Abstract
The hypotransferrinemic mouse (trf hpx) is a mutant strain exhibiting transferrin deficiency, marked anemia, hyperabsorption of iron, and elevated hepatic iron stores. We set out to investigate the relative roles of anemia and of transferrin in the malregulation of intestinal iron absorption in these animals. Transfusion of erythrocytes obtained from littermate controls increased hemoglobin levels and reduced reticulocyte counts in recipient animals. Although mucosal to carcass 59Fe transfer was reduced, total duodenal iron uptake was not significantly affected. Iron absorption in homozygotes, in contrast to littermate controls, was not reduced by hyperoxia. Mouse transferrin injections, in the short term, increased delivery of iron to the marrow and raised hemoglobin levels. Although mucosal transfer and total iron uptake were reduced at the higher transferrin doses, total uptake was still higher than in controls. Daily injections of mouse/human transferrin for 3 weeks from weaning, normalized hemoglobin values, and markedly reduced liver iron and intestinal iron absorption values in trf hpxanimals. When such daily-injected mice were left for a week to allow transferrin clearance, iron absorption values were significantly enhanced; hemoglobin or hepatic iron levels were, however, not significantly altered. These data indicate that hyperabsorption of iron in trf hpx mice is not solely because of the anemia; transferrin levels per se do affect iron absorption, possibly via a direct effect on the intestinal mucosa.
THE REGULATION of iron absorption remains poorly understood, in spite of the recent cloning of the hemochromatosis gene (HFe),1 a candidate component of the regulatory pathway. Subsequent investigations have shown that the transferrin-receptor and the HFe protein can interact with each other,2 3 suggesting a role for transferrin in the control of intestinal iron absorption.
The genetically hypotransferrinemic (trf hpx) mouse, first described by Bernstein,4 shows cytopathological features similar to the human conditions, idiopathic hemochromatosis,5 and congenital atransferrinemia.6,7 The mutant strain exhibits a virtual absence of circulating transferrin as a result of a splicing defect in transferrin mRNA8 and a hypochromic, microcytic anemia, despite marked hepatic parenchymal iron loading.4,9 Excess liver iron is attributed to the enhanced intestinal absorption of dietary iron combined with rapid and selective deposition of this iron in the liver. Although some degree of control over absorption is exercised by the surplus iron,9 little is known about the pathogenesis of the enhanced iron absorption in these animals.
One study10 claimed that transfusion of “washed” erythrocytes into trf hpx mice, normalized hematological parameters (ie, hematocrit and reticulocyte counts) and reduced the absorption of intragastrically administered iron to values comparable with those seen in normal balb/c mice. As the transfused homozygous animals had a marked reduction in serum transferrin, these workers concluded that anemia alone was responsible for the enhanced absorption of iron. Our more recent studies using other animal models of chronic anemia, namely β-thalassaemia11 and erythropoietin-deficiency,12 have, however, shown only modest changes in intestinal iron absorption as compared with values in littermate controls. Moreover, the changes in absorption were mainly because of increases in the mucosal uptake of radioiron. These findings thus indicate that factors other than the erythropoietic rate may be responsible for the enhanced absorption seen in trf hpxanimals. As transferrin is virtually absent in these animals, we decided to reinvestigate the possibility of whether the anemia or the lack of circulating transferrin accounts for the malregulation of intestinal iron absorption in the trf hpx mouse model.
MATERIALS AND METHODS
Reagents.
All chemicals and biochemicals were from either Sigma Chemical Co Ltd (Poole, Dorset, UK) or BDH Chemicals (Poole). Radio-iron (59FeCl3) was obtained from NEN-Du Pont (specific activity 0.19 to 2.78TBq/g; Stevenage, Herts, UK).
Animals.
The hypotransferrinemic mice originated from BALB/cJ background. Homozygous mice (trf hpx), which are phenotypically distinguishable at birth by their pale appearance, were maintained by weekly injections of mouse serum (150 μg to 1 mg transferrin) as described previously.9 Heterozygotes were differentiated from the wild-type controls by the reduction in serum transferrin levels. Experiments/manipulations on trf hpx mice were performed a week after the last serum maintenance dose, thus allowing for almost complete clearance of exogenously administered transferrin.13 Mice were 6 to 8 weeks of age at the time of study.
Transfusion of erythrocytes.
Control (ie, mixture of heterozygotes and wild types) or trf hpx animals were injected intraperitoneally (i.p.) with either 0.15 mol/L NaCl (saline) or washed, packed erythrocytes (∼250 μL) obtained from homozygotes, heterozygotes or wild-type mice, and studied 3 days post-single or -double (3-day interval between injections) transfusion. Washing of erythrocytes with saline 3 times was found to be adequate for removal of any plasma-associated transferrin. In some experiments, animals were given an injection of erythrocytes daily for 3 days and studied 2 to 5 days later. Preliminary experiments performed in animals that had been injected intravenously (IV) with cells/saline showed no differences compared with the i.p.-injected mice; the 2 groups have therefore been combined for analysis.
Transferrin injections.
Homozygous mice were injected i.p. with either a single or double dose of commercially obtained mouse transferrin (Chemicon Int, Temecula, CA), and studied 4 hours to 1 week later. In addition, some trf hpx mice were injected daily, starting from weaning, with a progressively increasing dose (1 to 3 mg) of human or mouse transferrin for 3 weeks; this injection regime was followed to account for mouse growth. Iron absorption studies were performed 6 to 18 hours or 1 week after the last injection. Mice injected on a daily basis for 3 weeks with human albumin received the usual weekly maintenance dose of transferrin (as serum).
Transferrin determination.
Serum levels were assayed by a radial immunodiffusion technique14 using 1% agar containing 0.02 mol/L sodium barbitone buffer (pH, 8.6) and appropriate amounts of either goat anti-human transferrin (Dynatech Labs, Billinghurst, Sussex, UK) or sheep anti-mouse transferrin (Chemicon Int). Purified human transferrin (Sigma) or mouse transferrin (Chemicon Int) was appropriately diluted and assayed at the same time to establish a calibration curve. The human and mouse antibodies did not cross-react with mouse or human transferrin, respectively.
Tissue oxygen levels.
Duodenal pO2 levels were determined polarographically in anesthetized mice by gently inserting a previously calibrated, thin, flexible wire-type electrode into the duodenal mucosa through a slit made distal to the point where the bile duct joins the small intestine. The body temperature was maintained by placing mice on a heated pad. Recordings on a Model pO2-100 monitor (composed of an ammeter with built-in power supply; Inter-Medical Ltd, Nagoya, Japan) were not commenced until the electrode was well positioned and secured. The measurement of pO2 in mammalian tissue via polarographic electrodes and other means is described in the review by Vanderkooi et al.15
Iron absorption.
In situ tied-off duodenal segments were used to determine intestinal iron absorption.9,11 A tracer dose of 59Fe (as a ferric chelate of nitrilotriacetate, 1:2, in physiological medium) was injected intraluminally into the tied-off segment of the anesthetized animal. The duodenal intraluminal contents were gently flushed-out with warm saline before the injection of radioiron solution. All experiments were performed under Vmaxconditions [Fe(III) = 250 μmol/L] and for an incubation period of 10 minutes. The segment was thereafter removed, flushed with ice-cold saline, weighed, and counted in a gamma counter (LKB Wallac Model 80000; Turku, Finland). Blood (cardiac puncture) and any other required tissues were also removed, weighed, and similarly counted. The carcass was counted in a well-type counter. Aliquots (10 μL) of the radioiron solution, acting as standard, were counted in both counters to normalize the 59Fe counts. The activity of 59Fe in the duodenal segment is referred to as “mucosal retention,” whereas the activity in the carcass reflects the “mucosal transfer.” The sum of the 2 parameters represents the “total mucosal uptake.” The activity of 59Fe associated with the blood was calculated assuming a blood volume of 1.5 mL/25 g body weight.16 For in vivo 51Cr-EDTA permeability studies, the physiological medium contained 100 μmol/L51CrEDTA and 300 μmol/L EDTA, in place of Fe(NTA)2.
Hematological parameters.
Five microliters of blood obtained by cardiac puncture was mixed with Drabkin’s reagent for hemoglobin determination.17Reticulocyte counts were performed independently by 2 people on methylene blue-stained blood smears.
Nonheme iron assays.
Data.
Shown as mean ± SD for (n) determinations. Statistical analysis was performed using unpaired t-test. Where the variances differed significantly, the Welch’s test was used.
RESULTS
Figure 1 shows the body weights and hemoglobin levels, and Table 1 shows the iron absorption parameters in control and homozygous hypotransferrinemic mice. The heterozygote and wild-type mice have been combined into a single control group because previous investigations11 have shown no significant differences in iron absorption between the 2 groups.
Body weights and hemoglobin levels in (a) controls and in homozygous (trf hpx) mice transfused with (b) nothing, (c) saline (×2), (d) trf hpx erythrocytes (×2), and (e through g) control erythrocytes (1 to 3 doses, respectively). All determinations were performed 3 days after the last transfusion except for the multiple-transfused group (g) which were performed 5 days postinjection. *P < .04; **P < .002; ***P< .007 as compared with untreated trf hpx mice (b).
Body weights and hemoglobin levels in (a) controls and in homozygous (trf hpx) mice transfused with (b) nothing, (c) saline (×2), (d) trf hpx erythrocytes (×2), and (e through g) control erythrocytes (1 to 3 doses, respectively). All determinations were performed 3 days after the last transfusion except for the multiple-transfused group (g) which were performed 5 days postinjection. *P < .04; **P < .002; ***P< .007 as compared with untreated trf hpx mice (b).
Iron Absorption Values in Untransfused and Transfused trfhpx Mice
| Group . | n . | Mucosal Retention . | Mucosal Transfer . | Total Uptake . | Mucosal Transfer (%) . |
|---|---|---|---|---|---|
| (pmol/mg/10 min) . | |||||
| Untransfused | |||||
| Control | 10 | 22.2 ± 8.1 | 10.1 ± 5.4 | 32.3 ± 12.6 | 30.1 ± 10.5 |
| trfhpx | 6 | 28.4 ± 10.7 | 63.2 ± 14.4† | 91.5 ± 12.9† | 69.0 ± 11.1* |
| Transfusion of trfhpx with | |||||
| saline (×2) | 4 | 42.5 ± 10.4 | 65.3 ± 13.8 | 108 ± 10.2 | 60.4 ± 9.9 |
| trfhpxErythrocytes (×2) | 3 | 36.6 ± 14.0 | 87.8 ± 26.8 | 124 ± 41 | 70.9 ± 1.8 |
| Control erythrocytes | |||||
| (×1) | 3 | 29.9 ± 9.8 | 72.0 ± 3.4 | 102 ± 6.9 | 71.0 ± 7.4 |
| (×2) | 5 | 51.0 ± 19.0‡ | 46.4 ± 25.3 | 97.4 ± 36.8 | 46.5 ± 15.6‡ |
| (×3)1-154 | 6 | 37.8 ± 17.7 | 30.7 ± 19.01-153 | 68.5 ± 34.7 | 42.8 ± 8.41-155 |
| Group . | n . | Mucosal Retention . | Mucosal Transfer . | Total Uptake . | Mucosal Transfer (%) . |
|---|---|---|---|---|---|
| (pmol/mg/10 min) . | |||||
| Untransfused | |||||
| Control | 10 | 22.2 ± 8.1 | 10.1 ± 5.4 | 32.3 ± 12.6 | 30.1 ± 10.5 |
| trfhpx | 6 | 28.4 ± 10.7 | 63.2 ± 14.4† | 91.5 ± 12.9† | 69.0 ± 11.1* |
| Transfusion of trfhpx with | |||||
| saline (×2) | 4 | 42.5 ± 10.4 | 65.3 ± 13.8 | 108 ± 10.2 | 60.4 ± 9.9 |
| trfhpxErythrocytes (×2) | 3 | 36.6 ± 14.0 | 87.8 ± 26.8 | 124 ± 41 | 70.9 ± 1.8 |
| Control erythrocytes | |||||
| (×1) | 3 | 29.9 ± 9.8 | 72.0 ± 3.4 | 102 ± 6.9 | 71.0 ± 7.4 |
| (×2) | 5 | 51.0 ± 19.0‡ | 46.4 ± 25.3 | 97.4 ± 36.8 | 46.5 ± 15.6‡ |
| (×3)1-154 | 6 | 37.8 ± 17.7 | 30.7 ± 19.01-153 | 68.5 ± 34.7 | 42.8 ± 8.41-155 |
Homozygous mice were transfused with saline or erythrocytes from trfhpx or littermate controls. Iron absorption studies were performed 3 days after the last transfusion except for “
,” where studies were performed 5 days after the last transfusion. Data: mean ± SD for (n) experiments. Control group includes heterozygote and wild-type mice.
Percent mucosal transfer = [mucosal transfer (pmol/mg/10 min)/total mucosal uptake (pmol/mg/10 min)] × 100.
P < .007.
P < .001 as compared with the control group.
P < .04.
P < .008.
P < .001 as compared with nontransfused trfhpx mice.
Homozygous mice had stunted growth as reflected by the reduced body weights and were markedly anemic with a high degree of reticulocytosis (23.2 ± 5.5 [6]%) as compared with the control group (1.3 ± 1.0[6]%). Intestinal iron absorption studies showed a marked enhancement in trf hpx mice mainly because of increased transfer of radioiron from the mucosa to the plasma. Permeability studies performed with 51CrEDTA, a stable nonabsorbed ecf marker,20 showed no difference between the control (8.7 ± 4.8 [3] pmol/mg/10 min) and trf hpx(8.7 ± 4.6 [3]) mice, thus indicating that the enhanced absorption of iron is specific and not attributable to increased intestinal permeability. Most of the absorbed radioiron (after a 10-minute incubation in a tied-off duodenal segment) in trf hpx mice was found in the liver (72 ± 8.1 [6]%), with little being incorporated into erythrocytes (0.63 ± 0.64 [6]%). In contrast, values for the liver and erythrocyte 59Fe incorporation in the control group were 16.3 ± 8.0 (10)% and 11.9 ± 12.6 (10)%, respectively. These values resemble those reported in control/mutant mice studied at 24 hours4 and 3 days5 postadministration of59FeCl3 in phosphate buffer via the IV and oral route, respectively. At the time of our study, transferrin could not be detected immunologically in the serum of trf hpx mice (ie, <.05 mg/mL).
Transfusion of trf hpx animals on 2 occassions (with a 3-day gap in between injections), with either saline or erythrocytes obtained from homozygous animals, did not cause any significant changes in the hemoglobin, mucosal transfer, or total uptake of59Fe in recipient mice. It is noteworthy that injection of trf hpx erythrocytes was performed as an additional control for the transfusion experiments and shows that injection of red blood cells per se does not affect iron absorption. In contrast, a single or double transfusion of erythrocytes from control animals resulted in significant changes in the hemoglobin level in recipient trf hpx mice, but neither treatment resulted in a decrease in the total mucosal uptake of iron. The doubly transfused group, though exhibiting a significant decrease in the percent mucosal transfer values, showed a significant increase in the mucosal retention of radioiron (Table 1); thus, no apparent change in overall uptake was evident. Reticulocyte counts decreased after the single- (15.1 ± 13.2 [3]%) or double-erythrocyte transfusion (3.6 ± 3.5[4]%) of control erythrocytes as compared with the saline-infused group (33.6 ± 7.4 [3]%).
Multiple transfusion of erythrocytes (3 times) induced a further small increase in the hemoglobin level; the values posttransfusion were comparable with the values in the control group. As the reduction in percent mucosal transfer was more prominent and the mucosal retention values were unaltered in the multiple-transfused group, the total absorption values showed a reduction, but the values failed to reach statistical significance (P < .2). It is noteworthy that the total uptake values were still significantly higher than those in the control group. Clearance of radioiron by the liver was marginally increased (82.5 ± 9.0 [6]%), whereas that by the erythrocytes was reduced (0.10 ± 0.24 [6]%) following the multiple transfusions. Mice studied 2 to 3 days after multiple transfusions also showed a similar, statistically insignificant reduction in total mucosal iron uptake (69.8 ± 17.4 [3] pmol/mg/10 min).
Liver and duodenal mucosal non-heme iron levels were unperturbed in the transfused groups compared with untreated trf hpx mice (Fig 2). When the hepatic data were corrected for liver weights, a small but progressive increase in total iron level was observed with increasing transfusion of control erythrocytes. Splenic iron levels were also moderately increased following the triple-transfusion (1.56 ± 0.61[6] nmol/mgv 1.04 ± 0.71 [6]). When allowance was made for the spleen weights, the total iron content values were comparable with those seen in the noninjected group (362 ± 133 [6] nmol v365 ± 263 [6]). Control animals multiply transfused with erythrocytes from heterozygote/wild-type littermates had elevated liver weights and hepatic iron content (2.4 ± 0.5 [5] μmol v1.9 ± 0.8 [10]) and exhibited small, insignificant, reductions in mucosal transfer (7.7 ± 1.6 [5] v 10.1 ± 5.4 [10] pmol/mg/10 min), percent mucosal transfer (27.6 ± 4.4 [5]%v 30 ± 10.5 [10]%), and total mucosal uptake (27.8 ± 4.6 [5] v 32.3 ± 12.6 [10] pmol/mg/10 min).
(A) Liver iron (total content or tissue concentration) and (B) duodenal iron in control and homozygous hypotransferrinemic mice; the latter group was either untransfused or given 1 to 3 transfusions of erythrocytes from control animals. All determinations were performed 3 days after the last transfusion except for the multiple-transfused group, which were performed 5 days postinjection. Data: mean ± SD for 5 to 10 animals in each group except for the (×1) transfused group where n = 3. *P < .04; **P< .001 as compared with the control group. $,P < .02 as compared with the nontransfused homozygous group.
(A) Liver iron (total content or tissue concentration) and (B) duodenal iron in control and homozygous hypotransferrinemic mice; the latter group was either untransfused or given 1 to 3 transfusions of erythrocytes from control animals. All determinations were performed 3 days after the last transfusion except for the multiple-transfused group, which were performed 5 days postinjection. Data: mean ± SD for 5 to 10 animals in each group except for the (×1) transfused group where n = 3. *P < .04; **P< .001 as compared with the control group. $,P < .02 as compared with the nontransfused homozygous group.
Effect of hyperoxia on iron absorption.
Trf hpx mice exposed to 40% O2 for 3 days failed to show any marked changes in the degree of reticulocytosis (18%, 21%, n = 2) or in intestinal iron absorption (total uptake = 108 ± 33[4] pmol/mg/10 min). Exposure for shorter periods (ie, 24 and 48 hours) was also without effect on iron absorption (109 and 168 pmol/mg/10 min, respectively). A statistically significant reduction in iron absorption was, however, evident in control mice similarly exposed to 3 days hyperoxia (20.0 ± 6.1 [6] v 32.3 ± 12.6 [10] pmol/mg/10 min,P < .05), owing to a reduction in both the mucosal retention (13.4 ± 4.0 [6] v 22.2 ± 8.1 [10] pmol/mg/10 min) and mucosal transfer (6.6 ± 3.6 [6] pmol/mg/10 min v 10.1 ± 5.4 [10]) of 59Fe.
Tissue oxygen levels.
No difference was evident in the duodenal pO2 levels between the trf hpx mice (21 mm Hg, n = 2) and the control group (20.5 ± 1.8 [3] mm Hg). When both groups were, however, made to inhale 10% O2 for 2 minutes via a small mouth piece and the measurements performed, a decrease of between 2 and 5 mm Hg was evident as compared with basal values. Conversely, inhalation of 40% O2 resulted in an increase of 2 to 8 mm Hg. The mucosal oxygen levels determined with the thin wire electrode are within the range of values observed in mammalian small intestine by surface and microelectrode measurements.21-23
Effect of transferrin injection(s) on intestinal iron absorption.
To monitor the effects of transferrin, trf hpx mice were studied at specific times after injection with various doses of mouse transferrin (Fig 3).
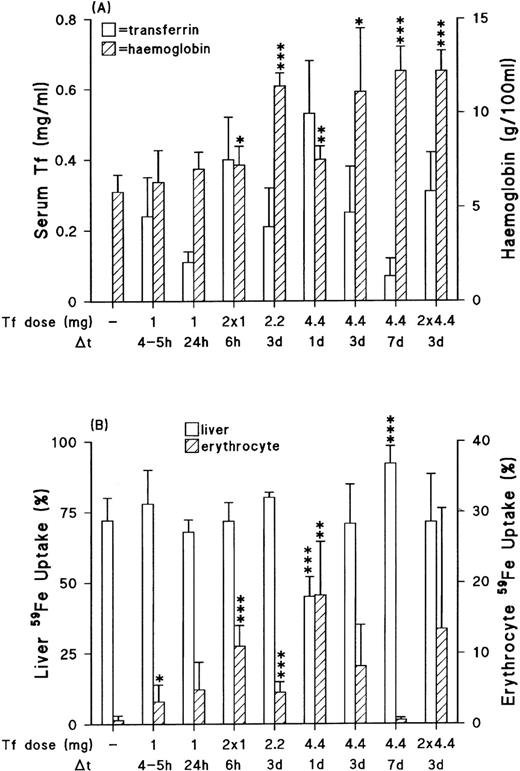
Effect of mouse transferrin injections on circulating levels, hemoglobin (A) and liver/erythrocyte uptake of the absorbed radioiron (B). The transferrin (Tf ) dose and sampling time (after last injection if more than one) are as indicated. Data: mean ± SD for 4 to 6 animals in each group except for the one studied 3 days post 2.2 mg dose, where n = 3. Detection limit for serum transferrin was 0.05 mg/mL. Any values below this level are therefore not indicated on the figure. *P < .05; **P < .02; ***P < .004 as compared with untreated homozygous mice.
Effect of mouse transferrin injections on circulating levels, hemoglobin (A) and liver/erythrocyte uptake of the absorbed radioiron (B). The transferrin (Tf ) dose and sampling time (after last injection if more than one) are as indicated. Data: mean ± SD for 4 to 6 animals in each group except for the one studied 3 days post 2.2 mg dose, where n = 3. Detection limit for serum transferrin was 0.05 mg/mL. Any values below this level are therefore not indicated on the figure. *P < .05; **P < .02; ***P < .004 as compared with untreated homozygous mice.
Circulating transferrin levels in trf hpx mice were at the time of study dependent on the dose given and the period postinjection (Fig 3A). Increased incorporation of absorbed radioiron by immature red blood cells was apparent 4 to 5 hours after transferrin injection, and peaked at about 24 hours; thereafter, the red blood cell59Fe incorporation values decreased and by 7 days the values had reverted to basal levels (Fig 3B). Alterations in the incorporation of iron into erythrocytes (and thus in hemoglobin values) were more striking with the larger transferrin doses. The increased59Fe incorporation by erythrocytes 24 hours postinjection with 4.4 mg transferrin was associated with a significant decrease in radioiron clearance by the liver. Thereafter, as the red blood cell incorporation of 59Fe decreased, the liver clearance values equalled or even exceeded values seen in untreated trf hpx mice. The injected group, however, still exhibited enhanced intestinal iron absorption (Table2). Homozygous mice given 8.8 mg (2 × 4.4 mg) of transferrin however, showed an appreciable reduction in total uptake, mainly because of reduced transfer of59Fe from the mucosa to the portal circulation (Table 2). The uptake values were still higher (P < .04) than those in wild-type/heterozygotes (total mucosal uptake, 32.3 ± 12.6 [10] pmol/mg/10 min). It is noteworthy that at the time of experiment there were no significant alterations in either clearance of radioiron by the liver or total liver iron content (23.4 ± 6.3 [4] μmolv 21.2 ± 3.5 [6]) after dosing with 8.8 mg of transferrin. The fact that circulating transferrin levels 3 days postinjection with either the single or double dose (with a 3-day gap in between injections) of 4.4 mg transferrin are similar, indicates that transferrin clearance is rapid (half-life < 24 hours), and is in agreement with previous observations.13
Effect of Transferrin (Tf) Injections on Intestinal Iron Absorption
| Tf Dose (mg) . | n . | Δt . | Mucosal Retention . | Mucosal Transfer . | Total Uptake . | Mucosal Transfer (%) . |
|---|---|---|---|---|---|---|
| (pmol/mg/10 min) . | ||||||
| Nil | 6 | — | 28.4 ± 10.7 | 63.2 ± 14.4 | 91.5 ± 12.9 | 69.0 ± 11.1 |
| 1 | 6 | 4-5 h | 39.3 ± 14.5 | 67.2 ± 12.0 | 107 ± 23 | 63.9 ± 7.9 |
| 1 | 4 | 24 h | 29.9 ± 12.9 | 57.3 ± 21.2 | 87.2 ± 31.9 | 66.1 ± 7.3 |
| 2 × 1 over 24 h | 5 | 6 h* | 47.2 ± 17.8 | 62.7 ± 12.1 | 110 ± 21.1 | 57.7 ± 10.6 |
| 2.2 | 3 | 3 d | 33.6 ± 19.0 | 80.8 ± 33.2 | 114 ± 52 | 71.7 ± 4.0 |
| 4.4 | 4 | 1 d | 30.0 ± 6.1 | 51.4 ± 13.0 | 81.4 ± 16.0 | 62.7 ± 6.3 |
| 4.4 | 4 | 3 d | 23.2 ± 18.6 | 53.6 ± 38.9 | 76.8 ± 57.2 | 71.1 ± 8.4 |
| 4.4 | 4 | 7 d | 33.0 ± 10.0 | 42.9 ± 27.7 | 75.9 ± 33.3 | 52.7 ± 14.1 |
| 2 × 4.4 over 1 wk | 4 | 3 d* | 23.5 ± 6.6 | 28.7 ± 14.2‡ | 52.2 ± 18.2† | 53.4 ± 10.6 |
| Tf Dose (mg) . | n . | Δt . | Mucosal Retention . | Mucosal Transfer . | Total Uptake . | Mucosal Transfer (%) . |
|---|---|---|---|---|---|---|
| (pmol/mg/10 min) . | ||||||
| Nil | 6 | — | 28.4 ± 10.7 | 63.2 ± 14.4 | 91.5 ± 12.9 | 69.0 ± 11.1 |
| 1 | 6 | 4-5 h | 39.3 ± 14.5 | 67.2 ± 12.0 | 107 ± 23 | 63.9 ± 7.9 |
| 1 | 4 | 24 h | 29.9 ± 12.9 | 57.3 ± 21.2 | 87.2 ± 31.9 | 66.1 ± 7.3 |
| 2 × 1 over 24 h | 5 | 6 h* | 47.2 ± 17.8 | 62.7 ± 12.1 | 110 ± 21.1 | 57.7 ± 10.6 |
| 2.2 | 3 | 3 d | 33.6 ± 19.0 | 80.8 ± 33.2 | 114 ± 52 | 71.7 ± 4.0 |
| 4.4 | 4 | 1 d | 30.0 ± 6.1 | 51.4 ± 13.0 | 81.4 ± 16.0 | 62.7 ± 6.3 |
| 4.4 | 4 | 3 d | 23.2 ± 18.6 | 53.6 ± 38.9 | 76.8 ± 57.2 | 71.1 ± 8.4 |
| 4.4 | 4 | 7 d | 33.0 ± 10.0 | 42.9 ± 27.7 | 75.9 ± 33.3 | 52.7 ± 14.1 |
| 2 × 4.4 over 1 wk | 4 | 3 d* | 23.5 ± 6.6 | 28.7 ± 14.2‡ | 52.2 ± 18.2† | 53.4 ± 10.6 |
Data: mean ± SD for (n) experiments.
Abbreviations: Δt, sampling time after the single or second injection.*
P < .02.
P < .006 as compared with untreated homozygous mice.
Daily injections of commercially available mouse transferrin (1 to 3 mg) over a 3-week period, starting from weaning, normalized hemoglobin levels, markedly reduced hepatic iron stores, and significantly decreased iron absorption in trf hpx mice (Tables 3 and4). The iron absorption values were, however, still higher than the control group. Mice treated similarly for 3 weeks with mouse transferrin and then left for a week (without injections) so that circulating transferrin levels decreased to undetectable/negligible levels, showed a marked increase in both the mucosal transfer and total uptake (P < .02) without any change in liver iron content or hemoglobin values. The total uptake values were similar to those seen in normally maintained trf hpx mice. Animals treated with human transferrin on a daily basis for 3 weeks exhibited a similar increase in the hemoglobin level and also showed normalized percent mucosal59Fe transfer and total mucosal uptake values. Liver iron stores were also reduced, though not to the same extent as in the mouse-transferrin treated group. As before, mice left untreated for a week after 3 weeks of daily injections had undetectable circulating transferrin (ie, <.05 mg/mL) and yielded a significant increase in both mucosal transfer and total uptake, even though hemoglobin levels were normal. Circulating transferrin levels at the end of the 3-week period of daily injections were similar in both groups, suggesting that the protein, though from different sources, is cleared at similar rates.
Effect of Daily Transferrin Injections on Circulating Transferrin, Hemoglobin, and Liver Iron in Homozygous Hypotransferrinemic Mice
| Group . | n . | Serum Transferrin (mg/mL) . | Hemoglobin (g/100 mL) . | Liver Iron (nmol/mg) . | |
|---|---|---|---|---|---|
| Mouse . | Human . | ||||
| Controls | 10 | 2.1 ± 0.6* | ND | 17.4 ± 1.1 | 1.9 ± 0.8 |
| trfhpx | 6 | <0.053-151 | ND | 5.8 ± 0.93-152 | 27.4 ± 3.13-152 |
| Daily mTf injections | 5 | 0.88 ± 0.253-153 | ND | 15.4 ± 3.13-155 | 6.5 ± 2.43-155 |
| Daily mTf–left for 1 wk | 6 | 0.13 ± 0.093-154 | ND | 14.6 ± 2.4 | 4.9 ± 3.0 |
| Daily hTf injections | 5 | <0.053-151 | 1.04 ± 0.6 | 14.9 ± 1.73-155 | 9.1 ± 4.53-155 |
| Daily hTf–left for 1 wk | 5 | <0.053-151 | <0.05 | 13.2 ± 1.1 | 11.2 ± 3.4 |
| Daily hAlb injections | 3 | <0.05 | ND | 4.9 ± 1.4 | 22.9 ± 7.3 |
| Group . | n . | Serum Transferrin (mg/mL) . | Hemoglobin (g/100 mL) . | Liver Iron (nmol/mg) . | |
|---|---|---|---|---|---|
| Mouse . | Human . | ||||
| Controls | 10 | 2.1 ± 0.6* | ND | 17.4 ± 1.1 | 1.9 ± 0.8 |
| trfhpx | 6 | <0.053-151 | ND | 5.8 ± 0.93-152 | 27.4 ± 3.13-152 |
| Daily mTf injections | 5 | 0.88 ± 0.253-153 | ND | 15.4 ± 3.13-155 | 6.5 ± 2.43-155 |
| Daily mTf–left for 1 wk | 6 | 0.13 ± 0.093-154 | ND | 14.6 ± 2.4 | 4.9 ± 3.0 |
| Daily hTf injections | 5 | <0.053-151 | 1.04 ± 0.6 | 14.9 ± 1.73-155 | 9.1 ± 4.53-155 |
| Daily hTf–left for 1 wk | 5 | <0.053-151 | <0.05 | 13.2 ± 1.1 | 11.2 ± 3.4 |
| Daily hAlb injections | 3 | <0.05 | ND | 4.9 ± 1.4 | 22.9 ± 7.3 |
Data: mean ± SD for (n) determinations except for “*” where n = 6. Mouse (mTf) or human (hTf) transferrin, as appropriate, was assayed in the serum of noninjected or injected hypotransferrinemic mice.
Abbreviations: ND, not determined; hAlb, human albumin.
P < .05.
P < .001 as compared with the control group.
P < .03.
P < .002 as compared with untreated trfhpxmice.
P < .002 as compared with values in the appropriate 3-week–treated group.
Iron Absorption in Hypotransferrinemic Mice Injected Daily for 3 Weeks With Either Mouse or Human Transferrin
| Group . | n . | Mucosal Retention . | Mucosal Transfer . | Total Uptake . | Mucosal Transfer (%) . |
|---|---|---|---|---|---|
| (pmol/mg/10 min) . | |||||
| trfhpx | 6 | 28.4 ± 10.7 | 63.2 ± 14.4 | 91.5 ± 12.9 | 69.0 ± 11.1 |
| Daily mTf injections | 5 | 23.1 ± 4.4 | 37.9 ± 13.54-150 | 61.0 ± 17.54-150,4-151 | 61.2 ± 4.9 |
| Daily mTf–left for 1 wk | 6 | 35.8 ± 13.7 | 72.5 ± 28.84-153 | 108 ± 304-153 | 65.6 ± 11.9 |
| Daily hTf injections | 5 | 22.7 ± 5.5 | 11.1 ± 5.4‡ | 33.9 ± 9.7‡ | 32.0 ± 8.5‡ |
| Daily hTf–left for 1 wk | 5 | 43.9 ± 15.64-153 | 59.9 ± 32.24-153 | 104 ± 424-153 | 54.0 ± 17.24-153 |
| Daily hAlb injections | 3 | 61.4 ± 15.64-151 | 81.5 ± 14.0 | 143 ± 284-151 | 57.3 ± 4.3 |
| Group . | n . | Mucosal Retention . | Mucosal Transfer . | Total Uptake . | Mucosal Transfer (%) . |
|---|---|---|---|---|---|
| (pmol/mg/10 min) . | |||||
| trfhpx | 6 | 28.4 ± 10.7 | 63.2 ± 14.4 | 91.5 ± 12.9 | 69.0 ± 11.1 |
| Daily mTf injections | 5 | 23.1 ± 4.4 | 37.9 ± 13.54-150 | 61.0 ± 17.54-150,4-151 | 61.2 ± 4.9 |
| Daily mTf–left for 1 wk | 6 | 35.8 ± 13.7 | 72.5 ± 28.84-153 | 108 ± 304-153 | 65.6 ± 11.9 |
| Daily hTf injections | 5 | 22.7 ± 5.5 | 11.1 ± 5.4‡ | 33.9 ± 9.7‡ | 32.0 ± 8.5‡ |
| Daily hTf–left for 1 wk | 5 | 43.9 ± 15.64-153 | 59.9 ± 32.24-153 | 104 ± 424-153 | 54.0 ± 17.24-153 |
| Daily hAlb injections | 3 | 61.4 ± 15.64-151 | 81.5 ± 14.0 | 143 ± 284-151 | 57.3 ± 4.3 |
Data: mean ± SD for (n) determinations.
Abbreviations: mTf, mouse transferrin; hTf, human transferrin; hAlb, human albumin.
P < .02.
P < .006.
P < .001 as compared with untreated homozygous mice.
P < .04 as compared with values in the appropriate 3-week–treated group.
Homozygous mice injected in a similar fashion for 3 weeks, but with human albumin, exhibited marked anemia, elevated hepatic iron stores, and enhanced mucosal retention, transfer, and total uptake values; these characteristics are similar to those seen in mice receiving maintenance serum injections only, and suggests that the effects of human transferrin on iron absorption are not because of possible induction of an immunologic reaction.
DISCUSSION
The trf hpx mouse, a genetic strain with a virtual absence of circulating transferrin, shows similarities to the human conditions hemochromatosis and atransferrinemia. It is thus a useful animal model for investigating not only the regulation of iron absorption but also the mechanism of iron toxicity.24
In spite of the surplus iron, the mutant strain exhibits a marked anemia of an iron-deficient nature; the sustained hyperabsorption of iron, though not surprising, is, however, intriguing because (1) animals lack transferrin, which is necessary for the delivery of iron as ‘iron-transferrin,’ the primary physiological source of iron for erythroid cells, and (2) changes in absorption are more marked than seen in other models with chronic anemia (ie, β-thalassemia,11 erythropoietin-deficient12) or with enhanced reticulocytosis (phenylhydrazine-treated25). In this study we have investigated the relative importance of both anemia and transferrin in the malregulation of intestinal iron absorption in homozygous hypotransferrinemic mice.
The role of anemia/oxygen delivery in the regulation of iron absorption in trf hpx mice.
Transfusion of erythrocytes from trf hpx animals resulted in a small, statistically insignificant increase in hemoglobin levels in recipient mice. Intestinal iron absorption values were unaffected. The lack of a significant effect on the hemoglobin level is surprising and may be attributable to the fact that erythrocytes from trf hpx animals are low in hemoglobin content.4 Transfusion of washed erythrocytes from littermate controls (wild-type/heterozygotes), however, caused even after a single dose, a significant increase in the hemoglobin level and a decrease in the reticulocyte count. Multiple transfusion of erythrocytes led to further increases in hemoglobin, with the values being comparable with those seen in the control group. Even though mucosal transfer (as percentage of total uptake) decreased appreciably after the double and triple transfusions, total mucosal uptake failed to exhibit a significant decrease as compared with the untreated group. The decrease in mucosal transfer could not be attributable to either radioiron dilution within the duodenal mucosa (because nonheme iron levels were unaltered even after the triple transfusion) or to alterations in 59Fe clearance by the liver. The decrease in mucosal transfer is in support of the findings of Buys et al.10 However, our data do not reach the same conclusion (ie, normalized absorption values posttransfusions). This discrepancy may be attributable to differences in the absorption method used (ie, tied-off intestinal segment for 10 minutes v whole-body retention 4 to 24 hours after gavage) or the iron complex used (FeNTAv iron-ascorbate). Moreover, our absorption studies were confined to the duodenum, the primary region where iron absorption occurs, and were unaffected by effects on intestinal transit.
Erythrocyte transfusions had 3 effects, namely increased hemoglobin, decreased erythropoiesis, and increased liver iron (presumably because of exogenous/endogenous red cell breakdown). Any of these, independently, could perhaps have resulted in a decrease in mucosal transfer and overall iron absorption. The importance of the change in liver iron was tested by injecting iron dextran into trf hpx mice. No significant effect on iron absorption was seen even though liver iron levels were comparable with those in erythrocyte-infused trf hpx mice. Increased hemoglobin is presumed to affect intestinal iron absorption via oxygen delivery to the mucosa, as oxygen level in the inspired air is known to influence iron absorption.26,27 However, exposure of homozygous mice to 40% oxygen for up to 3 days failed to alter iron absorption. Furthermore, intestinal mucosal pO2 levels in trf hpx mice did not differ appreciably from those in the control group. Studies in pigs have shown that intestinal mucosal pO2 levels remain fairly constant unless the hematocrit falls below 10%.23 In previous studies, we found that acute alterations (≤1 week) in reticulocyte levels affected mucosal transfer more than mucosal uptake,25 whereas chronic alterations (>1 week) in erythropoiesis affected mucosal uptake more than transfer.11,12 Mice with chronic anemia and reticulocytosis comparable with trf hpx animals, namely β-thalassaemic mice, did not show the massively enhanced absorption of iron seen in trf hpx mice.11 In an earlier study (unpublished observations, Simpson and Raja, January 1991) we found that exchange transfusion of blood (from the control group) into trf hpx mice modestly raised the hemoglobin levels and completely suppressed the reticulocyte response, without affecting iron absorption in these recipient mice. Based on these observations, it is unlikely that reticulocytosis is solely responsible for enhanced mucosal transfer and uptake in the trf hpxmice.
The tendency for the erythropoietic rate and iron absorption in genetically normal rodents to be decreased by hypertransfusion or hyperoxia26,27 and increased by bleeding28 or hypoxia26 is consistent with a regulatory mechanism, which is related to mucosal oxygen supply. In mice with lifelong anemia, as in genetic hypotransferrinemia, adaptive changes to the cardiovascular system and erythrocyte oxygen affinity will have occured to produce an animal able to maintain mucosal oxygen levels, as shown above, despite the anemia. We have previously shown that duodenal blood flux in homozygous animals is similar to that in the control group.29 It is unsurprising that transfusion with erythrocytes from normal mice may decrease iron absorption, because it will increase oxygen delivery to the tissues. The finding that hyperoxia does not depress iron absorption in these homozygous mice suggests that their adapted mucosal oxygen delivery system is, however, not responsive to increased inspired oxygen content. This highlights the fact that responses of trf hpx mice to experimental manipulation should be interpreted in light of their adaptation to chronic anemia.
Role of transferrin in the regulation of iron absorption in trf hpx mice.
Transferrin, though clearly not obligatory for the absorptive process, may have a role in the regulatory process. Injection of purified mouse transferrin (1.0 to 4.4 mg) into trf hpx mice markedly increased delivery of 59Fe to the bone marrow (and thence to red blood cells) and subsequently led to increases in the hemoglobin levels. The changes in 59Fe incorporation by erythrocytes, which were apparent within 4 to 5 hours posttransferrin injection, were bigger with the higher transferrin doses; the radioiron clearance values peaked at 24 hours postinfusion and thereafter decreased with time and by 7 days had reverted to basal levels. Liver 59Fe clearance values, in all cases but one, showed no significant reduction after transferrin injection(s). These findings show that the liver in trf hpx mice has a large capacity for clearance of nontransferrin bound iron, in support of previous findings.30 Erythrocytes, however, depend on transferrin as the main physiological route of iron delivery.
Although small decreases were apparent in the mucosal transfer of59Fe posttransferrin injection, the total mucosal uptake values did not change significantly in any of the treated groups except for the group given a double dose of 4.4 mg transferrin. The interpretation of the role of transferrin in the regulation of iron absorption is, however, hampered by the fact that hemoglobin values have increased concurrently. We conclude that iron absorption was reduced, but not normalized by acute correction of anemia, or by short-term injections of transferrin. We therefore investigated the involvement of transferrin in the control of iron absorption in trf hpx mice with long-term correction of anemia.
Transferrin (mouse/human) was injected daily for 3 weeks to normalize hemoglobin levels. This regime was calculated to produce transferrin levels which oscillated around a mean value equivalent to that seen in heterozygote mice (which show normal iron absorption rates11). These transferrin-injected mice exhibited markedly reduced liver iron stores and had transferrin levels comparable with values seen in heterozygous mice. Iron absorption values (especially in the human transferrin-injected group) were considerably lower than those in the normally maintained, anemic trf hpx mice. Mice left uninjected thereafter for a week, thus allowing adequate time for the clearance of transferrin,13 but not for the development of anemia, showed markedly increased mucosal transfer and iron absorption values in spite of no changes being evident in the hemoglobin levels and liver iron stores; the total uptake values in both the mouse and human transferrin-treated groups were similar to those seen in normally maintained trf hpx mice. It is feasible that, in spite of normalized hemoglobin levels, low transferrin levels may lead to ineffective (iron-deficient) erythropoiesis, which may in turn contribute to the enhancement of iron absorption. The failure of daily mouse transferrin injections to completely normalize iron absorption is probably attributable to insufficient transferrin being present at all times throughout the experimental period. The finding that daily injections of human transferrin were more effective at normalizing absorption was apparently not attributable to the protein half-life, nor to a nonspecific effect of a foreign protein. It is possible that human transferrin interacts with transferrin-receptor in a way that more effectively regulates iron absorption.
These data show that the greatly enhanced intestinal iron absorption in trf hpx mice, leading to the highest degree of iron overload reported so far in mice fed a standard rodent diet, is not solely explained by anemia or increased production of erythrocytes. Transferrin levels do, however, influence iron absorption (especially mucosal transfer) independently of effects on hemoglobin levels.
Recently, a transgenic mouse colony with the homologous HFe gene disrupted has been established.31 These mutant mice were hematologically normal, had almost fully saturated transferrin, and showed hepatic iron-loading even though their diet was not supplemented with iron. It has previously been suggested32 that the amount of iron absorbed via the intestine is a function of the programming of crypt cells depending on body iron status. As HFe is reported to be present in the crypts33 and to interact with transferrin receptors,2,3 it is reasonable that HFe is involved in the sensing mechanism: Disruption of the gene would result in the crypt cells not being appropriately programmed, thus resulting in the malabsorption of iron through over expression of genes for iron absorption such as the recently described DCT1/Nramp2 transporter protein.34-36 Our data support the involvement of transferrin in the control of iron absorption possibly through direct effects on the intestinal mucosa.
ACKNOWLEDGMENT
This is a contribution from the King’s College Centre for the Study of Metals in Biology and Medicine, London, UK.
Supported by a UK Medical Research Council Project Grant.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to K.B. Raja, PhD, Department of Clinical Biochemistry, King’s College School of Medicine, Denmark Hill, London SE5 9PJ, UK; e-mail: K.Raja@KCL.AC.UK.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal