoing treatment with IVIG. In all cases, the last booster immunization had been administered 3 to 6 months (n = 19) or 6 months to 3 years (n = 3) before analysis. Serum Ig concentrations were measured by nephelometry. IgG isotypes were determined by an immunoenzymatic method using monoclonal antibodies (MoAbs). Serum antibodies directed against polioviruses, tetanus, and diphtheria toxoids were determined by enzyme-linked immunosorbent assays. The determination of Ig allotypes was performed by an hemagglutination assay.
Antibodies.
The following MoAbs were used in immunofluorescence studies: anti-CD3: Leu 4 (IgG2a; Becton Dickinson, San Diego, CA); anti-CD4: Leu 3a (IgG1; Becton Dickinson); anti-CD8: Leu 2a (IgG1; Becton Dickinson); anti-CD19: J4 119 (IgG1; Immunotech, Marseille, France); anti-CD27: 1A4 (IgG1; Immunotech); anti-CD14: Leu M3 (Becton Dickinson); anti-CD16: 3G8 (IgG1; Immunotech); and anti-CD56: MY31 (IgG1; Becton Dickinson). Cells were fluorescence stained with phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated MoAbs. Cell fluorescence was measured with a FACScan flow cytometer (Becton Dickinson).
Cell isolation.
Leukocytes were isolated from fresh heparin-treated blood by Plasmagel (Roger Bellon Laboratories, Paris, France) sedimentation and separation by Lymphoprep (Nicomed Pharma, Oslo, Norway). Polymorphonuclear (PMN) cells sedimented in the pellet and peripheral mononuclear cells (PBMC) at the interface. E+ (rosette forming) and E− (no rosette) cells were obtained by treatment of PBMC with neuraminidase-treated (Berhing Werke, Marburg Lahn, Germany) red blood cells from sheep.
Monocytes (CD14+) and B lymphocytes (CD19+) on the one hand and T lymphocytes (CD3+) and NK cells (CD56+CD16+CD3−) on the other hand were sorted, respectively, from E− and E+ cells using a FACStar plus cell sorter (Becton Dickinson) after staining with the appropriate MoAb.
Chimerism studies.
DNA chimerism was studied using the patients’ sorted cells. Cells (0.1 mol/L) were lysed by incubation with 50 μL lysis buffer (10× Taq buffer [ATGC, Noisy le Grand, France], 0.5% Tween-20, and 0.1 mg/mL proteinase K at 56°C for 45 minutes, followed by heat inactivation of the enzyme at 94°C for 5 minutes). Polymerase chain reaction (PCR) was performed using 1 μL of the DNA preparation and primers specific for the dinucleotide, trinucleotide, or tetranucleotide repeat polymorphism at the D10S 206,38DXS101,39 or HPRT40 loci, respectively. All of the patient studies were informative for at least 1 of these 3 loci. One tenth of each reaction mixture was subjected to electrophoresis in a 5% polyacrylamide, 8 mol/L urea sequencing gel. The sensitivity of chimerism detection was 5%.
RESULTS
Chimerism Analysis
HLA-identical BMT.
Five patients received an HLA-identical BMT. Chimerism studies (Table 1) showed that, in all cases, T cells originated from the donor, whereas monocytes originated from the host. Four of these 5 patients exhibited B cells of host origin and 1 patient had B-cell mosaicism (50% donor cells). NK-cell chimerism was studied in 4 cases: NK cells were of donor origin in 2 cases, of host origin in 1 case, and undetectable in 1 case. T- and B-cell chimerism was previously studied in 2 patients during the first 2 years after BMT by using HLA typing in 1 case and by karyotyping in the other. These 2 patients with donor T cells and host B cells 5 and 6 months, respectively, after BMT exhibited the same chimerism pattern at last follow-up 14 and 21 years, respectively, after BMT.
Leukocyte Chimerism in 22 Patients With B+SCID After BMT
| . | UPN . | Molecular Deficiency . | Age at BMT (mos) . | Follow-Up (yrs) . | CR . | Chimerism . | |||
|---|---|---|---|---|---|---|---|---|---|
| T Cells . | B Cells . | Monocytes . | NK Cells . | ||||||
| HLA-identical BMT | 17 | γc | 1 | 23 | No | D | H | H | (−) |
| 116* | γc | 1 | 13 | No | D | H | H | D | |
| 261 | γc | 6.5 | 5.5 | No | D | H | H | ND | |
| 50 | Unknown | 4.5 | 13.5 | No | D | D/H | H | D | |
| 299 | Unknown | 10 | 4.5 | No | D | H | H | H | |
| HLA-nonidentical BMT | 51* | γc | 2.5 | 13 | No | D | H | H | ND |
| 122 | γc | 6.5 | 10 | No | D | D | D | ND | |
| 307 | γc | 93 | 3.5 | No | D | H | H | (−) | |
| 325 | γc | 14 | 3 | No | D | H | H | D | |
| 277 | γc | 8 | 4.5 | No | D | H | H | ND | |
| 57 | JAK3 | 9 | 13 | No | D | H | H | (−) | |
| 223 | JAK3 | 11 | 6.5 | No | D | D | D | ND | |
| 365 | JAK3 | 5 | 2 | No | D | H | H | D | |
| 168 | γc | 2 | 8.5 | Yes | D | H | H | ND | |
| 272b | γc | 29 | 3 | Yes | D | H | H | D | |
| 303 | γc | 1 | 4 | Yes | D | H | H | D | |
| 308 | γc | 8.5 | 3.5 | Yes | D | H | H | ND | |
| 318 | γc | 5.5 | 3 | Yes | D | H | H | ND | |
| 348 | γc | 1 | 2 | Yes | D | H | H | D | |
| 158 | Unknown | 4 | 9 | Yes | D | H | H | D | |
| 360 | Unknown | 10 | 2 | Yes | D | H | H | D | |
| 221 | JAK3 | 11 | 6.5 | Yes | D | D | D | D | |
| . | UPN . | Molecular Deficiency . | Age at BMT (mos) . | Follow-Up (yrs) . | CR . | Chimerism . | |||
|---|---|---|---|---|---|---|---|---|---|
| T Cells . | B Cells . | Monocytes . | NK Cells . | ||||||
| HLA-identical BMT | 17 | γc | 1 | 23 | No | D | H | H | (−) |
| 116* | γc | 1 | 13 | No | D | H | H | D | |
| 261 | γc | 6.5 | 5.5 | No | D | H | H | ND | |
| 50 | Unknown | 4.5 | 13.5 | No | D | D/H | H | D | |
| 299 | Unknown | 10 | 4.5 | No | D | H | H | H | |
| HLA-nonidentical BMT | 51* | γc | 2.5 | 13 | No | D | H | H | ND |
| 122 | γc | 6.5 | 10 | No | D | D | D | ND | |
| 307 | γc | 93 | 3.5 | No | D | H | H | (−) | |
| 325 | γc | 14 | 3 | No | D | H | H | D | |
| 277 | γc | 8 | 4.5 | No | D | H | H | ND | |
| 57 | JAK3 | 9 | 13 | No | D | H | H | (−) | |
| 223 | JAK3 | 11 | 6.5 | No | D | D | D | ND | |
| 365 | JAK3 | 5 | 2 | No | D | H | H | D | |
| 168 | γc | 2 | 8.5 | Yes | D | H | H | ND | |
| 272b | γc | 29 | 3 | Yes | D | H | H | D | |
| 303 | γc | 1 | 4 | Yes | D | H | H | D | |
| 308 | γc | 8.5 | 3.5 | Yes | D | H | H | ND | |
| 318 | γc | 5.5 | 3 | Yes | D | H | H | ND | |
| 348 | γc | 1 | 2 | Yes | D | H | H | D | |
| 158 | Unknown | 4 | 9 | Yes | D | H | H | D | |
| 360 | Unknown | 10 | 2 | Yes | D | H | H | D | |
| 221 | JAK3 | 11 | 6.5 | Yes | D | D | D | D | |
Abbreviations: H, cells of host origin; D, cells of donor origin; ND, not done; (−), cells not found.
These 2 patients had the same γc gene mutation.
HLA-nonidentical T-cell–depleted BMT.
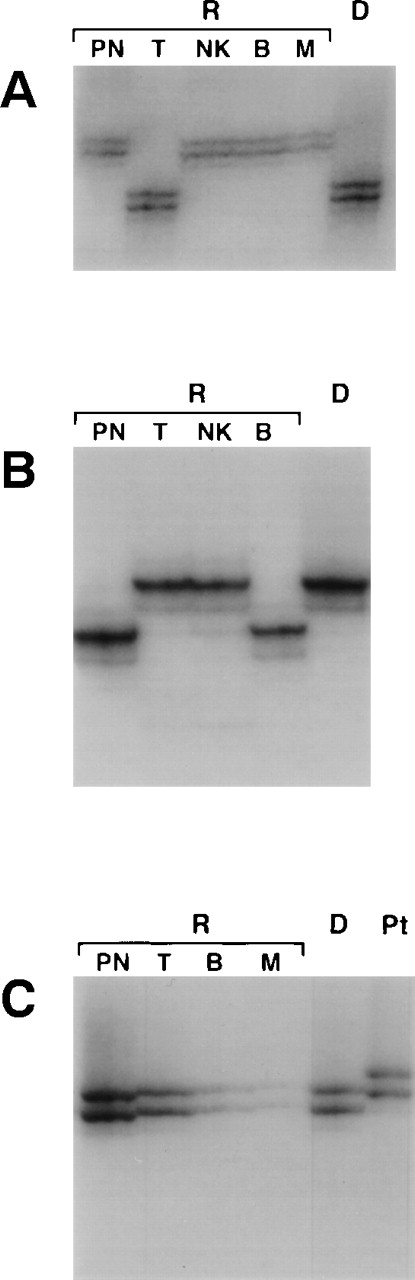
Seventeen patients received an HLA-nonidentical T-cell–depleted BMT. T cells originated from the donor in all patients. In 3 patients, both B cells and monocytes were exclusively of donor origin, whereas neither donor B cells nor donor monocytes were detected in 14 patients (Table 1and Fig 1). NK-cell chimerism was studied in 10 of these 17 patients. CD16+CD56+CD3− NK cells were of donor origin in 8, whereas no CD16+ cells were detected in the blood of 2 patients. Among the 8 patients with NK cells of donor origin, B cells and monocytes were of host origin in 7 and of donor origin in 1 (Table 1). T- and B-cell chimerism was previously studied in 3 patients during the first 2 years after BMT by using HLA typing. In all 3 cases, T and B cells were found to be of donor origin 6, 14, and 15 months, respectively, after BMT, whereas host B cells only were detected at the last follow-up 3, 9, and 2 years, respectively, after BMT.
Leukocyte chimerism of B+ SCID patients after BMT. Microsatellite typing of polymorphonuclear cells (PN), T lymphocytes (T), natural killer cells (NK), B lymphocytes (B), and monocytes (M) from recipients (R) or from donor cells (D) was performed at the D10S206 (A), DXS101 (B), or HPRT (C) loci, depending on how informative the locus was for each subject. (A) UPN 299; (B) UPN 272b; (C) UPN 223.
Leukocyte chimerism of B+ SCID patients after BMT. Microsatellite typing of polymorphonuclear cells (PN), T lymphocytes (T), natural killer cells (NK), B lymphocytes (B), and monocytes (M) from recipients (R) or from donor cells (D) was performed at the D10S206 (A), DXS101 (B), or HPRT (C) loci, depending on how informative the locus was for each subject. (A) UPN 299; (B) UPN 272b; (C) UPN 223.
Conditioning regimen treatment did not affect long-term chimerism of monocytes and B cells, because B cells and monocytes were of donor origin in 2 of 8 patients who did not receive any CR, whereas B cells and monocytes were of donor origin in 1 of 9 patients who did receive a CR. In contrast, all tested patients who received a CR treatment had NK cells of donor origin (n = 6), whereas NK cells were either of donor origin (n = 2) or undetectable (n = 2) in patients who did not receive any CR. Other characteristics of the BMT procedure, such as the method of T-cell depletion used, anti-LFA1 antibody treatment, and the number of nucleated cells and T cells infused, did not affect chimerism status (data not shown, P = not significant [NS]).
B-Cell Function Analysis
HLA-identical BMT.
All 5 patients exhibited normal blood B-cell counts (range, 150 to 672/μL; median, 505/μL). As shown in Table 2, at last follow-up, 3 patients were considered to have a normal B-cell function, because they had normal IgG concentrations, exhibited a normal antibody production, and did not require IVIG treatment. One of these 3 patients exhibited a low level of IgG2 and an absence of IgA and IgE. One patient (UPN 17) is considered to have a B-cell deficiency, because he never achieved a normal IgG concentration and attempts to stop IVIG treatment led to a much lower serum IgG concentration and to the recurrence of infections. One patient (UPN 50) had an isolated IgG2 deficiency with recurrent pulmonary infections and therefore required IVIG treatment despite antibody production to poliovirus and tetanus toxoid. In this patient, the B-cell deficiency could be the consequence of a low T-cell count and function.41
B-Cell Functions of 22 Patients With B+SCID After BMT
| . | UPN . | Diagnosis . | B-Cell Chimerism . | IVIG Treatment . | IgG (mg/mL) . | IgA (mg/mL) . | IgM (mg/mL) . | IgE (IU/mL) . | IgG Isotype . | Antibody Production . | CD3 (/mL) . | T-Cell Function . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA-identical BMT | 17 | γc | H | Yes | / | 0.9 | 1 | ND | / | / | 2,300 | N |
| 50 | Unknown | 50%D | Yes | 20.4 | 1.1 | 1.3 | 1,474 | No IgG2 | Normal | 1,200 | Low | |
| 116* | γc | H | No | 8 | 1.4 | 1 | 37 | Normal | Normal | 1,960 | N | |
| 261 | γc | H | No | 8.6 | 0 | 1 | 0 | Low IgG2 | Normal | 4,950 | N | |
| 299 | Unknown | H | No | 8.8 | 0.9 | 1 | 14 | Normal | Normal | 2,300 | N | |
| HLA-nonidentical BMT | 51* | γc | H | Yes | / | 0 | 1.3 | ND | / | / | 760 | N |
| 168 | γc | H | Yes | / | 0 | 1.4 | ND | / | / | 3,290 | N | |
| 272b | γc | H | Yes | / | 0.2 (−) | 0.9 | ND | / | / | 1,000 | N | |
| 277 | γc | H | Yes | / | 0 | 0.4 (−) | 0 | / | / | 2,320 | N | |
| 303 | γc | H | Yes | / | 1 | 2.8 (+) | 48 | / | / | 1,920 | N | |
| 307 | γc | H | Yes | / | 1.3 | 3.2 (+) | ND | / | / | 320 | Low | |
| 308 | γc | H | Yes | / | 0.2 (−) | 0.8 | 3 | / | / | 4,500 | N | |
| 325 | γc | H | Yes | / | 0.5 (−) | 0.3 (−) | 3 | / | / | 2,288 | N | |
| 348 | γc | H | Yes | / | 0 | 0.4 | 0 | / | / | 2,520 | N | |
| 318 | γc | H | No | 8 | 0.8 | 4 (+) | 11 | ND | Dissociated† | 2,370 | N | |
| 122 | γc | D | No | 10.7 | 0.8 | 1.2 | 20 | Normal | Normal | 1,700 | N | |
| 158 | Unknown | H | No | 5.2 | 1 | 0.9 | ND | Low IgG2 | Normal | 784 | N | |
| 360 | Unknown | H | No | 13.9 | 0 | 2 (+) | ND | ND | Normal | 2,145 | N | |
| 57 | JAK3 | H | No | 11.2 | 0 | 2.7 (+) | ND | Normal | Dissociated‡ | 2,000 | N | |
| 221 | JAK3 | D | No | 7.6 | 1.2 | 0.7 | 22 | ND | Normal | 3,198 | N | |
| 223 | JAK3 | D | No | 10 | 0.4 (−) | 1.5 | 25 | Normal | Dissociated2-153 | 2,500 | N | |
| 365 | JAK3 | H | No | 7.7 | 0 | 2.1 (+) | 37 | Normal | Normal | 3,120 | N |
| . | UPN . | Diagnosis . | B-Cell Chimerism . | IVIG Treatment . | IgG (mg/mL) . | IgA (mg/mL) . | IgM (mg/mL) . | IgE (IU/mL) . | IgG Isotype . | Antibody Production . | CD3 (/mL) . | T-Cell Function . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA-identical BMT | 17 | γc | H | Yes | / | 0.9 | 1 | ND | / | / | 2,300 | N |
| 50 | Unknown | 50%D | Yes | 20.4 | 1.1 | 1.3 | 1,474 | No IgG2 | Normal | 1,200 | Low | |
| 116* | γc | H | No | 8 | 1.4 | 1 | 37 | Normal | Normal | 1,960 | N | |
| 261 | γc | H | No | 8.6 | 0 | 1 | 0 | Low IgG2 | Normal | 4,950 | N | |
| 299 | Unknown | H | No | 8.8 | 0.9 | 1 | 14 | Normal | Normal | 2,300 | N | |
| HLA-nonidentical BMT | 51* | γc | H | Yes | / | 0 | 1.3 | ND | / | / | 760 | N |
| 168 | γc | H | Yes | / | 0 | 1.4 | ND | / | / | 3,290 | N | |
| 272b | γc | H | Yes | / | 0.2 (−) | 0.9 | ND | / | / | 1,000 | N | |
| 277 | γc | H | Yes | / | 0 | 0.4 (−) | 0 | / | / | 2,320 | N | |
| 303 | γc | H | Yes | / | 1 | 2.8 (+) | 48 | / | / | 1,920 | N | |
| 307 | γc | H | Yes | / | 1.3 | 3.2 (+) | ND | / | / | 320 | Low | |
| 308 | γc | H | Yes | / | 0.2 (−) | 0.8 | 3 | / | / | 4,500 | N | |
| 325 | γc | H | Yes | / | 0.5 (−) | 0.3 (−) | 3 | / | / | 2,288 | N | |
| 348 | γc | H | Yes | / | 0 | 0.4 | 0 | / | / | 2,520 | N | |
| 318 | γc | H | No | 8 | 0.8 | 4 (+) | 11 | ND | Dissociated† | 2,370 | N | |
| 122 | γc | D | No | 10.7 | 0.8 | 1.2 | 20 | Normal | Normal | 1,700 | N | |
| 158 | Unknown | H | No | 5.2 | 1 | 0.9 | ND | Low IgG2 | Normal | 784 | N | |
| 360 | Unknown | H | No | 13.9 | 0 | 2 (+) | ND | ND | Normal | 2,145 | N | |
| 57 | JAK3 | H | No | 11.2 | 0 | 2.7 (+) | ND | Normal | Dissociated‡ | 2,000 | N | |
| 221 | JAK3 | D | No | 7.6 | 1.2 | 0.7 | 22 | ND | Normal | 3,198 | N | |
| 223 | JAK3 | D | No | 10 | 0.4 (−) | 1.5 | 25 | Normal | Dissociated2-153 | 2,500 | N | |
| 365 | JAK3 | H | No | 7.7 | 0 | 2.1 (+) | 37 | Normal | Normal | 3,120 | N |
Abbreviations: H, cells of host origin; D, cells of donor origin; ND, not done; (−) and (+), respectively, low or high level of Ig.
These 2 patients had the same γc gene mutation.
Normal production of antibodies against Tetanus and Diphtheria toxoids, Polio 1 and 3, but not Polio 2 antigen.
Normal production of antibodies against Diphtheria toxoid, Polio 1 and 2, but not Polio 3 and Tetanus toxoid.
Normal production of antibodies against Diphtheria toxoid, Polio 2 and 3, but not Polio 1 and Tetanus toxoid.
HLA-nonidentical T-cell–depleted BMT.
Sixteen of the 17 patients exhibited normal to elevated blood B-cell counts (range, 140 to 2,280/μL; median, 550/μL). One patient had very low B-cell counts (40/μL) and low T-cell counts. At last follow-up, 9 patients were considered to have deficiencies in B-cell function, because they still required IVIG treatment and attempts to stop IVIG treatment led to a much lower serum IgG concentration and to the recurrence of infections (except for UPN 303). Eight patients were considered to have functional B-cell immunity, because they had normal IgG concentrations, produced antibodies, and did not require IVIG treatment. No recurrent infections occurred in these patients. However, in 3 of these patients, an IgA deficiency was found.
Analysis of the Factors Influencing B-Cell Function
B-cell chimerism status exerts an influence on B-cell function, because all 4 patients whose B cells were of donor origin (3 patients after an HLA-nonidentical BMT and 1 after an HLA identical BMT) had normal or close to normal B-cell function. Conversely, 10 of the 18 patients whose peripheral B cells were found exclusively of host origin (9 patients after an HLA-nonidentical BMT and 1 after an HLA-identical BMT) required IVIG treatment. These results confirm that engraftment of donor B cells offers the best chance to achieve development of normal B-cell function.
However, it was found that the exclusive detection of host peripheral B cells was associated with normal B-cell function in 8 other patients (5 after HLA-nonidentical BMT and 3 after HLA-identical BMT; Table 2). These results suggest that γc(−), JAK3(−), or other genetically deficient B cells could function once T-cell function is restored. However, a B-cell microchimerism cannot be strictly excluded and could account for development of in vivo B-cell function. We therefore studied the origin of serum Igs by determining several Ig heavy chain and κ-associated allotypes in 4 of these cases. Polymorphism for γ3 (γ3m21 and γ3m28) was found in 2 families, enabling us to determine that, in these 2 patients (UPN 261 and 158), IgG3 were of host origin. It was similarly found that IgG2 (γ2m23) were of host origin in patient UPN 365. It was also possible to assess whether host B cells, in patients with and without B-cell immunity, respectively, express the membrane marker CD27 that is associated with memory.42 It was found that, in 5 patients studied who had normal B-cell function, the percentage of CD27+CD19+ B cells was close to age-matched controls (range in patients, 8% to 29%; range in controls, 12% to 67%). In contrast, in the 3 patients studied who had no B-cell function, the fraction of CD27+ CD19 B cells was less than 4%. In 1 patient still under IVIG, a high fraction of CD27+CD19+ B cells was found (ie, 23%). It is noteworthy that IgA and IgM are detectable within the normal range in the serum of this patient (UPN 303; Table 2). His B-cell immunity is thus not absent. Overall, these results show that host memory B cells are detectable in patients who developed B-cell immunity after BMT.
After HLA-nonidentical BMT, the conditioning regimen used was found not to affect B-cell chimerism and therefore B-cell function outcome, because 4 of the 8 patients who did not receive any CR treatment and 4 of the 9 patients who did receive a CR treatment do not require IVIG treatment. Other factors, including age at BMT, occurrence and outcome of acute and chronic GVHD, method of T-cell depletion used, anti-LFA1 antibody treatment, and duration of follow-up, had no effect on B-cell function (data not shown, P = NS).
It is difficult to assess in this series of patients whether SCID diagnosis and possible intrinsic B-cell functional defects had an influence on B-cell function outcome after BMT because of the small number of JAK-3–deficient patients in this study. However, it was found that B cells from patients with SCID of unknown etiology and JAK-3 deficiency were functional (Table 3).
Molecular Deficiency Influence on B-Cell Function Outcome in the Absence of Donor B-Cell Development
| B-Cell Function . | γc (−) . | JAK-3 . | Unknown . |
|---|---|---|---|
| + | 3 | 2 | 3 |
| − | 10 | 0 | 0 |
| B-Cell Function . | γc (−) . | JAK-3 . | Unknown . |
|---|---|---|---|
| + | 3 | 2 | 3 |
| − | 10 | 0 | 0 |
Abbreviations: +, patients not requiring IVIG treatment; −, patients requiring IVIG treatment.
It is worth noting that patients UPN 51 and 116, who are first cousins and carry the same γc mutation, had a different B-cell function outcome after BMT, although T-cell function was found normal and B cells were of host origin in both cases. The only observed difference is that patient UPN 51 received an HLA-identical BMT whereas patient UPN 116 received an HLA-nonidentical BMT.
DISCUSSION
We report in this retrospective study the chimerism status and B-cell function of 22 patients with B+ SCID treated by a BMT at a single center who are still alive more than 2 years after BMT. All studies were performed in 1997 and 1998 in long-term survivors. The survival rate (data not shown) is similar to those reported in other studies.19-21,23,29,30 43
Chimerism studies showed that there was an engraftment of T cells in all of the patients, consistent with previous reports.20,21,23,26,27 44 However, circulating B cells and monocytes from 18 of the 22 patients were found to be of host origin. It is worth noting that repeated chimerism analysis performed in 5 patients from this study showed an apparent loss of donor B cells found in 3 patients in the first 2 years post-BMT. An initial myeloid engraftment followed by the gradual loss of the engrafted B cells may account for this finding. However, monocyte chimerism was not previously studied in any of these patients. Therefore, a transient peripheral expansion of the donor B-cell population in the initial months after BMT in the absence of myeloid engraftment cannot be entirely excluded. Whatever the case of this observation, it shows that lymphoid chimerism status can not be considered as stable before a long period of time after BMT performed in B+ SCID patients has elapsed.
The fact that T cells and NK cells were found to be of donor origin in most patients, whereas B cells and monocytes were of host origin, suggests that donor pluripotent stem cells do not develop into all cell lineages in these patients. This split chimerism may be due to the transfer of mature T cells from the marrow inoculum, producing a long-lived expansion in the pool of memory cells. This may partially account for the pattern of T-cell immunity development after HLA-identical BMT, but it cannot account for the chimerism status observed after HLA-nonidentical T-cell–depleted BMT. Indeed, the depletion of mature T cells from the marrow inoculum results in a delay of 3 to 6 months in the generation of peripheral blood T cells.17,25,28 This delay, consistent with the recapitulation of fetal thymopoiesis,45,46 suggests that T cells develop from transplanted hematopoietic stem cells rather than being transferred as mature T cells directly from the transplanted marrow. Similarly, naive CD45RA(+) T cells develop in X SCID dogs transplanted with a T-cell–depleted marrow inoculum.47 It is nevertheless possible that pluripotent stem cells can engraft in these patients. These donor-derived stem cells could result in the development of donor T cells and NK cells, given the selective advantage conferred to γc or JAK-3(+) T/NK lymphoid precursor cells, whereas normal monocytes and B-cell precursors that are less likely to benefit from selective advantage would be diluted out in the host population. Another possibility is that potential donor self-renewing progenitor cells migrate directly to the thymus without colonization of the marrow.
It has been suggested that conditioning regimen use could increase donor B-cell engraftment after HLA-nonidentical BMT and thereby increase the likelihood of humoral immune function development.20,21,23,26,27,44 However, we found that busulfan (8 mg/kg) and cyclophosphamide (200 mg/kg total dose) treatment did neither result in higher frequency of B-cell engraftment nor of B-cell function. This apparent discrepancy may be related to the fact that, in the above-mentioned studies, all types of SCID were considered rather than B+ SCID only. A detailed analysis of chimerism after BMT in B+ SCID patients has been reported in 2 studies. Dror et al26 have reported that B cells were of host origin in 5 of 6 cases and of unknown origin in 1. In van Leeuwen et al,23 B cells were found to be of host origin in 3 of 11, of donor origin in 3, and of mixed origin in 5. Because use of a CR consisting of 8 mg/kg busulfan and 200 mg/kg cyclophosphamide total doses does not improve survival,17,21,24,25 we propose that it should not be used any longer in B+ SCID patients receiving haploidentical BMT. However, we show here that B-cell engraftment is the best setting to observe B-cell immunity development. Because in most reported studies, including this one, the CR used was not myeloablative, the possibility remains that fully myeloblative CR would be of clinical benefit. In the European registry, 10 of 16 patients to whom 16 mg/kg busulfan was administered are alive, 9 of them with functional B cells.25 Unfortunately, chimerism data are not currently available for these patients. This strategy therefore remains a possible option, at least in patients who are not severely infected at the time of BMT.
In this study, autologous B cells and monocytes were detected together with donor T cells in 18 cases. Because T cells were functional in all but 1, it provides a unique opportunity to determine host B-cell function in these patients. B-cell function was found normal in 8 of these 18 patients. From these data, it cannot be fully excluded that a B-cell microchimerism accounts for antibody production in these patients. This hypothesis appears unlikely because, when tested and informative, Ig allotype determination showed that IgG isotypes were of host origin. In addition, memory B cells (CD27+ B cells)42 were detected among host B cells of patients with normal B-cell function but not among host B cells of patients with persisting B-cell immunodeficiency. Although not definitive, these data strongly argue in favor of the in vivo ability of γc-deficient or JAK-3–deficient B cells to produce antibodies in the presence of competent T cells. Cooperation via the shared HLA antigens or even T-cell education by host major histocompatibility complex (MHC) antigens expressed in the thymus can probably account for these results.48-52 B-cell function may rely on γc-independent cytokine pathways such as IL4/IL4RII or IL13/IL13R.31,32 However, 4 of these 8 patients do not make IgA, consistent with the results of Buckley et al17 and van Leeuwen et al,23 who found that half of the B+SCID patients with B cells of host origin did not synthesize IgA after BMT. Determining whether the molecular defect had a subtle influence on B-cell function would require a more thorough analysis of B-cell function in a larger group of patients.
However, B-cell function was deficient in 10 other patients with autologous γc(−) B cells. These results are consistent with some form of intrinsic B-cell defect, as also suggested by the subtle anomalies found in vitro in X-linked SCID B-cell activation53 and by the nonrandom X inactivation pattern of mature B lymphocytes from obligate XSCID carriers.54 There could be 2 possible reasons to explain why γc(−) B cells of some patients function normally, whereas others do not. First, the nature and severity of the γc mutation may affect B-cell function. However, this is ruled out by the fact that B cells from 2 related patients (first cousins) with the same γc gene mutation had different functional abilities in vivo. Alternatively, because patients receiving T-cell–depleted marrow from an HLA-identical donor develop more frequently normal B-cell function than those receiving T-cell–depleted nonidentical marrow, the expansion of a pool of mature T cells could exert an indirect effect on B-cell function. Mebius et al55have shown that the normal lymph node architecture of mice depends on a fraction of T-cell precursors (CD4+CD3−cells) that colonize lymph nodes during the last weeks of fetal development and during the first 3 or 4 days after birth that are γc-dependent in their growth. After HLA-identical BMT, the infusion of full marrow may lead to the rapid colonization of lymph nodes by similar cells, rescuing lymph nodes from involution. In HLA-nonidentical T-cell–depleted BMT, the maturation of the T cells could take too long to prevent at least some lymph node involution, preventing germinal center formation. Histology analysis of lymph node from transplanted B+ SCID patients would be useful to assess this hypothesis.
This retrospective analysis of B-cell function and chimerism demonstrates that BMT in B+ SCID patients is a reliable tool for studying in vivo the function of genetically deficient B cells and the mechanism of engraftment and hematopoiesis after BMT in the absence of a myeloablative conditioning regimen.
ACKNOWLEDGMENT
The authors thank the clinical staff for taking care of patients and Dr M. Daveau (Bois-Guillaume, France) for Ig allotype determination.
Supported by an institutional INSERM grant.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Elie Haddad, MD, Unité d’Immunologie et d’Hématologie Pédiatriques, Hôpital Necker-Enfants Malades, 149 rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: ehaddad@igr.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal