Abstract
Daclizumab, a humanized monoclonal IgG1 directed against the chain of the interleukin-2 receptor (IL-2R), is a competitive inhibitor of IL-2 on activated lymphocytes. To test the hypothesis that specific inhibition of activated lymphocytes in patients with ongoing acute graft-versus-host disease (GVHD) might ameliorate the process, we treated 43 patients with advanced or steroid-refractory GVHD with daclizumab. The first cohort of 24 patients was treated with daclizumab 1 mg/kg on days 1, 8, 15, 22, and 29. On day 43, the complete response (CR) rate was 29% (95% confidence interval [CI], 13%-51%). Survival on day 120 was 29% (95% CI, 13%-51%). A second cohort of 19 patients was treated with daclizumab 1 mg/kg on days 1, 4, 8, 15, and 22. For these patients, the CR rate on day 43 was 47% (95% CI, 24%-71%), and survival on day 120 was 53% (95% CI, 29%-76%). There were no infusion-related reactions and no serious side effects related to daclizumab. Following treatment, there was a reduction in serum concentrations of soluble IL-2R and peripheral blood CD3 + 25+ lymphocytes, but these changes were not predictive of response. Daclizumab has substantial activity for the treatment of acute GVHD, and the second regimen evaluated is recommended for a controlled study. (Blood, 2000; 95:83-89)
The high-affinity interleukin-2 receptor (IL-2R) is a heteromultimer comprised of the α (p55), β (p75) and γ (p64) chains.1,2 The IL-2Rβ and IL-2Rγ chains are expressed on the surface of most T, NK, and B cells as an intermediate-affinity receptor; signal transduction occurs via the IL-2Rβγ complex.2 The IL-2Rα chain is found predominantly on activated cytotoxic T cells and confers high-affinity properties on the heteromultimer.3,4 Interaction of IL-2R with its ligand IL-2 triggers activation of the ras pathway and results in enhancement of lymphocyte proliferation and differentiation.5
The limited distribution of IL-2Rα on activated lymphocytes suggested that it could be an appropriate target for strategies designed to eliminate antigen-specific alloreactive T cells. Indeed, in vitro treatment of alloantigen-stimulated lymphocytes with IL-2Rα immunotoxin conjugates or depleting IL-2Rα+ cells by immunomagnetic techniques resulted in a decreased reactivity of the purged population following secondary stimulation against the primary antigen, whereas third-party responses were largely maintained.6-8 In addition, in a murine model, such allodepleted lymphocytes had a reduced capacity to induce graft-versus-host disease (GVHD).9 Administration of anti-IL-2R antibody in vivo early after transplantation was also at least partially effective in preventing GVHD in an F1→P model and in a model with a minor H-2 disparity,10 11 but whether administration of anti-IL-2R in vivo would be effective in abrogating an ongoing GVH response has not been tested in an animal model.
Daclizumab (humanized anti-Tac, HAT) is a human monoclonal IgG1 that incorporates the complementarity-determining regions of a murine monoclonal antibody raised against the human IL-2Rα chain.12,13 In vitro daclizumab displayed specific binding to IL-2Rα+ cells and inhibited the T-cell proliferative response to tetanus toxoid as well as influenza antigen and alloantigen in a dose-dependent fashion, but it did not fix complement.12,13 The mechanism of action of daclizumab is thought to be the competitive inhibition of binding of IL-2 to its receptor.14 In preclinical primate studies, daclizumab was effective in prolonging cardiac allograft survival and in reducing inflammation in experimental uveitis,15,16 and subsequent phase I-III clinical trials demonstrated the safety and efficacy of daclizumab for prevention of renal allograft rejection.17 18
In a phase I study in patients with acute GVHD, Anasetti et al19 reported no serious side effects after administration of a single dose of daclizumab up to 1.5 mg/kg, and the t1/2β was approximately 3.5 days. Moreover, improvement in or resolution of acute GVHD was seen in 8 of 20 patients in the phase I study19 and in 3 of 5 patients in a subsequent single-dose pilot study.20 Herein we report the results of a multidose strategy with the use of daclizumab for treatment of severe or steroid-refractory GVHD.
Patients and methods
Patients
Forty-two patients were enrolled on the prospective study, and one additional patient was treated on a compassionate basis according to the protocol just before the start of the study. The diagnosis of GVHD was made clinically, and appropriate biopsies were obtained for histologic confirmation. Patients were eligible if they were < 100 days after allogeneic marrow or blood stem cell transplantation and had visceral GVHD or steroid-resistant GVHD (any stage of GVHD persisting for 3 days while receiving ≥ 2 mg/kg/d methylprednisolone or developing while on ≥ 1 mg/kg/d methylprednisolone). Mechanical ventilation, serum creatinine > 3 mg/dL, renal dialysis, total bilirubin > 12 mg/dL, use of vasopressors, and uncontrolled infections at the start of therapy were criteria for exclusion. The protocol was approved by the Institutional Review Board at each of the treatment centers, and written informed consent was obtained for each patient.
Treatment plan and monitoring
Daclizumab was administered at 1 mg/kg iv on study days 1, 8, 15, 22, and 29 (regimen 1) for the first 24 patients and at 1 mg/kg iv on study days 1, 4, 8, 15, and 22 (regimen 2) for 19 subsequent patients. Doses were calculated on actual body weight, and the maximum single dose was limited to 100 mg. The appropriate dose of daclizumab was diluted in 50 mL normal saline USP and infused over 30 minutes. Vital signs were recorded before and at 15, 30, and 90 minutes after start of infusion of daclizumab. Cyclosporine or tacrolimus was continued according to the patient's original prophylaxis protocol, and methylprednisolone was administered intravenously at 2 mg/kg in divided doses for at least 7 days before tapering. If there was no improvement in GVHD of the skin after 3 days or of the liver or gut after 7 days, or if there was substantial progression of GVHD after 2 days, then antithymocyte globulin (ATG) could be added at 10 mg/kg iv daily for at least 7 days for patients on regimen 1 or at 40 mg/kg iv daily for 4 days for patients on regimen 2. If there was no improvement in GVHD of the skin after 3 days of ATG or of the liver or gut after 7 days, or if there was substantial progression of GVHD after 2 days of ATG, then patients were considered a treatment failure and eligible for any available salvage therapy. Patients were evaluated for GVHD on study days 1, 8, 15, 22, 29, 36, and 43. GVHD was scored according to the consensus criteria.21 A complete blood count with differential, platelet count, and chemistry panel was done at least twice weekly through study day 29 and on study day 43.
Response criteria
Response was the primary end point of the study and was scored on study day 43; patients who received ATG or expired before study day 43 were considered nonresponders. Patients were evaluable for response in an organ if they had GVHD in that organ at the start of treatment with daclizumab or if GVHD developed after the start of daclizumab but before the time point of evaluation. A complete response (CR) in an organ was defined as stage 0, and a partial response (PR) required a reduction in at least one stage without additional therapy. All patients were evaluable for the overall response. For the overall assessment, a CR was defined as complete resolution of rash, normalization of bilirubin, and absence of diarrhea because of GVHD without the use of antimotility agents, and a partial response (PR) was defined as a decrease by at least one stage in at least one organ system without worsening in the other organ systems. Survival was a secondary end point. Survival was scored on study day 120.
Flow cytometry
Heparinized peripheral blood was collected on study days 1, 8, 15, 22, 29, 36, and 43 as well as at 2 and 3 months on study. CD3+ and CD3 + 25+ cells were enumerated by multiparameter flow cytometry22,23 with the use of fluorochrome-conjugated 2A3 (anti-CD25, Becton Dickinson, San Jose, CA; BD) that binds at or near the same epitope on p55 as daclizumab,24 and fluorochrome-conjugated SK7 (anti-CD3) (BD) that binds the epsilon chain of the T-cell receptor. For five normal volunteers, the median absolute lymphocyte count/μL was 1755 (range, 944-2397), the median absolute number of CD3+cells/μL was 1366 (range, 717-1457), and the median absolute number of C3 + 25+ cells/μL was 140 (range, 85-215). For patients at the M. D. Anderson Cancer Center (MDACC), absolute numbers of CD3+CD4+, CD3+CD8+, CD3+HLA-DR+, and CD3−CD56+ in peripheral blood samples were also determined at each study time point with the use of commercial antibodies (BD). In addition, the CD3+ cells in these patients were evaluated for staining with PE-conjugated daclizumab and with FITC-conjugated 7G7, a murine monoclonal antibody that binds p55 at an epitope not recognized by daclizumab.25
Cytokine and receptor ELISA
For patients at MDACC, serum was collected on study days 1, 8, 15, 22, 29, 36, and 43 as well as at 2 and 3 months on study, and they were tested for IL-2 and soluble IL-2 receptor (sIL-2R) levels with the use of ELISA kits (BioSource International, Camarillo, CA) according to the manufacturer's instructions. The sIL-2R ELISA uses an antibody against p55 that crossreacts with daclizumab; addition of as little as 100 ng of daclizumab totally suppressed detection of the 1000 pg/mL sIL-2R standard.
Statistical considerations
At the time of analysis, all patients had completed follow-up through study day 120. The protocol was originally designed as a two-step phase II study with sufficient power to detect a response rate of 20% or more with a standard error of 10%. When this part was completed, the protocol was modified to allow for treatment of a second cohort (regimen 2). The second regimen included an additional dose of daclizumab in the first week to increase the response rate, and salvage therapy was compressed to 4 days to minimize infectious complications from prolonged use of immunosuppression. Results are reported as a proportion; 95% CIs were calculated by the binomial distribution. Frequencies were compared by log-likelihood ratio or Fisher exact test with a two-sidedP < .05 considered significant. Serial measurements were compared with the use of the Friedman analysis of variance with a two-sided P < .01 considered significant. Medians were compared with the use of the signed rank test for matched samples and the Mann-Whitney U test for independent samples with a two-sided P < .05 considered significant. The statistical analysis was performed with the use of True Epistat v5.3 (True Epistat, Richardson, TX).
Results
Study participants
Patient characteristics are described in Table1. All patients had been transplanted for hematologic malignancies or for nonmalignant hematologic disorders, and all had received a myeloablative preparative regimen. There were 12 patients less than 18 years of age. The majority of the patients had received transplants from HLA-nonidentical donors. For prevention of GVHD, 31 patients had received cyclosporine or tacrolimus with methotrexate or methylprednisolone, whereas 12 received a T-cell–depleted transplant with or without cyclosporine. Eight patients had received ATG as part of GVHD prophylaxis, and six had received ATG as part of the preparative regimen before transplantation. Four patients received daclizumab as part of primary treatment of multiorgan GVHD, and the remainder of the patients were steroid refractory. The episode of GVHD treated on this protocol started at a median of 25 days posttransplant (range, 3-95 days), and the median duration of methylprednisolone use for treatment before study entry was 6 days (range, 0-35 days). The distributions of the stages and grades of GVHD are shown in Table 2. Eleven (46%) patients on regimen 1 and 9 (47%) patients on regimen 2 had grades 3-4 GVHD.
Patient characteristics
| Number of patients | 43 |
| Sex (male/female) | 25/18 |
| Median age, y (range) | 31 (1-53) |
| Diagnosis | |
| Leukemia/myelodysplastic syndrome | 32 |
| Malignant lymphoma | 9 |
| Aplastic anemia | 1 |
| Histiocytosis | 1 |
| Preparative regimen | |
| TBI-based | 33 |
| Busulfan-based | 10 |
| Transplant | |
| Marrow | 25 |
| Blood | 11 |
| Both | 7 |
| Donor | |
| HLA-identical sibling | 14 |
| Mismatched related | 15 |
| Unrelated | 14 |
| GVHD prophylaxis | |
| Tacrolimus-based | 18 |
| Cyclosporine-based | 13 |
| Cyclosporine + T-cell depletion | 7 |
| T-cell depletion + other | 5 |
| Daclizumab regimen | |
| 1 (days 1, 8, 15, 22, 29) | 24 |
| 2 (days 1, 4, 8, 15, 22) | 19 |
| Number of patients | 43 |
| Sex (male/female) | 25/18 |
| Median age, y (range) | 31 (1-53) |
| Diagnosis | |
| Leukemia/myelodysplastic syndrome | 32 |
| Malignant lymphoma | 9 |
| Aplastic anemia | 1 |
| Histiocytosis | 1 |
| Preparative regimen | |
| TBI-based | 33 |
| Busulfan-based | 10 |
| Transplant | |
| Marrow | 25 |
| Blood | 11 |
| Both | 7 |
| Donor | |
| HLA-identical sibling | 14 |
| Mismatched related | 15 |
| Unrelated | 14 |
| GVHD prophylaxis | |
| Tacrolimus-based | 18 |
| Cyclosporine-based | 13 |
| Cyclosporine + T-cell depletion | 7 |
| T-cell depletion + other | 5 |
| Daclizumab regimen | |
| 1 (days 1, 8, 15, 22, 29) | 24 |
| 2 (days 1, 4, 8, 15, 22) | 19 |
Distribution of graft-versus-host disease by organ stage or overall grade at the start of treatment with daclizumab
| Site . | Stage or Grade . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | 4 . | |
| Skin | 8 | 2 | 7 | 22 | 4 |
| Liver | 36 | 1 | 3 | 2 | 1 |
| Gut | 19 | 9 | 2 | 8 | 5 |
| Overall | 1 | 22 | 12 | 8 | |
| Site . | Stage or Grade . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | 4 . | |
| Skin | 8 | 2 | 7 | 22 | 4 |
| Liver | 36 | 1 | 3 | 2 | 1 |
| Gut | 19 | 9 | 2 | 8 | 5 |
| Overall | 1 | 22 | 12 | 8 | |
Study compliance
Ten patients received fewer than five doses of daclizumab. Five patients on regimen 1 failed to complete the course because of early death. On regimen 2, five patients did not get all doses. Three patients did not receive the fifth dose at the discretion of the attending physician; two of the three had achieved a complete response with the first four doses alone. The fourth patient developed Epstein-Barr virus lymphoproliferative disease (LPD) before completion of therapy. The fifth patient stopped receiving the drug after the fourth dose because of rising liver enzymes; this condition was shown by liver biopsy to be viral in etiology.
Response to therapy
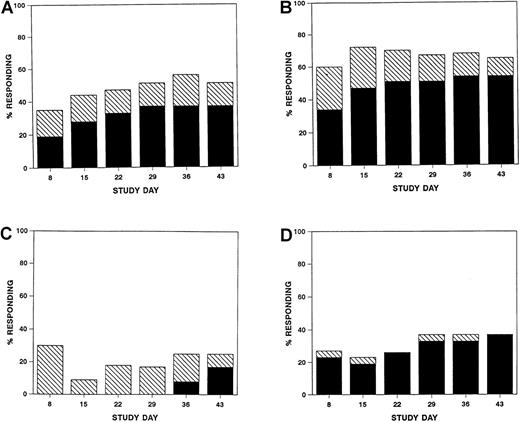
Responses were seen as early as study day 8 (Figure1), but overall response was scored on study day 43. Sixteen (37%; 95% CI, 23%-53%) patients achieved a complete response, and six (14%; 95% CI, 5%-30%) achieved a partial response for a total response rate of 51% (95% CI, 35%-67%) (Table3). Complete responses varied with the organ involved (P = .014) (Table 3) and with multiorgan involvement (P = .014), but there was no significant difference in the complete response rates when compared by initial grade of GVHD, age, or use of T-cell depletion. The four patients with untreated visceral GVHD failed to respond to daclizumab. Seven (29%, 95% CI, 13-51%) patients on regimen 1 and 9 (47%, 95% CI, 24-71%) patients on regimen 2 achieved a complete response. Six patients survived to the end of follow-up (study day 120) free of recurrent acute GVHD or chronic GVHD.
Percentage of evaluable patients at each study time point with a complete (solid bars) or partial (hatched bars) response overall (A) or in skin (B), liver (C), or gut (D).
Percentage of evaluable patients at each study time point with a complete (solid bars) or partial (hatched bars) response overall (A) or in skin (B), liver (C), or gut (D).
Response to treatment with daclizumab
| Response on day 43 | |
| Complete response | 16 of 43 (37%) |
| Partial response | 6 of 43 (14%) |
| Failed but alive | 13 of 43 (30%) |
| Death before day 43 | 8 of 43 (19%) |
| Complete response by organ involvement | |
| Skin | 20 of 37 (54%) |
| Liver | 2 of 12 (17%) |
| Gut | 10 of 27 (37%) |
| Complete response by extent | |
| Skin alone | 10 of 16 (63%) |
| Gut alone | 0 of 3 (0%) |
| Multiorgan | 6 of 24 (25%) |
| Complete response by baseline grade | |
| Grade 1-2 | 10 of 23 (43%) |
| Grade 3-4 | 6 of 20 (30%) |
| Complete response by age group | |
| < 18 y | 5 of 12 (42%) |
| ≥ 18 y | 11 of 31 (35%) |
| Complete response by regimen | |
| Regimen 1 | 7 of 24 (29%) |
| Regimen 2 | 9 of 19 (47%) |
| Response on day 43 | |
| Complete response | 16 of 43 (37%) |
| Partial response | 6 of 43 (14%) |
| Failed but alive | 13 of 43 (30%) |
| Death before day 43 | 8 of 43 (19%) |
| Complete response by organ involvement | |
| Skin | 20 of 37 (54%) |
| Liver | 2 of 12 (17%) |
| Gut | 10 of 27 (37%) |
| Complete response by extent | |
| Skin alone | 10 of 16 (63%) |
| Gut alone | 0 of 3 (0%) |
| Multiorgan | 6 of 24 (25%) |
| Complete response by baseline grade | |
| Grade 1-2 | 10 of 23 (43%) |
| Grade 3-4 | 6 of 20 (30%) |
| Complete response by age group | |
| < 18 y | 5 of 12 (42%) |
| ≥ 18 y | 11 of 31 (35%) |
| Complete response by regimen | |
| Regimen 1 | 7 of 24 (29%) |
| Regimen 2 | 9 of 19 (47%) |
Seventeen patients (40%) were treated with ATG; this included 13 (54%) patients on regimen 1 and four (21%) patients on regimen 2. Median time to start of ATG after daclizumab was 8 days (range, 2-19 days). Seven patients (41%) achieved a complete response to salvage therapy with ATG, two of two with skin GVHD alone and five of 15 with visceral involvement. All responders had been treated initially on daclizumab regimen 1.
Drug-related toxicities
There were no infusion-related side effects, and no serious clinical adverse events related to daclizumab were reported.
Laboratory correlates
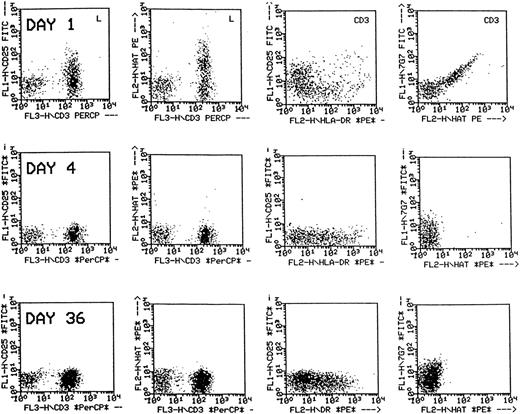
For 36 patients who completed follow-up through the end of therapy, there was no significant difference between the absolute lymphocyte count or absolute CD3+ count at baseline and at 2 weeks after the end of treatment, but the number of CD3 + 25+ lymphocytes detected was significantly lower after treatment with daclizumab (20 vs 0 CD3 + 25+ lymphocytes/μL;P < .0001). Response did not correlate with the elimination of detectable CD3 + 25+ lymphocytes as almost all patients had no detectable CD3 + 25+lymphocytes by study day 8. It was noted, however, that, although reactivity of peripheral blood lymphocytes with anti-CD25 or daclizumab was lost during the treatment period, CD3+ cells continued to display HLA-DR, an activation marker, and had a low level of positivity for 7G7, the antibody that binds p55 but does not crossreact with daclizumab (Figure 2).
Flow cytometric analysis of peripheral blood lymphocytes of a patient treated with daclizumab.
The dot plots in the first two columns are gated on lymphocytes, and those in the third and fourth columns are gated on CD3+cells. Following treatment, p55 is not detected by anti-CD25 nor by anti-humanized Tac (daclizumab) on CD3+ cells (first and second columns), but the CD3+ cells remain positive for HLA-DR (third column) and faintly positive for p55 using 7G7 (fourth column).
Flow cytometric analysis of peripheral blood lymphocytes of a patient treated with daclizumab.
The dot plots in the first two columns are gated on lymphocytes, and those in the third and fourth columns are gated on CD3+cells. Following treatment, p55 is not detected by anti-CD25 nor by anti-humanized Tac (daclizumab) on CD3+ cells (first and second columns), but the CD3+ cells remain positive for HLA-DR (third column) and faintly positive for p55 using 7G7 (fourth column).
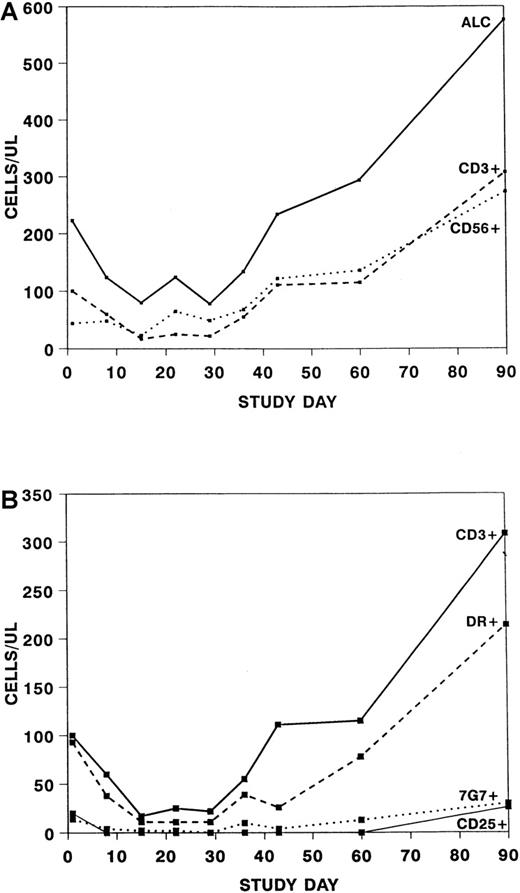
Over the course of the treatment period, a significant change was seen in the absolute lymphocyte count (P = .002) and the absolute CD3 + 56− cell count (P = .005) but no significant change in the absolute CD3−56+ cell count (Figure3A). The changes in the number of CD3+ cells affected both the CD4+ and CD8+ subsets (data not shown). When only patients who did not receive ATG within the first 15 days were assessed, no significant difference was seen in the absolute lymphocyte counts or absolute numbers of CD3+ cells from baseline to day 15, suggesting that the previous observation was because of ATG. CD3 + 25+ cells were essentially undetectable until study day 60, whereas 48% to 75% of patients had 7G7+ cells at various time points through the treatment period (Figure 3B).
Absolute numbers of cells after treatment with daclizumab.
Note the difference in scale of the y-axis between graphs. (A) Absolute number of lymphocytes (ALC) (solid line), CD3 + 56− (dashed line) and CD3 − 56+(dotted line) cells after treatment with daclizumab. (B) Absolute number of CD3 + 56− cells (solid line), CD3+DR+ cells (dashed line), CD3 + 7G7+ cells (dotted line), and CD3 + 25+ cells (thin line) after treatment with daclizumab.
Absolute numbers of cells after treatment with daclizumab.
Note the difference in scale of the y-axis between graphs. (A) Absolute number of lymphocytes (ALC) (solid line), CD3 + 56− (dashed line) and CD3 − 56+(dotted line) cells after treatment with daclizumab. (B) Absolute number of CD3 + 56− cells (solid line), CD3+DR+ cells (dashed line), CD3 + 7G7+ cells (dotted line), and CD3 + 25+ cells (thin line) after treatment with daclizumab.
Serum sIL-2R concentrations decreased following treatment with daclizumab (Table 4) and returned to near baseline by study day 90. No significant difference was seen between responders and nonresponders in the serum sIL-2R levels on study day 8 or on study day 15. Serum IL-2 concentrations did not vary significantly over the time period tested (Table 4).
Serum IL-2 and soluble IL-2 receptor concentrations after treatment with daclizumab
| Study Day . | IL-2 . | sIL-2R . | ||
|---|---|---|---|---|
| n . | pg/mL (range) . | n . | pg/mL (range) . | |
| 1 | 25 | 189 (21-1478) | 25 | 3095 (106-10 330) |
| 8 | 26 | 109 (11-1336) | 26 | 188 (37-2452) |
| 15 | 25 | 119 (14-1229) | 25 | 243 (51-1635) |
| 22 | — | — | 21 | 172 (42-1200) |
| 29 | — | — | 20 | 171 (53-738) |
| 36 | — | — | 20 | 185 (50-1920) |
| 43 | — | — | 12 | 307 (86-875) |
| 60 | — | — | 17 | 1432 (124-17 177) |
| 90 | — | — | 12 | 4668 (243-10 312) |
| Study Day . | IL-2 . | sIL-2R . | ||
|---|---|---|---|---|
| n . | pg/mL (range) . | n . | pg/mL (range) . | |
| 1 | 25 | 189 (21-1478) | 25 | 3095 (106-10 330) |
| 8 | 26 | 109 (11-1336) | 26 | 188 (37-2452) |
| 15 | 25 | 119 (14-1229) | 25 | 243 (51-1635) |
| 22 | — | — | 21 | 172 (42-1200) |
| 29 | — | — | 20 | 171 (53-738) |
| 36 | — | — | 20 | 185 (50-1920) |
| 43 | — | — | 12 | 307 (86-875) |
| 60 | — | — | 17 | 1432 (124-17 177) |
| 90 | — | — | 12 | 4668 (243-10 312) |
—, not done.
Survival
Twenty-six patients died by study day 120; the causes of death included active GVHD and infection (10), infection (7), active GVHD (3), EBV LPD (3), thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (1), relapse (1), and sudden death of unknown etiology (1). The median survival on study was 77 days, 70 days for patients on regimen 1 and more than 120 days for patients on regimen 2. Study day 120 survival was 40% (95% CI, 25%-56%), 29% (95% CI, 13%-51%) for patients on regimen 1, and 53% (95% CI, 29%-76%) for patients on regimen 2. There was no significant difference in study day 120 survival when compared by age (6/12 < 18 years vs 11/31 > 18 years, P = .49) or GVHD grade at baseline (12/23 grades 1-2 vs 5/20 grades 3-4, P = .12).
Discussion
Abrogation of steroid-refractory acute GVHD by blockade of the IL-2 binding site with the use of murine monoclonal antibodies directed against the IL-2R α chain has been reported by several groups.26-31 Complete response rates with the use of the murine antibodies varied from 10% to 88%, and high response rates were seen with high blood levels of antibody26 or prolonged administration of antibody.30,31 Maintaining therapeutic serum concentrations of bioavailable antibody has been difficult with murine antibodies, which have a relatively short half-life and substantial immunogenicity. By contrast, daclizumab, a humanized anti-IL-2Rα antibody, has a terminal half-life of 3.5 days and is much less immunogenic than its murine counterpart.19,32 In the initial studies of daclizumab for treatment of refractory acute GVHD, 7 of 25 patients achieved a complete response with one or two doses of the drug.19 20 With the use of multidose schedules in our study, we found that 29% of patients achieved a complete response with 5 weekly doses of daclizumab and 47% when doses were given on days 1, 4, 8, 15, and 22.
The optimal duration of therapy with daclizumab is unclear. The role of laboratory testing to guide treatment with anti–T-cell antibodies has been an area of controversy, although in the field of solid organ transplantation there is some evidence that after treatment with ATG or OKT3, it may be useful to monitor CD3+ cells, the target of therapy.33,34 In our study, we monitored CD3 + 25+ cells, the target of daclizumab, and found that elimination of detectable CD3 + 25+ was nearly universal and independent of response to therapy. Serum sIL-2R has been cited as a potential measure of GVHD activity,35-38 but neither we nor Tiberghien et al39 found a correlation between serum sIL-2R levels and response to treatment of GVHD with an anti-IL-2R antibody. Serum soluble CD8 and tumor necrosis factor levels may correlate with response,39 but prospective studies will be required to determine if these assays can identify patients in whom the subclinical graft-versus-host reaction has abated and antibody therapy can be discontinued.
We found no significant clinical adverse events attributable to daclizumab, a striking distinction from the side-effect profiles of ATG and OKT3. Others have also reported that daclizumab, as well as anti-IL-2R antibodies in general, has little or no clinical toxicity.18-20,26-31 Of concern, however, are the three patients who developed EBV LPD during or after treatment with daclizumab. All three patients had received T-cell–depleted transplants from alternative donors, which is associated with a high risk for developing EBV LPD.40 Although no increased risk of EBV LPD was reported in previous controlled trials of daclizumab for prevention of organ allograft rejection, we cannot exclude daclizumab as a contributing factor, and the potential for development of EBV LPD must be considered when use of daclizumab is contemplated in the high-risk marrow transplant population.40
Because the activity of daclizumab for treatment of GVHD was unknown at the start of this study, early salvage with ATG was allowed by design. The intention was not to test a sequential daclizumab-ATG regimen but rather to intervene as early as possible for treatment failures. The response rate to secondary salvage to ATG was 41%, including both patients with skin involvement only and 33% with visceral GVHD. These response rates are similar to those reported for primary salvage with ATG,41 suggesting that prior treatment with daclizumab does not eliminate subsequent responsiveness to ATG.
Whether the activity of daclizumab might impair the graft-versus-leukemia (GVL) effect of the allogeneic transplant is also of interest. With the use of an animal model, Weiss et al42found that pretreatment of allografts with anti-IL-2R antibody reduced the GVL effect after transplantation in comparison to untreated allografts. Further, Blaise et al43 reported a higher relapse rate in allogeneic marrow transplant patients given 33B3.1, a murine monoclonal anti-IL-2R antibody, for prevention of GVHD in comparison to control patients. Although only one of our patients relapsed within the study period, the heterogeneity of the patients and the short follow-up in our study preclude meaningful conclusions about the effect of daclizumab on GVL after treatment of GVHD.
We were disappointed to find that some patients with single-organ involvement failed to respond to treatment with daclizumab. Possible reasons for treatment failure include (1) functionally redundant cytokines, such as IL-4 and IL-15, that bypass IL-2Rα blockade16,44; (2) “cold target inhibition” by high levels of sIL2R 45; and (3) loss of activation-induced cell death (AICD).46 The potential lack of AICD has important implications for induction of tolerance. IL-2Rα knockout mice display an autoimmune disorder47,48 similar to that of humans with a truncated IL-2Rα chain.49 Activated T cells devoid of the IL-2Rα chain fail to trigger the pro-apoptotic pathway and are resistant to Fas-mediated lysis.46-48 Functionally redundant cytokines can reinduce AICD but only at supraphysiologic concentrations.46,47 Thus, excessive IL-2Rα blockade may predispose patients to recurrence of GVHD because of a lack of clonal deletion. This phenomenon may also explain why anti-IL-2Rα antibodies used for GVHD prophylaxis delayed but did not decrease the incidence of acute GVHD.43 49-52
In design of regimen 2, an additional dose of daclizumab was given early in the treatment period to saturate free sIL-2R and to maximize binding to cellular IL-2R, and immunosuppression was reduced earlier than in regimen 1 to avoid infectious complications. The dose-schedule of daclizumab 1 mg/kg iv days 1, 4, 8, 15, and 22 had a response rate of 47%, and a day 120 survival of 53% that compares favorably with ATG but without the toxicities of ATG.53 This regimen would be appropriate for a controlled trial.
Acknowledgments
We are grateful to Dr John Hakimi, who supplied the fluorochrome-conjugated daclizumab and 7G7 for the flow cytometry studies, and to Dr Claudio Anasetti for helpful advice throughout the project.
Supported in part by grants from Hoffmann-La Roche, Inc., and from the National Institutes of Health (CA16672).
Reprints:Donna Przepiorka, Baylor College of Medicine, Center for Cell and Gene Therapy, 6565 Fannin St., M964, Houston, TX 77030; e-mail: donnap@bcm.tmc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal