Abstract
CD8+ cells have an important role in controlling Epstein-Barr virus (EBV) infection. We adapted the interferon-γ ELISPOT assay to the quantitative analysis of EBV-specific CD8+ cells. Using peripheral blood mononuclear cells (PBMCs) from healthy donors, we measured both the aggregate response to the virus, using EBV-transformed lymphoblastoid cell lines (LCLs) as stimulators, and the specific responses to 2 A2-restricted peptide epitopes: the subdominant latency membrane protein-2 (LMP2) peptide CLGGLLTMV and the early lytic BMLF1 peptide GLCTLVAML. LCL-responsive CD8+ cells were detected in all EBV-seropositive donors (range 954 to 37 830 spots/106CD8+ cells). LMP2 peptide-responsive CD8+cells were detected in 10 of 11 healthy seropositive A2 donors (range 11 to 83 spots/106 PBMC). BMLF1 peptide-responsive CD8+ cells were detected in all seropositive A2 donors examined (range 13 to 943 spots/106 PBMC). Cytotoxic T-lymphocyte (CTL) lines generated with weekly stimulation of LCLs for therapeutic purposes were also studied. Relative to PBMCs, these CTL lines showed a marked increase in the level of LCL-responsive and LMP2 peptide-responsive CD8+ cells and a lesser degree of expansion of BMLF1 peptide-responsive CD8+ cells. Finally, we applied the ELISPOT assay to monitor adoptive infusion of EBV CTL lines. In 2 patients examined, a transient increase in LCL-responsive CD8+ cells could be detected after infusion. Thus, the ELISPOT assay can be applied to the analysis of CD8+responses to EBV antigens in PBMCs, in ex vivo expanded CTL lines, and in PBMCs from patients treated with ex vivo expanded CTL lines. (Blood. 2000;95:241-248)
CD8+ T cells have an important role in controlling viral infection by recognizing small peptides derived from intracellular pathogens presented on the surface of infected cells by major histocompatibility complex (MHC) class I molecules.1The present study concerns the CD8+ response to Epstein-Barr virus (EBV), a ubiquitous herpesvirus associated with several human malignancies including Burkitt's lymphoma, nasopharyngeal carcinoma (NPC), Hodgkin's disease, and posttransplant lymphoproliferative disease (PTLD).2-4 Studies suggest that tumor cells in NPC, Hodgkin's disease, and PTLD are sensitive or should be to CD8+-mediated killing.5-9Furthermore, adoptive cellular immunotherapy is effective in treating or preventing PTLD in some settings.10 With the ability to manipulate CD8+ responses rapidly advancing, the importance of assays to measure EBV-specific T-cell responses increases.
Standard methods of detecting and characterizing EBV antigen-specific CD8+ T cells that rely on initial in vitro expansion in response to stimulation with EBV-immortalized lymphoblastoid cell lines (LCLs) have inherent limitations. The amplified repertoire of T cells may be skewed, responses to subdominant antigens obscured by responses to dominant antigens,6 and responses to lytic cycle antigens missed altogether inasmuch as these are generally not expressed by LCLs.
The ELISPOT assay for the detection of antigen-specific T cells offers an alternative approach.11-14 This assay relies on the visualization of cytokine secretion by individual T cells following in vitro stimulation with antigen. The assay is more sensitive than enzyme-linked immunosorbent assay15 16 and does not require in vitro expansion of specific T cells before testing. In the present study we adapted the interferon-γ (IFN-γ) ELISPOT assay to the quantitative detection of EBV-specific CD8+ cells. We assessed the aggregate CD8+ response to EBV latency antigens expressed by LCLs, to a peptide derived from the subdominant latency membrane protein 2 (LMP2), and to a peptide derived from the lytic cycle antigen BMLF1. Responses were assayed in both unstimulated peripheral blood mononuclear cells (PBMCs) and in polyclonal cytotoxic T-lymphocyte (CTL) lines. Finally, we used the assay to monitor adoptive cellular immunotherapy in vivo.
Materials and methods
PBMCs and isolation of specific cell populations
The PBMCs from healthy platelet donors and laboratory personnel with known HLA type and known EBV antibody status were studied under a protocol approved by a human investigations committee. PBMCs were isolated by density gradient centrifugation using Ficoll-Hypaque 1.077 (Biochrom, Berlin, Germany) and cryopreserved immediately. CD8+, CD4+, or CD19+ cells were positively selected from cryopreserved PBMCs using immunomagnetic beads (Dynal, Oslo, Norway). Beads were detached from isolated cells by using respective DetachaBead (Dynal). CD56+ cells were isolated with CD56 MicroBeads (Miltenyi Biotec, CA).
Establishment of LCL
The PBMCs (5 × 106) were incubated with 2 mL infectious supernatant from the B95.8 cell line for 2 hours at 37°C. An equal volume of RPMI-FBS (RPMI 1640, 2 mMl-glutamine, 10 mM HEPES, 100 IU/mL penicillin, 100 μg/mL streptomycin, 10% vol/vol fetal bovine serum [FBS]) was added directly to PBMCs and the culture was supplemented with 25 U/mL interleukin 2 (IL-2) (Proleukin, Cetus, Emeryville, CA) and 5 μg/mL phytohemagglutinin (PHA) (Sigma). Thereafter cells were maintained in RPMI-FBS.17
Generation of Staphylococcus aureus–activated B cells
Staphylococcus aureus Cowan I strain (SAC; Calbiochem, San Diego, CA) activated B-cell blasts were used as controls in some ELISPOT assays. For generation of SAC-activated B-cell blasts, CD19+ B cells were isolated from cryopreserved PBMCs and cultured at 2 × 106/mL in RPMI-FBS medium supplemented with 0.01% SAC and 100 U/ml IL-2 for 3 days. The activated B cells were then depleted of SAC and dead cells by density gradient centrifugation using Ficoll-Hypaque 1.077.
Synthetic peptides
Peptides (Macromolecular Resources, Fort Collins, CO) used in our study corresponded to LMP2 residues 426-434 (CLGGLLTMV),18 and BMLF1 residues 280-288 (GLCTLVAML).19 Both peptides are HLA-A*0201-restricted CTL epitopes. A peptide corresponding to LMP2 residues 409-417 (ILTEWGSGN) known not to be recognized by CTLs from A*0201 individuals18 was studied as a negative control. All peptides were dissolved in dimethyl sulfoxide (Sigma) at a concentration of 10 mg/mL and further diluted in appropriate assay media for individual experiments.
ELISPOT assay
The 96-well multiscreen HA filtration plates (MAHA S4510, Millipore, Bedford, MA) were coated with 50 μL of mouse antihuman IFN-γ mAb (1598-00, R&D System, Minneapolis, MN; 4 μg/mL in PBS). After incubation overnight at room temperature, wells were washed 4 times with PBS. Remaining protein-binding sites were blocked by incubating plates with 200 μL/well RPMI-AB medium (RPMI 1640, 2 mMl-glutamine, 10 mM HEPES, 100 IU/mL penicillin, 100 μg/mL streptomycin, 10% vol/vol heat-inactivated human AB-serum (Sigma) for 2 hours at 37°C. Autologous LCL (1 × 105/well) or peptide (10 μg/mL, final concentration) was plated with responder cell populations. Responder cell populations were seeded across a range of concentrations to achieve 10 to 100 spots/well so as to facilitate accurate and reproducible counting. For LCL stimulators with PBMC responders, the concentration used was 2 × 103 to 4 × 104 PBMC/well; for LCL stimulators with CD8+ responders, the concentration used was 5 × 102 to 2 × 104CD8+ cells/well; for LCL stimulators with CTL line responders, this was 50 to 800 CTLs/well. For peptide stimulators (LMP2 or BMLF1 peptide) with PBMC responders, cells were plated at 2 × 105 PBMC/well and a total of 2 to 5 × 106 PBMC plated. CTL line responders with peptide stimulators were plated at 1 × 103 to 4 × 104 CTLs/well and 1 × 105autologous PBMCs were added to each well to serve as antigen presenters. The culture medium for the ELISPOT assay was RPMI-AB supplemented with 100 U/mL IL-2 (final volume 200 μL/well). After undisturbed incubation for 24 hours at 37°C, plates were washed 4 times with PBS containing 0.05% Tween 20 (PBS-Tw). Wells were incubated with 100 μL polyclonal rabbit antihuman IFN-γ antibody (IP-500, R&D System; 1:250 dilution in PBS-Tw) at 4°C overnight and washed 4 times with PBS-Tw. Then 100 μL polyclonal peroxidase-conjugated goat anti-rabbit IgG antibody (P-0448, DAKO, Carpinteria, CA; 1: 625 dilution in PBS-Tw) was added and incubated for 3 hours at 37°C. Wells were washed 3 times with PBS-Tw and 3 times with PBS. Peroxidase substrate was prepared by dissolving 20 mg 3-amino-9-ethycarbazol (Sigma) in 2 mL dimethylformamide (Sigma). The solution was diluted 1:30 in 14.5 mL of 50 mmol/L sodium acetate buffer at pH 5.0. Immediately before use, 7.5 μL 30% H2O2 was added. The substrate solution was filtered through a 0.22 filter and added to wells at 100 μL/well. After incubation at room temperature for 8 minutes, the reaction was stopped by discarding the substrate solution and washing the plates under running water. The plates were then air dried and colored spots counted using a stereomicroscope.
Data analysis
Frequencies of antigen-specific IFN-γ-secreting cells were calculated based on the numbers of responder cells and the number of spots per well after subtraction of background. The procedure for estimating background was tailored to the particular combination of stimulator and responder. For ELISPOT assays with LCL stimulators, background was the sum of IFN-γ spots associated with LCLs alone and those associated with responders alone. For ELISPOT assays with peptide stimulators and PBMC responders, background was obtained by incubating PBMC in the presence of a control peptide. For ELISPOT assays with peptide stimulators and polyclonal CTL responders, background spots include those from incubating CTL with control peptide and those from incubating autologous PBMC with the peptide of interest. Correlation analysis of ELISPOT and 51Cr-release assays was carried out using Microsoft Excel software.
Establishment of EBV-specific polyclonal CTL lines
Polyclonal CTL lines were established according to previously published methods.20 Briefly, 2 × 106PBMC were co-cultured with γ-irradiated (8000 rad) autologous LCLs at a responder/stimulator ratio of 40:1 in wells of 24-well plates. These were restimulated on days 7 and 14. On day 21, CTL cultures were restimulated again but at a new responder/stimulator ratio (4:1), and IL-2 was added to a final concentration of 25 U/mL. CTLs were harvested on day 14 or day 28, cryopreserved, and stored at −135°C until being tested in ELISPOT or 51Cr-release assays. All CTL cultures were maintained in lymphocyte expansion medium, LyEM (45% RPMI 1640, 45% EHAA, 2 mM glutamine, 10 mM HEPES, 100 IU/mL penicillin, 100 μg/mL streptomycin, 10% vol/vol FBS).
51Cr-release assays
PHA blasts were generated by culturing PBMCs at a concentration of 2 × 106/mL in the presence of 5 μg/mL PHA (Sigma) in RPMI-FBS for 3 days. PHA blasts were washed 4 times and then cultured for at least 4 more days in RPMI-FBS containing 100 U/mL IL-2 before being used in 51Cr-release assays. LCLs or PHA blasts were incubated with 51CrO4 for 90 minutes. Labeled cells were washed and resuspended to 1 × 105/mL in RPMI-FBS, and 50 μL was added to wells of 96 V-bottom plates containing 50 μL/well RPMI-FBS with or without 40 μg/mL of the LMP2 or BMLF1 peptide and incubated for 2 hours at 37°C. Then cryopreserved CTLs were thawed and added to each well (1.5 × 106/mL, 100 μL/well, E/T ratio 30:1) for the subsequent 5-hour incubation. The concentration of peptide in the final assay volume (200 μL) was 10 μg/mL.
Patients and CTL infusion protocol
Two patients developed PTLD after solid organ transplantation. They received EBV-specific CTL infusions from HLA partially matched donors at a dose of 5 × 107 CTL/m2. Blood was drawn before and at different time points after infusion. CD8+ cells were used as responders in ELISPOT assays with the patient's LCLs as stimulators. Patient 1 had active PTLD and patient 2 was in remission at the time of infusion. The donor EBV-specific CTL lines were generated by weekly restimulation with irradiated donor LCLs for 4 weeks.
Results
Detection of LCL-responsive CD8+ cells
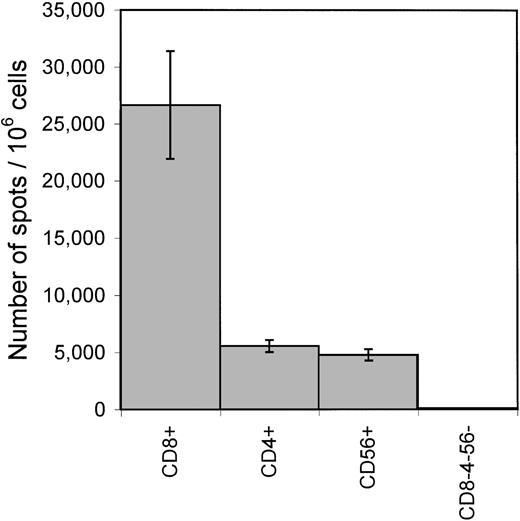
To characterize the cellular sources of LCL-stimulated IFN-γ production, PBMCs from a healthy seropositive donor were fractionated into CD8+, CD4+, CD56+, and CD8−CD4−CD56−populations by positive selection with immunomagnetic beads. ELISPOT assays with autologous LCL stimulators were performed on each population. Spots were found in CD8+, CD4+, and CD56+ populations but not in the CD8−CD4−CD56−population (Figure 1). The sum of the numbers of spots from CD8+, CD4+, and CD56+ populations was similar to that produced by the corresponding number of unfractionated PBMCs tested in parallel.
Cellular sources of IFN-γ secretion in response to autologous LCLs.
PBMCs from a healthy EBV-seropositive donor FT were sequentially fractionated into CD8+, CD4+, CD56+, and CD8−CD4−CD56−cell populations using CD8 and CD4 Dynal-beads and CD56 Microbeads. Each cell population was incubated with autologous LCL stimulators for the detection of IFN-γ secretion in an ELISPOT assay. Each bar represents the mean ± SD of triplicate wells. The numbers of spots/1 × 106 cells of the respective cell population are shown on the Y axis.
Cellular sources of IFN-γ secretion in response to autologous LCLs.
PBMCs from a healthy EBV-seropositive donor FT were sequentially fractionated into CD8+, CD4+, CD56+, and CD8−CD4−CD56−cell populations using CD8 and CD4 Dynal-beads and CD56 Microbeads. Each cell population was incubated with autologous LCL stimulators for the detection of IFN-γ secretion in an ELISPOT assay. Each bar represents the mean ± SD of triplicate wells. The numbers of spots/1 × 106 cells of the respective cell population are shown on the Y axis.
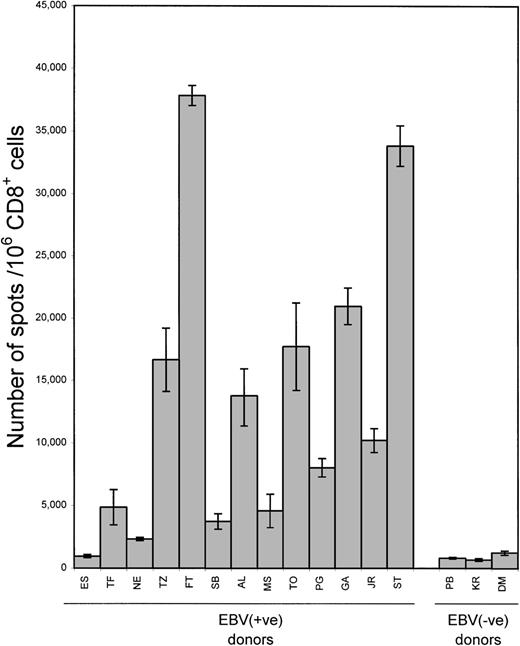
To measure the aggregate CD8+ response to EBV latency antigens, we used isolated CD8+ cells from a series of healthy donors with autologous LCL stimulators. In seropositive donors the frequency of LCL-responsive IFN-γ-producing cells ranged from 954 to 37 830 spots/106 CD8+ cells (mean 13 489) (Figure 2). This corresponded to 0.1% to 3.8% (mean 1.3%) of the CD8+ population. In 3 seronegative donors, the frequency of LCL-responsive cells ranged from 680 to 1231 spots/106 CD8+ cells (mean 908, 0.1% of CD8+ population). Background produced by CD8+ cells alone was < 7 spots/106CD8+ cells (mean 1). Background associated with LCLs alone varied from 0 to 16 spots/105 LCL (mean 3.2).
Frequency of LCL-responsive CD8+ cells.
Isolated CD8+ cells from 13 healthy EBV-seropositive and 3 seronegative donors were incubated with autologous LCL stimulators in ELISPOT assays. Each bar represents the mean ± SD as determined in 2 separate experiments; data from each experiment represent the average of triplicate wells.
Frequency of LCL-responsive CD8+ cells.
Isolated CD8+ cells from 13 healthy EBV-seropositive and 3 seronegative donors were incubated with autologous LCL stimulators in ELISPOT assays. Each bar represents the mean ± SD as determined in 2 separate experiments; data from each experiment represent the average of triplicate wells.
To determine whether the CD8+ responses to LCLs were EBV specific or responses to the activated B-cell phenotype, SAC-activated B-cell blasts generated from 3 seropositive donors and 1 seronegative donor were used as negative controls in ELISPOT assays. No CD8+ response to SAC-activated B blast cells was found (data not shown).
Detection of peptide-responsive CD8+ cells
The LCL-responsive CD8+ cells consist of a mixed population of peptide-specific CD8+ cells. Responses to individual epitopes were assayed by using synthetic peptides as stimulators. We studied 2 peptides: CLGGLLTMV from the LMP2 and GLCTLVAML from the early lytic protein BMLF1. Recognition of both peptides is restricted through HLA-A*0201.18 Peptide stimulators elicited IFN-γ spots in the CD8+ population and in unfractionated PBMCs, but not in the CD8+-depleted population (data not shown). Therefore, in further experiments, unfractionated PBMCs were used as responder cells for the detection of peptide-responsive CD8+ cells. We assayed PBMCs at various peptide concentrations. No differences in the number of IFN-γ spots were observed at concentrations of peptide ranging from 1 to 100 μg/mL, so 10 μg/mL was used in further experiments.
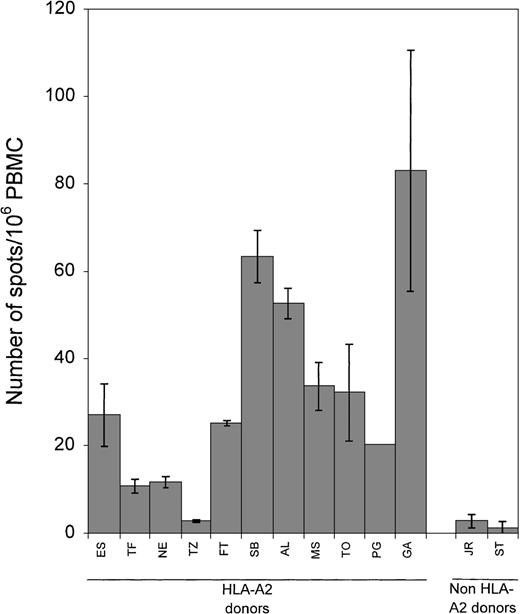
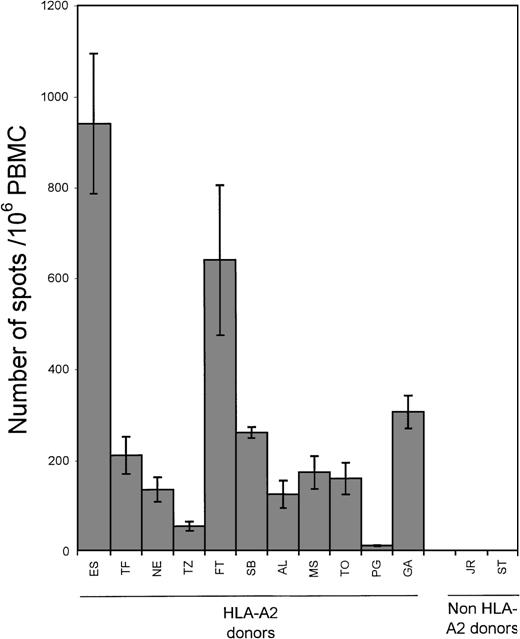
The PBMCs from 13 healthy EBV-seropositive donors, 11 HLA-A2 and 2 non-A2, were tested for their reactivity to the LMP2 peptide (Figure3). Ten of the 11 EBV-seropositive HLA-A2 donors showed a response to the peptide (range 11 to 83 spots/106 PBMC; mean 33). In contrast, the 2 EBV-seropositive non-HLA-A2 donors generated few IFN-γ spots (1 and 3 spots/106 PBMC, respectively). Responses to the BMLF1 peptide were measured in the same 13 seropositive donors (Figure4). All donors who were HLA-A2 showed a response to the BMLF1 peptide (range 13 to 943 spots/106PBMC, mean 276). Donors who were EBV-seropositive but not HLA-A2 showed no response (0 spots/106 PBMC). Background reactivity for peptide stimulators was determined by incubating PBMC in the presence of a control peptide corresponding to LMP2 residues 409-417 known not to elicit a CD8+ response. No difference in the number of spots was observed when PBMCs were incubated alone or with the control peptide. The number of spots ranged from 0 to 33 spots/106PBMC (mean 9).
Frequency of LMP2 peptide-responsive CD8+ cells.
PBMC from 13 healthy EBV-seropositive donors, 11 HLA-A2 and 2 non-HLA-A2, were incubated with 10 μg/mL of the LMP2 peptide (residues 426-434) in ELISPOT assays. Each bar represents the mean ± SD as determined in 2 separate experiments; data from each experiment represent the average of more than 12 wells.
Frequency of LMP2 peptide-responsive CD8+ cells.
PBMC from 13 healthy EBV-seropositive donors, 11 HLA-A2 and 2 non-HLA-A2, were incubated with 10 μg/mL of the LMP2 peptide (residues 426-434) in ELISPOT assays. Each bar represents the mean ± SD as determined in 2 separate experiments; data from each experiment represent the average of more than 12 wells.
Frequency of BMLF1 peptide-responsive CD8+ cells.
PBMC from 13 healthy EBV-seropositive donors, 11 HLA-A2 and 2 non-HLA-A2, were incubated with 10 μg/mL of the BMLF1 peptide (residues 280-288) in ELISPOT assays. Each bar represents the mean ± SD as determined in 2 separate experiments; data from each experiment represent the average of triplicate wells.
Frequency of BMLF1 peptide-responsive CD8+ cells.
PBMC from 13 healthy EBV-seropositive donors, 11 HLA-A2 and 2 non-HLA-A2, were incubated with 10 μg/mL of the BMLF1 peptide (residues 280-288) in ELISPOT assays. Each bar represents the mean ± SD as determined in 2 separate experiments; data from each experiment represent the average of triplicate wells.
To get a sense of the relative strength of responses to LCLs, to the subdominant LMP2 peptide, and to the lytic BMLF1 peptide, we compared the frequencies of CD8+ cells responsive to these stimulators (Table 1). In EBV seropositive HLA-A2 donors, CD8+ cells specific for the LMP2 peptide accounted for a minority (0.2% to 15.0%, mean 4.6%) of LCL-responsive CD8+ cells. This was consistent with the notion that LMP2 is a subdominant antigen. Responses to the lytic BMLF1 peptide were markedly higher than those to the LMP2 peptide in 10 of 11 HLA-A2 donors (range 2- to 35-fold, mean 13-fold).
Frequency of CD8+ responders to EBV antigen stimulation
| . | LCL-responsive . | LMP2 pept-responsive . | BMLF1 pept-responsive . | |||
|---|---|---|---|---|---|---|
| Healthy donors . | Spots/106 CD8+cells (Mean ± SD)* . | % of CD8+ population . | Estimated Spots/106PBMC† . | Spots/106 PBMC (Mean ± SD) . | % of LCL-resp CD8+ cells . | Spots/106PBMC (Mean ± SD) . |
| EBV(+ve) HLA-A2 | ||||||
| ES | 954 ± 133 | 0.1 | 180 (18.9 ± 0.1%) | 27 ± 7 | 15.0 | 943 ± 154 |
| TF | 4 855 ± 1406 | 0.5 | 730 (15 ± 0.1%) | 11 ± 2 | 1.5 | 213 ± 41 |
| NE | 2 317 ± 145 | 0.2 | 199 (8.6 ± 0.4%) | 12 ± 1 | 5.8 | 138 ± 27 |
| TZ | 16 655 ± 2539 | 1.7 | 1577 (9.5 ± 0.5%) | 3 ± 0 | 0.2 | 57 ± 10 |
| FT | 37 830 ± 789 | 3.8 | 6545 (17.3 ± 1.8%) | 25 ± 1 | 0.4 | 641 ± 165 |
| SB | 3 721 ± 615 | 0.4 | 522 (14.0 ± 0.0%) | 63 ± 6 | 12.1 | 262 ± 12 |
| AL | 13 756 ± 2170 | 1.4 | 2553 (18.6 ± 1.2%) | 52 ± 4 | 2.1 | 126 ± 30 |
| MS | 4 565 ± 1322 | 0.5 | 607 (13.3 ± 0.6%) | 33 ± 6 | 5.5 | 174 ± 36 |
| TO | 17 705 ± 3514 | 1.8 | 2540 (14.3 ± 3.2%) | 32 ± 11 | 1.3 | 161 ± 35 |
| PG | 8 015 ± 733 | 0.8 | 560 (7.0 ± 1.4%) | 20 ± 0 | 3.6 | 13 ± 0 |
| GA | 20 961 ± 1472 | 2.1 | 2748 (13.1 ± 0.1%) | 83 ± 28 | 3.0 | 306 ± 36 |
| Average | 33 | 4.6 | 276 | |||
| EBV(+ve) non-A2 | ||||||
| JR | 10 197 ± 952 | 1.0 | 1977 (19.4 ± 0.6%) | 3 ± 1 | 0.1 | 0 ± 1 |
| ST | 33 822 ± 1616 | 3.4 | 3485 (10.3 ± 0.0%) | 1 ± 1 | 0.0 | 0 ± 0 |
| Average | 13 489‡ | 1.3† | 1863† | 2 | 0.1 | 0 |
| EBV(−ve) | ||||||
| PB | 813 ± 67 | 0.1 | 71 (8.7 ± 0.7%) | |||
| KR | 680 ± 103 | 0.1 | 102 (14.9 ± 0.1%) | |||
| DM | 1231 ± 165 | 0.1 | 241 (19.6 ± 2.2%) | |||
| Average | 908 | |||||
| . | LCL-responsive . | LMP2 pept-responsive . | BMLF1 pept-responsive . | |||
|---|---|---|---|---|---|---|
| Healthy donors . | Spots/106 CD8+cells (Mean ± SD)* . | % of CD8+ population . | Estimated Spots/106PBMC† . | Spots/106 PBMC (Mean ± SD) . | % of LCL-resp CD8+ cells . | Spots/106PBMC (Mean ± SD) . |
| EBV(+ve) HLA-A2 | ||||||
| ES | 954 ± 133 | 0.1 | 180 (18.9 ± 0.1%) | 27 ± 7 | 15.0 | 943 ± 154 |
| TF | 4 855 ± 1406 | 0.5 | 730 (15 ± 0.1%) | 11 ± 2 | 1.5 | 213 ± 41 |
| NE | 2 317 ± 145 | 0.2 | 199 (8.6 ± 0.4%) | 12 ± 1 | 5.8 | 138 ± 27 |
| TZ | 16 655 ± 2539 | 1.7 | 1577 (9.5 ± 0.5%) | 3 ± 0 | 0.2 | 57 ± 10 |
| FT | 37 830 ± 789 | 3.8 | 6545 (17.3 ± 1.8%) | 25 ± 1 | 0.4 | 641 ± 165 |
| SB | 3 721 ± 615 | 0.4 | 522 (14.0 ± 0.0%) | 63 ± 6 | 12.1 | 262 ± 12 |
| AL | 13 756 ± 2170 | 1.4 | 2553 (18.6 ± 1.2%) | 52 ± 4 | 2.1 | 126 ± 30 |
| MS | 4 565 ± 1322 | 0.5 | 607 (13.3 ± 0.6%) | 33 ± 6 | 5.5 | 174 ± 36 |
| TO | 17 705 ± 3514 | 1.8 | 2540 (14.3 ± 3.2%) | 32 ± 11 | 1.3 | 161 ± 35 |
| PG | 8 015 ± 733 | 0.8 | 560 (7.0 ± 1.4%) | 20 ± 0 | 3.6 | 13 ± 0 |
| GA | 20 961 ± 1472 | 2.1 | 2748 (13.1 ± 0.1%) | 83 ± 28 | 3.0 | 306 ± 36 |
| Average | 33 | 4.6 | 276 | |||
| EBV(+ve) non-A2 | ||||||
| JR | 10 197 ± 952 | 1.0 | 1977 (19.4 ± 0.6%) | 3 ± 1 | 0.1 | 0 ± 1 |
| ST | 33 822 ± 1616 | 3.4 | 3485 (10.3 ± 0.0%) | 1 ± 1 | 0.0 | 0 ± 0 |
| Average | 13 489‡ | 1.3† | 1863† | 2 | 0.1 | 0 |
| EBV(−ve) | ||||||
| PB | 813 ± 67 | 0.1 | 71 (8.7 ± 0.7%) | |||
| KR | 680 ± 103 | 0.1 | 102 (14.9 ± 0.1%) | |||
| DM | 1231 ± 165 | 0.1 | 241 (19.6 ± 2.2%) | |||
| Average | 908 | |||||
The mean and standard deviations are derived from results on two separate experiments; the results from each experiment represent the average of triplicate wells (or more than 12 wells in the case of LMP2 peptide-responsive CD8+ cells) with the same number of responders per well.
The frequency of LCL-responsive CD8+ cells is also presented as the number of spots/106 PBMC for comparison with peptide-responsive CD8+ cells. This is estimated by: (number of spots/106 CD8+ cells) × (% of CD8+ cells among PBMC). The percentage of CD8+cells among PBMC is obtained by positive selection of CD8+cells with Dynal-beads. The mean and standard deviation of 2 CD8+ cell selection are presented in parentheses.
This average is derived from both HLA-A2 and non HLA-A2 EBV (+ve) donors.
Detection of EBV-specific CD8+ cells in polyclonal CTL lines
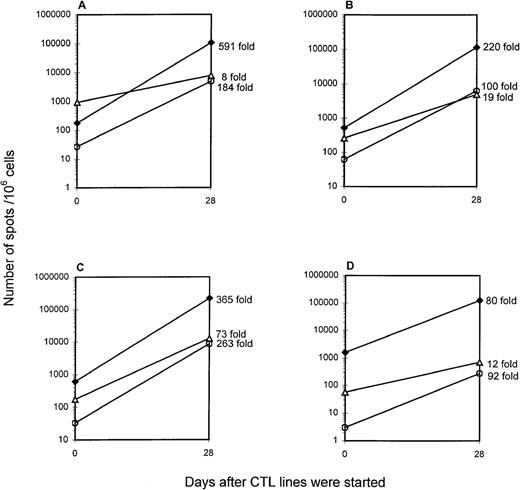
Polyclonal LCL-stimulated EBV-specific CTL lines are being used in ongoing clinical studies of adoptive cellular immunotherapy at our center and others. We sought to evaluate these T-cell expansions using the ELISPOT methodology. EBV-specific polyclonal CTL lines were generated from 4 healthy HLA-A2 donors by weekly restimulation with irradiated autologous LCLs for 4 weeks. The phenotype of these CTL lines as determined by flow cytometry was predominantly CD8+ (on average 73.2% CD8+, 23.2% CD4+, and 0.8% CD56+). EBV-specific CD8+ responses were measured in unstimulated PBMCs and the polyclonal CTL lines they yielded (Figure5). Compared with unstimulated PBMCs, polyclonal CTL lines showed an average 314-fold increase in LCL-responsive CD8+ cells. This corresponded to 11% to 22% of the CTL population (106 383 to 223 214 spots/106cells in the CTL line). The LMP2 peptide-responsive and BMLF1 peptide-responsive CD8+ cells showed an average increase of 160-fold (275 to 8673 spots/106 cells in the CTL line) and 28-fold (700 to 12 755 spots/106 cells in the CTL line), respectively, compared with PBMCs.
Increases in the number of EBV-specific CD8+ cells over the course of polyclonal CTL line generation.
EBV-specific polyclonal CTL lines from HLA-A2 donors ES (A), SB (B), MS (C), and TZ (D) were established. Cells were harvested on day 28 and cryopreserved. On the day of the ELISPOT assay, aliquots of CTL lines were thawed and incubated with stimulators in multiscreen HA plate. Stimulators for ELISPOT assay included autologous LCLs (1 × 105/well), and autologous PBMCs (1 × 105/well) in medium containing the LMP2 or BMLF1 peptide (10 μg/mL, final concentration). The fold-increase in the number of LCL-responsive (filled diamonds), LMP2 peptide-responsive (open circles), and BMLF1 peptide-responsive CD8+ cells (open triangles) is indicated to the right of the day 28 data point.
Increases in the number of EBV-specific CD8+ cells over the course of polyclonal CTL line generation.
EBV-specific polyclonal CTL lines from HLA-A2 donors ES (A), SB (B), MS (C), and TZ (D) were established. Cells were harvested on day 28 and cryopreserved. On the day of the ELISPOT assay, aliquots of CTL lines were thawed and incubated with stimulators in multiscreen HA plate. Stimulators for ELISPOT assay included autologous LCLs (1 × 105/well), and autologous PBMCs (1 × 105/well) in medium containing the LMP2 or BMLF1 peptide (10 μg/mL, final concentration). The fold-increase in the number of LCL-responsive (filled diamonds), LMP2 peptide-responsive (open circles), and BMLF1 peptide-responsive CD8+ cells (open triangles) is indicated to the right of the day 28 data point.
To test whether the number of EBV-specific CD8+ cells as measured by ELISPOT correlates with the function of these cells, cytotoxic activities of the polyclonal CTL lines were measured by standard 51Cr-release assays. A positive correlation (R = 0.9) was found between the frequency of EBV antigen-responsive CD8+ cells and the cytolytic activity of CTL lines (Figure6).
Positive correlation between the number of IFN-γ-secreting cells and cytolytic activity in CTL lines.
EBV-specific CTL lines from 4 HLA-A2 donors were generated by weekly restimulation with irradiated autologous LCLs for 2 to 4 weeks. The CTL lines were then assayed as responders/effectors in a paired ELISPOT/51Cr-release assay. Stimulators for the ELISPOT assay or targets for 51Cr-release assay included autologous LCLs for both the ELISPOT and 51Cr-release assays (filled diamonds), autologous PBMCs in medium containing peptide (LMP2 or BMLF1 peptide) as stimulators for ELISPOT assays, and autologous PHA blasts in medium containing peptide as targets for 51Cr-release assay (open diamonds).
Positive correlation between the number of IFN-γ-secreting cells and cytolytic activity in CTL lines.
EBV-specific CTL lines from 4 HLA-A2 donors were generated by weekly restimulation with irradiated autologous LCLs for 2 to 4 weeks. The CTL lines were then assayed as responders/effectors in a paired ELISPOT/51Cr-release assay. Stimulators for the ELISPOT assay or targets for 51Cr-release assay included autologous LCLs for both the ELISPOT and 51Cr-release assays (filled diamonds), autologous PBMCs in medium containing peptide (LMP2 or BMLF1 peptide) as stimulators for ELISPOT assays, and autologous PHA blasts in medium containing peptide as targets for 51Cr-release assay (open diamonds).
Application of ELISPOT assay to the monitoring of changes in LCL-responsive CD8+ cells following adoptive CTL infusion
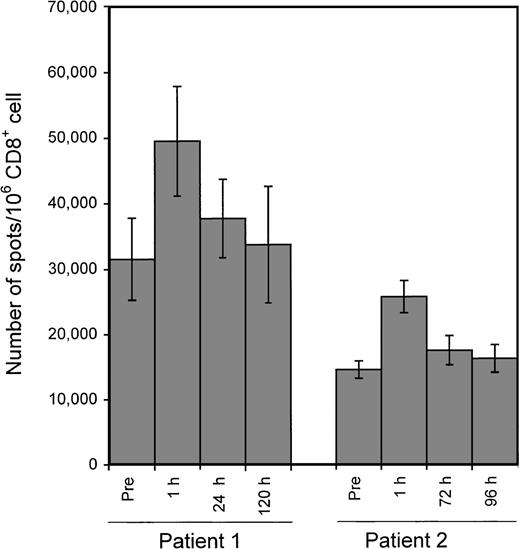
In an ongoing clinical trial, patients with EBV-associated tumors were infused with in vitro expanded EBV-specific CTL products from HLA partially matched donors (Ambinder et al, in preparation). We sought to determine whether changes in the frequency of LCL-responsive CD8+ cells could be detected by ELISPOT assay following adoptive CTL infusion. Two patients receiving an infusion of 5 × 107 CTL/m2 from unrelated donors were included in the pilot experiments. A transient increase in LCL-responsive CD8+ cells was detected after infusion in both patients examined (Figure 7).
Application of the ELISPOT assay to the monitoring of changes in the number of LCL-responsive CD8+ cells following adoptive CTL infusion.
Two patients with PTLD received EBV-specific polyclonal CTL infusion from partially matched donors. The HLA types of patients and donors were: patient 1 (A1, 24, B7, 8), donor for patient 1 (A1, 33, B8, 17); patient 2 (A2, 32, B13, 27), donor for patient 2 (A2, 3, B7, 27). Blood was drawn immediately before and at the indicated time points after infusion. CD8+ cells were used as responders in ELISPOT assays with the patient's LCLs as stimulators. Each bar represents the mean ± SD of triplicate wells.
Application of the ELISPOT assay to the monitoring of changes in the number of LCL-responsive CD8+ cells following adoptive CTL infusion.
Two patients with PTLD received EBV-specific polyclonal CTL infusion from partially matched donors. The HLA types of patients and donors were: patient 1 (A1, 24, B7, 8), donor for patient 1 (A1, 33, B8, 17); patient 2 (A2, 32, B13, 27), donor for patient 2 (A2, 3, B7, 27). Blood was drawn immediately before and at the indicated time points after infusion. CD8+ cells were used as responders in ELISPOT assays with the patient's LCLs as stimulators. Each bar represents the mean ± SD of triplicate wells.
Discussion
We have adapted the ELISPOT assay to the quantitative detection of EBV antigen-specific CD8+ cells. This assay is sensitive and does not require prior expansion of specific T cells. In its various formats the assay is suitable for the analysis of the aggregate CD8+ response to EBV-infected lymphocytes (see Figure 2), and responses to individual peptide epitopes including subdominant antigens such as the LMP2-derived peptide (see Figure 3) and lytic cycle antigens such as the BMLF1-derived peptide (see Figure 4). Finally, it appears well suited for monitoring expansion of EBV-specific T cells for adoptive cellular immunotherapy in vitro and in vivo.
We used whole cell (LCL) stimulators in the ELISPOT assay described here. Several features of LCLs make them suitable as stimulators. First, LCLs are phenotypically activated B cells with high surface expression of MHC class I and other molecules involved in various immune processes and are thus potent antigen presenters. Second, LCLs express the full spectrum of EBV latent cycle antigens, namely, the Epstein-Barr nuclear antigens (EBNAs) 1, 2, 3A, 3B, 3C,-LP, and the LMPs 1, 2A, and 2B.21 A comparable aggregate assessment of EBV-responsive T cells would require a mixture of many peptides tailored to each individual's HLA type. Third, insofar as the spectrum of viral antigen expression in LCLs corresponds generally with that in PTLD, assessment of response may be directly relevant to the pathogenesis or treatment of this disease.
In the present study we aimed at measuring the CD8+responses to EBV by detection of IFN-γ secretion. CD8+ T cells, CD4+ T cells, and natural killer cells have been identified by previous investigators as producers of IFN-γ.22-26 When LCLs are used as stimulators, cells from the CD8+, CD4+, and CD56+populations secrete IFN-γ, whereas PBMCs depleted of these populations (CD8−CD4−CD56−cells) do not (see Figure 1). In EBV seropositive individuals, non-CD8+ responders account for 20% to 81% (mean 62%) of PBMC responders producing spots in response to LCL stimulation (data not shown), whereas in EBV-seronegative individuals, non-CD8+ cells account for a higher portion of the responses (84% to 89%, mean 86%). Previous investigators have also reported a high frequency of LCL-reactive CD56+ cells in cord blood and seronegative donors.27
Peptides were also used as stimulators to characterize responses to single epitopes. The first peptide chosen in the present study was the LMP2-derived peptide restricted through HLA-A*0201. ELISPOT assays reproducibly detected responses to the subdominant LMP2 peptide in most healthy EBV-seropositive HLA-A2 donors (see Figure 6). Consistent with previous reports that LMP2 is a subdominant antigen, responses to this peptide accounted for about 5% of the CD8+ response to autologous LCLs. Because LMP2 is expressed in most types of EBV tumors8,28-30 and is restricted through a common HLA type,31 assays of responses to this antigen may provide a window on immune responses to viral tumor antigens in patients with these malignancies.
The second peptide tested is derived from the early lytic protein BMLF1. Recent work by Steven et al showed that primary EBV infection is accompanied by unusually strong responses to lytic cycle antigens.19 Responses to the BMLF1 peptide were as strong as those to an immunodominant latency antigen epitope. Callan et al reported a high frequency of BMLF1 peptide-responsive CD8+cells during primary infection with tetramer staining technique.32 Using the ELISPOT assay, we could reproducibly detect CD8+ cells specific for this early lytic BMLF1 peptide in all healthy EBV-seropositive HLA-A2 donors tested. The number of BMLF1-responsive cells is 13-fold higher on average than the number of LMP2 peptide-responsive cells. Similar results have recently been published by Tan et al.14 These authors also found good responses to a B8 restricted epitope in the lytic BZLF1 protein. The frequency of responses to these lytic epitopes was similar to the frequency of responses to the immunodominant EBNA3A and EBNA3B epitopes. This demonstrates not only that lytic cycle antigen-specific T cells persist in the healthy carrier state, but also that they persist at high frequencies. Other investigators have called attention to the cellular immune response to lytic epitopes.33,34Thus, lytic cycle antigen-specific T cells may have a role in controlling lytic reactivation of EBV infection in the carrier state. Inasmuch as PTLD is often associated with lytic antigen expression,35-37 the ability to monitor these responses may be important in understanding the pathogenesis of these disorders and in developing new approaches to therapy.
The ELISPOT assay detects antigen-specific T cells in unstimulated PBMCs and in CTL lines. Our study of EBV-specific CTL lines showed that LCL-responsive CD8+ cells constitute 11% to 22% of the total cellular population in the product used for adoptive cellular immunotherapy. It should be noted that these data were obtained with frozen CTL lines that were thawed immediately before the ELISPOT assay. Limited data with fresh CTL lines have yielded higher estimates of the frequency of LCL-responsive CD8+ cells (60% to 100%, data not shown). In EBV-specific polyclonal CTL lines, LMP2 peptide-responsive CD8+ cells, although increased one- to several hundred-fold, constitute only a small minority of cells, averaging about 4% of LCL-responsive CD8+ cells. This explains why CTL responses to this LMP2 peptide are sometimes obscured in the analysis of cell lines but can be detected more readily by analysis of individual T-cell clones.6,38 The lesser degree of expansion of BMLF1 peptide-responsive CD8+ cells supports the idea that the repertoire of EBV-specific T cells in vivo may be skewed during in vitro expansion with LCLs toward latency antigens.21 However, BMLF1 peptide-specific CD8+ cells still increased significantly in all of the polyclonal CTL lines tested, indicating that the occasional lytic events in LCLs are capable of reactivating CD8+ cell responses to lytic antigens. The presence of lytic antigen-specific T cells exemplified by BMLF1-responsive CD8+ cells suggests that polyclonal CTL products may also be effective in controlling lytic EBV infection and the spread of virus.
One of the potential applications of the ELISPOT assay is in monitoring therapeutic interventions. Several investigators have reported adoptive cellular immunotherapy with donor lymphocytes from allogeneic bone marrow donors or with EBV-activated lymphocyte populations expanded in vitro from allogeneic and autologous sources. Generally these infusions have been monitored by limiting dilution assays (LDA).39-42The ELISPOT assay with LCL stimulators presents an alternative approach that requires fewer cells and less time for the assay. In addition, our preliminary data show that values obtained from ELISPOT with LCL stimulators are 2- to 10-fold higher than values obtained from LDAs (data not shown). Other investigators also reported a 5.3-fold higher frequency of epitope-specific CD8+ T cells as measured by ELISPOT assays than those measured by LDAs.14 Using the ELISPOT assay, we show that in patients with a history of PTLD, adoptive T cell immunotherapy from HLA partially matched allogeneic donors led to transient increase in frequency of CD8+ cells responsive to EBV. Analysis of the fate of infused T cells in a series of patients treated with EBV-specific T cells from partially matched allogeneic donors is ongoing.
Other methods have also been developed for the detection of antigen-specific T cells. Among them, direct staining of the antigen-specific CD8+ set with multimeric (tetrameric or dimeric) complexes of MHC class I glycoprotein with peptide43-47 has attracted great interest. The multimer staining technique and the ELISPOT assay using peptide have in common high specificity that allows analysis of unfractionated PBMCs. However, whereas the ELISPOT assay can be adopted to characterize an aggregate response to viral antigens in fractionated cell populations as shown here using the assay with LCL stimulators, there is as yet no comparable approach to the detection of aggregate responses using the multimer staining technique. The multimer staining technique and the ELISPOT assay take advantage of different aspects of the T-cell–antigen interaction. The multimer staining technique uses the specific physical interaction between T-cell receptor (TCR) and peptide-bound MHC class I molecules and thus is capable of detecting the physical presence of antigen-specific T cells without any requirement for function and might detect anergic cells.48The ELISPOT assay, on the other hand, relies on the ability of T cells to secrete cytokine on activation. Our results show that IFN-γ secretion correlates with specific target cell killing (see Figure 6). Similar conclusions have been reported by previous investigators.49
Tan et al compared the ELISPOT assay with tetramer staining for the detection of EBV epitope-specific T cells.14 They reported that on average tetramer staining yielded 4.4-fold higher frequencies than did ELISPOT assay, presumably reflecting the presence of T cells that carried TCR of appropriate specificity, but did not produce IFN-γ on exposure to peptide. Thus at higher T-cell frequencies, tetramer staining yields higher estimates than ELISPOT assay. However, at T-cell frequencies of 20/106 PBMC or lower as measured by ELISPOT assay, tetramer staining failed to yield signal, presumably reflecting sensitivity limits imposed by the flow cytometry detection method. In our hands, antigen-specific T cells at frequencies as low as 1/100 000 PBMCs could be reproducibly detected by ELISPOT assay.
In conclusion, the ELISPOT technique may be particularly useful in the analysis of low-frequency responses. The ability to analyze aggregate responses to whole cells suggests the possibility of a much broader range of applications than has previously been explored, especially in the monitoring of immunotherapeutic interventions as illustrated here in the adoptive immunotherapy trial.
Acknowledgment
We thank Dr Hyam I. Levitsky for advice.
Supported by NIH grant PO1 CA15396.
Reprints:R. F. Ambinder, Johns Hopkins Oncology Center, 418 North Bond Street, Baltimore, MD 21231; email: rambind@jhmi.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal