THE RAS FAMILY comprises H-Ras, K-Ras 4A, K-Ras 4B, N-Ras, and other homologous proteins such as R-Ras, TC21, Rap, and Ral. Ras protein function is controlled by a guanosine triphosphate-guanosine diphosphate (GTP-GDP) cycle that is regulated by at least 2 distinct classes of regulatory proteins.1 First, a GTPase-activating protein recognizes the active GTP-bound protein and stimulates the intrinsic GTPase activity of Ras to form the inactive GDP-bound protein. Second, guanine nucleotide exchange factors promote the formation of the active GTP-bound state.2
Ras proteins are proto-oncogene products that are critical components of signaling pathways leading from cell-surface receptors to the control of cellular proliferation, differentiation, or cell death. Ligand-stimulated activation of the cell-surface receptor, receptor-associated tyrosine kinases, or agonist mediated through G protein–coupled receptors results in the activation of Ras proteins.3,4 Activated Ras, in turn, stimulates a cascade of serine/threonine kinases to initiate transcriptional activation of genes. Several proteins with Src homology domains (SH2 and SH3), which mediate protein-protein interaction, have been implicated as connectors of the pathway.5
In this review, we will provide an overview of our current knowledge of the role of Ras proteins in signal transduction leading to proliferation or apoptotic cell death. We will discuss recent observations concerning the functional role of Ras modifications and the regulatory proteins that control Ras activity as well as the intracellular signaling pathways that are mediated by Ras proteins, with special mention of hematopoietic cells.
RAS C-TERMINAL POSTTRANSLATIONAL MODIFICATIONS AND THEIR FUNCTIONAL SIGNIFICANCE
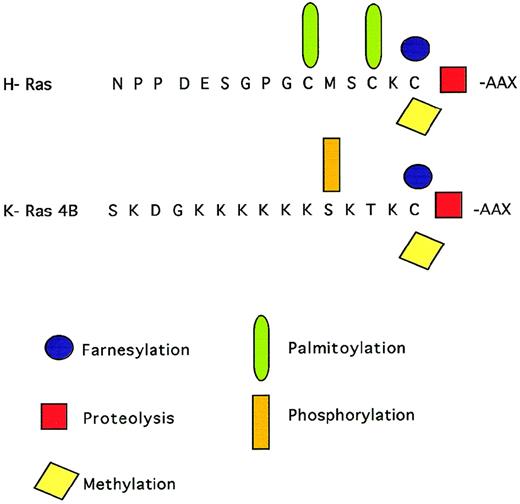
Ras proteins are posttranslationally modified by prenylation, a process that involves the addition of a 15-carbon farnesyl isoprenoid moiety to a conserved cysteine residue in a C-terminal CAAX motif by a farnesyl protein transferase (FPT). After prenylation, the C-terminal tripeptide is removed by proteolysis and the newly exposed C-terminal is methylated (Fig1). Ras prenylation is thought to facilitate membrane targeting and to be essential for Ras function.6,7 In addition, this modification can have important consequences for protein-protein interactions.8,9 In the same context, several reports have presented biochemical evidence for a prenylation-dependent interaction of Ras proteins with protein acceptors in the cytoplasmic membrane and with guanine nucleotide exchange factors and effectors.10Ras isoprenylation appears not to be essential for transformation, because it can be replaced by a different type of plasma membrane targeting signal, such as the addition of a transmembrane domain.11
C-terminal modifications of Ras proteins. A farnesyl group is added to the cysteine of the C-terminal CAAAX motif. The C-terminal tripeptide is removed by proteolysis and the newly exposed cysteine residue is methylated. Ras proteins can be further palmitoylated or phosphorylated.
C-terminal modifications of Ras proteins. A farnesyl group is added to the cysteine of the C-terminal CAAAX motif. The C-terminal tripeptide is removed by proteolysis and the newly exposed cysteine residue is methylated. Ras proteins can be further palmitoylated or phosphorylated.
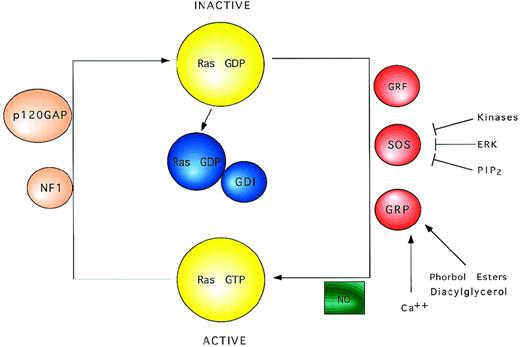
Schematic view of Ras regulatory factors. Ras proteins cycle between the active GTP-bound and the inactive GDP-bound state. Exchange factors catalyze the activation of Ras inducing the dissociation of GDP. NO may also promote the formation of Ras-GTP. Ras remains active until bound GTP is hydrolyzed to GDP, a process that is accelerated by GTPase activating proteins.
Schematic view of Ras regulatory factors. Ras proteins cycle between the active GTP-bound and the inactive GDP-bound state. Exchange factors catalyze the activation of Ras inducing the dissociation of GDP. NO may also promote the formation of Ras-GTP. Ras remains active until bound GTP is hydrolyzed to GDP, a process that is accelerated by GTPase activating proteins.
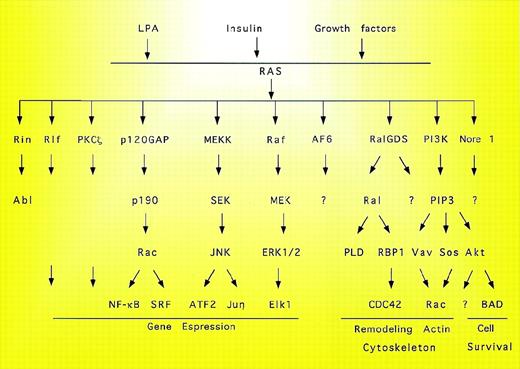
Summary of candidate Ras effectors. The complexity of the signal pathways triggered by Ras is evidenced by the multiple downstream effectors. PLD, phospholipase D; PIP, phosphatidylinositol phosphate; SRF, serum response factor.
Summary of candidate Ras effectors. The complexity of the signal pathways triggered by Ras is evidenced by the multiple downstream effectors. PLD, phospholipase D; PIP, phosphatidylinositol phosphate; SRF, serum response factor.
The various Ras proteins also present some differences in their posttranslational processing. Prenylated H-Ras and N-Ras proteins can be further lipidated by palmitoylation, a reversible modification that could improve the association of these proteins to the plasma membrane. In contrast, K-Ras proteins are not palmitoylated, but possess a polybasic domain that can be reversibly phosphorylated.12Ras proteins also differ in their affinity for FPT in vitro and in their sensitivity to FPT inhibitors, because K- and N-Ras can be alternatively geranylgeranylated in cells treated with FPT inhibitors.13-17 In addition, K-Ras can be both geranylgeranylated or farnesylated in vivo.18 Both farnesyltransferase and geranylgeranyltransferase inhibitors are required for inhibition of oncogenic K-Ras prenylation, but each alone is sufficient to suppress human tumor growth in the nude mouse.19 Interestingly, nonfarnesylated H-Ras can be palmitoylated and trigger differentiation and transformation, suggesting that farnesyl is not needed as a signal for palmytate attachment and that palmytate can support H-Ras membrane binding and 2 different biological functions.20
Recently, novel mechanisms for the regulation of Ras processing have been proposed. Induction of isoprenoid biosynthetic pathways by lipoprotein depletion can upregulate the farnesylation and membrane association of Ras.21 Conversely, cholesterol enrichment may lead to a reduction in Ras farnesylation and membrane association.
REGULATORS OF THE RAS-GTP/GDP CYCLE
In addition to posttranslational modifications, Ras proteins require binding of GTP to develop functional activity. Switching between the active GTP-bound and the inactive GDP-bound state is regulated by binding to guanine nucleotide. Although Ras proteins possess intrinsic GTPase and GDP/GTP exchange activities, they are too low to account for the rapid and transient GDP/GTP cycling that occurs during mitogenic stimulation. Instead, a complete model for Ras function includes regulatory proteins that control the GTP/GDP cycling rate.22 These regulatory proteins include GTPase activating proteins (GAPs), which stimulate hydrolysis of bound GTP to GDP,23 and guanine nucleotide exchange factor proteins, which promote the replacement of bound GDP with GTP24 (Fig2).
Two distinct GAPs for Ras proteins have been identified: p120GAP, a predominantly cytosolic protein, with a catalytic C-terminal domain that contains the Ras-binding domain and interacts with the Ras effector domain. The N-terminal domain regulates the activity of the catalytic domain and interacts with downstream effectors. This domain has 2 SH2 and 1 SH3 domain and a pleckstrin homology (PH) domain. In addition to their roles as negative regulators of Ras, it is believed that GAPs can operate as downstream effectors of Ras.25 The first evidence for a role of GAPs as Ras effectors came from the observation that oncogenic Ras mutants still require GAP interaction for their transforming activity. In addition, Ras-transforming activity can be blocked by Rap1 by competing for binding to p120GAP. The effector function of p120GAP is located in the N-terminal regulatory domain, which interacts with receptor and nonreceptor tyrosine kinases, as well as with phosphorylated proteins.26 27 All of these results are incorporated into a proposed model that suggests binding of Ras-GTP to the catalytic domain of p120GAP. This binding results in conformational changes that expose the SH2/SH3 domains for interaction with downstream effectors.
The RasGAP NF1 shares both sequence identity and substrate specificity with the p120GAP C-terminal catalytic domain. Less is known about the functions of NF1 and it can be assumed that each protein mediates distinct pathways. While growth factor stimulates tyrosine phosphorylation of p120GAP, serine and threonine phosphorylation has been reported for NF1. In addition to its role as a negative regulator of Ras activity, NF1 regulates proliferation and survival of precursors and lineage-restricted myeloid progenitors in response to multiple cytokines by modulating Ras output.28 Loss of NF1 gene is found in some patients with juvenile chronic myelogenous leukemia (JCML). Deficiency in NF1 also induces myeloproliferative disease through Ras-mediated hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF).29Likewise, NF1−/− mouse embryos show an aberrant growth of hematopoietic cells, suggesting that NF1 is required to downregulate Ras activation in myeloid cells exposed to GM-CSF, interleukin-3 (IL-3), or stem cell factor (SCF).30 Finally, NF1 inactivation cooperates with N-Ras in lymphogenesis by a mechanism independent of its GTPase activity.31 The observed cooperation emphasizes the importance of searching for additional functions of NF1. Another RasGAP, Gap1m, with specific GTPase activity for H- and R-Ras, stimulates the GTPase activity of Ras better than it does that of R-Ras. The high affinity of Gap1m for the substrates and its membrane localization suggests that Gap1m may regulate the basal activity of both H- and R-Ras.32
The third class of regulatory proteins controlling the RasGDP/GTP cycle includes the guanine nucleotide dissociation inhibitor (Ras GDI). Ras GDI is a negative regulator of Ras activity because of its potent ability to inhibit dissociation of bound GDP. This factor inhibits GDS, but not GAP, activity of Ras.33
As mentioned above, Ras proteins have low intrinsic exchange activity, increased by the binding of positive regulators. The first regulatory factor isolated that enhances and controls RasGDP/GTP exchange was the yeast cdc25 gene.34 Cdc25 activates H-Ras in vivo, but not N- or K-Ras. Selective activation of a single Ras homologue by cdc25 suggests that each Ras protein participates in a different signal transduction pathway. Sos1 and 2, which couple tyrosine kinase receptors with Ras activation, are also guanine nucleotide exchange factors. Sos activity is regulated by intracellular interactions35-37 and by phosphorylation after growth-factor stimulation of the cells. In addition to MAK kinases, p90Rsk-2 can phosphorylate Sos.38 Finally, Sos activity is inhibited in vitro by binding of phosphatidylinositol 4, 5-P2 to the PH domain.39
The direct posttranslational modification of Ras by nitric oxide (NO) promotes Ras activation. Ras can be single nitrosylated at Cys 118 by NO resulting in stimulation of guanine nucleotide exchange and activation of downstream signaling,40 possibly by destabilizing interaction between residues in the GDP-binding pocket and the nucleotide. This suggests that Ras function may be regulated directly by changes in the redox state of the cell.
A guanyl nucleotide-releasing protein for Ras, Ras-GRP, has been described recently, which has a calcium and diacylglycerol binding domain, activates Ras, and causes transformation. RasGRP may couple changes in diacylglycerol and possibly calcium concentrations to Ras activation.24,41 Finally, contradictory data exist concerning the role of Vav as a RasGDS. While some groups describe Vav as a RasGDS that activates Ras after T-cell receptor activation,42 other groups suggest that Vav cooperates with Ras in transformation but is not a GDP/GTP exchange factor for Ras.43
CANDIDATE EFFECTORS OF RAS
Signaling pathways transmitted through Ras further activate Ras effector molecules, the best characterized of which is the serine/threonine kinase Raf. Through interaction with Raf, Ras activates the MEK1 and 2 kinases and, in turn, the ERK1 and 2 kinases. ERKs phosphorylate cytoplasmic targets such as Rsk, Mnk, and phospholipase A244-46 and translocate to the nucleus, where they stimulate the activity of various transcription factors (Fig 3).
The Raf zinc finger is not required for plasma recruitment by Ras, but is essential for full activation of Raf at the cytoplasmic membrane, suggesting that Ras has 2 separate roles in Raf activation: recruitment of Raf to the plasma membrane through interaction with the Ras-binding domain, and activation of membrane-localized Raf via a mechanism that requires the Raf zinc finger.47 It has been shown that Ras interacts through the effector domain with 2 distinct N-terminal regions of Raf,48,49 suggesting that Ras promotes more than membrane translocation of Raf.50 Among other components that contribute to Raf activation, we can include the 14-3-3 proteins and phospholipids.51 The MAPK kinase pathway is critical in mediating signals from Ras/Raf; however, Ras mutants have shown that the PI3 kinase pathway synergizes with the Raf pathway to induce proliferation and loss of contact inhibition.52 Similarly, it has been shown that activation of Raf and ERK is not needed for Ras to induce membrane ruffling, suggesting that Ras could regulate both Raf-dependent and Raf-independent signals.53
Ras isoforms vary in their ability to activate Raf; K-Ras recruits Raf to the plasma membrane more efficiently than does H-Ras, and H-Ras is a more potent activator of PI3 kinase than is K-Ras. This suggests that activation of different Ras isoforms can have distinct biochemical consequences for the cell.54 In this context, it has been suggested that the subcellular distribution of Ras proteins could be related to differential participation of various Ras homologues in signaling processes. Raf-1 is not only activated in mitogenic pathways leading to cell-cycle entry, but also during mitosis. Transient expression experiments have shown that, in contrast to growth-factor–dependent activation of Raf-1, mitotic activation of Raf-1 is Ras-independent. In mitosis, activated Raf-1 is located predominantly in the cytoplasm, in contrast to mitogen-activated Raf-1, which is bound to the plasma membrane. Mitotic activation of Raf-1 is partially dependent on tyrosine phosphorylation and does not signal via the MAP kinase pathway.55
Using mutants of Ras and Raf that affect physical association, it has been shown that activated Ras stimulates the kinase activity of membrane-targeted Raf only when both molecules interact physically.56 The mechanism by which Ras interaction with Raf enhances Raf activity may operate by induction of conformational changes in Raf, exposing residues that are substrates for activation of kinases; alternatively, Ras may participate in the assembly of a signaling complex between Raf and other proteins. It has been also shown that Raf activation by Ras can occur in the absence of phosphorylation. In contrast, Raf activation by Src kinase is accompanied by tyrosine phosphorylation, suggesting that activation of Raf by Ras or Src occurs through different mechanisms.57Finally, Rap1A, which has an effector domain identical to that of Ras, cannot activate Raf and even antagonizes several Ras functions in vivo. Rap1A interferes with Ras-dependent activation of Raf by inhibiting Ras binding to a cysteine-rich region of Raf.58 On the contrary, it has been reported that Rap1 mediates sustained MAP kinase activation induced by nerve growth factor via activation of B-Raf.59
In addition to controlling Raf kinases, Ras also regulates other proteins such as PI3 kinase.60 Ras interacts with at least 4 different p110 subunits of PI3 kinase. The domain of PI3 kinase interacting with Ras is located between amino acids 133 and 314. Mutants in this region show differential impairment of effector interaction providing information concerning the contribution of Ras effectors to Ras function.61 This interaction in turn activates the serine/threonine kinase Akt/PKB.62 PI3 kinase-dependent activation of Ras also controls the activity of Rac and p70s6k. In addition to Raf and PI3 kinase, other Ras effectors have been described. These include Rin1,63p120GAP,25 AF6,64,65 Ral GDS,66Nore1,67 Rlf,68 and PKCζ.69PKCζ is an atypical protein kinase C isoform that is calcium-independent and unresponsive to phorbol esters. PKCζ is structurally similar to Raf and has been reported to have mitogenic effects in Ras-dependent oocyte maturation. Regulatory regions of PKCζ associate with Ras-GTP, suggesting that Ras-GTP localizes PKCζ to the plasma membrane, where it may be activated by PtdIns P3. Raf activation by PKC was not blocked by dominant negative Ras, indicating that PKC activates Raf by a mechanism distinct from that initiated by activation of receptor tyrosine kinases.70
Rin1 directly interacts in vivo with H-Ras in a GTP- and effector domain-dependent fashion and competes with Raf for in vitro binding to Ras. The domain of Rin1 that binds Ras also binds the 14-3-3 protein, suggesting that Rin1 can interact with multiple signaling molecules. Rin1 also interacts with Abl and Bcr through a domain distinct from the Ras binding domain.71,72 Nore1 has recently been identified as a potential Ras effector. Nore1 interacts directly with Ras in vitro in a GTP-dependent manner; this interaction also requires an intact Ras effector domain.67 Ras/Nore1 association also occurs in vivo after EGF receptor activation. Rlf has been described as an effector of Ras that functions as an exchange factor for Ral.68 A constitutively active form of Rlf can stimulate transcriptional activation and cell growth. AF6 was identified by the yeast 2-hybrid screening.64,65 The N-terminal domain of AF6 interacts with Ras-GTP and this interaction interferes with the binding of Ras to Raf. It has recently been shown that stimulation of EGF receptor results in a rapid activation of Ral, that correlates with the activation of Ras.73 Finally, Sos facilitates the exchange of Ras nucleotide and couples Ras to Rac through its Dbl and pleckstrin homology domains (PH) in a PI3 kinase-dependent manner.74
Other Ras effectors have been identified that could contribute to Ras regulation. Ras interacts with the N-Jun amino-terminal kinase (JNK).75 Ras also interacts with MEK kinase,76Bcl-2,77,78 REKS (Ras-dependent extracellular signal-regulated kinase kinase stimulator),79 and KSR (kinase suppressor of Ras or ceramide-activated protein kinase).80,81 KSR is a positive regulator of Ras signaling that functions between Ras and Raf or in a parallel pathway to Raf.82 KSR is a potent modulator of a signaling pathway essential to cell growth and development.83 This kinase contains 5 consensus sites of phosphorylation by mitogen-activated protein kinase, suggesting that KSR is an in vivo substrate of MAP kinases.84 It has been shown that KSR, the dimeric protein 14-3-3, and Raf form an oligomeric signaling complex that positively regulates the Ras signaling pathway.85 Finally, using the yeast 2-hybrid method, we have shown that Ras interacts with the transcription factor Aiolos. IL-2 deprivation induces Ras/Aiolos association and, consequently, inhibition of Bcl-2 expression, resulting in apoptotic cell death. One of the functional consequences of Ras/Aiolos interaction is the translocation inhibition of Aiolos from the cytoplasm to the nucleus. Our results suggest a novel role for Ras as a blocker of Bcl-2 expression through the cytoplasmic sequestering of Aiolos.86
Other important targets for Ras signals are the transcription factors NFAT and NFκB. NFAT proteins are cytosolic but, in response to receptor stimulation, they translocate to the nucleus, where they form transcriptionally active complexes with proteins of the Jun and Fos family of transcription factors. Activation of NFAT requires the coordinated interaction of the Ras signaling and the calcium/calcineurin pathways, suggesting that activation of NFAT requires the action of multiple Ras effector pathways.87,88NF-κB is activated in response to many extracellular stimuli and is involved in the regulation of cytokine, chemokine, and growth-factor genes.89 NF-κB has been shown to have antiapoptotic effects. In NF-κB–deficient cells, as well as in cells expressing a dominant negative IκBα, the apoptotic responses to external stimuli are enhanced.90 The proposed mechanism for the antiapoptotic effect of NF-κB is the transcriptional regulation of specific genes that are antiapoptotic. The ability of activated Ras to transform p53 null cells is dependent on the ability of Ras to activate NF-κB. Thus, there are cell death pathways that can be initiated by Ras after the inactivation of NF-κB.91 Oncogenic H-Ras activates NF-κB, which is required for cellular transformation, suggesting that NF-κB is a critical downstream mediator of H-Ras signaling.92 There is also evidence that, in some cell types, NF-κB can be a proapoptotic molecule.
ANTIAPOPTOTIC AND PROAPOPTOTIC RAS-MEDIATED PATHWAYS
Ras proteins have been involved in both the protection and the promotion of apoptosis. This apparent contradiction is solved by the ability of Ras to regulate multiple signaling pathways through the interaction with different effectors.93 Ras activation results in the induction of cyclin D1 expression.94-96 Ras also plays an important role in the downregulation of the cdk inhibitor p27 kip, possibly through the MAPK-mediated phosphorylation of p27kip, which prevents binding of the cdk2 inhibitor and may induce p27kip degradation.97,98 Recent studies link Ras function to the retinoblastoma (Rb) cell-cycle checkpoint,99,100establishing a link between Ras and cdk/Rb/E2F pathway.101Oncogenic Ras also causes growth arrest and premature senescence associated with upregulation of p53 and p16 ink.102
Ras also mediates the signaling pathway responsible for phosphorylation and activation of the cdc25 phosphoserine phosphatase. To become activated, cdks need to be dephosphorylated by the cdc25 phosphatases A, B, and C, regulating the progression through G2/M transition.103 All 3 phosphatases have been found in association with Raf, an interaction that may be facilitated by the 14-3-3 protein.104 Finally, using dominant negative and constitutive active Ras mutants, it has been shown that Ras regulates c-Myc expression.105 Coexpression of Ras and Myc induces cyclin-E–dependent kinase activity and transition to S phase.106
On the other hand, Ras can also mediate antiproliferative effects. Ras activation can induce p21cip expression and G1 arrest.107In PC12 cells, the extent and duration of Ras activation determines whether cells proliferate or differentiate. Treatment of cells with EGF leads to transient activation of Ras and proliferation while stimulation with NGF results in a sustained activation of Ras, which leads to differentiation.108,109 It it has been shown that NGF acts via Ras and PI3 K in sensory neurons.110 Finally, activated Ras is detected in growth-factor–stimulated T and B cells.111,112 The antiapoptotic activity of Ras has been linked to its ability to activate PI3 K. The PI3K-mediated survival signal is mediated by the activation of Akt/PKB, a serine/threonine kinase activated by PtdIns-3,4 P2.113-115 However, there is also evidence that Akt/PKB can be activated in a PI3K-independent fashion, thus raising the possibility that Akt/PKB-mediated protection from apoptosis can also occur without PI3K activation. Akt/PKB activation is involved in prevention of apoptosis in IL-4–stimulated cells because overexpression of wild-type or constitutively active Akt mutants protect cells from IL-4 deprivation-induced apoptosis. Moreover, overexpression of a constitutively active Akt mutant in IL-4–deprived cells correlates with inhibition of JNK2 activity.115 Akt/PKB inhibits the activation of caspases, which are required for the apoptotic response to serum withdrawal.116 One mechanism for Akt/PKB protection against apoptosis is the phosphorylation and inactivation of Bad, a proapoptotic Bcl-2 family member.117,118 PI3K/Akt is also implicated as a key mediator of the aberrant survival of Ras transformed cells in the absence of attachment and mediates matrix-induced survival of normal cells.119,120Ras-regulated expression of the transcription factor NFIL3 inhibits apoptosis without affecting Bcl-x expression in pro-B lymphocytes, indicating that multiple independent pathways mediate survival of developing B cells.121 In agreement, recent studies have shown that different downstream Ras pathways mediate the antiapoptotic function of Ras in IL-3–dependent hematopoietic cells.122Oncogenic Ras also causes resistance to the growth inhibitor insulinlike growth factor binding protein-3 (IGFBP-3), a possible factor involved in the dysregulation of breast cancer cell growth.123 Finally, IL-2– and IL-3–dependent cells are protected from starvation-induced apoptosis by activated Ras through upregulation of Bcl-2 and Bcl-X expression.124,125 This protection is probably due to the association Raf/Bcl-2.126 127
The interaction between Ras and JNK in relation to the induction of apoptosis is not clear. JNK activation may promote different cellular consequences depending on the cell type or the activation of complementary pathways. It is not completely understood whether JNK activation is a cause or consequence of apoptosis.128-130IL-2 deprivation correlates with an increase in JNK1 activity directly related to the induction of apoptosis.131 By contrast, activation of the ERK pathway suppresses the activity of JNK and promotes cell survival.128 However, it has also been shown that inhibition of JNK activation can impair Ras transformation, suggesting a growth-promoting role for this kinase.132
Ras activation has also been involved in the induction of apoptosis. Ras mediates signals triggered by activation of the cell death receptor Fas133 and overexpression of activated Ras leads to increased Fas ligand expression.134 Ras activation is also linked to the induction of apoptosis in the phaechromocytoma cell line PC12, which are rescued from apoptosis after expression of a dominant negative Ras mutant.135 In T cells, Ras is activated following both IL-2 stimulation and deprivation, leading either to cell proliferation or apoptosis, depending on whether other stimuli are acting simultaneously.136
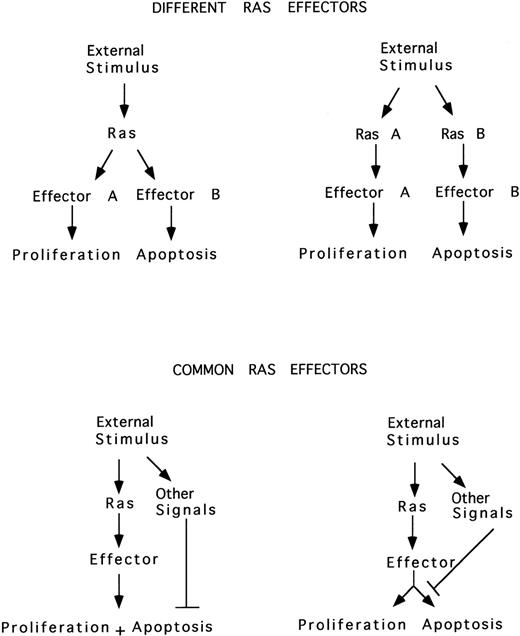
In parallel with the model proposed for the proto-oncogene c-Myc, it is possible that 2 different Ras-mediated pathways may be triggered by an external stimulus, 1 involved in proliferation and the other in apoptosis. Alternatively, Ras may simultaneously induce both proliferation and apoptosis, the latter blocked by the action of survival factors, or Ras may induce either proliferation or apoptosis, depending on external signals (Fig 4).
Ras induces proliferation or apoptosis. Two different Ras-mediated pathways may be triggered by an external stimulus, one involved in proliferation and the other in apoptosis. Alternatively, Ras may simultaneously induce both proliferation and apoptosis, the latter blocked by the action of survival factors, or Ras may induce either proliferation or apoptosis, depending on other simultaneous external signals.
Ras induces proliferation or apoptosis. Two different Ras-mediated pathways may be triggered by an external stimulus, one involved in proliferation and the other in apoptosis. Alternatively, Ras may simultaneously induce both proliferation and apoptosis, the latter blocked by the action of survival factors, or Ras may induce either proliferation or apoptosis, depending on other simultaneous external signals.
HOMOLOGUE-SPECIFIC ROLES OF RAS PROTEINS
The H-, N-, and K-Ras genes are ubiquitously expressed in mammalian cells. A number of recent works suggest that the different Ras homologues could preferentially mediate distinct cellular processes. K-Ras, but not H- or N-Ras, plays an essential role in mouse development.137-139 K-Ras is induced during differentiation of pluripotent embryonal stem cells. Its expression during early embryogenesis is limited temporally in a tissue-specific distribution.140 K-Ras−/− mice have defects in myocardial cell proliferation and neuronal programmed cell death. Erythroid cells from these embryos are able to achieve end-stage differentiation within the hepatic microenvironment. K-Ras has been described to specifically interact with microtubules141 and disrupts basolateral polarity in colon epithelial cells.142Selective activation of H-Ras by Ras-GRF has been reported, suggesting the potential participation of each Ras homologue in different signaling pathways.143 This hypothesis is supported by recent findings showing a differential ability of the 4 Ras homologues to induce focus formation, cell migration, or anchorage-dependent cell growth.144 It has recently been found that Ras homologues vary in their ability to activate the key effectors Raf-1 and PI3K,145 with K-Ras being more effective as a recruiter and activator of Raf-1 and H-Ras being more effective as an activator of PI3K.99 In addition, selective activation of K-Ras expression attenuates the ability of EGF receptor to activate MAP kinase pathway by interfering with the receptor autophosphorylation146; K-Ras also modulates the cell cycle via both positive and negative regulatory pathways.147Finally, K-Ras amplification was detected in mammary tumor progression.148 H-Ras, but not N-Ras, is involved in the IL-3–dependent signaling pathway implicated in integrin activation.149 Moreover, H-Ras stimulates tumor angiogenesis by 2 distinct pathways.150 The activated H-Ras–induced factor-independent growth of myeloid cells requires the activation of at least 2 pathways, 1 inhibiting factor-withdrawal apoptosis and other causing cell-cycle progression.151Moreover, the transforming activity of Ras can be suppressed through ERK dephosphorylation.152 Cell-specific differences in the intrinsic transforming potential of N-, H-, and K-Ras153 as well as in the different capacity of H- and N-Ras to regulate MAP kinase activity154 have also been reported. In this context, results from our laboratory have evidenced distinct behavior of Ras homologues in T cells undergoing apoptosis in response to IL-2 deprivation, with K-Ras present in mitochondria only in IL-2–stimulated cells and H-Ras being observed in mitochondria only upon IL-2 deprivation.78 Mutations of N-Ras may be involved in the pathogenesis of JCML155 as well as in acute myelogenous leukemia (AML),156 suggesting that point mutations in Ras gene might affect signal transduction through GM-CSF. In addition, N-Ras mutation induces myeloproliferative disorders and apoptosis in bone marrow repopulated mice.157 The results are consistent with a model in which antiproliferative effects are the primary consequence of N-Ras mutations and secondary transforming events are necessary for the development of AML. Moreover, erythroid progenitor cells expressing mutated N-Ras exhibit a proliferative defect resulting in an increased cell doubling time and a decrease in the proportion of cells in S/G2 phase of the cell cycle.158Finally, activated K-Ras–mediated signals are involved in the SEK-JNK pathway that are distinct from that involved in MEK-ERK activation in human colon cancer cells. The imbalance between ERK and JNK activity caused by activated K-Ras may play a critical role in human tumorigenesis.159
ACKNOWLEDGMENT
We thank Drs P. Martinez for critical reading of the manuscript and C. Mark for editorial assistance.
The Department of Immunology and Oncology was founded and is supported by the Spanish Research Council (CSIC) and Pharmacia & Upjohn.
REFERENCES
Author notes
Address reprint requests to Angelita Rebollo, Centro Nacional de Biotecnologı́a, Department of Immunology and Oncology, Universidad Autónoma, Campus de Cantoblanco, E-28049 Madrid, Spain; e-mail: arebollo@cnb.uam.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal