Abstract
We describe here that lineage phenotype- negative (Lin)−c-kit+ hematopoietic progenitor cells (HPCs) from day 13 postcoitus (dpc) murine fetal liver (FL) can generate dendritic cell (DC) precursors when cultured in vitro in the presence of PA6 stromal cells plus granulocyte/macrophage colony-stimulating factor (GM-CSF) + stem cell factor (SCF) + Flt3 ligand (Flt3L) for 12 to 14 days, and develop into mature DCs when stimulated with GM-CSF plus mouse tumor necrosis factor (mTNF) for an additional 3 to 5 days. A transwell culture system showed that the generation of DC precursors depended on the support of PA6 cell-secreted soluble factor(s). The mature DCs derived from 13 dpc FL Lin−c-kit+ HPCs showed characteristic morphology and function of DCs and expressed high levels of Ia, CD86, and CD40 molecules, low levels of DEC205, E-cadherin, and F4/80 molecules, but barely detectable CD11c antigen. Once FL-derived HPCs were cultured without GM-CSF, NK1.1+ cells developed in the presence of PA6 cells + SCF + Flt3L. These NK1.1+ cells could develop into DC precursors at an earlier stage of differentiation by reculturing with PA6 cells + SCF + Flt3L + GM-CSF, but they would be irreversibly committed to NK cell precursors without GM-CSF after 3 days, suggesting that GM-CSF plays a critical role in controlling the transition of DC and NK cell precursors from 13 dpc FL-derived Lin−c-kit+ HPCs. This study represents the first success in generating mature DCs in vitro from murine FL HPCs. (Blood. 2000;95:138-146)
Dendritic cells (DCs) are professional antigen-presenting cells that are distributed throughout tissues and organs, where they show functional and phenotypic heterogeneity.1-4 DCs can uptake, process, and present antigens on major histocompatibility complex (MHC) class II to induce T cell immune responses, in particular to initiate primary antigen-specific immune reaction.5,6 They also participate in B cell-mediated immune responses,7-9 constituting an integral part of the immune system.10 Recent studies also suggest an important role for DCs in the induction of T cell tolerance. When antigen-bearing DCs are directly injected into the developing thymus or fetal thymic organ cultures, reactive T cells were selectively deleted.11 Moreover, if MHC class II molecules were only expressed by cortical epithelium, but not by DCs in thymic medulla, the propensity to autoimmunity increases.12 All these data suggest that DCs may be involved in the induction of tolerance by deleting autoreactive T cells and the tolerance of T cells to self-antigens during the fetal development.
During embryonic development of hematopoiesis, hematopoietic progenitor cells (HPCs) sequentially appear in yolk sac, paraaortic splanchnopleura, aorta-gonad-mesonephros region (AGM), fetal liver (FL), and finally in the bone marrow (BM).13-18 The FL is considered to be the principle hematopoietic organ during the murine fetal stage until the early neonatal stage, and serves as a reservoir of founder hematopoietic cells generated at early hematopoietic sites within the conceptus.13,17-19 It has been reported that the progenitor cells derived from FL can differentiate in vitro into T lymphocytes,20 natural killer (NK) cells, B lymphocytes, macrophages and mast cells in mouse,21 and T lymphocytes and thymic NK cells in human.22 However, it remains elusive whether murine FL-derived HPCs can differentiate into mature DCs.
It is well established now that DCs can be generated from blood monocytes,23 BM-derived HPCs24-26 and cord blood HPCs27 stimulated with granulocyte/macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor α (TNFα). In addition, CD4lo thymic precursors obtained from adult thymus can develop into T cells and thymic DCs bearing CD8α molecule after intrathymic transplantation into irradiated congeneic mice,28,29 indicating the existence of lymphoid-committed DC progenitors. In the fetus, thymic DCs start to develop in the rat embryonic thymus as early as day 17.30 All these observations imply that the development of different DC subsets from embryonic HPCs may be regulated under distinct mechanisms from those of adult BM HPCs.
Because the thymus and spleen form relatively late in gestation and are involved in the production of highly differentiated more mature hematopoietic cells, HPCs presumably seed these organs from other embryonic hematopoietic sites, mostly FL.18 31 To explore the capacity of generating DC from embryonic HPCs, lineage phenotype-negative (Lin)−c-kit+HPCs were isolated from day 13 postcoitus (dpc) FL, and cultured in the presence of growth factors and stromal cell line PA6. We found that these FL HPCs possess the capacity to generate DC precursors, which can finally differentiate into mature DCs, in the presence of GM-CSF + stem cell factor (SCF) + Flt3 ligand (Flt3L) and PA6 cells. We also describe that DC precursors in FL-derived HPCs may share progenitor cells in common with NK cells.
Materials and methods
Cytokines, antibodies, and cell lines
Recombinant murine SCF and anti-c-kit antibody (ACK-2) were kindly provided by Dr T. Sudo, Basic Research Institute of Toray Co (Kanagawa, Japan), murine GM-CSF by Kirin Brewery Co (Tokyo, Japan), murine macrophage colony-stimulating factor (M-CSF) by Morinaga Co (Tokyo, Japan), and Flt3L by Immunex Co (Seattle, WA). Mouse TNFα (mTNFα) was produced in house.32 These cytokines were used at the optimal concentrations as previously described.26,33 DEC-205 (NLDC145), a rat monoclonal antibody (MoAb) to murine DCs, was a generous gift of Dr R. M. Steinman (Rockefeller University, New York, NY).34 35 The MoAb to mouse E-cadherin was purchased from Dainipon Pharmaceutical Co (Osaka, Japan). Other MoAbs and reagents used for immunostaining were obtained from PharMingen (San Diego, CA) unless otherwise indicated.
BM-derived stromal cell line, PA6 and M-CSF defective stromal cell line, OP9 were kindly provided by Dr S. Nishikawa (Kyoto University, Kyoto, Japan) and Dr T. Nakano (Osaka University, Osaka, Japan), respectively.
Mice
C57BL/6 and BALB/c mice were obtained from CLEA Animal Co (Tokyo, Japan) and maintained under specific pathogen-free conditions in the Animal Facility of the Department of Molecular Preventive Medicine, School of Medicine, The University of Tokyo, Tokyo, Japan. FL were obtained from 13 dpc fetuses of female BALB/c or C57BL/6 mice mated with male C57BL/6 mice. The presence of a postcoital plug was used to determine day 0 of the embryonic age. All animal experiments complied with the standards set out in the Guideline for Care and Use of Laboratory Animals of The University of Tokyo.
Suspension culture of Lin−c-kit+HPCs
BM Lin−c-kit+ HPCs were obtained and cultured to induce DCs as previously described.26,33 FL cells were obtained by aspirating FL from 13 dpc murine embryos. Lin−c-kit+ HPCs were isolated from FL mononuclear cells (MNCs) using a cell sorter (EPICS ELITE, Coulter Electronics, Hialeah, FL) as previously described.26 33 In brief, FL MNCs were subjected to indirect staining using a biotin-conjugated anti-c-kit MoAb and phycoerythrin (PE)-labeled streptavidin, followed by a set of fluorescein isothiocyanate (FITC)-labeled MoAbs to CD3ε(145-2C11), CD4 (H129.19), CD8α(53-6.7), B220 (RA3-6B2), Gr-1 (Ly-6G), CD11a(2D7), and CD11b (M1/70). The degree of contamination by other types of cells in these preparations was consistently < 0.5% as revealed by an immunofluorescence analysis.
Purified FL-derived Lin−c-kit+HPCs were incubated at a cell concentration of 3 × 104 cells/mL in Iscove's modified Dulbecco's medium (IMDM; GiBCO, Rockville, MD), supplemented with 20% fetal calf serum (FCS), penicillin G (100 U/mL), and streptomycin (100 μg/mL) in the presence of GM-CSF (4 ng/mL) + SCF (10 ng/mL) + Flt3L (10 ng/mL) + mTNFα (50 ng/mL). Optimal conditions were maintained by splitting these cultures at day 7 and every 2 to 3 days replacing 50% of the medium with a new medium containing fresh cytokines. At the indicated time intervals, morphologic observation with an inverted microscope and immunophenotypical analyses were performed on the cultured cells.
FL-derived Lin−c-kit+ HPCs were cocultured with PA6 or OP9 stromal cell line as shown in Figure1. In some experiments, FL-derived HPCs were cultured with or without stromal cells in the presence or absence of M-CSF (100 ng/mL) under the above described conditions. In other experiments, the NK 1.1+ cell subset was sorted at day 3, 5, and 7 from Lin−c-kit+ HPCs, which had been cocultured with PA6 cells in the upper compartments of transwell plates in the presence of SCF and Flt3L. The purity of the sorted cells was more than 99%. The sorted NK1.1+ cell subset was recultured for 10 to 12 days, supplemented with GM-CSF. These cells were collected for analyses after restimulation by GM-CSF + mTNFα for an additional 3 to 5 days. Meanwhile, the sorted NK1.1+ cell subset was also cultured continuously in the absence of GM-CSF for an additional 10 to 12 days. All the staining and sorting procedures were performed in the presence of 1 mM EDTA to avoid cell aggregation.
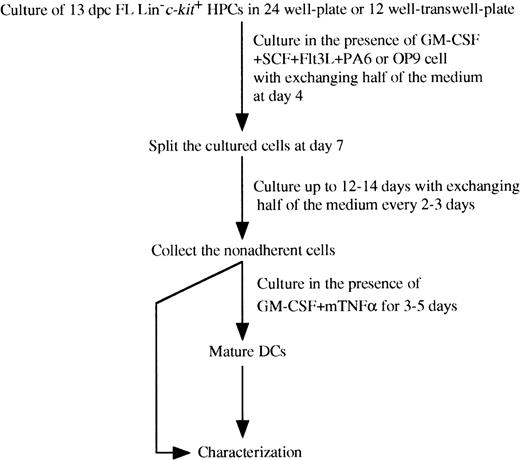
Schematic representation of culture conditions of purified murine FL-derived Lin−c-kit+ HPCs.
Schematic representation of culture conditions of purified murine FL-derived Lin−c-kit+ HPCs.
Immunofluorescence analysis
Immunofluorescence analyses were performed as previously described.26 33 In 2-color analyses, 4 × 105 cells were sequentially incubated with optimal concentrations of biotinylated hamster anti-CD86 or anti-CD11c, and rat anti-DEC-205, anti-E-cadherin or anti-F4/80 MoAbs, followed by FITC-labeled streptavidin and by FITC-conjugated goat antirat IgG (Fab′)2 antibodies (Caltag, Camarillo, CA), respectively, or directly with FITC-labeled anti-CD8α or NK1.1 MoAbs. These cells were finally stained with PE-conjugated mouse antimouse Ia MoAb (AF6-120.1). In other experiments, the cells were first incubated with the appropriate concentration of biotinylated anti-Ly49c MoAb and subsequently labeled by PE-conjugated streptavidin. Then the cells were stained with FITC-conjugated anti-NK1.1 MoAb. In tri-color analysis, the cells were sequentially incubated with rabbit antimouse asialo-GM1 antibody (Wako Co, Osaka, Japan) and PE-conjugated goat antirabbit IgG (Fab′)2 antibody, followed by the incubation with FITC-labeled anti-CD3ε or CD4 and biotinylated anti-NK1.1 MoAbs. The cells were finally stained with Cy-chrome-conjugated streptavidin. The instrument compensation was set in each experiment using single-color or 2-color stained samples. In some experiments, the corresponding cell subpopulations were isolated using a cell sorter.
Endocytosis
The endocytosis experiment was performed as previously described.36,37 Briefly, in the endocytosis test the cells were incubated with 0.1 mg/mL FITC-Dextran (FITC-DX; 4,000 daltons; Sigma Chemicol Co, St Louis, MO) at 0°C or 37°C for 60 minutes. The reaction was stopped by adding ice-cold phosphate-buffered saline (PBS) containing 5% bovine serum albumin and 0.02% sodium azide, and the cells were washed 3 times with 2.5% FCS-0.02% sodium azide-PBS, respectively. Finally, the percentage and density of FITC-positive cells were examined with a cell sorter as previously described.33
Mixed leukocyte reaction (MLR)
Splenic MNCs were prepared from allogeneic mice as previously described.33 The adherent cells were first removed by incubating them at 37°C for 60 minutes in IMDM medium containing 10% FCS. To obtain highly purified T cells, the nonadherent splenic MNCs were incubated with superparamagnetic MicroBeads conjugated with hamster anti-mouse CD4 MoAb (Miltenyi Biotec, Germany), thereby isolating the CD4+ T cells by magnetic cell sorting. After treatment with mitomycin C (MMC; 15 μg/mL),38 the indicated stimulator cells from FL Lin−c-kit+ cell culture, BM Lin−c-kit+ cell culture or peritoneal macrophages (from 100 to 3 × 104 cells) were added to the T cells (3 × 105) in wells of 96-well round-bottomed microtest tissue-culture plates (Nunc, Roskilde, Denmark). After incubating at 37°C for 4 to 5 days, cell proliferation was determined by using 3-(4,5-dimethylthiazolyl-2yl-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical Co). In brief, 15 μL of MTT (5 mg/mL in PBS) was added into each well and the plates were incubated at 37°C for an additional 4 hours. The resultant absorbance at 550 nm was read by a microplate immunoreader.
Nonspecific esterase (NSE) staining
Cells were cytocentrifuged for 5 minutes at 500 rpm on a microscope slide and used for NSE staining (a-naphthyl acetate esterase staining kit; Sigma Chemical Co), according to the instructions of the manufacturer.
Statistical analysis
Significant differences were evaluated with the Student's ttest. P < .05 were considered to be statistically significant.
Results
Culture conditions for generating DCs from 13 dpc FL-derived Lin−c-kit+HPCs
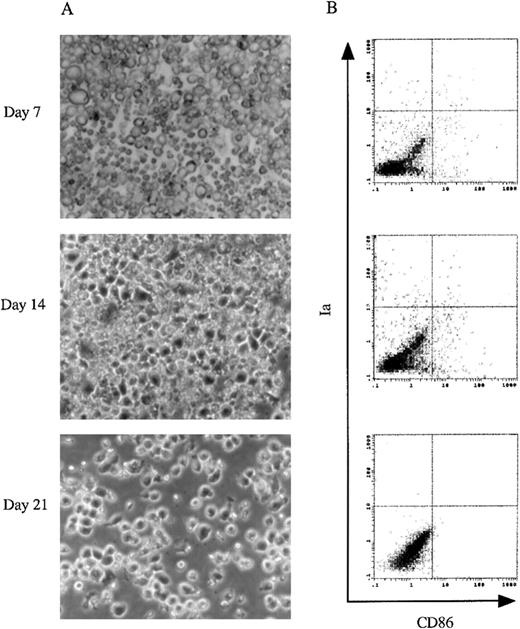
We first determined whether culture conditions that lead to the differentiation of adult murine BM HPCs into DCs would also be applicable in FL-derived progenitor cells. The capacity to generate DCs from 13 dpc FL-derived Lin−c-kit+HPCs in the presence of GM-CSF + SCF + Flt3L and mTNFα was examined after culturing for 7, 14, and 21 days. As shown in Figure2A, there was no typical DC aggregate formation in the cultures. The immunofluorescence analysis showed that < 1.5% cells expressed Ia and CD86 antigens, characteristics of mature DCs (Figure 2B). These results indicated that the combination of GM-CSF + SCF + Flt3L + mTNFα could not induce the generation of mature DCs from 13 dpc FL-derived Lin−c-kit+ HPCs, whereas combination of these growth factors could induce mature DC differentiation from adult murine BM Lin−c-kit+ HPCs as reported previously by us.26
The culture of murine 13 dpc FL-derived Lin−c-kit+ HPCs.
(A) Using a phase contrast microscope, morphologic analyses were performed on cultured murine 13 dpc FL-derived Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + Flt3L + mTNFα at day 7, 14, and 21. Original magnifications: × 200. (B) 2-color immunofluorescence analysis was performed on the cultured cells at the indicated time points. The cells were sequentially stained with biotinylated CD86 MoAb and PE-labeled Ia MoAb. CD86 was revealed by FITC-streptavidin. The quads were set up on the isotype-matched control dot plot. These results are representative of 3 independent experiments.
The culture of murine 13 dpc FL-derived Lin−c-kit+ HPCs.
(A) Using a phase contrast microscope, morphologic analyses were performed on cultured murine 13 dpc FL-derived Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + Flt3L + mTNFα at day 7, 14, and 21. Original magnifications: × 200. (B) 2-color immunofluorescence analysis was performed on the cultured cells at the indicated time points. The cells were sequentially stained with biotinylated CD86 MoAb and PE-labeled Ia MoAb. CD86 was revealed by FITC-streptavidin. The quads were set up on the isotype-matched control dot plot. These results are representative of 3 independent experiments.
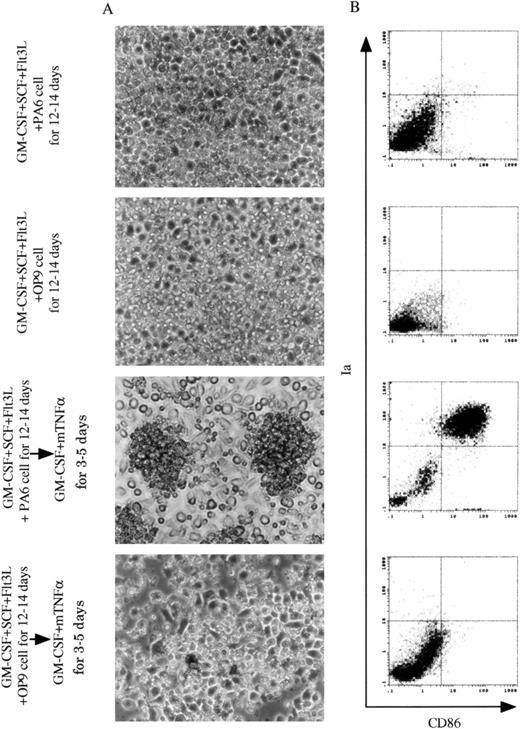
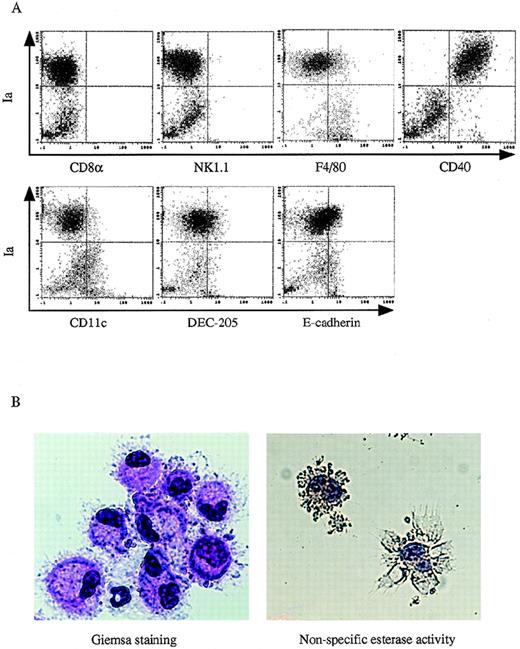
To explore what conditions might be essential for inducing DCs from FL-derived Lin−c-kit+ HPCs, we first cocultured them with BM-derived stromal cell line PA6 or M-CSF defective stromal cell line OP9 in the presence of GM-CSF + SCF + Flt3L for 12 to 14 days. Because addition of mTNFα significantly inhibited the proliferation of FL Lin−c-kit+ HPCs (data not shown), this was excluded from the cultures. On the coculture with the PA6 or OP9 cells in the presence of GM-CSF + SCF + Flt3L, FL-derived HPCs did not develop DC aggregates (Figure 3A), and only a few cells were Ia+CD86+ cells (Figure3B). Because our previous findings showed that mTNFα plays a critical role in stimulating the differentiation of DCs from adult murine BM-derived Lin−c-kit+HPCs,26 the nonadherent cells from FL-derived HPCs cocultured with PA6 stromal cells in the presence of GM-CSF + SCF + Flt3L were stimulated with GM-CSF + mTNFα for an additional 3 to 5 days. As shown in Figure 3A, this resulted in the development of DC-like aggregates surrounded by cells with large sheet-like processes in the periphery. Phenotypically, such cells expressed high levels of Ia, CD86, and CD40, and low levels of F4/80, DEC205, and E-cadherin antigens. However, CD11c molecule was barely detected, and CD8α and NK1.1 antigens were negative (Figures 3B and4A). Giemsa-Wright staining showed that the aggregated cells were morphologically DCs with eccentric nuclei and polarized lamellipodia. They were negative for nonspecific esterase activity (Figure 4B).39 Functionally, these cells could enhance the proliferation of allogeneic T lymphocytes in an MLR assay at a comparable level with DCs generated from adult BM Lin−c-kit+ HPCs (Figure5). On the contrary, cells derived from OP9 cells + GM-CSF + SCF + Flt3L-induced FL-derived HPCs did not differentiate into mature DCs when restimulated with GM-CSF + mTNFα (Figure 3). These results suggested that DC precursors were generated from murine FL-derived Lin−c-kit+ HPCs cocultured with PA6 stromal cells in the presence of GM-CSF + SCF + Flt3L, whereas mTNFα and GM-CSF were essential for the final maturation of DCs from these precursors.
Generation of DCs from 13dpc FL-derived Lin−c-kit+ HPCs.
13 dpc FL-derived Lin−c-kit+ HPCs were cocultured with stromal cell lines PA6 or OP9 cells in the presence of GM-CSF + SCF + Flt3L for 12 to 14 days, and restimulated by GM-CSF + mTNFα for an additional 3 to 5 days. (A) A phase contrast microscopic observation was performed on cocultured FL-derived HPCs with PA6 or OP9 cells in the presence of GM-CSF + SCF + Flt3L for 12 to 14 days before and after replanted into new plates and restimulated by GM-CSF + mTNFα for an additional 3 to 5 days. Original magnification: × 200. (B) Immunophenotypic detection was performed on nonadherent cells from FL-derived HPC cultures at the indicated time points and culture conditions. These cells were sequentially stained with biotinylated CD86 MoAb and PE-labeled Ia MoAb, whereas CD86 was revealed by FITC-streptavidin. The quads were set up on the isotype-matched control dot plot. These results are representative of 3 independent experiments.
Generation of DCs from 13dpc FL-derived Lin−c-kit+ HPCs.
13 dpc FL-derived Lin−c-kit+ HPCs were cocultured with stromal cell lines PA6 or OP9 cells in the presence of GM-CSF + SCF + Flt3L for 12 to 14 days, and restimulated by GM-CSF + mTNFα for an additional 3 to 5 days. (A) A phase contrast microscopic observation was performed on cocultured FL-derived HPCs with PA6 or OP9 cells in the presence of GM-CSF + SCF + Flt3L for 12 to 14 days before and after replanted into new plates and restimulated by GM-CSF + mTNFα for an additional 3 to 5 days. Original magnification: × 200. (B) Immunophenotypic detection was performed on nonadherent cells from FL-derived HPC cultures at the indicated time points and culture conditions. These cells were sequentially stained with biotinylated CD86 MoAb and PE-labeled Ia MoAb, whereas CD86 was revealed by FITC-streptavidin. The quads were set up on the isotype-matched control dot plot. These results are representative of 3 independent experiments.
Immunophenotypic and morphologic analyses of DC-like cells.
13dpc FL-derived Lin−c-kit+ HPCs were first supplemented with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days and then stimulated with GM-SCF + mTNFα for an additional 3 to 5 days. (A) The phenotype of the nonadherent cells was analyzed by 2-color immunofluorescence staining as described in the Materials and Methods. The indicated FITC-labeled MoAbs (CD8α, NK1.1, F4/80, CD40, CD11c, DEC205, and E-cadherin) were used to demonstrate the phenotypic characteristics of the generated DCs. The quads were set up on the isotype-matched control dot plot. (B) Giemsa staining and nonspecific esterase activity analyses were performed on GM-CSF + mTNFα-stimulated DC precursors at day 3 to day 5 that were derived from FL-derived HPCs cocultured with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days. Original magnification: × 400. These results are representative of 3 independent experiments.
Immunophenotypic and morphologic analyses of DC-like cells.
13dpc FL-derived Lin−c-kit+ HPCs were first supplemented with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days and then stimulated with GM-SCF + mTNFα for an additional 3 to 5 days. (A) The phenotype of the nonadherent cells was analyzed by 2-color immunofluorescence staining as described in the Materials and Methods. The indicated FITC-labeled MoAbs (CD8α, NK1.1, F4/80, CD40, CD11c, DEC205, and E-cadherin) were used to demonstrate the phenotypic characteristics of the generated DCs. The quads were set up on the isotype-matched control dot plot. (B) Giemsa staining and nonspecific esterase activity analyses were performed on GM-CSF + mTNFα-stimulated DC precursors at day 3 to day 5 that were derived from FL-derived HPCs cocultured with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days. Original magnification: × 400. These results are representative of 3 independent experiments.
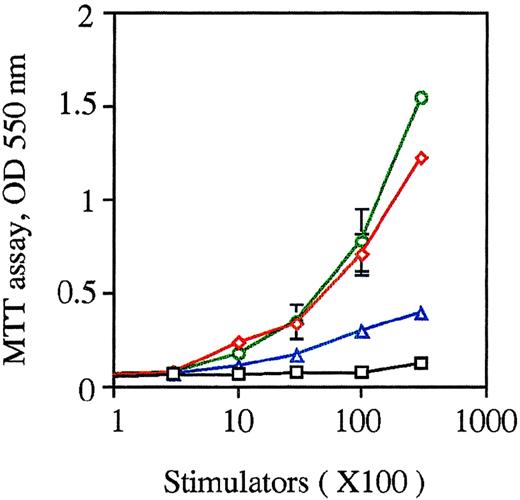
The capacity of the cultured cells to enhance allogeneic MLR.
Allogeneic MLR was performed using purified T cells (3 × 105 cells/well in 96-round-well plate) as responder cells. The unfractionated nonadherent cells, which were generated from FL-derived HPCs cocultured with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days before and after restimulation by GM-CSF + mTNFα for an additional 3 to 5 days, were treated by MMC and used as stimulator cells at the indicated cell numbers. DCs derived from BM-derived HPCs stimulated with SCF + Flt3L + GM-CSF + mTNFα, and peritoneal macrophages were used as controls. The proliferation of T cells was measured using MTT after 5 days of culture. Results are expressed as the mean ± 1 SD of triplicate cultures and are representative of 3 independent experiments. Black squares indicate macrophages; red diamonds, DCs from BM HPCs; green circles, DCs from FL HPCs; and purple triangles, FL-derived DC precursors.
The capacity of the cultured cells to enhance allogeneic MLR.
Allogeneic MLR was performed using purified T cells (3 × 105 cells/well in 96-round-well plate) as responder cells. The unfractionated nonadherent cells, which were generated from FL-derived HPCs cocultured with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days before and after restimulation by GM-CSF + mTNFα for an additional 3 to 5 days, were treated by MMC and used as stimulator cells at the indicated cell numbers. DCs derived from BM-derived HPCs stimulated with SCF + Flt3L + GM-CSF + mTNFα, and peritoneal macrophages were used as controls. The proliferation of T cells was measured using MTT after 5 days of culture. Results are expressed as the mean ± 1 SD of triplicate cultures and are representative of 3 independent experiments. Black squares indicate macrophages; red diamonds, DCs from BM HPCs; green circles, DCs from FL HPCs; and purple triangles, FL-derived DC precursors.
Mediators required for generating DC precursors from FL-derived Lin−c-kit+HPCs
To elucidate whether the cell-cell contact is required for the generation of DC precursors, transwell plates with 0.4-μm pore size were used in the coculture system. The direct interaction of HPCs and stromal cells could be blocked in the transwell system, while soluble factors could diffuse into the cell cultures. After coculturing with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days, the nonadherent cells in the upper compartments of transwell plates from FL-derived HPCs were transferred into a new 6-well plate and restimulated with GM-CSF + mTNFα. As shown in Figure 6, DC precursors could be generated in the transwell culture system, and these cells could subsequently differentiate into mature DCs by restimulation with GM-CSF + mTNFα.
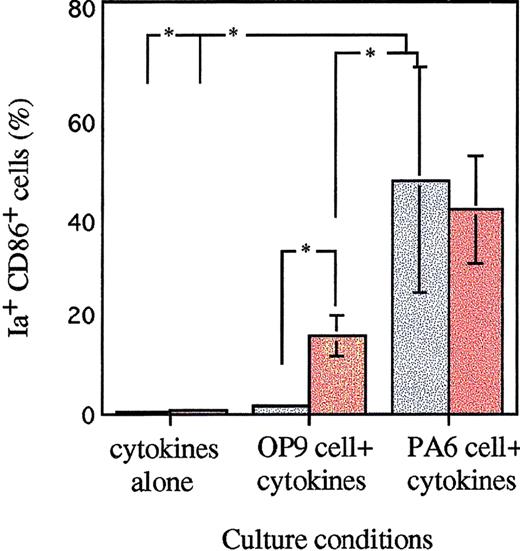
The role of stromal cells in the induction of DCs from FL-derived Lin−c-kit+ HPCs.
13 dpc FL-derived HPCs were cultured in the presence of cytokines, GM-CSF + SCF + Flt3L, with or without stromal cell line OP9 or PA6 cells in a transwell culture system. M-CSF was added into these cultures as indicated. After 12 to 14 days of culture, the nonadherent cells were replanted in a new 6-well plate, restimulated with GM-CSF + mTNFα for an additional 3 to 5 days and then collected for immunofluorescence analysis. Ia+CD86+ cells were examined by staining with PE-labeled Ia MoAb and biotinylated CD86 MoAb, and revealed by FITC-streptavidin. Results are expressed as the mean ± SD of 3 independent experiments. *P < .05 significance compared with cultures lacking stromal cells or M-CSF addition, or between different stromal cells. Gray bars represent cultures without M-CSF; colored bars, with M-CSF.
The role of stromal cells in the induction of DCs from FL-derived Lin−c-kit+ HPCs.
13 dpc FL-derived HPCs were cultured in the presence of cytokines, GM-CSF + SCF + Flt3L, with or without stromal cell line OP9 or PA6 cells in a transwell culture system. M-CSF was added into these cultures as indicated. After 12 to 14 days of culture, the nonadherent cells were replanted in a new 6-well plate, restimulated with GM-CSF + mTNFα for an additional 3 to 5 days and then collected for immunofluorescence analysis. Ia+CD86+ cells were examined by staining with PE-labeled Ia MoAb and biotinylated CD86 MoAb, and revealed by FITC-streptavidin. Results are expressed as the mean ± SD of 3 independent experiments. *P < .05 significance compared with cultures lacking stromal cells or M-CSF addition, or between different stromal cells. Gray bars represent cultures without M-CSF; colored bars, with M-CSF.
In contrast, coculture with OP9 cells in the same condition could not generate DC precursors from FL-derived Lin−c-kit+ HPCs. Because OP9 cells are deficient in the production of functional M-CSF, we asked whether M-CSF might account for the generation of DC precursors in PA6-stimulated culture. Addition of M-CSF to OP9 cells + GM-CSF + SCF + Flt3L resulted in significant induction of FL HPCs-derived DC precursors that subsequently differentiated into mature DCs by restimulation with GM-CSF + mTNFα. However, the effect of M-CSF on the generation of DC precursors was significantly less than that of PA6 cells + GM-CSF + SCF + Flt3L-stimulated culture. Moreover, addition of M-CSF alone to the combination of cytokines, GM-CSF + SCF + Flt3L, could not induce the generation of DC precursors from FL-derived HPCs. Furthermore, addition of M-CSF to PA6 cells + GM-CSF + SCF + Flt3L-induced culture did not enhance the generation of DC precursors (Figure 6).
Further characterization of DC precursors derived from 13 dpc FL Lin−c-kit+HPCs
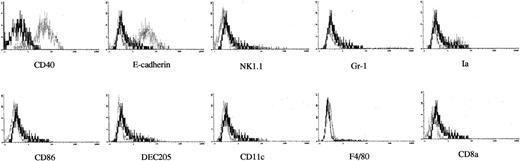
To better understand the cellular basis for mature DC development from 13 dpc FL-derived Lin−c-kit+HPCs, we analyzed the features of the cells generated at day 12 to day14 in culture with PA6 cells + SCF + Flt3L + GM-CSF. Morphologically, these cells showed monocyte-like cells. Phenotypic analysis demonstrated that these cells expressed high level of CD11b (data not shown). When these nonadherent cells were further examined with a panel of MoAbs, the analysis revealed that these cells expressed high levels of CD40 and E-cadherin and low levels of NK1.1 and Gr-1, but lacked certain DC-associated markers Ia, CD86, and DEC205, whereas CD11c and F4/80 molecules were barely detected. However, CD8α antigen was negative (Figure7). The above evidence indicates that culture with PA6 cells + GM-CSF + SCF + Flt3L is not enough to support the generation of mature DCs from 13 dpc FL-derived Lin−c-kit+ HPCs.
Immunofluorescence analysis on DC precursors generated from murine FL-derived Lin−c-kit+HPCs.
Immunofluorescence analysis was performed on the nonadherent cells from FL-derived HPCs cocultured with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days. The indicated markers of FITC-labeled MoAbs were used to demonstrate the phenotypic characteristics of the DC precursors. Solid and dotted lines indicated the immunofluoresence intensity of cells as a control and the test of MoAbs, respectively. Representative results from 3 independent experiments are shown.
Immunofluorescence analysis on DC precursors generated from murine FL-derived Lin−c-kit+HPCs.
Immunofluorescence analysis was performed on the nonadherent cells from FL-derived HPCs cocultured with PA6 cells + GM-CSF + SCF + Flt3L for 12 to 14 days. The indicated markers of FITC-labeled MoAbs were used to demonstrate the phenotypic characteristics of the DC precursors. Solid and dotted lines indicated the immunofluoresence intensity of cells as a control and the test of MoAbs, respectively. Representative results from 3 independent experiments are shown.
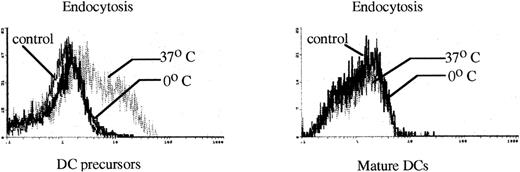
Functionally, these DC precursors were active in endocytic uptake of FITC-DX at 37°C, but not at 0°C (Figure8). These cells were not effectively able to stimulate allogeneic T-cell proliferation in MLR (Figure 5). When these DC precursors were further stimulated by GM-CSF and mTNFα to differentiate into mature DCs, such endocytic capacity was significantly reduced (Figure 8), and the capacity to stimulate T cell proliferation in allogeneic MLR was considerably enlarged (Figure 5).
Endocytic activity of FL HPC-derived DC precursors and mature DCs.
The capacity of FITC-DX uptake was analyzed by a cell sorter as described in “Materials and Methods.” The FITC intensity of cells as a control or the test of FITC-DX uptake was indicated. The results are representative of 3 independent experiments.
Endocytic activity of FL HPC-derived DC precursors and mature DCs.
The capacity of FITC-DX uptake was analyzed by a cell sorter as described in “Materials and Methods.” The FITC intensity of cells as a control or the test of FITC-DX uptake was indicated. The results are representative of 3 independent experiments.
DCs derived from FL Lin−c-kit+HPCs share progenitor cells in common with NK cells
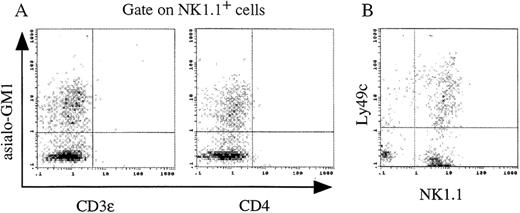
Previous reports demonstrated that Sca-1+c-kit+ FL cells could differentiate into lymphocytes.20 21 In our studies, culture of FL-derived Lin−c-kit+HPCs with PA6 alone for 7 days led to the generation of NK1.1+ cells accounted for 75% of the suspended cells. Addition of SCF + Flt3L further increased the proliferation of NK1.1+ cells. However, GM-CSF significantly inhibited the generation of NK1.1+ cells in PA6 cells + SCF + Flt3L cocultures (Figure 9). The cultured cells derived from 13 dpc FL Lin−c-kit+HPCs in the presence of PA6 cells + SCF + Flt3L at day 14 were immunophenotypically analyzed. These NK1.1+ cells were CD3ε and CD4 negative, and expressed similar level of asialo-GM1 antigen (Figure 10A) to that of adult murine spleen NK cells (data not shown). They also partly expressed a mature NK cell marker, Ly49c antigen (Figure 10B). The results suggest that these cultured cells possess characteristics of NK cells, but not of NK T cells.
Generation of NK1.1+ cells from FL-derived Lin−c-kit+ HPCs.
13 dpc FL-derived Lin−c-kit+ HPCs were cocultured with PA6 cells in the presence or absence of SCF + Flt3L with or without GM-CSF in a transwell culture system. The nonadherent cells were collected at the indicated time points and stained with FITC-conjugated anti-NK1.1 MoAb. The data represent mean value ± SD of NK1.1+ cell percentage in the cultures from 5 independent experiments. Black squares represent PA6 cells; red diamonds, PA6 cells with SCF and Flt3L; and green circles, PA6 cells with GM-CSF, SCF, and Flt3L.
Generation of NK1.1+ cells from FL-derived Lin−c-kit+ HPCs.
13 dpc FL-derived Lin−c-kit+ HPCs were cocultured with PA6 cells in the presence or absence of SCF + Flt3L with or without GM-CSF in a transwell culture system. The nonadherent cells were collected at the indicated time points and stained with FITC-conjugated anti-NK1.1 MoAb. The data represent mean value ± SD of NK1.1+ cell percentage in the cultures from 5 independent experiments. Black squares represent PA6 cells; red diamonds, PA6 cells with SCF and Flt3L; and green circles, PA6 cells with GM-CSF, SCF, and Flt3L.
Immunophenotypic analysis on NK1.1+ cells generated from FL-derived Lin−c-kit+ HPCs.
Lin−c-kit+ HPCs from 13 dpc FL were cultured in the presence of PA6 cells + SCF + Flt3L for 14 days. The phenotypes of the nonadherent cells were analyzed by 2- and tri-color immunofluorescence staining as described in “Materials and Methods.” (A) In the tri-color analyses, expression of asialo-GM1 and CD3ε or CD4 antigens on NK1.1+ cells was analyzed. (B) The 2-color analysis was performed on expression of NK1.1 and Ly49c antigens from the cultured cells. The quads were set up on the isotype-matched control dot plot and the results are representative of 3 independent experiments.
Immunophenotypic analysis on NK1.1+ cells generated from FL-derived Lin−c-kit+ HPCs.
Lin−c-kit+ HPCs from 13 dpc FL were cultured in the presence of PA6 cells + SCF + Flt3L for 14 days. The phenotypes of the nonadherent cells were analyzed by 2- and tri-color immunofluorescence staining as described in “Materials and Methods.” (A) In the tri-color analyses, expression of asialo-GM1 and CD3ε or CD4 antigens on NK1.1+ cells was analyzed. (B) The 2-color analysis was performed on expression of NK1.1 and Ly49c antigens from the cultured cells. The quads were set up on the isotype-matched control dot plot and the results are representative of 3 independent experiments.
To clarify whether DCs share a common progenitor with NK cells, we sorted NK1.1+ cells from PA6 cells + SCF + Flt3L-stimulated FL Lin−c-kit+ HPCs cultures at the indicated time points, cultured them again in the presence of the PA6 cells + SCF + Flt3L + GM-CSF for an additional 10 to 12 days, and restimulated them with GM-CSF + mTNFα. Mature DCs (> 26%) could be generated from the NK1.1+ cells sorted at day 3 of original culture, but not from a prolonged identical culture of more than 5 days duration (Figure 11). In contrast, flow cytometry analysis revealed that more than 92% of the cells expressed NK1.1 antigen from the continuous culture of the sorted NK1.1+ cells in the absence of GM-CSF for an additional 10 to 12 days. These results suggest that FL Lin−c-kit+ HPC-derived DCs may share a common progenitor with NK cells.
Capacity to generate DC precursors from NK1.1+ cells.
13 dpc FL-derived HPCs were co-cultured with PA6 cells + SCF + Flt3L in transwell plates. At the indicated time points, the nonadherent cells were collected and NK1.1+ cells were sorted and recultured with PA6 cells + GM-CSF + SCF + Flt3L. After being recultured for 10 to 12 days, the nonadherent cells were restimulated with GM-CSF + mTNFα for an additional 3 to 5 days. The expression of Ia and CD86 antigens on these cells was examined as described in “Materials and Methods.” Solid and dotted lines indicated the immunofluoresence intensity of cells stained with a control and the test MoAbs, respectively. The quads were set up on the isotype-matched control dot plot. Representative results from 3 independent experiments are shown.
Capacity to generate DC precursors from NK1.1+ cells.
13 dpc FL-derived HPCs were co-cultured with PA6 cells + SCF + Flt3L in transwell plates. At the indicated time points, the nonadherent cells were collected and NK1.1+ cells were sorted and recultured with PA6 cells + GM-CSF + SCF + Flt3L. After being recultured for 10 to 12 days, the nonadherent cells were restimulated with GM-CSF + mTNFα for an additional 3 to 5 days. The expression of Ia and CD86 antigens on these cells was examined as described in “Materials and Methods.” Solid and dotted lines indicated the immunofluoresence intensity of cells stained with a control and the test MoAbs, respectively. The quads were set up on the isotype-matched control dot plot. Representative results from 3 independent experiments are shown.
Discussion
Identification of the progenitors of DCs in the early stages of fetal hematopoiesis is of particular interest in understanding the origin and development of DCs in the establishment of the immune system.1,10-12,40,41 We have described here that purified murine 13 dpc FL Lin−c-kit+ HPCs can differentiate into DC precursors in vitro after coculture with PA6 stromal cells for 12 to 14 days in the presence of GM-CSF + SCF + Flt3L. These DC precursors can subsequently be induced to develop into mature DCs by further stimulation with GM-CSF + mTNFα for an additional 3 to 5 days. The resultant DCs not only show typical morphologic characteristics and immunophenotype of DCs, but also markedly stimulate T cell proliferation in allogeneic MLR as effectively as adult murine BM Lin−c-kit+ HPC-derived DCs do,26 suggesting that these cells are mature DCs with antigen-presenting function.
Generation of DC precursors from 13 dpc murine FL Lin−c-kit+ HPCs in vitro depends on the support of PA6 stromal cell line even in the presence of a cytokine mixture of GM-CSF + SCF + Flt3L, which suffices to induce adult BM Lin−c-kit+ HPCs to develop into DC precursors.26 In transwell culture, we showed that some soluble factors secreted by PA6 stromal cells together with the cytokine cocktail could induce FL-derived HPCs to develop into DC precursors. Previous studies have shown that the number of Langerhans cells (LCs) is reduced in the skin of osteopetrotic (op/op) mice which is defective in the production of functional M-CSF protein.42 This implied that OP9 stromal cells might induce 13 dpc FL-derived Lin−c-kit+ HPCs to develop into DCs by the addition of M-CSF. Addition of M-CSF to OP9 cells + GM-CSF + SCF + Flt3L generated DC precursors from FL-derived HPCs to a much less extent than that of PA6 cells and the cytokines-stimulated culture. This result indicates that M-CSF partially compensates for the incapability of OP9 cells to induce the differentiation of DC precursors from FL-derived HPCs. However, the addition of M-CSF to the culture of PA6 cells + GM-CSF + SCF + Flt3L did not enhance the yield of DCs generated from FL-derived Lin−c-kit+ HPCs. Moreover, M-CSF plus the cytokines GM-CSF + SCF + Flt3L without stromal cells could not induce FL-derived HPCs into DC precursors, indicating that M-CSF cannot substitute for the role of PA6-derived soluble factor(s) which play an essential role in stimulating FL Lin−c-kit+ HPCs to differentiate into DC precursors.
Several other cytokines have been considered to be the candidates accounting for the generation of DC precursors from FL-derived HPCs. For example, IL-4 and TGF-β have been shown to be essential factors for DC differentiation.43,44 However, addition of IL-4 to the combination of GM-CSF + SCF + Flt3L failed to generate DC precursors from FL-derived HPCs, or anti–IL-4 MoAb did not block the generation of FL-derived DC precursors in the PA6 cells and cytokine cocktail culture system (data not shown). We have recently observed that TGF-β polarizes adult murine BM-derived HPCs to differentiate into monocytes/macrophages that can eventually differentiate into LC-like DCs expressing high level of E-cadherin.45Interestingly, DC precursors generated in our cultures expressed E-cadherin, which is believed to be a specific marker expressed on LCs and to be tightly regulated by TGF-β.46 However, without PA6 cells, addition of TGF-β to the combination of GM-CSF + SCF + Flt3L did not stimulate the generation of DC precursors from FL-derived HPCs. Moreover, both OP9 and PA6 cells expressed IL-4 mRNA, whereas TGF-β mRNA was undetectable in either of them (data not shown). Taken together, these observations indicate that neither IL-4 nor TGF-β substitutes for PA6 cells to induce the generation of DCs from FL-derived HPCs. Thereafter, the collaborative role of IL-4 or serum-derived TGF-β with some PA6 cell-secreted factors may not be ruled out.
Most recently, it has been demonstrated that generation of T cells, NK cells, and DCs in vitro from human FL-derived CD34+D38− cells required the fetal thymic organ cultures,47 in- consistent with our findings that the generation of DC precursors from murine FL HPCs needs the support of PA6 cells. Obviously, the culture conditions reported here are artificial and might not exactly reflect the physiological situation in vivo. PA6 stromal cells were derived from BM.48 It has been shown that PA6 cells can promote the proliferation of BM hematopoietic cells through a short range cell-to-cell interaction by providing an in vitro microenvironment similar to that for in vivo hematopoiesis.49 It is anticipated that the unidentified factor produced by PA6 cells may be available in bone marrow or thymus environments that may skew development of HPCs toward the DC lineage. Biochemical purification of the factor(s) that are responsive for the generationof DC precursors from FL HPCs is in progress, but so far unsuccessful.
FL HPC-derived DCs in this study displayed low levels of DEC205 and E-cadherin and barely detectable CD11c antigen. Previous studies demonstrated that murine splenic DCs express high level of CD11c and can be classified into at least 2 subpopulations CD8α+DEC205+ and CD8α−DEC205− cells. On culture in vitro, these mature splenic DCs express high level of DEC205.2 This phenotype of splenic DCs significantly differs from that of mature DCs or their precursors generated from FL-derived Lin−c-kit+ HPCs in our culture system. We have recently reported that the development of BM Lin−c-kit+ HPC-derived DCs could be mediated through CD11b−/dullCD11c+ and CD11b+/hi CD11c+ precursors.33 When we evaluated FL Lin−c-kit+ HPCs, it was found that the cytokerastic kinetics differ between BM- and FL-derived Lin−c-kit+ HPCs. FL-derived DCs were generated from the precursors on which CD11c antigen was barely expressed. Except for Gr-1 marker, these cells lacked the phenotypic characteristics of other hematopoietic cell lineages, whereas BM Lin−c-kit+HPC-derived ones expressed high levels of DEC205 and CD11c molecules.26,33 These findings would be reminiscent of the generation of CD11c− DC from FL-derived Lin−c-kit+ HPCs. In human blood, CD11c+ and CD11c− DC subsets have been identified.50A recent study demonstrated that CD11c+ and CD11c− DC subsets possibly localize in the B-cell and T-cell areas, respectively, and may exert distinct roles in initiating cellular and humoral immune responses.50 Further characterization of the phenotype of DC precursors in vivo in murine fetus will be helpful for understanding DC development and its function in educating the immune system during fetal stage.
Evidence has accumulated indicating that CD40 molecule, a member of TNF receptor family, could be expressed on tonsil DCs,51 blood DCs,52 and LCs53 in human and some murine DCs.33 Moreover, triggering of CD40 ligand and CD40 could stimulate human CD34+ HPCs to differentiate into mature DCs and activate the function of mature DCs.54 Interestingly, our results show that CD40 molecule was highly expressed on DC precursors generated from FL Lin−c-kit+ HPCs in the presence of PA6 cells + GM-CSF + SCF + Flt3L. Because CD40 and other members of TNF receptor family can bind to their ligands expressed on activated and memory T cells, it is presumed that the stimulatory effects of DCs and T cells are mutual because DCs induce T cells into immune or tolerance state by presenting antigens,1,11,12,54and T cells in turn promote the development of precursors into functionally mature DCs.10 54-56
It has been reported that NK cells are developmentally close to lymphoid cells in mouse57-59 and in human.60Murine FL-derived Sca-1+c-kit+ cells generated in vitro mixed NK colonies with B220+cells.21 A T cell precursor subset in murine thymus has been shown to be tripotential with full potential to generate DCs and also NK cells although the ability to develop B cells has been lost.59,61,62 Further study has demonstrated that NK cells and DCs may branch off the T cell lineage from a common intermediate bipotential progenitor in human postnatal thymus.63However, it remains to be elucidated which factors may determine the branch-off of DCs from NK and T cells. A remarkable feature from our findings is that murine FL-derived HPCs are able to differentiate into either DC or NK cell precursors, depending on the presence of GM-CSF. When cocultured with PA6 cells, murine FL-derived HPCs could generate DC precursors in the presence of GM-CSF, whereas the development of NK1.1+ cells was drastically suppressed. In the absence of GM-CSF, a large number of NK1.1+ cells proliferated in PA6 cells + SCF + Flt3L-stimulated cultures. Interestingly, the generated NK1.1+ cells could revert to DC precursors at an early stage of differentiation by reculturing with GM-CSF + PA6 cells + SCF + Flt3L. These observations reveal that unlike IL-2, which can simultaneously induce bipotential differentiation of human thymic progenitors into DCs and NK cells in similar proportion in vitro,63 GM-CSF plays a critical role not only in promoting the viability, proliferation, survival, function, and mobilization of DC precursors and DCs, but also in the commitment into DC precursors at the expense of NK cell precursors from FL-derived HPCs. The transition between DC and NK cell precursors suggests that DCs and NK cells may share a common progenitor of fetal hematopoietic origin. Thus, owing to the relationship of NK cells and DCs as well as their dependence on stromal cells in differentiation, we speculate that 13 dpc murine FL Lin−c-kit+ HPCs may be able to generate thymic DCs under appropriate culture condition. It is believed that CD8α+ DCs represent murine lymphoid DCs that may derive from lymphoid progenitor cells.64 However, it remains to establish a culture system for generating CD8α+ lymphoid DCs in vitro.65 We therefore could not directly compare the NK1.1+ precursor-derived DCs with CD8α+ lymphoid DCs. Further experiments will be performed in vivo to address the relationship of NK1.1+precursor-derived DCs with CD8α+ lymphoid DCs; these experiments are in progress in our laboratory.
It has been demonstrated that the primary stromal cultures from 16 dpc rat embryonic thymus can differentiate into morphologically and phenotypically mature DCs after several days of culture.30In agreement with these results, our findings suggest that FL HPCs gain the capacity to generate DCs as early as 13 dpc. Probably, the embryonic DC precursors have developed in the early embryonic hematopoietic organs such as fetal liver and thymus possibly with the phenotype different from that of the adult counterparts; for example, embryonic DC precursors barely display CD11c molecule. However, the differentiation of these DC precursors may require other factors during the embryonic development. FL Lin−c-kit+ HPCs may migrate into various tissues where they produce essential factors required for DC development and function in the fetal stage, to generate diverse DC precursors. This study would provide a novel insight into the mechanism of DC ontogeny and their role in the establishment of the immune system.
Acknowledgments
We express our gratitude to Dr R. M. Steinman (Rockefeller University, New York, NY) for his kind gift of MoAb to DEC-205 (NLDC 145); and Dr Tohru Nakano (Osaka University, Osaka, Japan) and Dr Shin-ichi Nishikawa (Kyoto University, Kyoto, Japan) for their generous gifts of PA6 and OP9 stromal cell lines. We highly appreciate Dr J. J. Oppenheim (NCI-FCRDC, Frederick, MD) for his critical review of the manuscript. We also thank Drs V. Christian, H. Iizasa, and Hongyan Dong for their kind assistance.
Reprints:Kouji Matsushima, Department of Molecular Preventive Medicine, School of Medicine, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal