Abstract
Peripheral monocytes are short-lived and are replenished from hematopoietic stem cells whose proliferation is believed to be confined to the bone marrow. Human peripheral monocytes are assumed not to be able to proliferate. In this study we show that CD137 (ILA/4-1BB), a member of the tumor necrosis factor receptor family, induces a widespread and profound proliferation of human peripheral monocytes. Macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor are essential, but not sufficient for proliferation. Additional soluble autocrine factors induced by CD137 are required. Induction of proliferation is mediated via reverse signaling through a CD137 ligand, expressed constitutively by peripheral monocytes. The ability of CD137 to induce proliferation in human peripheral monocytes is not shared by any other known molecule.
CD137, ORIGINALLY NAMED 4-1BB and induced by lymphocyte activation (ILA), is a tumor necrosis factor (TNF) receptor family member and was identified in screens for receptors expressed on activated lymphocytes.1-3 CD137 is expressed by activated lymphocytes and monocytes and expression in primary cells is strictly activation dependent.4 In several nonimmune cells, expression is inducible by proinflammatory cytokines.5,6 Soluble forms of CD137 are generated by differential splicing and can be detected at enhanced concentrations in sera of patients with rheumatoid arthritis.7 The gene for human CD137 resides on chromosome 1p36, in a cluster of related genes.8
Crosslinking of CD137 costimulates proliferation of T lymphocytes,9-11 and CD137 ligand expressed by B lymphocytes costimulates T-cell proliferation synergistically with B7.12 Injected, agonistic anti-CD137 antibodies activate T cells sufficiently for rejection of tumors and allogeneic transplants in mice.13 14
While agonistic antibodies and the ligand to CD137 enhance lymphocyte activation, CD137 protein has the opposite effect. It inhibits proliferation of activated T lymphocytes and induces programmed cell death. These T-cell inhibitory activities of CD137 require immobilization of the protein, indicating transmission of a signal through the ligand.11 Expression of the human CD137 ligand is inducible in T lymphocytes and is constitutive in monocytes.3
Reverse signaling through the CD137 ligand also takes place in monocytes. Immobilized recombinant CD137 protein leads to activation of the monocytes with enhanced expression of proinflammatory cytokines, inhibition of the anti-inflammatory cytokine IL-10, and induction of activation markers such as intercellular adhesion molecule-1 (ICAM).15 CD137 also prolongs monocyte survival via induction of macrophage colony-stimulating factor (M-CSF).16
Monocytes are key regulators of immune responses, essential for the generation of beneficial antitumor and antipathogen immune reactions. Monocytes also participate in the development of harmful autoimmune diseases.17 They migrate from the circulation to sites of inflammations, attracted by signals such as chemokines, and the accumulation of monocytes/macrophages is a characteristic feature of chronic inflammation. This accumulation is further enhanced by cytokines released at inflammatory sites, such as M-CSF, granulocyte-macrophage CSF (GM-CSF), and interleukin-3 (IL-3), which prolong the survival of monocytes/macrophages.18-20 The accumulation of monocytes/macrophages at sites of inflammation has been attributed so far solely to immigration and the extension of cell survival, because human peripheral monocytes/macrophages have been considered not to be able to proliferate.19 21
In this study we show that CD137 is able to induce a substantial degree of proliferation/endomitosis in a large proportion of human peripheral monocytes. This proliferative activity is not shared by M-CSF or any other known molecule.
MATERIALS AND METHODS
Reagents.
M-CSF and neutralizing anti–M-CSF, anti–GM-CSF, and anti–IL-3 antibodies were obtained from R&D (Wiesbaden, Germany). Anti–M-CSF: clone 26730,11, protein A–purified IgG fraction of ascites fluid of murine hybridoma; anti–GM-CSF: clone 3209.1, murine monoclonal, IgG1; and anti–IL-3: protein A–purified IgG fraction of ascites fluid of murine hybridoma. Anti–HLA-DR was obtained from Becton Dickinson (Heidelberg, Germany). The antibodies were free of sodium azide. CD137-Fc protein was purchased from Alexis (Grünberg, Germany). Human IgG1 Fc protein was obtained from Accurate Chemical and Scientific Corp (Westbury, NY). TNFRII-Fc protein was a gift from Daniela Maennel (University of Regensburg).
Cells and cell culture.
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy volunteers. Buffy coats were diluted with 2 equal volumes of phosphate-buffered saline (PBS), overlaid onto an equal volume of Histopaque (Sigma, Deisenhofen, Germany), and spun for 20 minutes at 1,200g. PBMCs that accumulated as a white layer at the Percoll boundary were recovered and erythrocytes were lysed with 2 mL of 200 mmol/L NH4Cl, 10 mmol/L NaHCO3, and 10 mmol/L EDTA pH 7.4 for 2 minutes at room temperature. Cells were washed 2 times with PBS, pelleted at 250g, and resuspended in RPMI, 5% fetal calf serum (FCS). Primary monocytes were isolated by elutriation.22 Elutriated monocytes were more than 95% pure and contaminating T lymphocytes were less than 3% as estimated by morphology and antigenic phenotype (CD14, CD3, CD4, and CD8 expression). Cells were cultured in polystyrene dishes (Becton Dickinson, Franklin Lakes, NJ) in RPMI 1640 supplemented with 5% FCS at a concentration of 106/mL. The cell lines HL60 and MonoMac were obtained from ATCC (Manassas, VA).
Enzyme-linked immunosorbent assay (ELISA).
ELISA kits were purchased from R&D Systems (Wiesbaden, Germany) and performed according to the manufacturer’s instructions. Cytokine concentrations were determined in triplicate and are expressed as mean ± standard deviation.
Cell survival and apoptosis.
The number of living cells was determined by counting cells in 4 representative fields using an ocular with an engraved grid to ensure equal sizes of the evaluated fields. Dead and live cells were distinguished by trypan blue exclusion.
Apoptosis was determined via DNA fragmentation by the ‘Cell Death Detection ELISA Plus’ (Boehringer Mannheim, Mannheim, Germany) following instructions provided by the manufacturer. Measurements were performed in triplicate.
Cell proliferation.
For measurement of proliferation of individual cells the ‘In situ proliferation kit’ (Boehringer Mannheim) was used. A total of 3 × 105 cells were seeded per chamber of an 8-chamber slide (Falcon; Becton Dickinson, Heidelberg, Germany), coated with Fc or CD137-Fc protein and grown for 10 days. Bromodeoxyuridine (BrdU), 10 μmol/L, was added for 60 minutes. Incorporated BrdU was visualized according to kit instructions, by staining with mouse anti-BrdU and sheep anti-mouse–fluorescein isothiocyanate (FITC). Monocytes were identified by staining with a phycoerythrin-labeled, anti-CD14 antibody (2 μg/mL; Immunotech, Marseille, France). Chromatin was stained by 5 μg/mL Hoechst 33342 (Sigma, Deisenhofen, Germany) for 5 minutes.
Proliferation of cell populations was determined in 96-well microtiter plates. 105 monocytes per well were pulsed for 24 hours with 0.5 μCi 3H-thymidine, harvested, and evaluated on the TopCount microplate scintillation counter (Packard, Meriden, CT). Each condition was performed in triplicate and results are depicted as means ± standard deviation.
Statistical analysis.
Statistical significance was evaluated by the Mann Whitney U test using SPSS software (SPSS Inc, Chicago, IL).
RESULTS
CD137 induces apoptosis in monocytes.
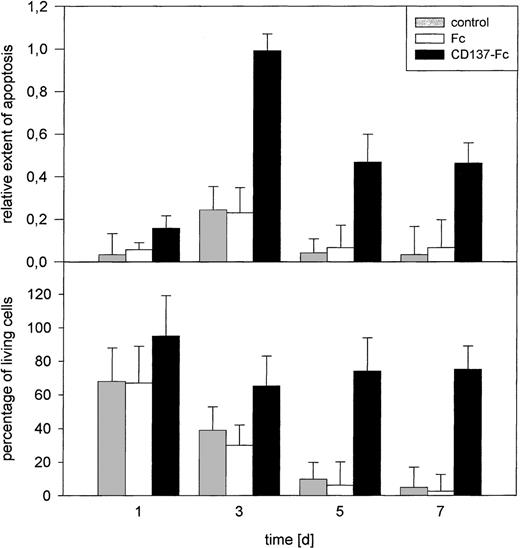
CD137 has been shown to substantially prolong the survival of human primary peripheral monocytes.16 To investigate the mechanism of this activity, we tested whether CD137 prevents apoptotic cell death. Monocytes were cultured on tissue culture dishes coated with a fusion protein consisting of the extracellular domain of CD137 and the constant domain of human immunglobulin G1 (Fc). Coating was performed with a solution of 1 μg/mL protein in PBS at 4°C overnight. Untreated and Fc protein-coated plates were used as controls. Monocytes in these control groups died due to lack of stimulation and/or survival signals.23-25 In the CD137-Fc protein-coated wells, the number of living monocytes was significantly higher from day 5 of culture onward (Fig 1).
Induction of monocyte apoptosis by CD137. 105primary monocytes were cultured on immobilized Fc or CD137-Fc protein or on untreated plates (control). The extent of apoptosis (top) and the number of living cells, determined by trypan blue exclusion (bottom) were evaluated at days 1, 3, 5, and 7. Error bars indicate standard deviation. The differences in rates of apoptosis and the number of living cells between Fc and CD137-Fc treated cultures were significant starting at days 3 and 5, respectively, with P < .05. Comparable results were obtained in 3 separate experiments.
Induction of monocyte apoptosis by CD137. 105primary monocytes were cultured on immobilized Fc or CD137-Fc protein or on untreated plates (control). The extent of apoptosis (top) and the number of living cells, determined by trypan blue exclusion (bottom) were evaluated at days 1, 3, 5, and 7. Error bars indicate standard deviation. The differences in rates of apoptosis and the number of living cells between Fc and CD137-Fc treated cultures were significant starting at days 3 and 5, respectively, with P < .05. Comparable results were obtained in 3 separate experiments.
The extent of apoptosis in the same cultures was quantified by measuring the amount of fragmented DNA (Fig 1). Quite unexpectedly, we found a higher degree of apoptosis in monocyte cultures treated with CD137 protein, compared to untreated cultures or cultures treated with the Fc control protein.
CD137 induces proliferation of peripheral monocytes.
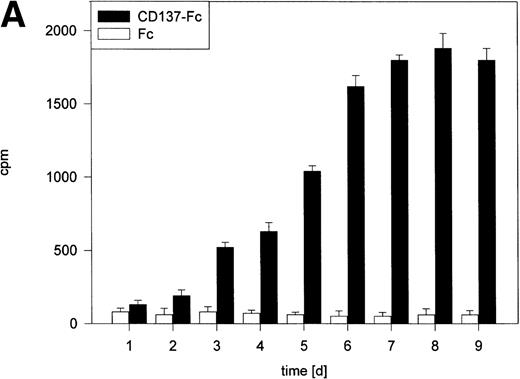
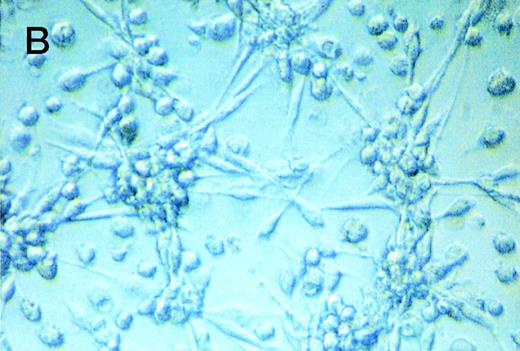
Despite induction of apoptosis by CD137, the fact remained that CD137 prolonged the survival of the monocyte population. These seemingly contradictory results could best be reconciled by the assumption that CD137 induces proliferation of monocytes, compensating for the loss of cells through apoptosis. As measured by 3H-thymidine incorporation, CD137 induced, in fact, a strong proliferation of monocytes (Fig 2A). Proliferation correlated positively with the time monocytes were grown in the presence of CD137 protein and reached maximum levels between day 7 to 10, with a 30-fold or even higher increase in 3H-thymidine incorporation compared with control cells. There was no difference between monocytes in untreated and Fc protein-coated wells (not shown). Also, in the CD137-Fc–treated monocyte cultures, but not in control cultures, colonies or cell aggregates were observed, which could wholly or partly result from proliferating monocytes (Fig 2B).
CD137 induces proliferation of peripheral monocytes. (A) 105 monocytes were cultured on 96-well plates coated with Fc or CD137-Fc protein. Proliferation was determined daily by a 24-hour pulse with 0.5 μCi 3H-thymidine and differed significantly from day 3 onward, with P < .02. (B) Colonies/aggregates formed on CD137-treated monocytes. Photographs were taken at day 10 of culture at a magnification of 200×.
CD137 induces proliferation of peripheral monocytes. (A) 105 monocytes were cultured on 96-well plates coated with Fc or CD137-Fc protein. Proliferation was determined daily by a 24-hour pulse with 0.5 μCi 3H-thymidine and differed significantly from day 3 onward, with P < .02. (B) Colonies/aggregates formed on CD137-treated monocytes. Photographs were taken at day 10 of culture at a magnification of 200×.
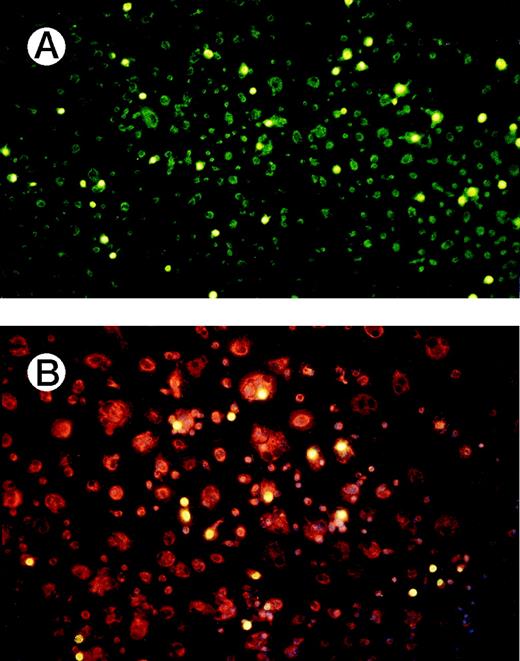
In line with the substantial increase in 3H-thymidine incorporation is that CD137-induced proliferation was rather wide-spread. Upon labeling CD137 treated monocytes with BrdU for only 1 hour, and subsequent detection with fluoresceine-labeled, anti-BrdU antibodies, 9.3% of the cells (55 ± 6 of 589 ± 43) were found to replicate their DNA (Fig 3A). Peripheral monocytes grown on Fc-coated dishes did not show incorporation of BrdU (not shown).
Verification of proliferating cells as monocytes. (A) Monocytes were grown for 8 days with immobilized CD137 protein and labeled for 60 minutes with 10 μmol/L BrdU (yellow). (B) Monocytes were cultured on immobilized CD137-Fc protein on chamber slides. After 10 days the cells were labeled for 60 minutes with 10 μmol/L BrdU (yellow). Monocytes were identified by staining for CD14 (red), and nuclei were visualized by Hoechst 33342 (blue). Similar results were obtained in 3 independent experiments for (A) and (B), respectively.
Verification of proliferating cells as monocytes. (A) Monocytes were grown for 8 days with immobilized CD137 protein and labeled for 60 minutes with 10 μmol/L BrdU (yellow). (B) Monocytes were cultured on immobilized CD137-Fc protein on chamber slides. After 10 days the cells were labeled for 60 minutes with 10 μmol/L BrdU (yellow). Monocytes were identified by staining for CD14 (red), and nuclei were visualized by Hoechst 33342 (blue). Similar results were obtained in 3 independent experiments for (A) and (B), respectively.
We confirmed by immunocytochemistry that the proliferating cells were in fact monocytes and not other blood cells. Proliferation was determined by BrdU-incorporation and the identity of monocytes was verified by simultaneously staining for CD14, a cell surface protein specific to monocytic cells. Nuclei were visualized by staining with the DNA intercalating dye Hoechst 33342. As shown in Fig 3B, the proliferating cells have features characteristic of monocytes: they are CD14-positive, are multi-nucleated, and have the morphological appearance of monocytes/macrophages.
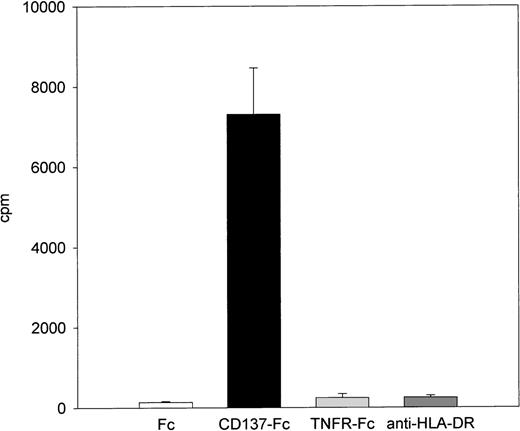
Other molecules that also bind to constitutively expressed molecules on monocytes, like TNFRII-Fc or anti–HLA-DR were not able to induce proliferation (Fig 4). Furthermore, CD137 had no effect on proliferation of the monocytic cell lines HL60 and MonoMac (Table 1), nor did it induce adherence and spreading of these nonadherent cells (not shown). However, the spontaneous rate of proliferation of the two lines was more than an order of magnitude higher than the rate of primary monocytes that were stimulated with CD137 for 8 days (Table1).
Specificity of CD137-induced monocyte proliferation. 105 peripheral monocytes were cultured on plates coated with 1 μg/mL of Fc, CD137-Fc protein, anti–HLA-DR antibody, or a fusion protein consisting of the extracellular domain of the type 2 TNF receptor and the constant domain of immunoglobulin G1 (TNFRII-Fc). Proliferation was determined at day 8 by 3H-thymidine incorporation and comparable results were obtained in 2 independent experiments.
Specificity of CD137-induced monocyte proliferation. 105 peripheral monocytes were cultured on plates coated with 1 μg/mL of Fc, CD137-Fc protein, anti–HLA-DR antibody, or a fusion protein consisting of the extracellular domain of the type 2 TNF receptor and the constant domain of immunoglobulin G1 (TNFRII-Fc). Proliferation was determined at day 8 by 3H-thymidine incorporation and comparable results were obtained in 2 independent experiments.
Proliferative Response of Primary and Immortalized Monocytes to CD137
| . | Uncoated . | Fc . | CD137-Fc . |
|---|---|---|---|
| Primary monocytes | 0.4 ± 0.2 | 0.5 ± 0.2 | 5.1 ± 1.2 |
| HL-60 | 89.7 ± 2.7 | 95.5 ± 4.8 | 92.0 ± 12.8 |
| MonoMac | 224.0 ± 10.4 | 203.8 ± 28.9 | 225.1 ± 23.0 |
| . | Uncoated . | Fc . | CD137-Fc . |
|---|---|---|---|
| Primary monocytes | 0.4 ± 0.2 | 0.5 ± 0.2 | 5.1 ± 1.2 |
| HL-60 | 89.7 ± 2.7 | 95.5 ± 4.8 | 92.0 ± 12.8 |
| MonoMac | 224.0 ± 10.4 | 203.8 ± 28.9 | 225.1 ± 23.0 |
105 primary monocytes or Monomac or HL-60 cells were cultured on 96-well plates coated with Fc or CD137-Fc protein. Proliferation was determined at day 8 by a 24-hour pulse with 0.5 μCi3H-thymidine. Numbers represent means ± standard deviation of cpm × 1,000. This experiment was repeated 3 times with comparable results.
M-CSF and GM-CSF are essential but not sufficient for CD137-induced monocyte proliferation.
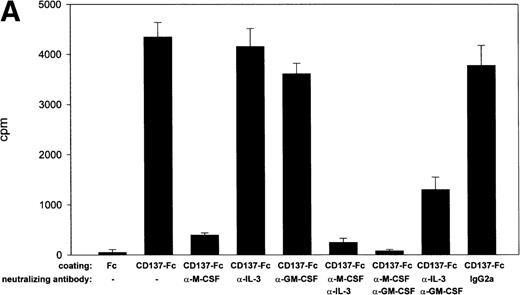
Because M-CSF is an essential monocyte survival factor,26,27 and because CD137 prolongs survival of monocytes via induction of M-CSF,16 it is conceivable that M-CSF would also be required for CD137-induced proliferation of monocytes. Confirming this hypothesis, neutralizing anti–M-CSF antibodies reduced CD137 induced proliferation almost completely (Fig 5A).
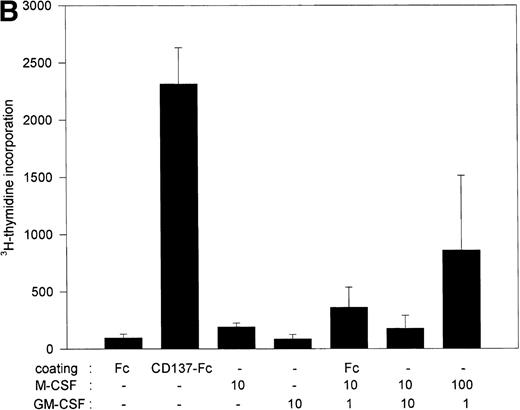
M-CSF and GM-CSF are essential, but not sufficient, for CD137-induced monocyte proliferation. (A) 105 peripheral monocytes were cultured on immobilized Fc or CD137-Fc protein. Neutralizing anti–M-CSF antibody (2 μg/mL), anti–GM-CSF antibody (2 μg/mL), and anti–IL-3 antibody (2 μg/mL) or isotype control (IgG2a; 2 μg/mL) were added where indicated. Proliferation was determined at day 10 by 3H-thymidine incorporation in triplicate conditions. (B) M-CSF and GM-CSF do not induce proliferation of monocytes. Peripheral monocytes were cultured on 96-well plates coated with Fc or CD137-Fc protein (1 μg/mL) or M-CSF and GM-CSF at indicated concentrations (ng/mL). Proliferation was determined at day 10 by 3H-thymidine incorporation. Similar results were obtained in 3 separate experiments for (A) and (B).
M-CSF and GM-CSF are essential, but not sufficient, for CD137-induced monocyte proliferation. (A) 105 peripheral monocytes were cultured on immobilized Fc or CD137-Fc protein. Neutralizing anti–M-CSF antibody (2 μg/mL), anti–GM-CSF antibody (2 μg/mL), and anti–IL-3 antibody (2 μg/mL) or isotype control (IgG2a; 2 μg/mL) were added where indicated. Proliferation was determined at day 10 by 3H-thymidine incorporation in triplicate conditions. (B) M-CSF and GM-CSF do not induce proliferation of monocytes. Peripheral monocytes were cultured on 96-well plates coated with Fc or CD137-Fc protein (1 μg/mL) or M-CSF and GM-CSF at indicated concentrations (ng/mL). Proliferation was determined at day 10 by 3H-thymidine incorporation. Similar results were obtained in 3 separate experiments for (A) and (B).
Besides M-CSF, GM-CSF and IL-3 have been reported to be important for monocyte survival.18 Complete inhibition of monocyte proliferation was achieved by neutralizing M-CSF and GM-CSF. Neutralizing GM-CSF or IL-3 alone had only a slight (18% reduction) or no effect on CD137-induced proliferation, respectively. The sequestration of both GM-CSF and IL-3 together synergistically reduced proliferation to one third (Fig 5A).
These experiments established M-CSF and GM-CSF as being essential for CD137-induced proliferation of peripheral monocytes. However, they were not sufficient. M-CSF and GM-CSF at concentrations induced by CD137 elicited only a negligible proliferation (Fig 5B). M-CSF is induced by CD137 up to 10 ng/mL,16 whereas no GM-CSF could be measured (not shown). In vivo, M-CSF and GM-CSF are detectable around 10 ng/mL and 50 pg/mL, respectively.28-30 Even at significantly higher concentrations, ie, at 100 and 1 ng/mL, respectively, M-CSF and GM-CSF induced only a fraction of the proliferation observed with CD137 (Fig 5B). This may point to the existence of other, additional factors that are induced by CD137 in monocytes, and that contribute in an autocrine fashion to CD137-induced proliferation of monocytes.
CD137-induced monocyte proliferation is mediated by soluble autocrine factor(s).
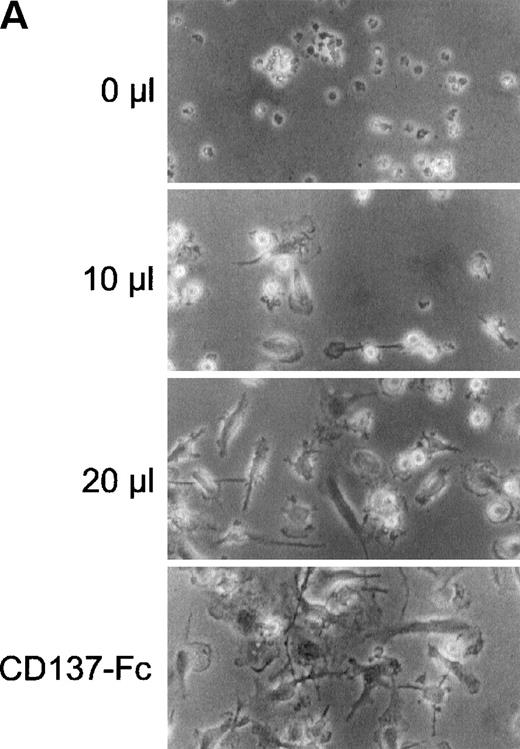
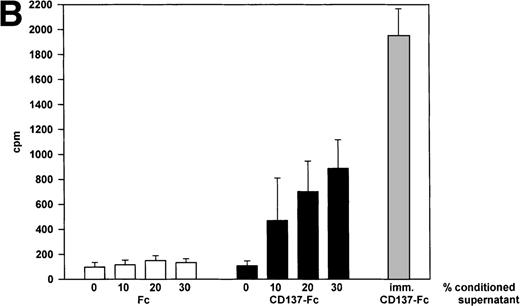
Next, we wanted to evaluate whether these additional factors required for monocyte proliferation were soluble or cell-surface–bound. Supernatants of monocytes cultured for 24 hours on immobilized CD137 protein were harvested and cells were removed by centrifugation for 5 minutes at 12,000g. Transfer of this conditioned medium to untreated monocytes from the same donor dose-dependently induced cell growth (Fig 6A) and proliferation (Fig 6B). Addition of neutralizing anti–M-CSF antibodies (2 μg/mL) to conditioned medium blocked induction of cell growth and proliferation (not shown).
CD137 induces proliferation of monocytes via autocrine induction of soluble factor(s). 105 peripheral monocytes were cultured on immobilized Fc or CD137-Fc protein for 24 hours. 0, 10, 20, or 30 μL of conditioned supernatant of these cultures were transferred to 100 μL of new cultures with untreated monocytes. (A) After 8 days, the cells were photographed at a magnification of 300×. The bottom panel depicts monocytes grown on immobilized CD137-Fc protein for the 8 days. (B) Proliferation of the cultures from (A) was determined at day 8 by a 24-hour pulse with 0.5 μCi3H-thymidine. ( ) Proliferation of monocytes on immobilized CD137-Fc protein. Cultures supplemented with conditioned supernatant from CD137-Fc–activated monocytes proliferated significantly stronger; P < .007. This experiment was repeated 3 times with similar results.
) Proliferation of monocytes on immobilized CD137-Fc protein. Cultures supplemented with conditioned supernatant from CD137-Fc–activated monocytes proliferated significantly stronger; P < .007. This experiment was repeated 3 times with similar results.
CD137 induces proliferation of monocytes via autocrine induction of soluble factor(s). 105 peripheral monocytes were cultured on immobilized Fc or CD137-Fc protein for 24 hours. 0, 10, 20, or 30 μL of conditioned supernatant of these cultures were transferred to 100 μL of new cultures with untreated monocytes. (A) After 8 days, the cells were photographed at a magnification of 300×. The bottom panel depicts monocytes grown on immobilized CD137-Fc protein for the 8 days. (B) Proliferation of the cultures from (A) was determined at day 8 by a 24-hour pulse with 0.5 μCi3H-thymidine. ( ) Proliferation of monocytes on immobilized CD137-Fc protein. Cultures supplemented with conditioned supernatant from CD137-Fc–activated monocytes proliferated significantly stronger; P < .007. This experiment was repeated 3 times with similar results.
) Proliferation of monocytes on immobilized CD137-Fc protein. Cultures supplemented with conditioned supernatant from CD137-Fc–activated monocytes proliferated significantly stronger; P < .007. This experiment was repeated 3 times with similar results.
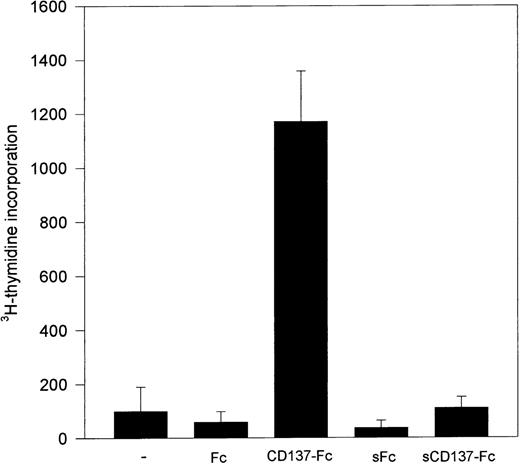
Immobilization of CD137 protein is necessary for monocyte proliferation.
Induction of monocyte proliferation by CD137 required immobilization of the CD137 protein, eg, by coating of the protein to the surface of the tissue culture dishes (Fig 7). If CD137 was given as a soluble protein, after preventing its immobilization by prior coating of the dishes unspecifically with bovine serum albumin, the proliferative activity of CD137 was absent. Therefore, induction of monocyte proliferation by CD137 seems to be mediated by crosslinking of a CD137 ligand expressed by monocytes.
Immobilized, but not soluble, CD137 induces monocyte proliferation. 105 peripheral monocytes were cultured on immobilized Fc or CD137-Fc protein. Proliferation was determined at day 10 by 3H-thymidine incorporation. Identical results were obtained in 3 independent experiments.
Immobilized, but not soluble, CD137 induces monocyte proliferation. 105 peripheral monocytes were cultured on immobilized Fc or CD137-Fc protein. Proliferation was determined at day 10 by 3H-thymidine incorporation. Identical results were obtained in 3 independent experiments.
DISCUSSION
Based on the survival enhancing activity of CD137,16 it was quite surprising to find a higher rate of apoptosis in CD137-treated monocytes. Considering that CD137 causes activation in monocytes,15 this situation resembles very much the phenomenon of activation induced cell death, originally described for lymphocytes. Cell activators that induce activation and proliferation simultaneously induce apoptosis in a certain percentage of the cells, but as long as proliferation outweighs cell death, the cell population expands.31
The contradictory results of the simultaneous induction of cell survival and apoptosis led us to test whether CD137 is able to induce proliferation of monocytes. The finding that human peripheral monocytes in fact do proliferate came as a surprise. The generally held opinion is that monocytes originate from hematopoietic stem cells, the proliferation of which is confined to the bone marrow. Peripheral monocytes and macrophages are assumed not to be able to proliferate.19,21 This notion was supported by experiments measuring only marginal proliferation of peripheral monocytes, even after treatment with monocyte activators and survival factors. None or only 0.3% of peripheral monocytes were found to undergo DNA replication in response to M-CSF and GM-CSF during a 20-hour labeling period.19,25 This low percentage of proliferating cells was in line with no increase or an increase by only a factor of 2 in incorporation of 3H-thymidine induced by M-CSF.19 25 Our results confirm these earlier findings. But in contrast to M-CSF, CD137 induced a strong proliferation in a large proportion of the cells. Upon labeling for only 1 hour, 9.3% of the cells had incorporated bromodeoxyuridine into their DNA, implying that a substantial percentage of the cells were proliferating. Furthermore,3H-thymidine incorporation at its maximum was 30- to 80-fold higher in CD137-treated monocytes compared with control cells. By demonstrating that the DNA replicating cells were CD14-positive, had multiple nuclei, and the morphological appearance of monocytes, we could show unequivocally that the proliferating cells were in fact monocytes.
Further, CD137 has been shown to inhibit proliferation and to induce apoptosis in T lymphocytes.11 Therefore, these cells would not, even if present, be able to contribute to the observed proliferation.
Endomitosis and cell fusion have been shown to occur in monocytes.32,33 Proliferation of monocytes induced by CD137 likely results, to some extent, in endomitosis. Many polynucleated cells are observed in CD137-protein–treated monocyte cultures. There are two indications that they arise at least partly from endomitosis rather than solely from cell fusion: First, replicating nuclei are present in already polynucleated cells that do not show signs of cell division. Second, a decrease in the cell number, as would be expected with cell fusion, is not observed. The few small BrdU-positive cells present in CD137-treated cultures may represent monocytes that just have undergone cell division. Generally, the simultaneously ongoing processes of proliferation, endomitosis, apoptosis, and cell fusion make it impossible to quantify the number of newly emerging and dying cells. The fact that the rate of proliferation of the monocytic cell lines was more than an order of magnitude larger than that of the primary cells may be due to an in vitro selection for rapid growth and an acquisition of mutations further supporting a high proliferation rate.34 35
For monocyte survival, M-CSF has been reported to be essential and sufficient.26 27 For proliferation of monocytes, M-CSF is also essential because sequestration of M-CSF is able to abolish proliferation almost completely. However, M-CSF is not sufficient, because at physiological and even at higher concentrations it is not able to induce proliferation of monocytes on its own. Also, the combination of M-CSF and GM-CSF failed to replicate the proliferative activity of CD137. Therefore, additional factor(s) contributing to monocyte proliferation must exist.
The ability of conditioned medium from CD137-treated monocytes to induce adherence, spreading, and proliferation in other monocytes proves (1) the soluble, and (2) the autocrine nature of these additional factor(s). Adherence and spreading can be induced by M-CSF, which is upregulated by CD137, and sequestration of M-CSF by neutralizing antibodies proves that M-CSF is also an essential factor in the conditioned medium. But M-CSF did not induce proliferation. Therefore, these additional factors may be novel cytokines or a combination of M-CSF and known cytokines.
Although significant proliferation of monocytes in isolation has not been demonstrated before, the concept that peripheral monocytes are incapable of proliferating has already been questioned by two recent studies. Local proliferation of peripheral monocytes in vivo, in a granuloma, a specialized inflammatory reaction, and in vitro, after coculturing of monocytes with aortic endothelial cells, has been reported.36,37 In both systems M-CSF was essential but not sufficient for proliferation of monocytes, a situation identical with CD137-induced monocyte proliferation. Since CD137 is expressed at sites of inflammation,4 and can be expressed by endothelial cells (H.S., unpublished observation, March 1997), CD137 could have been the factor enabling monocyte proliferation in these systems. Generally, it would be sites of inflammation, where CD137 is expressed and could induce activation and proliferation of monocytes, and thereby enhance inflammatory reactions.
It needs to be emphasized that CD137 is a cell surface receptor and the activities described here are mediated by crosslinking of a CD137 ligand and take place in the ligand-bearing monocyte. Dimerization of CD137 ligand is not sufficient to induce proliferation, because the CD137-Fc protein used in these studies dimerizes via its Fc domains and the soluble dimeric CD137 protein is not able to induce monocyte proliferation. Higher-order multimerization of the ligand—achieved by immobilization of the CD137 protein to the tissue culture plates—is required. Transduction of a signal through a CD137 ligand is also responsible for activation of monocytes and for inhibition of proliferation and induction of apoptosis in T lymphocytes.11,15 Because, in the TNF receptor and ligand families, the receptors as well as the ligands are membrane-bound molecules, a bidirectional transduction of signals is possible. Bidirectional transduction of signals through the receptor as well as the ligand has also been described for the OX40, CD40, and CD30 receptor/ligand systems, three other members of the TNF receptor and ligand families.38-41 A CD137 ligand has been isolated that is expressed constitutively by monocytes,3 thus enabling resting monocytes to respond to CD137 protein.
The proliferative activity of CD137 on peripheral monocytes is not shared by other molecules binding to cell surface proteins on monocytes, nor by well-established monocyte growth factors, like M-CSF, GM-CSF, nor any other known molecule, and constitutes an activity so far unique to CD137. Given the central position of monocytes in the regulation of immune responses, inhibition of CD137 may represent a new way to interfere with destructive autoimmune reactions. Accordingly, protective immune responses against pathogens and malignant cells may be enhanced by CD137-mediated activation of monocytic and dendritic cells, cells that are intensively studied for immune therapy for cancer.42-44
ACKNOWLEDGMENT
We thank Gitte Krause for excellent technical assistance, and M. Kreutz and R. Andreesen for peripheral monocytes.
Supported by the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Herbert Schwarz, Department of Pathology, University of Regensburg, 93042 Regensburg, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal