Abstract
The molecular and cellular requirements for the development of different populations of human dendritic cells (DC) were studied. Conditions were defined that support DC production from lymphoid progenitors but that fail to induce DC formation from peripheral monocytes. The production of these lymphoid-related DC was severely blocked when hematopoietic progenitors overexpressed Ik7, a mutant dominant-negative Ikaros protein. In contrast, Ik7 did not block the formation of DC in conditions supporting the development of monocyte-derived DC. Furthermore, Ik7 did not block the formation of monocyte/macrophages and enhanced granulopoiesis. One of the molecular mechanisms mediated by Ik7 appears to be down-regulation of the flt3-receptor mRNA. Thus, distinct signals control the formation of DC demonstrating that some aspects of DC diversity are determined in part by distinct molecular cues at the hematopoietic level. (Blood. 2000;95:128-137)
Dendritic cells (DC) are rare cells that are principally involved in the presentation of antigen and stimulation of lymphocytes.1 Variations among the tissue distribution of DC and differences in their phenotype and function indicate the existence of a heterogeneous population of DC (reviewed in reference 2). Recently, it has been recognized that various populations of DC in mice or man are able to induce distinct types of immune responses,3-5 prompting questions about the role of their origin in determining functional heterogeneity. An important question is how this heterogeneity arises at the developmental level.
Ample evidence suggests that DC receive an array of stimuli that can change their activation, migration, or survival.6-9Although some of these signals regulate DC development at relatively late stages, the molecules involved in the control of the early stages of DC formation are not entirely well defined. Understanding this process is complicated by the existence of several types of DC precursors. Peripheral monocytes (CD14+CD34−)10,11 and hematopoietic progenitors (CD14−CD34+)12,13 are both able to generate DC. Yet, the development of the more primitive hematopoietic progenitors into DC does not entirely overlap with that of monocytes; therefore, it was suggested that different pathways of DC hematopoiesis may exist.14
Lymphoid progenitors can give rise to the so-called lymphoid-related DC. Lymphoid progenitors with a relatively high ability to commit toward differentiation into lymphocytes but also capable of differentiating into DC are found in the thymus or bone marrow (BM) of mice and humans.15-18 In mice, the differentiation requirements for lymphoid-related DC are distinguishable from those of other DC as determined, in part, by cytokine responses and reliance on relB proteins.19-21 Yet, the molecular requirements controlling the hematopoiesis of lymphoid-related DC have not been entirely defined. We previously identified a human BM lymphoid progenitor cell of phenotype CD34+ Lin−CD10+ that differentiates into all classes of lymphocytes (T, B, natural killer [NK] lymphocytes) in the appropriate experimental systems.16 Multipotential clones with B cell, NK cell, and DC differentiation potential exist in this lymphoid progenitor population, which is markedly depleted of precursors for monocytes, macrophages, or myeloid cells16 and is therefore more endowed with distinct developmental options than peripheral blood monocytes. Consequently, lymphoid progenitors and monocytes appear to each represent a prototypical DC precursor and the signals regulating their respective developmental programs remain to be defined.
Little is known of the molecules that regulate the early stages of DC development but the zing finger DNA-binding transcription factor Ikaros has been implicated in DC hematopoiesis in mice. Homozygous mice for an Ikaros null allele have severe alterations in B- and NK lymphoid-cell development accompanied by specific alterations in T-cell development and a strong reduction in numbers of DC in lymphoid organs.22 Deletions of the DNA-binding domain from the mouse germline that generate an Ikaros mutation with dominant-negative properties (DN-/-) cause more serious lymphoid and DC defects.23,24 Proteins produced by the dominant-negative locus can interact and interfere with proteins produced by the wild-type Ikaros locus or with other family members and compromise their activity.25-27 Hematopoietic defects in DN-/-animals include a severe block in lymphopoiesis and a general depletion of DC in lymphoid organs although monocytes are abundantly present.22,24 Yet, DN-/-animals have DC in the skin, suggesting that several signaling pathways, in which Ikaros is differentially involved, regulate DC development in vivo. The human equivalent of Ikaroswas cloned and is highly homologous to its murine counterpart.28 Ikaros mRNA is detectable in human CD34+ cells,29 suggesting that Ikaros proteins may play a role in human hematopoiesis. Based on the high conservation between mouse and human Ikaros28,30 and almost complete identity in the DNA-binding region and protein interaction domains, we reasoned that murine dominant-negative proteins would interfere with human Ikaros family members. One of these dominant-negative proteins, Ik7, is the product of gene targeting deletion of exons 3 and 4 causing a strong reduction in the DNA-binding ability of heterocomplexes formed between Ik7 and other members of the Ikaros family of proteins through their C-terminal zing finger modules.25 Therefore, we used Ik7 in an overexpression system to test the role of Ikaros proteins in human hematopoiesis.
To determine the different cellular and molecular requirements of human DC hematopoiesis, we established contrasting culture systems that could distinguish between the development of lymphoid progenitors and of monocytes. The differential dependence for Ik7 was demonstrated by retroviral-mediated gene transfer of Ik7 in multipotential CD34+ progenitors. Therefore, we conclude that DC formation occurs along several pathways involving distinct cells and signals. Thus, DC heterogeneity is in part intrinsically determined at the hematopoietic level and this decision involves the intimate interaction between Ikaros family members.
Materials and methods
Source of cells
Human adult BM cells were isolated either from rib fragments removed from patients undergoing thoracic surgery or from the residual cells of screen filters after BM transfusion. Whole blood from normal volunteers was obtained from the American Red Cross, Detroit, MI. Mobilized peripheral blood (MPB) samples were obtained from patients with metastatic stage IV breast cancer after consent. All tissues were obtained according to institutional guidelines. Mononuclear cells (MNC) d < 1.077g/mL were prepared from blood or BM by centrifugation through Ficoll (Pharmacia) and cells were cryopreserved with 10% dimethyl sulfoxide in liquid nitrogen.
Isolation of CD34+ progenitor cell subsets by flow cytometry sorting
Frozen BM MNC were thawed in the presence of DNAse (100 U/mL) and heparin (10 U/mL) (Sigma, St Louis, MO) and spun over a Ficoll gradient to remove dead cells. Lineage-positive (Lin+) B cells, T cells, phagocytes, and erythrocytes were removed with magnetic beads (sheep anti-mouse Ig magnetic beads, Dynal Inc., Lake Success, NY) following incubation with mouse monoclonal antibodies (mAbs) against CD40 (G28.5), CD2 (RPA2.1), CD32 (IV3), CD143C10, and glycophorin A (10F7MN) (ATCC and kind gift from Dr G. Aversa, Novartis, Vienna, Austria). Remaining Lin-depleted cells were incubated with fluorochrome-conjugated mAbs: sulforhodamine (SR) anti-CD34 (PR3, kind gift of Dr B. Hill, SyStemix Inc., Palo Alto, CA), fluorescein isothiocyanate (FITC) anti-CD3 (7D6, Caltag, Burlingame, CA), FITC anti-CD15 (PR9, kind gift of Dr B. Hill), FITC anti-CD19 (HIB19, Pharmingen, San Diego, CA), and phycoerythrin (PE) anti-CD10 (HI10a, Pharmingen). Propidium iodide (PI) (Sigma) (5 μg/mL) was added to enable the detection of live and dead cells. Some cells were stained with irrelevant mAbs or with positive markers conjugated with each of the fluorochromes to serve, respectively, as negative or compensation controls. Cells were sorted on a Vantage sorter (Becton Dickinson, San Jose, CA) using an Argon laser tuned to 488 nm and a dye pump laser tuned to 590 to 600 nm. Electronic gating was set to select cells that are PI−, FITC (Lin)−, and CD34+, separating them into CD10+ and CD10− cell subsets. Reanalysis of sort purity showed > 95% purity in the CD34+ Lin−CD10− cell population, whereas CD34+Lin− CD10+ cells, being rare (1%-0% of CD34+ cells16), were typically 50% to 85% pure. When indicated, CD34+ Lin−CD10+ cells were run again on the sorter using the same settings and gates to obtain a highly purified cell population.
Isolation of blood monocytes
Plastic adherence of about 4 × 107 MNC in 75 cm2 tissue culture flasks (Corning Costar Corp., Oneonta, NY) in Dulbecco's modified Eagle's medium (Gibco, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS) (Hyclone, Logan, UT) for 2 hours, was used to isolate adherent cells. After washing, adherent cells were further incubated overnight in the same medium before being washed and detached by incubation in ice-cold Ca++Mg++-free phosphate-buffered saline (PBS) for 10 to 20 minutes and tapping. Flow cytometric analysis showed > 95% CD14+ expression in the resulting population.
Dendritic cell cultures
Subsets of CD34+ Lin− cells (CD10+ or CD10− cells) or monocytes were cultured in 24-well plates (Corning) in RPMI-1640 medium (Gibco) containing 10% FCS, penicillin streptomycin (100 U/mL and 100 μg/mL, respectively), l-glutamine (2 mM), 2-mercaptoethanol (2-ME) 2 × 10−5 mol/L, and cytokines at 37°C 5% CO2 for the indicated periods of time. The cytokine-containing medium was changed by demidepletion twice a week.
Cytokines
The following human recombinant cytokines were used: flt3-ligand (F), c-kit ligand (K), granulocyte/macrophage colony-stimulating factor (GM-CSF; Gm) (kind gift of Dr B. Hill, SyStemix Inc) (25 ng/mL each), interleukin (IL)-4(4) (100 UI/mL) (kind gift of Dr H. Yssel, DNAX, Palo Alto, CA), recombinant tumor necrosis factor (rTNF)-α (T) (25 ng/mL), IL-1βb(1) and IL-7(7) (10 ng/mL each) (R&D Systems, Minneapolis, MN).
Flow cytometric analysis
Nonspecific Fc receptor binding was blocked after saturation with 1 mg/mL human gamma globulin for 10 minutes (Gamimune, Miles, Eckhart, IL). Negative controls included directly labeled IgG1 and IgG2a irrelevant mAbs. Compensation controls and negative controls were used to determine the boundaries of regions in 2-color dot plots such that > 98% of the cells would be contained in the respective regions. Analysis was performed on live cells (PI−) using the PC Lysis software (Becton Dickinson). Cells were stained with the following mAbs: PE anti-CD1a (HIT2) (Pharmingen), PE anti-CD83 (HB15A) (Immunotech), FITC anti-CD14 (PR4, kind gift of Dr B. Hill, Systemix Inc.), or anti-CD14 (3C10, ATCC) detected with PE-conjugated goat anti-mouse Ig (Chemicon, Temecula, CA).
Allogeneic T-cell stimulation
Purified allogeneic blood T cells (0.5 to 1 × 105 cells/well) were prepared by negative selection after removal of B cells and phagocytes by incubation with G28-5 and IV.3 mAbs and panning on plastic dishes coated with goat anti-mouse immunoglobulins (Sigma). Nonadherent cells were further depleted with sheep anti-mouse IgG-coated beads (Dynal) following incubation with mAbs to HLA-DR, CD19 (Caltag), CD14, and CD15. The resulting cell population routinely contained > 98.5% CD3+ T cells. Purified T cells were incubated for 6 days in 96-well microtiter plates (Corning) in RPMI-1640 medium with 10% FBS, antibiotics, glutamine, and 2-ME at 37°C, 5% CO2 with variable percentages of irradiated APC (4000 cGy with a Cs137 source, JL Shepherd, San Fernando, CA). During the last 18 hours of T-cell cultures, 1 μCi of 3H thymidine (DuPont NEN, Boston, MA) was added to each well to determine cellular incorporation after harvesting cells on glass fibers and liquid scintillation counting. Results are expressed as average counts per minute (cpm) of triplicate wells ± SD. Secretion of IL-2 was measured at day 5 after removing an aliquot in the culture supernatant fluid that was analyzed by human IL-2-specific enzyme-linked immunosorbent assay (ELISA) (CYTImmune Sciences, College Park, MD) according to manufacturer's instructions.
Retroviral-mediated delivery of Ik7
A bi-cistronic retroviral vector was used to express murine Ik7 and enhanced green fluorescence protein (EGFP) in human cells. Characteristics of the vector have been reported31,32 but briefly Ik7 was cloned from pCDM8Ik1/2 into LZRS-IRES EGFP using standard techniques33 and sequenced to confirm that the vector was in-frame and correctly oriented. The control plasmid vector LZRS-IRES EGFP lacking Ik7 insert was also prepared. Virus-producing cells were prepared by transfection of the respective plasmids into Ampho-ΦNX cells (kind gift from Dr G. Nolan, Stanford University, Palo Alto, CA) and puromycin selection. Control and Ik7-containing virus stocks of equivalent titers, around 5 × 105CFU EGFP/mL were used. Titers were determined by infection of HCT116 cells colon carcinoma cells with dilutions of virus stocks in the presence of 8 μg/mL protamine sulfate and measurement of EGFP+ cells by flow cytometry at 488 nm on the FL1 channel of a Facscan (Becton Dickinson).
Reverse transcriptase-polymerase chain reaction analysis
Total RNA (RNA isolator, Genosys) was reverse-transcribed with MMLV (200 U/reaction, Promega) and random hexamer priming (1nM/reaction), and cDNA was amplified with Taq polymerase (Perkin Elmer) for 30 cycles (94°C/1 min; 55°C/2 min; 72°C/1 min) (Biometra personal cycler, Tampa, FL) in the presence of 2.5 mM MgCl2 and 0.25 μM each of the HEX2F 5′ CCCCCTGTAAGCGATA 3′ and EX7R 5′GATGGCTTGGTCCATCACGTGGGGA 3′ Ikaros primers.28 Reverse transcriptase-polymerase chain reaction (RT-PCR) products were transferred on a nylon membrane (Hybond) and probed with a 32P-labeled Ikaros-1 cDNA probe cloned from human thymus in our laboratory. Multiple isoforms of Ikaros are amplified by RT-PCR with HEX2F and EX7R primers. The mutant Ik7 is recognized by a specific 467 bp size. To amplify mRNA for flt3/STK or IL-2Rγ genes, we used, respectively, 1 μM of each of these primers, STK3 5′ AAAGCATCCCAGTCAATCAG 3′ and STK4 5′ GGTATCCATCCGAGAAACAG 3′, IL2R-3 5′ CCAGCCTACCAACCTCACTC 3′, and IL2R-4 5′TCCAGCCAGAAATACACACA 3′. In all experiments we verified the integrity and correct amount of cDNA by amplifying β2 microglobulin or GAPDH transcripts.
Methylcellulose colony-stimulating assays
Sorted CD34+ cell subsets were cultured for 1 to 2 1/2 weeks at 37°C, 5% CO2, at the density of 1500 to 2000 cells per 35 × 10 mm dishes (Corning) in 1.5 mL Methocult H4431 methylcellulose complete with erythropoietin and lymphocyte-conditioned medium (Stem Cell Technologies Inc., Vancouver). Colonies were counted microscopically and representative colonies were picked, spun on slides, and stained with Giemsa to determine the nature of cells in the colony.
Results
Production of lymphoid-related DC
Lymphoid-related DC can be generated from the CD34+Lin− CD10+ lymphoid progenitors found in human BM.16 Growth and differentiation signals supporting DC development from these cells can be provided by the combination of cytokines flt3-ligand+c-kit ligand+GM-CSF+IL-1β +IL-7 (FKGm17) in the absence of stroma.29 These cytokines have not been traditionally used to generate DC in vitro and it is not known how other hematopoietic progenitors respond. We therefore divided uncommitted CD34+ Lin− hematopoietic progenitor (Lin− cells lack lineage-specific antigens CD2, CD14, CD19, or CD15 to exclude myeloid or lymphoid-committed cells) into 2 subsets: CD34+ Lin−CD10+ cells representing 4.7% ± 3.5% of CD34+ Lin− cells and CD34+Lin− CD10− cells accounting for the rest of CD34+ cells, that is, about 95%, and compared their DC differentiation potential in FKGm17. DC were identified by morphology, large size as measured by the flow cytometric forward and side scatter (FSC, SSC) parameters, expression of cell surface antigens such as CD1a or CD83, which are relatively specific for DC in these culture conditions,34 and lack of markers specific for other hematopoietic lineages such as CD14, a marker normally expressed on monocytes (reviewed in reference 2). We also tested for a hallmark property of DC, which is the ability to stimulate the proliferation and IL-2 secretion of allogeneic T cells in mixed leukocyte reaction (MLR).35 This DC activity correlates well with expression of CD1a or CD83 on the cells in culture36 (and our own observations).
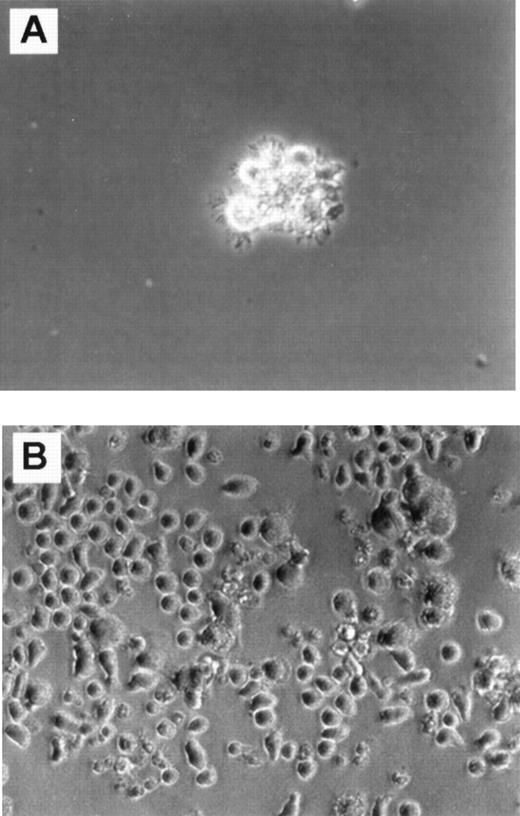
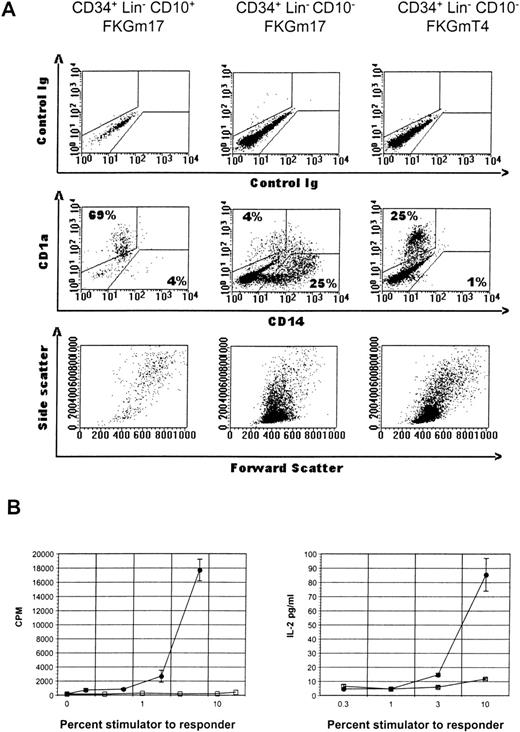
A limited cellular expansion was observed in cultures of CD34+ Lin− CD10+ cells treated with FKGm17 over 2 weeks (1 to 18-fold, n = 5) with cells rapidly differentiating, increasing in size, and acquiring dendrites as early as day 4. After about a week, most of the cells were dendritic, floating as aggregates (Figure 1A), and most cells had large FSC and SSC characteristics (Figure2A, left bottom panel). Around days 11 to 14, cells expressed high levels of surface CD1a with background levels of CD14 in a similar fashion as DC. Two separate experiments representative of 4 illustrate that the progeny of CD34+Lin− CD10+ cells has functional activity in MLR stimulating detectable T-cell responses at stimulator-to-responder concentrations of about 2% to 3%, which are typically in the range of activity displayed by “professional” antigen-presenting cells like DC (Figure 2B). No myeloid cells or macrophages were visually recognizable at any time in cultures initiated with highly purified (double-sorted) CD34+Lin− CD10+ cells (as in Figures 1A and2A). The rapid and relatively uniform differentiation of these cells into CD1a+ CD14−DC indicates their potential for a rapid commitment into DC. It is consistent with a high degree of commitment in this population as already demonstrated in other assays for NK, B-, and T-cell lineages.16 37
Cultures in FKGm17.
Phase contrast photomicrograph (20 × ) shows cultures of CD34+ Lin− CD10+ cells (A) and CD34+ Lin− CD10−cells (B) in FKGm17 at day 11.
Cultures in FKGm17.
Phase contrast photomicrograph (20 × ) shows cultures of CD34+ Lin− CD10+ cells (A) and CD34+ Lin− CD10−cells (B) in FKGm17 at day 11.
Phenotype and function of CD34+ cell subset progeny.
(A) Flow cytometric analysis of various cultures of hematopoietic progenitor cell subsets. CD34+Lin− CD10+ cells in FKGm17 (left panel), or CD34+ Lin− CD10−cells in FKGm17 (middle panel), or CD34+Lin− CD10− cells in FKGmT4 (right panel) were analyzed 2 weeks after the start of culture. The top row shows background values and the middle row shows the correlated expression of FITC-CD14 and PE-CD1a markers. Numbers indicate the percentage of cells in the respective regions. The bottom row shows forward scatter (FSC) and side scatter (SSC) characteristics of the cells. (B) Two representative and distinct experiments show the stimulation of purified allogeneic T cells in MLR with irradiated progenies of CD34+ Lin− CD10+cells (closed circle) or of CD34+ Lin−CD10−cells (open square) in MLR. Proliferation (left panel) was measured by 3H-thymidine incorporation and IL-2 secretion in the medium (right panel) by ELISA.
Phenotype and function of CD34+ cell subset progeny.
(A) Flow cytometric analysis of various cultures of hematopoietic progenitor cell subsets. CD34+Lin− CD10+ cells in FKGm17 (left panel), or CD34+ Lin− CD10−cells in FKGm17 (middle panel), or CD34+Lin− CD10− cells in FKGmT4 (right panel) were analyzed 2 weeks after the start of culture. The top row shows background values and the middle row shows the correlated expression of FITC-CD14 and PE-CD1a markers. Numbers indicate the percentage of cells in the respective regions. The bottom row shows forward scatter (FSC) and side scatter (SSC) characteristics of the cells. (B) Two representative and distinct experiments show the stimulation of purified allogeneic T cells in MLR with irradiated progenies of CD34+ Lin− CD10+cells (closed circle) or of CD34+ Lin−CD10−cells (open square) in MLR. Proliferation (left panel) was measured by 3H-thymidine incorporation and IL-2 secretion in the medium (right panel) by ELISA.
In contrast, parallel cultures of CD34+Lin− CD10− cells expanded abundantly in FKGm17 (11 to 135-fold in 2 weeks, n = 4) differentiating into myeloid cells with large granular cytoplasm (Figure 1B) and into monocytes expressing CD14 as detected by flow cytometry (Figure 2A, middle panel). Some adherent cells were found and most cells were single rather than aggregated, unlike in cultures of CD10+ cells (Figure 1A). An abundant population of medium-sized blasts (intermediate FSC and SSC) was recognizable by flow cytometry (Figure 2A, middle panel, bottom row). A few aggregates of cells resembling DC were seen and flow cytometric analysis detected 2.1% ± 1.5% (n = 4) cells with a CD1a+CD14−DC phenotype. Because there were few DC in these cultures, little T-cell stimulatory activity was detected (Figure 2B), but further purification by cell sorting confirmed that DC produced in these cultures were active antigen-presenting cells (data not shown). Such scarcity of DC contrasted with the prominence of DC in cultures of CD34+ Lin− CD10+ cells stimulated with FKGm17 and with the relatively high proportions of DC that can be obtained in general by stimulating hematopoietic progenitors with cytokines other than FKGm17. The cytokines flt3-ligand + c-kit ligand + GM-CSF + TNF-α + IL-4 (FKGmT4) have been effective at inducing DC differentiation of total CD34+ cells of adult BM or of mobilized peripheral blood.11 These cytokines support the development of monocytes and also of their precursors because the cytokines TNF-α+ GM-CSF+ c-kit ligand have been shown to support the CD14-dependent pathway of DC differentiation14,38 and IL-4 induces the phenotypic conversion of monocyte into DC.39 With these cytokines, CD34+ Lin− CD10− cells produced about 25% to 40% CD1a+ CD14−DC thus demonstrating that this subset of CD34+Lin− CD10− cells is not devoid of DC precursors (Figure 2A, right panel). These data suggest that DC formation by hematopoietic progenitors is induced by distinct cytokine signals. From CD34+ Lin−CD10+ cells, which are highly enriched in lymphoid precursors,16 the FKGM17 cytokine signals induce DC differentiation effectively.
Monocytes do not become DC in response to signals supporting lymphoid-related DC
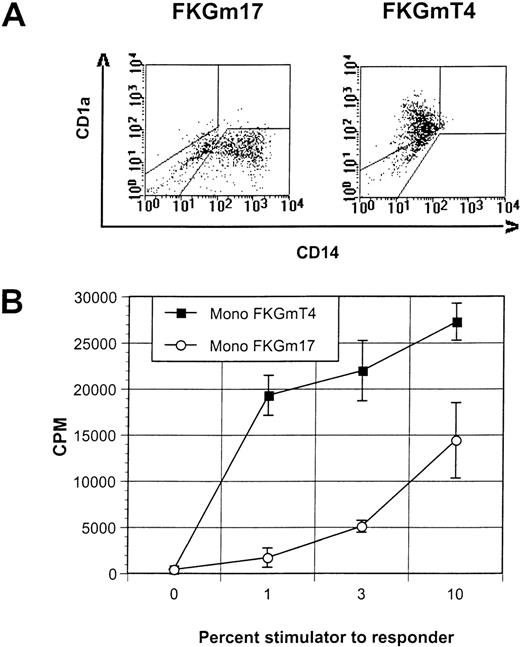
Peripheral blood monocytes, which represent prototypical DC precursors, undergo a rapid phenotypic and functional conversion into DC when cultured with cytokines such as FKGmT4 (Figure3A and B). This in vitro differentiation appears to involve almost all cells that acquire DC markers synchronously.11 So, like lymphoid progenitors, blood monocytes constitute a highly enriched, homogeneous, and rapidly committing population of DC precursors. We examined if mature blood monocytes could differentiate into DC with signals supporting lymphoid-related DC development. The FKGm17 cytokines did not induce the morphologic or phenotypical conversion of monocytes that is typical of DC at any time point of culture (tested up to 3 weeks). Cells were adherent and some resembled macrophages (not shown). Flow cytometric analysis showed that most cells remained CD14 (Figure 3A) and these cells were poor stimulators in MLR (Figure 3B). Monocytes from the blood of 2 separate normal blood donors and a cancer patient treated with granulocyte colony-stimulating factor gave the same results. We therefore conclude that the signals provided by the FKGm17 cytokines are not able to trigger the DC phenotype and the functional conversion of peripheral monocytes. Nevertheless, in the early hematopoietic progenitor compartment, certain cells can respond to these signals by differentiating into DC as well as cells responding to signals inducing monocyte-derived DC formation. The heterogeneity in response that is documented at the progenitor level is lost at the monocytic level, thus indicating the existence of 2 distinct developmental pathways supported by distinct signals. Two distinct prototypical DC precursors exist. Lymphoid progenitors (CD34+ Lin−CD10+ BM cells) respond to cytokines such as FKGm17 and differentiate along a lymphoid-related DC developmental pathway. Monocytes and their precursors respond efficiently to cytokines such as FKGmT4 and produce DC along a myeloid-related developmental pathway.
FKGm17 cytokines do not trigger DC conversion in monocytes.
(A) This representative experiment of 3 shows the flow cytometric correlation of CD14 and CD1a markers in cultures of peripheral blood monocytes in FKGm17 or FKGmT4. (B) Proliferation of allogeneic T cells in MLR measured by 3H-thymidine incorporation after stimulation with various percentages of monocyte-derived DC generated in FKGmT4 (closed squares) and monocyte-derived cells obtained in FKGm17 (open circles).
FKGm17 cytokines do not trigger DC conversion in monocytes.
(A) This representative experiment of 3 shows the flow cytometric correlation of CD14 and CD1a markers in cultures of peripheral blood monocytes in FKGm17 or FKGmT4. (B) Proliferation of allogeneic T cells in MLR measured by 3H-thymidine incorporation after stimulation with various percentages of monocyte-derived DC generated in FKGmT4 (closed squares) and monocyte-derived cells obtained in FKGm17 (open circles).
Ik7 blocks lymphoid-related DC hematopoiesis
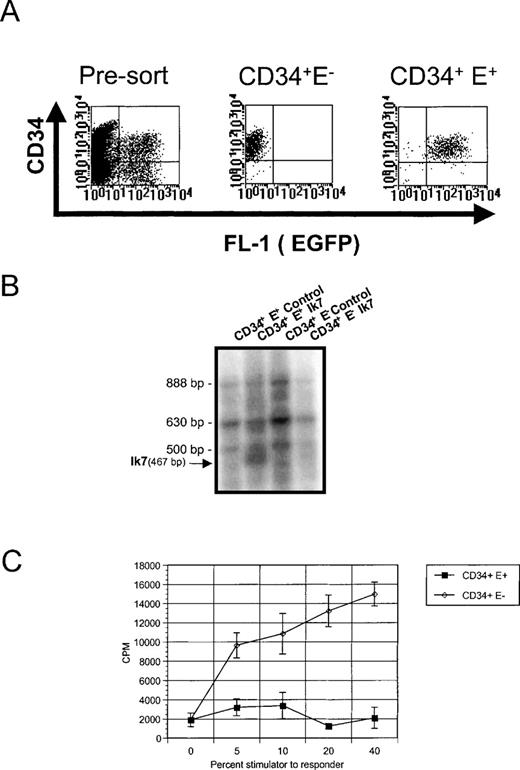
To further delineate the pathways that generate different DC at the molecular level, we examined the molecular requirements involved in DC differentiation from hematopoietic progenitors. A few molecules have been implicated in the formation of DC and a good candidate is Ikaros, a zing finger transcription factor critical for the development of murine DC in lymphoid organs but not essential for the development of skin DC or of monocytes.23 Because Ikaros mRNA is found in human CD34+ cells,29 we assessed whether Ikaros was important for the production of different DC populations by testing the effects of a dominant negative Ikaros protein, Ik7. This mutant form of Ikaros reduces the DNA-binding and transcriptional activity of wild-type Ikaros proteins and of Ikaros family members25-27and blocks lymphopoiesis and DC development when introduced in the germline of mice.23 Retroviral-mediated gene transfer with LZRS-based vectors was chosen to overexpress Ik7 in CD34+BM cells, based on prior successful overexpression of a dominant-negative transcription factor, Id3, into thymic CD34+ cells.32 We constructed the bi-cistronic LZRS-Ik7-IRES-EGFP retroviral vector encoding Ik7 and EGFP as well as the control vector LZRS-IRES-EGFP encoding EGFP only. Total BM CD34+ cells were infected with the respective viruses after a short culture in IL-3, c-kit-ligand, and IL-6 as described for the retroviral-mediated infection of primitive BM hematopoietic progenitors with multilineage clonogenic potential.40 After infection, cells were stained with anti-CD34 mAbs to re-isolate infected hematopoietic progenitors and to test their differentiation potential (Figure 4A). Controls consisted of CD34+ EGFP− cells retrieved from cultures incubated with Ik7 or with control viruses. RT-PCR analysis confirmed the presence of the Ik7 transcript specifically in CD34+EGFP+ cells isolated from the Ik7-infected culture (Figure4B). The DC differentiation potential of these different cell populations was tested in response to different signals.
Analysis of Ik7-infected CD34+ cells.
(A) Representative flow cytometric analysis of CD34+ cells cultured for a total of 4 days and having been infected with virus for 2 days (pre-sort). Reanalysis of CD34+EGFP− and EGFP+ cells from these cultures (CD34+ E− and CD34+E+), which are being used to assay DC hematopoiesis. (B) RT-PCR analysis of Ikaros mRNA in the sorted CD34+ cell populations showing the presence of the multiple endogenous Ikaros isoforms Ik1 (888 bp), Ik2/3 (630 bp), Ik4 (500 bp), and the 467 bp-specific product corresponding to Ik7. (C) Detection of DC activity by MLR. Stimulator cells were obtained after Ik7 infection and culture in FKGm17 of CD34+ EGFP+ cells (closed squares) or CD34+ EGFP− cells (open diamonds) and the response of purified T cells was measured as CPM after3H-thymidine incorporation.
Analysis of Ik7-infected CD34+ cells.
(A) Representative flow cytometric analysis of CD34+ cells cultured for a total of 4 days and having been infected with virus for 2 days (pre-sort). Reanalysis of CD34+EGFP− and EGFP+ cells from these cultures (CD34+ E− and CD34+E+), which are being used to assay DC hematopoiesis. (B) RT-PCR analysis of Ikaros mRNA in the sorted CD34+ cell populations showing the presence of the multiple endogenous Ikaros isoforms Ik1 (888 bp), Ik2/3 (630 bp), Ik4 (500 bp), and the 467 bp-specific product corresponding to Ik7. (C) Detection of DC activity by MLR. Stimulator cells were obtained after Ik7 infection and culture in FKGm17 of CD34+ EGFP+ cells (closed squares) or CD34+ EGFP− cells (open diamonds) and the response of purified T cells was measured as CPM after3H-thymidine incorporation.
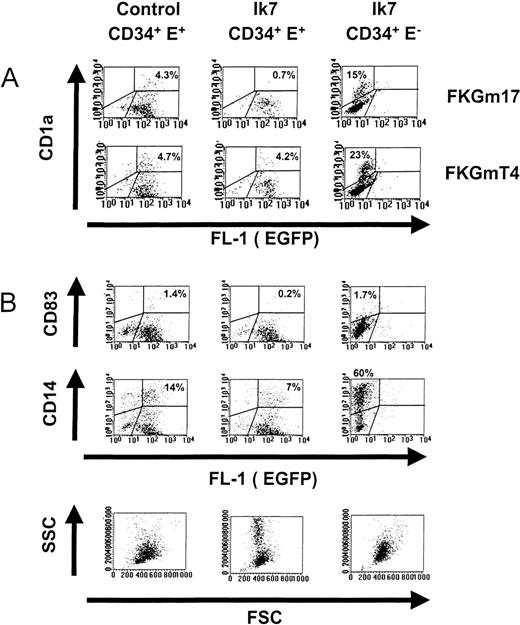
Progenitors were stimulated by the cytokines FKGm17 to support lymphoid-related DC development. Cells having the typical characteristics of DC were not visible in Ik7-infected cultures (Figures 4C and 5A and B). MLR showed an almost complete lack of T-cell stimulatory activity by the progeny of Ik7-infected CD34+cells, whereas the progeny of uninfected CD34+EGFP− cells contained DC activity (Figure 4C). Flow cytometry only detected 0.2% to 0.7% cells expressing the DC markers CD1a or CD83 above background in cultures of Ik7-infected cells (Table1). Both markers were used to identify DC separately in the same experiments. The small number of cells expressing CD1a or CD83 often expressed lower levels of markers and may not represent true DC. Other characteristics of DC such as CD11c expression (not shown) or large FSC and SSC (Figure5B) were markedly absent even at late time points of culture (day 17). In contrast, the progeny of control virus-infected CD34+ EGFP+ cells contained spiky DC often aggregating in typical clusters and flow cytometry detected the presence of 1% to 4% CD1a+ or CD83+ cells (Figure 5A and B). Using flow cytometry, it was determined that Ik7-infected cultures in FKGm17 contained about 5 times less DC based on proportion of cells and produced about one third of the absolute numbers of DC in control cultures. The proportion and absolute numbers of DC were respectively 16% ± 8% and 34% ± 15% (n = 4) of controls (Table 1). These differences between Ik7 cultures and their controls were statistically significant (P < 0.01, paired t test). The absence of DC in Ik7-infected cultures was not caused solely by the process of retroviral infection because DC were present in control-virus-infected cultures. An effect of Ik7 virus supernatant was excluded because EGFP− cells exposed to this medium differentiated into DC (Figure 5A). Noticeably, EGFP− cultures contained higher percentages of DC and of CD14+ cells than EGFP+ cultures (both in Ik7 or control-virus conditions), a phenomenon partly due to differences in calculating positive cells in the dot plots but also possibly reflecting some effects of retroviral or of EGFP expression on hematopoietic cell differentiation. An inhibitory effect by cells growing in Ik7-infected cultures was ruled out by testing CD34+ EGFP− cells either alone or mixed with Ik7-infected CD34+ EGFP+cells and showing that DC production from EGFP− cells was not affected in mixed cultures (data not shown). The strong reduction of DC in FKGm17 cultures infected by Ik7 was not caused by the relative expansion of other cells because the total number of DC was reduced compared to controls. These data strongly suggest that Ik7 severely blocked DC hematopoiesis from the lymphoid-related pathway.
Effects of Ik7 on DC hematopoiesis
| Exp. # . | Conditions . | FKGmT4 . | FKGm17 . | ||
|---|---|---|---|---|---|
| Percentage of DC* . | Number of DC† . | Percentage of DC . | Number of DC . | ||
| 1 | Control‡ | 4.7 | 100 | 4.3 | 100 |
| Ik7 | 4.2 | 198 | 0.7 | 17 | |
| 2 | Control | 4.6 | 100 | 1.6 | 100 |
| Ik7 | 0.7 | 34 | 0.1 | 46 | |
| 3 | Control | 11 | 100 | 1.0 | 100 |
| Ik7 | 7.3 | 100 | 0.2 | 26 | |
| 4 | Control | N.T. | N.T. | 2.0 | 100 |
| Ik7 | N.T. | N.T. | 0.5 | 50 | |
| Average response1-153 | 57 ± 31 | 110 ± 67 | 16 ± 8 | 34 ± 15 | |
| Ik7 vs. control (%) ± SD | P > 0.05 | P > 0.05 | P < 0.001 | P < 0.01 | |
| Exp. # . | Conditions . | FKGmT4 . | FKGm17 . | ||
|---|---|---|---|---|---|
| Percentage of DC* . | Number of DC† . | Percentage of DC . | Number of DC . | ||
| 1 | Control‡ | 4.7 | 100 | 4.3 | 100 |
| Ik7 | 4.2 | 198 | 0.7 | 17 | |
| 2 | Control | 4.6 | 100 | 1.6 | 100 |
| Ik7 | 0.7 | 34 | 0.1 | 46 | |
| 3 | Control | 11 | 100 | 1.0 | 100 |
| Ik7 | 7.3 | 100 | 0.2 | 26 | |
| 4 | Control | N.T. | N.T. | 2.0 | 100 |
| Ik7 | N.T. | N.T. | 0.5 | 50 | |
| Average response1-153 | 57 ± 31 | 110 ± 67 | 16 ± 8 | 34 ± 15 | |
| Ik7 vs. control (%) ± SD | P > 0.05 | P > 0.05 | P < 0.001 | P < 0.01 | |
Percentages of DC are calculated by flow cytometric measurement of CD1a- or CD83-positive cells after 7 to 11 days of culture. Results are expressed as percentages of live cells excluding propidium iodide and expressing the DC markers after subtraction of the percentage of live cells stained with isotype-matched irrelevant mAb.
Numbers of DC are calculated by multiplying the percentage of DC with the total number of live cells (excluding trypan blue). Results of Ik7-infected cultures are expressed relative to control cultures that are assigned an arbitrary value of 100.
Control conditions are CD34+ EGFP+control-infected cells compared to Ik7 conditions, ie, CD34+ EGFP+ Ik7-infected cells.
Responses to Ik7 in each experiment were calculated as a percentage relative to control, which was assigned an arbitrary value of 100% and averaged ± SD.
Effects of Ik7 on DC differentiation.
Representative flow cytometric analyses of cultures of CD34+ EGFP+ cells infected with control virus (left panels) or of CD34+ EGFP+ cells infected with Ik7 virus (middle panels) and of CD34+EGFP− control cells obtained from cultures infected with Ik7-containing virus (right panels). Progenitor cells were obtained as indicated in Figure 4 and cultured with different cytokines. (A) Dot plots show the correlated expression of the CD1a marker and EGFP correspond to 1 experiment where cells were cultured for 7 days with FKGmT4 or FKGm17. (B) A separate experiment in which cells were stimulated with FKGm17 for 11 days and analyzed for the correlated expression of CD83, CD14, EGFP, FSC, and SSC parameters. In all dot plots, the analysis is carried on live cells excluding propidium iodide. Percentages of cells comprised within defined regions are indicated on the plots.
Effects of Ik7 on DC differentiation.
Representative flow cytometric analyses of cultures of CD34+ EGFP+ cells infected with control virus (left panels) or of CD34+ EGFP+ cells infected with Ik7 virus (middle panels) and of CD34+EGFP− control cells obtained from cultures infected with Ik7-containing virus (right panels). Progenitor cells were obtained as indicated in Figure 4 and cultured with different cytokines. (A) Dot plots show the correlated expression of the CD1a marker and EGFP correspond to 1 experiment where cells were cultured for 7 days with FKGmT4 or FKGm17. (B) A separate experiment in which cells were stimulated with FKGm17 for 11 days and analyzed for the correlated expression of CD83, CD14, EGFP, FSC, and SSC parameters. In all dot plots, the analysis is carried on live cells excluding propidium iodide. Percentages of cells comprised within defined regions are indicated on the plots.
The effects of Ik7 were also examined under conditions supporting myeloid DC differentiation, which were obtained by culturing hematopoietic progenitors with the FKGmT4 cytokines. On stimulation with FKGmT4, Ik7-infected progenitors produced DC recognizable by their morphology (not shown) and flow cytometric analysis detected 0.7% to 7% of cells expressing DC markers in a similar range to control cultures (5%-11% DC) (Table 1). Results of 3 separate experiments based on flow cytometric determination showed that Ik7-infected cultures in FKGmT4 contained on average 57% ± 31% of the proportion of DC and 110% ± 67% of absolute numbers of DC relative to control cultures. There was no statistically significant difference between Ik7 cultures grown in the presence of FKGmT4 and their controls (P > 0.05, paired ttest). These results show that Ik7 did not block DC hematopoiesis in the FKGmT4 conditions. Thus, the overexpression of Ik7 in CD34+ cells blocked DC formation induced by conditions supporting lymphoid-related DC but did not abrogate DC formation in conditions supporting myeloid DC development. Therefore, we identified new molecular requirements during the development of different types of DC. This provides formal proof that there are intrinsic molecular differences in DC progenitors and precursors able to give rise to distinct developmental pathways of DC.
Other hematopoietic effects of Ik7 in FKGm17
Myeloid cells constitute the majority in cultures of hematopoietic progenitors stimulated by FKGm17 and we examined the effects of Ik7 on these cells. The proportion of CD33+ myeloid cells did not appear to be affected by Ik7 (data not shown). The proportion of CD14+ cells was reduced from 2- to 8-fold in cultures of Ik7 infected cells compared to control-infected cells but the absolute numbers were similar to those of control cultures. Thus the formation of CD14+ monocytic cells was not blocked. It is known that CD14 can be modulated at the surface of cells by various mechanisms41; therefore, we also measured the formation of monocyte/macrophages in standard methylcellulose clonogenic cultures. Results show that Ik7 infection did not significantly (P > 0.05 in paired t test) affect the formation of macrophage colonies or numbers of clonogenic precursors in the different myeloid lineages (Table 2). When cultures were analyzed at early times, we detected erythroid cells in both control- and Ik7-infected cultures, indicating that there were probably no major alterations in erythroid lineage differentiation by Ik7 in vitro. The analysis was essentially focused on the later time points to examine the differentiation of myeloid colonies, particularly of macrophage colonies. We found that their number, size, and appearance were not affected significantly. We noticed, however, in FKGm17-supported liquid cultures, that Ik7-infected cells expanded 5 to 10 times more than in EGFP+ control-infected cells. The Ik7-infected cultures contained large amounts of granulocytic cells recognized by their typical low FSC with high SSC (Figure 5B, lowest middle panel) and by the presence of azurophilic secondary granules strongly positive for myeloperoxidase (not shown). In one experiment, we detected about 20% of myeloperoxidase-positive cells in Ik-7 infected cultures compared to only 6% to 10% in control cultures. In addition, granulocytes in Ik7-infected cells had more secondary granules than in control cultures, suggesting a possible effect of Ik7 on granulocyte maturation. We conclude that Ik7 did not block the formation of the monocytic lineage. However, Ik7 had hematopoietic effects on myeloid cells, enhancing granulopoiesis in vitro. Thus, overexpression of Ik7 differentially affects myelopoiesis at the granulocyte/monocyte developmental point.
Effects of Ik7 on colony-forming units by CD34+ EGFP+ cells
| Exp. # . | Time (d) . | Conditions . | Number of Colonies/1000 Cells Plated . | ||
|---|---|---|---|---|---|
| Multilineage Colonies* . | Single-Lineage Colonies . | ||||
| Erythroid . | Macrophage† . | ||||
| 1 | 7 | Control | 3.8 | 0.8 | 1.5 |
| Ik7 | 19.5 | 1.3 | 3.3 | ||
| 2 | 18 | Control | 19.6 | 0 | 9 |
| Ik7 | 22 | 0 | 18 | ||
| 3 | 17 | Control | 6.3 | 0 | 3 |
| Ik7 | 10 | 0 | 2.3 | ||
| 4 | 17 | Control | 10.3 | 0 | 14 |
| Ik7 | 20.6 | 0 | 11.6 | ||
| Exp. # . | Time (d) . | Conditions . | Number of Colonies/1000 Cells Plated . | ||
|---|---|---|---|---|---|
| Multilineage Colonies* . | Single-Lineage Colonies . | ||||
| Erythroid . | Macrophage† . | ||||
| 1 | 7 | Control | 3.8 | 0.8 | 1.5 |
| Ik7 | 19.5 | 1.3 | 3.3 | ||
| 2 | 18 | Control | 19.6 | 0 | 9 |
| Ik7 | 22 | 0 | 18 | ||
| 3 | 17 | Control | 6.3 | 0 | 3 |
| Ik7 | 10 | 0 | 2.3 | ||
| 4 | 17 | Control | 10.3 | 0 | 14 |
| Ik7 | 20.6 | 0 | 11.6 | ||
Based on Giemsa stain, these colonies contained monocytes, granulocytes with or without erythroblasts.
Large to very large cells with vacuolated cytoplasm and ex-centered nucleus.
Regulation of gene expression in CD34+ hematopoietic progenitor cells by Ik7
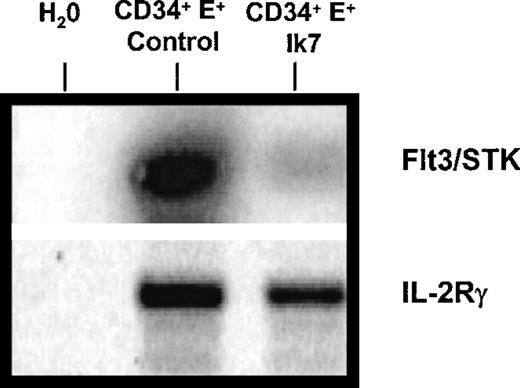
The effects of Ik7 on DC hematopoiesis and on myelopoiesis suggest that Ik7 controls the expression of genes involved in hematopoietic cell differentiation, prompting an analysis of cytokine receptor gene expression by RT-PCR. The tyrosine kinase receptor flt3/STK is known to be expressed by CD34+ hematopoietic progenitor cells,42 to be critical for murine lymphopoiesis and primitive stem cell activity,43 and to bind to flt3-ligand, a cytokine promoting the expansion of DC in vivo.44 RT-PCR analysis showed that Ik7-infected CD34+ cells expressed markedly less flt3/STK mRNA than control-infected CD34+cells (Figure 6). These reduced mRNA levels were seen in 4 different experiments and we confirmed that equal amounts of total RNA were analyzed by verifying equal amplification of mRNA for housekeeping genes such as GAPDH (not shown). Another gene, the IL-2 receptor gamma chain, was analyzed because, like flt3, IL2-Rγ is also critically important for lymphoid development,45 but results showed no significant alteration by Ik7 overexpression (Figure 6) and confirm the specific down-regulation of flt-3 mRNA. Thus, overexpression of Ik7 affects gene expression by interfering with proteins that normally govern transcriptional regulation in hematopoietic cells. These results suggest that the down-regulation of specific genes such as flt3/STK receptor on hematopoietic progenitor cells constitute one of the mechanisms responsible for the differential effects of Ik7 on lymphoid-related DC hematopoiesis while retaining the production of DC from the monocyte-derived pathway.
RT-PCR reaction.
One representative RT-PCR reaction of 4 shows a reduction in the levels of mRNA for flt3/STK mRNA in Ik7-infected CD34+ cells compared to control-infected CD34+ cells. The RNA was obtained after flow cytometry sorting of the cells as in Figure 4. The PCR product was blotted and hybridized with a 32P-labeled cDNA probe. In this experiment, the same amount of cDNA was amplified with primers specific for IL-2Rγ and results show no significant alteration of mRNA levels by Ik7.
RT-PCR reaction.
One representative RT-PCR reaction of 4 shows a reduction in the levels of mRNA for flt3/STK mRNA in Ik7-infected CD34+ cells compared to control-infected CD34+ cells. The RNA was obtained after flow cytometry sorting of the cells as in Figure 4. The PCR product was blotted and hybridized with a 32P-labeled cDNA probe. In this experiment, the same amount of cDNA was amplified with primers specific for IL-2Rγ and results show no significant alteration of mRNA levels by Ik7.
Discussion
In this study we demonstrate that there are 2 distinct pathways that give rise to populations of DC, which have their developmental origin in lymphoid progenitors or in myeloid precursor cells, respectively. We established their distinct cytokine requirements for differentiation and demonstrated that they exhibit differences at the transcriptional level. The differential dependence of DC differentiation pathways for proteins of the Ikaros family of transcription factors suggests the existence of distinct programs of gene expression that underlie these developmental processes.
Most of the culture conditions that have been defined for the generation of human DC in vitro support the differentiation of monocytes into DC.9,12,13,39,46,47 We provide evidence that alternative signaling pathways are involved in the formation of lymphoid-related DC suggesting that distinct molecular programs may control their development. Lymphoid progenitors, unlike monocytes and their precursors, can effectively develop in the FKGm17 conditions, and therefore do so by using a different signaling system than these former populations. Presumably, the cytokine flt-3-ligand and IL-7 are important factors for lymphoid-related DC because they have demonstrated positive effects in rodent systems.20,48Overexpression of Ik7 blocks the signals provided by the FKGm17 cytokines to instruct DC differentiation. One of the molecular mechanisms by which Ik7 may act in that capacity is by down-regulating levels of Flt3 receptor mRNA. The mutant dominant-negative protein Ik7 is known to reduce the transcriptional activity of murine proteins with which it associates and presumably does so with the human homologues. This suggests the possibility that some members of the Ikaros family of proteins may act as transcriptional regulators of the flt3/STK gene. Already described partners of Ik7 include other Ikaros proteins as well as the B-cell homologue Aiolos26 and Helios.27Both Ikaros and the human homologue of Aiolos are found in CD34+ cells (A. Galy, unpublished observations). Levels of wild-type Ikaros mRNA are not significantly affected by Ik7 in CD34+ cells (as seen in Figure 4), but it will have to be determined how Ikaros family members and the genes that they control are affected by Ik7 overexpression into DC.
Our results suggest that Ik7 differentially controls the hematopoiesis of lymphoid progenitors and of myeloid progenitors, therefore supporting the existence of 2 separate hematopoietic lineages of DC. Lymphoid progenitors and monocytes represent 2 distinct DC precursor/progenitor cells that appear to be unrelated based on the inability of lymphoid progenitors to generate CD14+monocytic cells in response to FKGm17 and because CD34+Lin− CD10+ lymphoid progenitors do not survive in the presence of c-kit ligand and macrophage colony-stimulating factor , cytokines that support monocytic development (data not shown). The differential effect of Ik7 on the development of lymphoid-related DC and on monocytes is compatible with a hematopoietic lineage-specific alteration as was suggested by Ikaros mutant mice lacking lymphocytes but having abundant numbers of monocyte/macrophages.22 23 The differential effect of Ik7 on DC progenitors in FKGm17 and FKGmT4 conditions might therefore be explained by a preferential response of distinct subsets of progenitor populations to each of these signals.
An alternative interpretation for our results is that they show distinct DC developmental options as the result of various signaling pathways in DC progenitors. Understanding why FKGm17 cytokines fail to support DC differentiation in monocytes might help explain the effects of Ik7 on DC hematopoiesis. We noticed that CD1a+/lowCD14+ cells are being generated in FKGm17 cultures of CD34+ Lin− CD10− cells (as seen in Figure 2A, middle panel), which appear to represent intermediate stages of DC development described in other studies.38 Yet, most CD14+ cells or CD1a+ CD14+ cells never become DC in FKGm17. Cultures tested up to 18 days accumulated up to 75% CD14+cells but still only contained about 0.1% DC as measured by CD1a expression (data not shown). We conclude that the FKGm17 conditions fail to provide the necessary signals to support the CD14+cell-dependent DC developmental pathway that has been described in other studies.14,38 It is possible that TNF-α which was shown by antibody neutralization studies to be essential for the development of CD1a+ CD14+ cells into DC, is at insufficient levels in the culture thus preventing their survival or differentiation.38 The difference in signals provided by FKGmT4 and FKGm17 may explain the different effects of Ik7. Indeed, we were able to partially rescue DC formation in Ik7-infected FKGm17 cultures by adding TNF-α thus mimicking most of the conditions found in FKGMT4 cultures (A. Galy, unpublished observations). A consequence of the existence of different signaling pathways in DC is the prediction that several types of mature DC probably exist. Indeed separate populations of DC have been described in mice with the characterization of “lymphoid-related” DC expressing the CD8-α marker and of “myeloid-derived” DC, which are CD8-α−. These DC differ not only at the level of their phenotype but in their requirements for GM-CSF for growth and differentiation,4,49 in transcription regulation underlined by Rel/B molecules,21 and in the types of immune responses that they elicit.4,5 Reconstitution experiments with thymic or BM precursors have suggested that these 2 types of DC derive from distinct hematopoietic precursors.50 Thus we propose that at least 1 aspect contributing to the functional heterogeneity of DC is determined by molecular events occurring at the hematopoietic level. Overall, as a model, Ik7 overexpression appears to be useful to understand the biology of the Ikaros family of molecules in human cells. Granulopoiesis was enhanced by Ik7 during the in vitro period of culture and these observations are compatible with the enlarged spleens and extramedullary hematopoiesis described in DN-/- mutant mice.23 Morphologic changes were seen in granulocytes suggesting a possible effect of Ik7 on their maturation with perhaps induction of some eosinophilic characteristics. Further studies, beyond the scope of this paper, will be required to understand the effects of Ik7 on this lineage of cells.
Altogether, our data indicate that the process of hematopoiesis in human DC occurs along several possible developmental pathways regulated by distinct signals. We conclude that different developmental lineages of DC exist and that lymphoid-related DC constitute a separate entity. The existence of non-overlapping signals and of distinct cellular developmental pathways for DC hematopoiesis may have important implications for the regulation of DC function and homeostasis. Most likely, regulating DC formation in normal and pathologic circumstances or for therapeutic purposes will be complex.
Acknowledgments
We acknowledge the support of the Flow Cytometry Core Facility at the Karmanos Cancer Institute and are thankful to Dr Dan at Harper Hospital, Detroit, for help with the cytology. Many thanks also to the staff in the operating room and pathology at Harper Hospital for their help in procurement of human rib marrow samples.
Supported by American Cancer Society grants, IRG162K and RPG-98-183-01.
Reprints:Anne Galy, Karmanos Cancer Institute, 110 Warren Avenue, Detroit, MI 48201; e-mail: galya@kci.wayne.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal