Abstract
Previous experiments in humans and mice have shown that allogeneic donors can serve as a source of cytotoxic T lymphocytes (CTL) specific for proteins, such as cyclin-D1 and mdm-2, expressed at elevated levels in tumor cells. In vitro, allo-major histocompatibility complex (MHC)–restricted CTL against these proteins selectively killed allogeneic tumor cells, including lymphoma, but not normal control cells. This suggested that these CTL may be useful for adoptive tumor immunotherapy, provided that they (1) survive in MHC-disparate hosts, (2) maintain their killing specificity, and (3) do not attack normal host tissues. Here, we used cloned allo-restricted CTL isolated from BALB/c mice (H-2d) that killed H-2b–derived tumor cells expressing elevated levels of the mdm-2 target protein. When these CTL were injected into bone marrow transplanted (BMT) C57BL/6 (H-2b) recipients, they consistently engrafted and were detectable in lymphoid tissues and in the bone marrow (BM). Long-term survival was most efficient in spleen and lymph nodes, where CTL were found up to 14 weeks after injection. The administration of CTL did not cause graft-versus-host disease (GVHD) normally associated with injection of allogeneic T cells. These data show that allo-restricted CTL clones are promising reagents for antigen-specific immunotherapy in BMT hosts, because they engraft and retain their specific killing activity without causing GVHD.
TWO WELL-DOCUMENTED immune reactions mediated by donor lymphocytes play an important role in bone marrow transplanted (BMT) leukemia patients. The graft-versus-host reaction is mediated by donor T lymphocytes recognizing antigenic determinants expressed by host cells. This can lead to destruction of normal host tissues, resulting in severe graft-versus-host disease (GVHD).1 The graft-versus-leukemia (GVL) reaction was first descibed in murine models,2-4 and it was subsequently found in humans that donor T lymphocytes can recognize antigenic determinants expressed by recipient leukemic cells.5 It has been shown that the GVL reaction can significantly improve the prognosis of patients suffering from leukemia, particularly chronic myeloid leukemia.6
The molecular nature of the antigenic determinants triggering GVHD and GVL has not yet been fully identified. However, it is clear that host HLA molecules presenting immunogenic peptide epitopes to donor T lymphocytes are critically important. In the case of minor histocompatibility antigen (mHA) mismatches, the peptide epitopes are derived from cellular proteins that differ between donor and host.7 To date, the peptide epitopes of 3 human8-10 and 7 murine11-17 mHAs have been identified, but it is unclear whether expression of any of these epitopes is restricted to hematopoietic cells and leukemias. This would provide a rationale for immunotherapy in BMT leukemia patients using donor-derived T cells specific for mHA expressed in recipient hematopoietic cells.
In the case of major histocompatibility mismatches, serologically undetectable polymorphism mapping to the peptide binding groove of HLA molecules is likely to change the nature and conformation of HLA-bound peptides.18,19 Consequently, donor T lymphocytes are stimulated by a variety of novel peptide epitopes bound to the groove of serologically matched host HLA molecules. In some cases, the peptide epitopes recognized by human allo-reactive T cells have been identified,20-22 but there is no evidence that these epitopes might be preferentially expressed on leukemic cells compared with normal host tissues. Thus, conventional allo-reactive T cells are likely to attack both leukemic cells and normal tissues.
The identification of T-cell–stimulating peptide epitopes selectively expressed in transformed cells is a prerequisite for dissociating GVL from GVHD. We have recently developed a strategy of directing cytotoxic T lymphocytes (CTL) of major histocompatibility complex (MHC)-mismatched donors against selected peptide epitopes presented by host MHC class I molecules.23,24 The selected peptide epitopes were derived from proteins, such as cyclin-D1 and mdm-2, known to be expressed at elevated levels in various human tumors, including leukemia. Because these proteins are physiologically expressed at low levels in normal tissues, the autologous immune system was found to be tolerant and unable to mount efficient CTL responses.25 In contrast, human and murine allo-MHC–restricted CTL against cyclin-D1 and mdm-2 were found to kill tumor cells expressing these proteins but not normal control cells in vitro.
The in vitro specificity of allo-restricted CTL suggested that they might be suitable for antigen-specific immunotherapy in BMT leukemia patients, resulting in a GVL reaction without triggering GVHD. However, there is currently no information on whether allo-restricted CTL can survive in MHC-mismatched hosts and whether they retain their peptide-specific killing activity without causing damage to normal host tissues. To address these issues we have designed experiments to study the fate of BALB/c-derived allo-restricted CTL clones injected into BMT C57BL/6 recipients. The results show that the CTL engraft, that they retain their killing specificity, and that they do not cause GVHD.
MATERIALS AND METHODS
Mouse strains.
C57BL/6 (MHC haplotype Kb, IAb, Db), B10.A(4R) (MHC haplotype Kk, IAk, IEk, Db), and BALB/c mice (MHC haplotype Kd, IAd, IEd, Dd, Ld) were purchased from Harlan, UK Ltd (Bicester, Oxfordshire, UK), and housed in the animal facility of Imperial College School of Medicine, Hammersmith site (London, UK). Animals were used for experiments at an age of 6 to 8 weeks.
CTL clones and cell lines.
In a previous study we described the isolation of allo-MHC–restricted CTL clones specific for a peptide epitope, mdm100 (YAMIYRNL), derived from the mdm-2 protein.24 The CTL were of BALB/c origin and recognized the mdm100 peptide presented by H-2Kb class I molecules. For this study, the CTL clone 6A5D was chosen. In vitro, this CTL clone showed good killing of RMA lymphoma cells (H-2b) expressing mdm-2 endogenously, but not Con-A–activated normal lymphoid cells from C57BL/6 mice. RMA is a lymphoma cell line isolated from C57BL/6 mice, and RMA-S cells were derived from RMA by in vitro mutagenesis and selection with anti-MHC class I antibodies.26 RMA-S cells have a mutation in the TAP-2 gene,27 impairing peptide transport from the cytosol into the ER, which results in inefficient loading of Kb and Db class I molecules with endogenous peptides. However, the class I molecules of RMA-S cells can be efficiently loaded with exogenously provided peptides.
BMT.
C57BL/6 mice received total body irradiation of 1,000 Rad, and the following day they were transplanted with T-cell–depleted BM from B10.A(4R) donors. T-cell depletion was achieved by mixing BM cells with DYNAL beads (DYNAL UK Ltd, Bromborough, Merseyside, UK) coated with anti–Thy-1 antibodies followed by magnetic removal of Thy-1–positive T cells. All BM samples were treated twice with DYNAL beads and successful removal of T cells was confirmed by fluorescence-activated cell sorting (FACS) analysis using antibodies specific for CD4 and CD8. In a typical depletion experiment, the percentage of T cells in the BM was reduced from 0.57% to 0.05% (data not shown). T-cell–depleted BM cells were injected intravenously (IV) at a dose of 2 × 107 per recipient. For the following 3 weeks, recipient mice were observed daily for signs of weight loss and other signs of poor health.
Antibodies.
Directly labeled anti-CD4-phycoerythrin (PE) and anti-CD8-PE antibodies were purchased from Sigma (Poole, Dorset, UK). To identify cells of C57BL/6, B10.A(4R), and BALB/c origin, antibodies against H-2Kb (AF6.88), H-2Kk (11-4.1), and H-2Ld (HB31), respectively, were used. These antibodies were used in combination with fluorescein isothiocyanate (FITC)-labeled antimouse Ig antibodies as a second layer. AF6.88 was a gift from Dr D. Gray (University of Edinburgh, Edinburgh, UK), 11-4.1 was a gift from Dr H. Reiser (I.C.S.M., Dept. of Immunology, London, UK), and HB31 was purchased from the American Tissue Culture Collection (Rockville, MD).
Limiting dilution assay (LDA) and bulk CTL cultures.
This assay was used to detect mdm100-specific CTL in the spleen, lymph nodes, thymus, and BM of C57BL/6 recipient mice. To validate this assay, pilot experiments were performed by mixing mdm100-specific CTL and C57BL/6 splenocytes in vitro at a ratio of 1 in 300. Decreasing numbers of cells taken from this mixture were stimulated under limiting dilution conditions in 96-well plates with irradiated peptide-loaded RMA-S stimulator cells (104 per well) and irradiated C57BL/6 splenocytes (2 × 105 per well) in 200 μL microcultures containing 10 U/mL recombinant interleukin-2 (IL-2). After 10 days, microcultures were restimulated using the same number of stimulator cells and feeder cells, and after 5 days, each well was tested in a 51chromium-release killing assay against RMA-S target cells coated with the mdm100 peptide or a Kb-binding control peptide.25 Microcultures that killed mdm100-coated targets at least 10% more efficiently than control targets were counted as positive. Using these conditions, the calculated frequency in the mixture containing 1 CTL/300 splenocytes was 1/273 (χ2 = 1.4; P = .682). This indicated that the assay was suitable to accurately measure the frequency of mdm100-specific CTL in cell mixtures isolated from BMT mice.
Similar conditions were used for measuring mdm100-specific CTL in bulk cultures. Cultures were set up in 24-well plates, with each well containing 2 × 106 irradiated C57BL/6 splenocytes as feeders and 105 irradiated mdm100 peptide-loaded RMA-S cells in 2 mL to which 106 cells isolated from spleen, lymph nodes, thymus, or BM of transplanted mice were added. After one restimulation using the same number of stimulator cells and feeder cells, CTL activity was tested against RMA-S targets coated with the mdm100 peptide or a Kb-binding control peptide.
Histology of tissues from recipient mice.
Samples of skin, stomach, liver, and colon of 4 BMT mice injected with CTL and 4 control mice without CTL were fixed in 10% neutral buffered formalin, processed routinely, and stained with hematoxylin and eosin (H&E).
RESULTS
Experimental design.
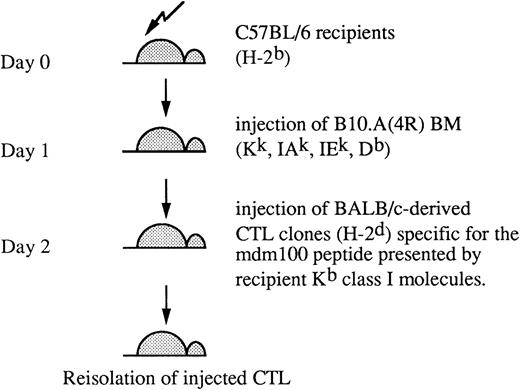
We have previously generated allo-MHC–restricted CTL clones from BALB/c mice against a Kb-presented peptide, mdm100, derived from the mdm-2 protein. High avidity CTL clones were shown to kill H-2b–derived tumor cells, including lymphomas, but not normal cells of H-2b origin.24 For the in vivo study described here, we selected a CTL clone of intermediate avidity that displayed efficient killing of RMA lymphoma cells (eg, see Fig4C). To test these CTL in vivo, they were injected into C57BL/6 mice (H-2b) transplanted the previous day with BM from B10.A(4R) donors (Fig 1). This experimental set up was chosen to mimic a planned immunotherapy protocol for leukemia patients.28 In the human immunotherapy, allo-restricted CTL lines are generated from HLA-A2–negative donors and directed against tumor-associated peptide epitopes presented by HLA-A2 class I molecules.23 Once a leukemia-reactive CTL clone is established, it would be ideal to use it for treatment of all HLA-A2–positive leukemic patients, avoiding generation of new CTL clones for each individual patient. Consequently, in some patients, there may be a complete MHC-mismatch between injected CTL and host. To mimic such a complete mismatch, BALB/c-derived CTL were injected into C57BL/6 hosts in the murine model used in this study. B10.A(4R) BM donors were chosen to mimic a partially mismatched transplant situation that sometimes occurs intentionally or unintentionally in humans. The H-2K locus mismatch is functionally important, because it renders B10.A(4R) donor cells expressing the Kk allele unrecognizable by the injected CTL that are specific for the mdm100 peptide presented by Kb molecules (Fig 1).
Murine BMT model. Experimental model used in this study involves a partial MHC-mismatch between recipient mice and BM donors and a complete MHC-mismatch between recipients and CTL donors. CTL are allo-MHC–restricted and recognize peptides presented by recipient H-2Kb class I molecules. BM donor cells expressing H-2Kk are not recognized by CTL.
Murine BMT model. Experimental model used in this study involves a partial MHC-mismatch between recipient mice and BM donors and a complete MHC-mismatch between recipients and CTL donors. CTL are allo-MHC–restricted and recognize peptides presented by recipient H-2Kb class I molecules. BM donor cells expressing H-2Kk are not recognized by CTL.
Analysis of BMT mice.
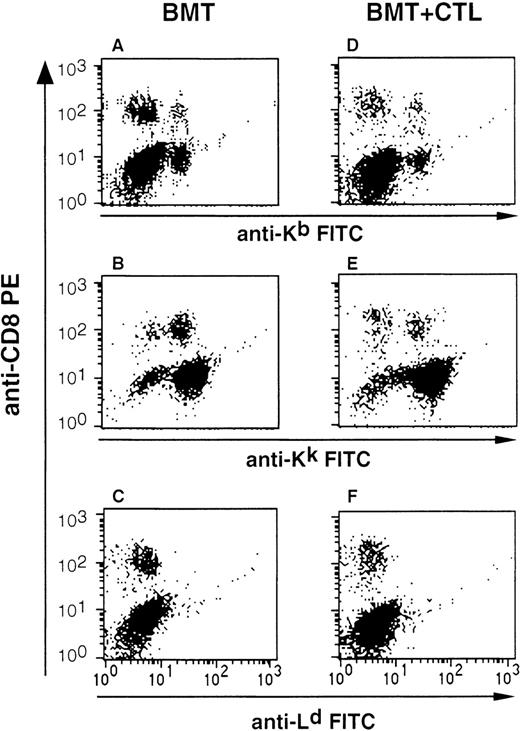
C57BL/6 mice underwent BMT, and after 4 to 14 weeks, mice were analyzed by double-staining of lymphocytes with CD8 antibodies and antibodies specific for Kb and Kk to identify cells of C57BL/6 host and B10.A(4R) donor origin, respectively. FACS analysis showed that the majority of the lymphoid cells were of B10.A(4R) origin (Fig 2A and B). However, it was consistently observed that a proportion of lymphocytes, including CD8 T cells, stained with anti-Kb antibodies, indicating that they were of C57BL/6 host origin (Fig 2A). It is likely that these cells represent mature T lymphocytes that escaped total body irradiation, raising the possibility that these residual host T cells might prevent engraftment of BALB/c-derived CTL clones.
FACS analysis to monitor BM engraftment and to search for injected CTL. Shown is the analysis of splenocytes isolated from C57BL/6 mice that either received BM from B10.A(4R) donors (BMT group) or BM and 1.5 × 107 cloned allo-restricted CTL of BALB/c origin (BMT+CTL group). Splenocytes were stained with PE-labeled CD8 antibodies and with antibodies specific for the MHC class I alleles Kb, Kk, and Ld. The cells displayed in all panels were acquired through a lymphocyte gate set according to the size (FSC) and granularity (SSC) of lymphocytes. Shown is a representative analysis performed 6 weeks after BMT. Similar data were obtained with mice analyzed between 4 and 14 weeks after BMT.
FACS analysis to monitor BM engraftment and to search for injected CTL. Shown is the analysis of splenocytes isolated from C57BL/6 mice that either received BM from B10.A(4R) donors (BMT group) or BM and 1.5 × 107 cloned allo-restricted CTL of BALB/c origin (BMT+CTL group). Splenocytes were stained with PE-labeled CD8 antibodies and with antibodies specific for the MHC class I alleles Kb, Kk, and Ld. The cells displayed in all panels were acquired through a lymphocyte gate set according to the size (FSC) and granularity (SSC) of lymphocytes. Shown is a representative analysis performed 6 weeks after BMT. Similar data were obtained with mice analyzed between 4 and 14 weeks after BMT.
Engraftment of allo-restricted CTL in BMT recipients.
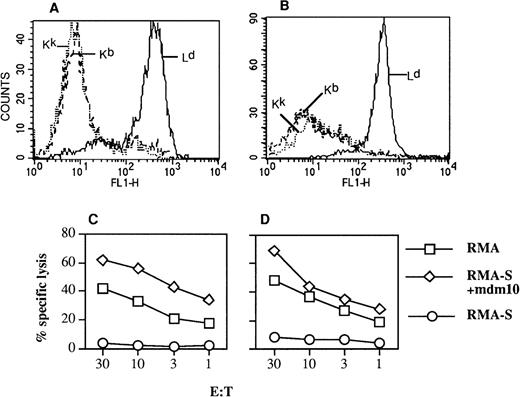
C57BL/6 mice were transplanted with B10.A(4R) BM, and the following day, one group of mice was injected IV with 4 × 106BALB/c-derived CTL specific for the mdm100 peptide presented by H-2Kb class I molecules. A control group did not receive any CTL. Injection of an additional dose of 5 × 106CTL was repeated after 9 days in the CTL group. Three weeks after the second CTL injection, mice in each group were killed and lymphocytes isolated from spleen, thymus, lymph nodes, and BM were stimulated in vitro with RMA-S cells presenting the mdm100 peptide in the context of Kb class I molecules. Figure 3shows that strong peptide-specific CTL activity was detected in all tissues from BMT mice that had been injected with CTL. In contrast, BMT mice that were not injected with CTL failed to generated any peptide-specific CTL activity (Fig 3E). We further investigated whether the observed CTL activity was caused by BALB/c-derived CTL. In an independent experiment, the CD8 cells in cultures displaying peptide-specific killing activity were stained with antibodies specific for Ld, Kb, and Kk to identify cells of BALB/c, C57BL/6, and B10.A(4R) origin, respectively. Nearly all of the CD8 cells expressed the Ld molecule (Fig 4A), suggesting that BALB/c-derived CTL accounted for the observed killing activity.
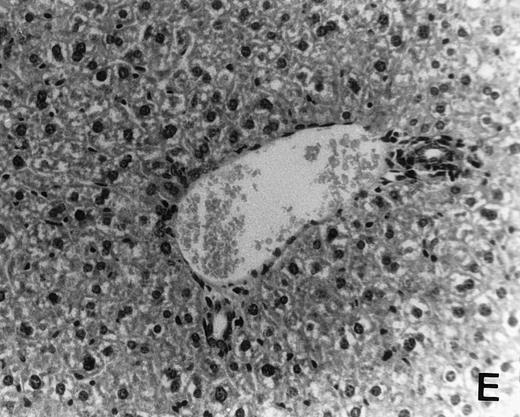
Reisolation of mdm100-specific CTL activity from BMT recipients. C57BL/6 mice received a BMT from B10.A(4R) donors followed by injection of 4 × 106 and 5 × 106mdm100-specific CTL 1 and 10 days after the BMT (BMT+CTL group). Control mice received a BMT but no CTL (BMT group). Three weeks after the second CTL injection, mice were killed and cells isolated from the spleen (A), thymus (B), lymph nodes (C), and BM (D) were stimulated in vitro with irradiated RMA-S cells loaded with mdm100 peptides as described in Materials and Methods. Peptide-specific CTL activity was discovered in all tissues from BMT+CTL mice (A through D), and similar results were obtained in independent experiments. In contrast, no peptide-specific CTL activity was observed in tissues from BMT mice. ([E] shows a representative result obtained with BM cells. Similar results were obtained when spleen, thymus, and lymph nodes were analyzed.)
Reisolation of mdm100-specific CTL activity from BMT recipients. C57BL/6 mice received a BMT from B10.A(4R) donors followed by injection of 4 × 106 and 5 × 106mdm100-specific CTL 1 and 10 days after the BMT (BMT+CTL group). Control mice received a BMT but no CTL (BMT group). Three weeks after the second CTL injection, mice were killed and cells isolated from the spleen (A), thymus (B), lymph nodes (C), and BM (D) were stimulated in vitro with irradiated RMA-S cells loaded with mdm100 peptides as described in Materials and Methods. Peptide-specific CTL activity was discovered in all tissues from BMT+CTL mice (A through D), and similar results were obtained in independent experiments. In contrast, no peptide-specific CTL activity was observed in tissues from BMT mice. ([E] shows a representative result obtained with BM cells. Similar results were obtained when spleen, thymus, and lymph nodes were analyzed.)
MHC phenotype and killing specificity of CTL reisolated from BMT recipients. C57BL/6 mice were transplanted with BM from B10.A(4R) mice and injected the following day with mdm100-specific CTL isolated from BALB/c mice. (A) After 4 weeks, mice were killed and cells from lymph nodes were stimulated in bulk cultures as described in Fig 3, resulting in mdm100-specific CTL activity similar to that seen in Fig 3C. (B) After 4 weeks, mice were killed and cells from lymphoid tissues were analyzed in LDA assays as described in Fig 5. The CTL bulk cultures (A) or the CTL expanded from positive wells of LDA assays (B) were then double-stained with PE-labeled CD8 antibodies and antibodies specific for Kb, Kk, and Ld. Staining with the anti-class I antibodies was detected with FITC-labeled secondary antibodies (see Materials and Methods). Shown is the staining of gated CD8 cells with the indicated anti-class I antibodies. Similar results were obtained in independent experiments. (C and D) CTL killing of RMA-S target cells coated with mdm100 and of RMA lymphoma cells expressing naturally processed mdm100. (C) Analysis of CTL used for injection into BMT mice and (D) analysis of CTL rescued from mice and expanded from LDA plates as in (B).
MHC phenotype and killing specificity of CTL reisolated from BMT recipients. C57BL/6 mice were transplanted with BM from B10.A(4R) mice and injected the following day with mdm100-specific CTL isolated from BALB/c mice. (A) After 4 weeks, mice were killed and cells from lymph nodes were stimulated in bulk cultures as described in Fig 3, resulting in mdm100-specific CTL activity similar to that seen in Fig 3C. (B) After 4 weeks, mice were killed and cells from lymphoid tissues were analyzed in LDA assays as described in Fig 5. The CTL bulk cultures (A) or the CTL expanded from positive wells of LDA assays (B) were then double-stained with PE-labeled CD8 antibodies and antibodies specific for Kb, Kk, and Ld. Staining with the anti-class I antibodies was detected with FITC-labeled secondary antibodies (see Materials and Methods). Shown is the staining of gated CD8 cells with the indicated anti-class I antibodies. Similar results were obtained in independent experiments. (C and D) CTL killing of RMA-S target cells coated with mdm100 and of RMA lymphoma cells expressing naturally processed mdm100. (C) Analysis of CTL used for injection into BMT mice and (D) analysis of CTL rescued from mice and expanded from LDA plates as in (B).
How many CTL are present in different tissues?
To determine the frequency of injected CTL, single-cell suspensions prepared from various tissues were double-stained with antibodies to CD8 and Ld, Kb, or Kk. The proportion of CD8 T cells staining with anti-Kb and anti-Kk antibodies was similar in BMT mice and in mice that were additionally injected with BALB/c-derived CTL (Fig 2A, B, D, and E). Staining with anti-Ld antibodies showed no difference between the 2 groups of mice, indicating that FACS analysis was not sufficiently sensitive to detect the injected CTL (Fig 2C and F). Therefore, a more sensitive assay was used. Single-cell suspensions from spleen, thymus, lymph nodes, and BM were analyzed by LDA to measure the frequency of CTL specific for the mdm100 peptide. A high frequency of CTL (up to 1/481) was detected in lymphoid tissues of mice 3 weeks after CTL injection (Fig 5A and B). Weekly analysis showed good long-term survival of CTL in spleen and lymph nodes: at week 10, the frequency in spleen and lymph nodes was only 2- to 5-fold lower than at week 3 (Fig 5A). The longest follow-up in our experiments was 14 weeks postinjection, at which time CTL were still present in spleen and lymph nodes (Fig 5B). Most importantly, in all recipient mice, CTL were consistently found in the BM, a site that is likely to contain residual leukemic cells in BMT patients. After 3 weeks, the CTL frequency in BM was similar to that seen in lymphoid tissues, followed by a more rapid decrease at weeks 6 and 7 in the BM (Fig 5A and B). Analysis of (C57BL/ 6 × BALB/c) F1 mice transplanted with BM from littermates showed that the frequency of engrafted CTL and their decrease in the BM was similar to that observed in C57BL/6 recipients (Fig 5A). Thus, there is no evidence that engraftment of the BALB/c-derived, mdm100-specific CTL was impaired in MHC-mismatched C57BL/6 hosts.
Frequency of injected CTL in tissues of BMT recipients. C57BL/6 mice were transplanted with 2 × 107T-cell–depleted B10.A(4R) BM cells. Animals then received a single dose (1.5 × 107) of mdm100-specific CTL at day 1 post-BMT (A) or 2 doses (4 × 106 and 5 × 106) of CTL at days 1 and 10 post-BMT (B). Mice in (B) were divided into a group that did receive 2 doses of 50,000 U IL-2 at the time of CTL injection and into a group that received CTL without IL-2. At the indicated weeks post-CTL injection, mice were killed (1 or 2 mice per time point) and single-cell suspensions were prepared from spleen (□), thymus (○), lymph nodes (◊), and BM (▵). These cell suspensions were used in LDA experiments as described in Materials and Methods to determine the frequency of mdm100-specific CTL. Each data point in (A) and (B) shows the calculated frequency of 1 LDA experiment. The solid symbols in (A) show the frequency data of (C57BL/6 × BALB/c) F1 mice transplanted with BM from littermates and injected with a dose of 1.5 × 107 mdm100-specific CTL (▪, F1 mice: spleen; ⧫, F1 mice: lymph nodes). At weeks 3 and 5, the frequency was analyzed only in the spleen, and at weeks 12 and 13, the frequency was analyzed in the spleen, lymph nodes, thymus, and BM. Injected mdm100-specific CTL were undetectable in thymus and BM at weeks 12 and 13. The frequency was calculated using zero-linear regression analysis, and all data had statistically acceptable χ2 less than 11 and P values greater than 0.1, except for 2 experiments (spleen at 8 weeks [A] had a χ2 of 17.5, and the lymph nodes at 3 weeks+IL-2 [B] had a χ2 of 22.5). No data points are shown for experiments in which the frequency was less than the detection limit of the LDA assay (eg, thymus and BM at 7 and 14 weeks in [B]).
Frequency of injected CTL in tissues of BMT recipients. C57BL/6 mice were transplanted with 2 × 107T-cell–depleted B10.A(4R) BM cells. Animals then received a single dose (1.5 × 107) of mdm100-specific CTL at day 1 post-BMT (A) or 2 doses (4 × 106 and 5 × 106) of CTL at days 1 and 10 post-BMT (B). Mice in (B) were divided into a group that did receive 2 doses of 50,000 U IL-2 at the time of CTL injection and into a group that received CTL without IL-2. At the indicated weeks post-CTL injection, mice were killed (1 or 2 mice per time point) and single-cell suspensions were prepared from spleen (□), thymus (○), lymph nodes (◊), and BM (▵). These cell suspensions were used in LDA experiments as described in Materials and Methods to determine the frequency of mdm100-specific CTL. Each data point in (A) and (B) shows the calculated frequency of 1 LDA experiment. The solid symbols in (A) show the frequency data of (C57BL/6 × BALB/c) F1 mice transplanted with BM from littermates and injected with a dose of 1.5 × 107 mdm100-specific CTL (▪, F1 mice: spleen; ⧫, F1 mice: lymph nodes). At weeks 3 and 5, the frequency was analyzed only in the spleen, and at weeks 12 and 13, the frequency was analyzed in the spleen, lymph nodes, thymus, and BM. Injected mdm100-specific CTL were undetectable in thymus and BM at weeks 12 and 13. The frequency was calculated using zero-linear regression analysis, and all data had statistically acceptable χ2 less than 11 and P values greater than 0.1, except for 2 experiments (spleen at 8 weeks [A] had a χ2 of 17.5, and the lymph nodes at 3 weeks+IL-2 [B] had a χ2 of 22.5). No data points are shown for experiments in which the frequency was less than the detection limit of the LDA assay (eg, thymus and BM at 7 and 14 weeks in [B]).
The possiblity that antigenic stimulation might prevent CTL decrease in the BM will be addressed in future experiments. The observed homing of CTL to the thymus raised the interesting possibility that they might tolerize recipient mice against the allogeneic MHC molecules expressed by the injected CTL.
To confirm that the mdm100-specific killing activity observed in the LDAs was due to BALB/c-derived CTL, cells from positive wells from some LDA plates were expanded to obtain sufficient cells for double-staining with antibodies against CD8 and Ld, Kb, or Kk. FACS analysis indicated that the CD8 T cells expanded from LDA cultures were of BALB/c origin (Fig 4B).
Finally, we explored the possibility that low-level mdm-2 expression in normal tissues in vivo might downmodulate the ability of injected CTL to recognize naturally processed mdm100 antigen. Thus, CTL rescued from BMT mice were tested for their ability to recognize RMA tumor cells presenting naturally processed mdm100.24 The efficiency of RMA killing by rescued CTL was similar to the killing by CTL before injection into BMT hosts (Fig 4C and D). Thus, injected CTL retained the ability to lyse mdm-2–expressing lymphoma cells.
Does IL-2 improve CTL engraftment?
This question was addressed in BMT mice that were injected twice with CTL. The first injection was performed 1 day after BMT, followed by a booster injection 10 days later. In one group of mice, both IV CTL injections were performed in combination with subcutaneous injections of 50,000 U of recombinant IL-2 in an oil emulsion to achieve gradual IL-2 release. The control group was treated identically, except that IL-2 was omitted. Analysis 3 and 7 weeks after the second CTL injection showed that the frequency of mdm100-specific CTL in different tissues was similar in IL-2–treated mice compared with the control group (Fig 5B). Thus, IL-2 did not detectably increase the frequency of injected CTL, nor was it required for long-term CTL survival.
Allo-restricted CTL engraft without causing GVHD.
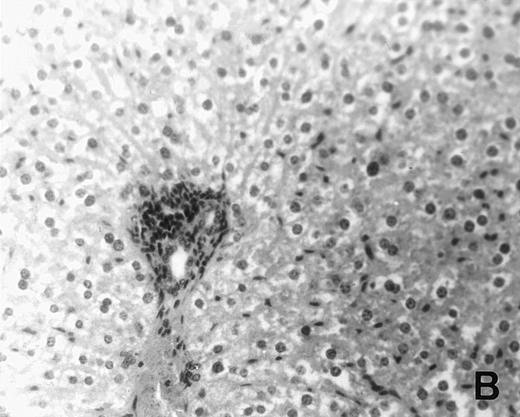
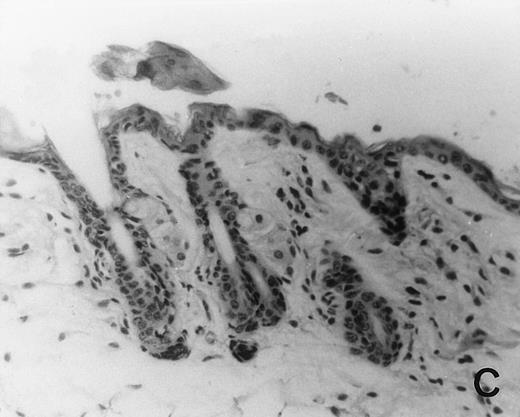
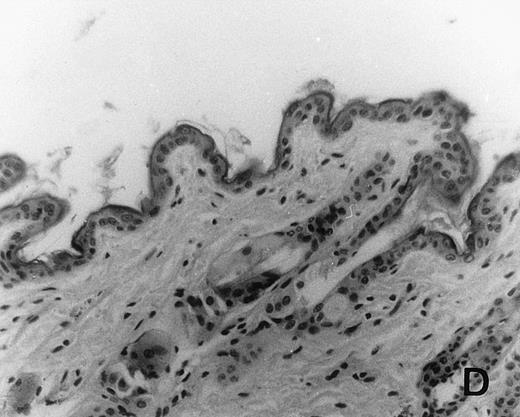
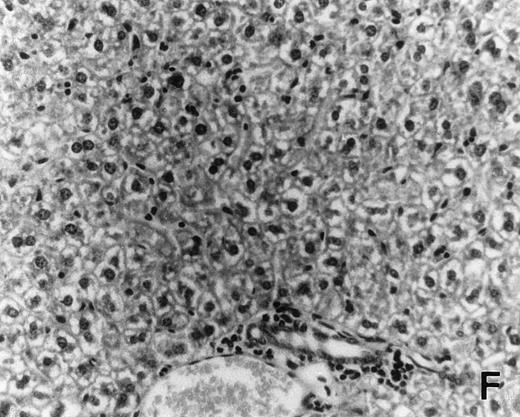
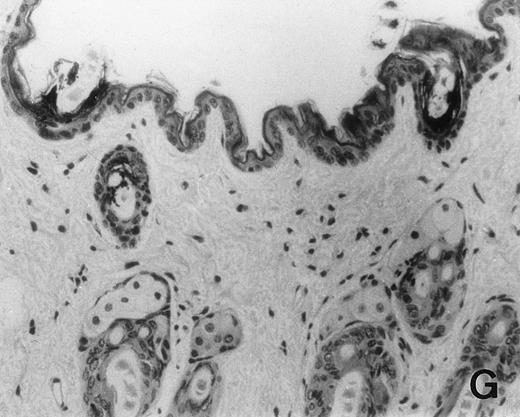
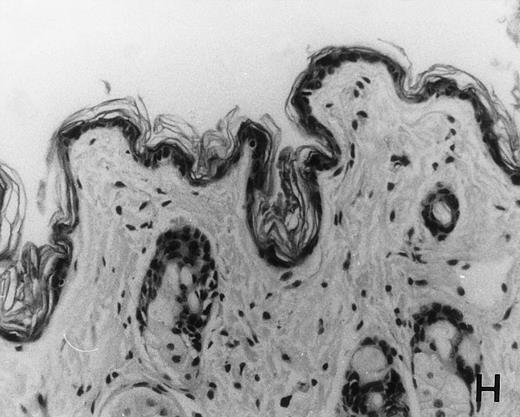
Previous in vitro studies showed that mdm100-specific, allo-restricted CTL killed H-2b tumor cells, including lymphoma cells, but not normal cells.24 However, only a limited selection of normal cells such as dendritic cells and Con-A–activated lymphocytes was available for in vitro tests. Thus, it remained unclear whether some normal tissues in vivo express sufficient levels of mdm-2 to trigger killing by the injected CTL. Several lines of evidence suggested that injected CTL did not attack normal host tissues. Firstly, none of the mice showed any acute side effects after CTL injection. Secondly, the recovery from BMT and the general health status were indistinguishable in mice that received CTL compared with control mice. Thirdly, histological analysis of liver, gut, and skin from 4 CTL treated and 4 untreated mice showed that these tissues, which are normally affected by GVHD, showed similar histology in mice with or without CTL administration (Fig 6). This indicated that CTL injection did not cause the tissue damage that is frequently seen in BMT individuals after infusion of allogeneic T lymphocytes.
Histology of BMT mice. (A through D) The histology of C57BL/6 mice transplanted with B10.A(4R) BM. One group of mice was injected with allo-restricted CTL while the control group did not receive CTL. (A) Liver from a CTL-treated mouse 3 weeks posttransplant, with no significant inflammation or necrosis. (B) Liver from a control mouse 3 weeks posttransplant, with mild periportal inflammation. Skin from a CTL-treated mouse (C) and a control mouse (D) 3 weeks posttransplant, with no significant inflammation or keratinocyte necrosis. Tissues were also examined at 4, 5, and 6 weeks posttransplant and showed no evidence of GVHD in either CTL-treated or control mice (not shown). (E-H) show the histology of (C57BL/6 × BALB/c) F1 mice, transplanted with BM from littermates. One group of mice was injected with allo-restricted CTL and a control group did not receive CTL. (E) Liver from a CTL-treated mouse 4 weeks posttransplant, with no significant inflammation or necrosis. (F) Liver from control mouse 4 weeks posttransplant, with no significant inflammation and necrosis. Skin from a CTL-treated mouse (G) and a control mouse (H) 4 weeks posttransplant, with no significant inflammation or keratinocyte necrosis. Tissues were also examined at 3 and 5 weeks posttransplant and showed no evidence of GVHD in either CTL-treated or control mice (not shown). (All H&E-stained sections, original magnification × 20.) Sections from colon and stomach also showed no significant inflammation (not shown).
Histology of BMT mice. (A through D) The histology of C57BL/6 mice transplanted with B10.A(4R) BM. One group of mice was injected with allo-restricted CTL while the control group did not receive CTL. (A) Liver from a CTL-treated mouse 3 weeks posttransplant, with no significant inflammation or necrosis. (B) Liver from a control mouse 3 weeks posttransplant, with mild periportal inflammation. Skin from a CTL-treated mouse (C) and a control mouse (D) 3 weeks posttransplant, with no significant inflammation or keratinocyte necrosis. Tissues were also examined at 4, 5, and 6 weeks posttransplant and showed no evidence of GVHD in either CTL-treated or control mice (not shown). (E-H) show the histology of (C57BL/6 × BALB/c) F1 mice, transplanted with BM from littermates. One group of mice was injected with allo-restricted CTL and a control group did not receive CTL. (E) Liver from a CTL-treated mouse 4 weeks posttransplant, with no significant inflammation or necrosis. (F) Liver from control mouse 4 weeks posttransplant, with no significant inflammation and necrosis. Skin from a CTL-treated mouse (G) and a control mouse (H) 4 weeks posttransplant, with no significant inflammation or keratinocyte necrosis. Tissues were also examined at 3 and 5 weeks posttransplant and showed no evidence of GVHD in either CTL-treated or control mice (not shown). (All H&E-stained sections, original magnification × 20.) Sections from colon and stomach also showed no significant inflammation (not shown).
We explored whether immune responses of BMT C57BL/6 hosts against BALB/c-derived CTL prevented the induction of GVHD by injected CTL. If this were the case, we predicted that CTL injection into (C57BL/6 × BALB/c) F1 hosts would result in GVHD. Thus, (C57BL/6 × BALB/c) F1 mice were transplanted with BM from littermates using the same conditioning protocol that was used for all experiments in this study. One group of transplanted mice was injected with 1.5 × 107 allo-restricted CTL and a control group did not receive any CTL. Histology of skin, liver, stomach, and gut performed after 3, 4, and 5 weeks showed that injected CTL did not cause GVHD in these F1 hosts (Fig 6E through H). Thus, lack of GVHD was not dependent on host immune responses against injected CTL.
DISCUSSION
Allo-MHC–restricted CTL are promising reagents for treatment of BMT leukemia patients. Currently, infusion of lymphocytes from BM donors is used to successfully treat leukemic relapse in these patients.6 29-40 However, in many cases, the donor lymphocytes cause not only GVL but also GVHD. Because the antigen specificity of infused donor lymphocytes is unknown, it is difficult to selectively direct them against leukemic cells. In contrast, allo-restricted CTL clones can be raised against peptide epitopes that are preferentially expressed in leukemia.
Immunotherapy with allo-restricted CTL will always involve at least one MHC-class I locus mismatch between injected CTL and recipient host. To avoid generation of CTL clones for individual patients, it would be desirable to use one established clone specific for a tumor-associated peptide epitope presented by a common MHC class I allele, such as HLA-A0201. This clone could then be used in the treatment of all A0201-positive patients. However, in some cases, this may involve a complete MHC-mismatch between host and injected CTL. For this reason, we have investigated in the described murine model the fate of BALB/c-derived CTL clones injected into MHC-mismatched, BMT C57BL/6 hosts.
Our data showed that CTL consistently engrafted and were detectable in spleen, lymph nodes, thymus, and BM. Even in the absence of antigenic stimulation, a high frequency of CTL was detected in lymphoid tissues 3 weeks postinjection, followed by a gradual decrease. This is similar to the kinetics of antigen-specific immune responses in which, after initial clonal expansion and death of effector CTL, the frequency of memory CTL gradually decreases over time. Like memory CTL, injected allo-restricted CTL responded readily to antigenic stimulation in vitro and displayed peptide-specific killing activity. These results are promising; further experiments will assess whether the injected CTL expand and develop cytotoxic effector function when they encounter their peptide antigen in vivo.
Although the frequency of CTL was lower in BM than in lymphoid tissues, it was probably higher than the number of leukemic cells present in the BM of patients undergoing BMT after myeloablative therapy. In the experiments described here, the frequency of CTL in the BM 3 weeks postinjection was 1/1,821 to 1/12,550. For comparison, reverse-transcriptase-polymerase chain reaction (RT-PCR) used for detection of BCR/ABL-positive cells in the BM of CML patients can detect 1/105 to 1/106 leukemic cells.41 Thus, in patients with minimal residual disease as defined by RT-PCR, a high ratio of CTL over leukemic cells may result in efficient elimination of malignant cells and prevent relapse.
Histological examination indicated that injection of allo-restricted CTL against peptide epitopes expressed at elevated levels in transformed cells did not trigger GVHD. It is currently unclear whether the expected low level of peptide display in normal tissues can deliver weak stimulatory signals to enhance the survival of injected CTL without triggering effector function.42 Alternatively, low-level peptide display may be ignored, or it may cause downmodulation of the TCR and/or CD8 molecules.43 Future studies will investigate these alternate possibilities.
Because the allo-restricted T-cell strategy is not limited by immunological tolerance, it provides an opportunity to target almost any protein that is expressed in leukemic cells. These include hematopoietic transcription factors, such as WT-1 and gata-1, and differentiation antigens, such as myeloperoxidase and CD45. Allo-restricted CTL clones against these proteins would be expected to function as proficient GVL effectors and attack leukemic cells. The CTL may also attack normal hematopoietic cells expressing the relevant protein, but not nonhematopoietic tissues, which are usually the target of GVHD. The patients’ hematopoietic system could be protected from CTL attack by selecting allogeneic BM donors who do not express the HLA class I allele that is required for antigen recognition by the injected CTL clones.28
Supported by grants to H.J.S. from the Leukaemia Research Fund and the Cancer Research Campaign.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hans J. Stauss, MD, ICSTM, Hammersmith Hospital, Department of Immunology, Du Cane Road, London W12 0NN, UK; e-mail: hstauss@rpms.ac.uk.

![Fig. 3. Reisolation of mdm100-specific CTL activity from BMT recipients. C57BL/6 mice received a BMT from B10.A(4R) donors followed by injection of 4 × 106 and 5 × 106mdm100-specific CTL 1 and 10 days after the BMT (BMT+CTL group). Control mice received a BMT but no CTL (BMT group). Three weeks after the second CTL injection, mice were killed and cells isolated from the spleen (A), thymus (B), lymph nodes (C), and BM (D) were stimulated in vitro with irradiated RMA-S cells loaded with mdm100 peptides as described in Materials and Methods. Peptide-specific CTL activity was discovered in all tissues from BMT+CTL mice (A through D), and similar results were obtained in independent experiments. In contrast, no peptide-specific CTL activity was observed in tissues from BMT mice. ([E] shows a representative result obtained with BM cells. Similar results were obtained when spleen, thymus, and lymph nodes were analyzed.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/9/10.1182_blood.v94.9.2999/4/m_blod42112003x.jpeg?Expires=1763595605&Signature=z7JDAvsmt3g~lNzSDJnD80HQT~S9EDKgHsuk3AXRbQB2r7Wg3G13wnHDRuYgIP9ZV89YPLXr3N5V2MqhsZ1aUVLi6TJeHDei84pRMDY5D3OpMmUa8lFZZiOLDeaQrOIQ5oVwkQCeJXxzVHYORHITZaLdGo-icD8K4Erq-3HkWNWdVZAARWekrddf5cKOWoUrzNvHz3DwqtrNR1mnFzmxZEXoAOQfV-cz73gnuBNxQ5E0cTtfRQLIPV6A8t3HMzbXyLQxuy86eT4oRQ6yRlkVoeXyQA9O0rcM3RJgdjjOkVtTItDD-~AtXbvWG0hvW7alAmy3cHJzJg5bnyr8xzZmqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Frequency of injected CTL in tissues of BMT recipients. C57BL/6 mice were transplanted with 2 × 107T-cell–depleted B10.A(4R) BM cells. Animals then received a single dose (1.5 × 107) of mdm100-specific CTL at day 1 post-BMT (A) or 2 doses (4 × 106 and 5 × 106) of CTL at days 1 and 10 post-BMT (B). Mice in (B) were divided into a group that did receive 2 doses of 50,000 U IL-2 at the time of CTL injection and into a group that received CTL without IL-2. At the indicated weeks post-CTL injection, mice were killed (1 or 2 mice per time point) and single-cell suspensions were prepared from spleen (□), thymus (○), lymph nodes (◊), and BM (▵). These cell suspensions were used in LDA experiments as described in Materials and Methods to determine the frequency of mdm100-specific CTL. Each data point in (A) and (B) shows the calculated frequency of 1 LDA experiment. The solid symbols in (A) show the frequency data of (C57BL/6 × BALB/c) F1 mice transplanted with BM from littermates and injected with a dose of 1.5 × 107 mdm100-specific CTL (▪, F1 mice: spleen; ⧫, F1 mice: lymph nodes). At weeks 3 and 5, the frequency was analyzed only in the spleen, and at weeks 12 and 13, the frequency was analyzed in the spleen, lymph nodes, thymus, and BM. Injected mdm100-specific CTL were undetectable in thymus and BM at weeks 12 and 13. The frequency was calculated using zero-linear regression analysis, and all data had statistically acceptable χ2 less than 11 and P values greater than 0.1, except for 2 experiments (spleen at 8 weeks [A] had a χ2 of 17.5, and the lymph nodes at 3 weeks+IL-2 [B] had a χ2 of 22.5). No data points are shown for experiments in which the frequency was less than the detection limit of the LDA assay (eg, thymus and BM at 7 and 14 weeks in [B]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/9/10.1182_blood.v94.9.2999/4/m_blod42112005x.jpeg?Expires=1763595605&Signature=KroeqygouUyLALTQo3kXOXOpWWi3qJwHwtl9W6PbAFZheayXhwKP8Bh5wYJKP27adRpFGRzEpvUwXXEG6JEkVIYwtXvpP0FrjHokFMsff8adSRYAjKTKgzWW~ePy8l7vQ07HfR3Pq9XZ72nBitZ3m8EU0J5BvIZ9fwYTtA72dE2npgfrO8RenASmMzizD7KO-e7rOm-4Tm1GpBuK0PUOlZANANG2X4zDMdnowYV0ENn7HKPQ~KIBdJNqAaZKG4GoJuOwYp8TAB-Y1XQtrwft7jHnZzDI9j7PnioUFmnK7dAupBTXSNbGrkR2Bjqan7TarSl918PhUEgw6M2JGEOnQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)