Abstract
Mutations in the gene encoding the common cytokine receptor gamma chain (γc) are responsible for human X-linked severe combined immunodeficiency disease (SCIDX1). We have used a γc-deficient mouse model to test the feasibility and potential toxicity of γc gene transfer as a therapy for SCIDX1. A retrovirus harboring the murine γc chain was introduced into γc-deficient bone marrow cells, which were then transplanted into alymphoid RAG2/γcdouble-deficient recipient mice. Circulating lymphocytes appeared 4 weeks postgraft and achieved steady-state levels by 8 weeks. The mature lymphocytes present in the grafted mice had integrated the γc transgene, expressed γc transcripts, and were able to proliferate in response to γc-dependent cytokines. The γc-transduced animals demonstrated (1) normal levels of immunoglobulin subclasses, including immunoglobulin G1 (IgG1) and IgG2a (which are severely decreased in γc- mice); (2) the ability to mount an antigen-specific, T-dependent antibody response showing effective in vivo T-B cell cooperation, and (3) the presence of gut-associated cryptopatches and intraepithelial lymphocytes. Importantly, peripheral B and T cells were still present 47 weeks after a primary graft, and animals receiving a secondary graft of γc-transduced bone marrow cells demonstrated peripheral lymphoid reconstitution. That γc gene transfer to hematopoietic precursor cells can correct the immune system abnormalities in γc- mice supports the feasibility of in vivo retroviral gene transfer as a treatment for human SCIDX1.
One of the most studied cytokine receptor deficiencies in man is X-linked severe combined immunodeficiency disease (SCIDX1), which results from defects in the common γ chain (γc). SCIDX1 accounts for 50%-60% of all cases of SCID (reviewed in 1) and is characterized by the complete absence of mature T and natural killer (NK) cells, whereas B cells are frequently present in increased numbers. The thymus and peripheral lymphoid organs are severely hypoplastic in patients with SCIDX1, suggesting an early block in T-cell differentiation. Following the co-localization of the gene encoding the γ chain of the interleukin-2 receptor (IL-2R; now denoted γc) to the SCIDX1 locus at Xq12-13.1, γc mutations were identified in a number of these patients,2 thereby demonstrating the essential roles of γc-dependent cytokines in human lymphoid development (reviewed in 3).
The γc chain was initially isolated as a functional component of the intermediate and high-affinity IL-2R.4Disruption of IL-2 mediated signaling, however, could not account for the SCIDX1 phenotype, because IL-2 deficiency was compatible with T-cell development.5 Further studies established that γc also participated in the receptors for IL-4, IL-7, IL-9, and IL-15 (reviewed in 6). The SCIDX1 phenotype, therefore, results from combined defects in these 5 cytokine systems. A T-cell developmental block similar to that seen in SCIDX1 can result from IL-7Rα-deficiency in man,7 suggesting that IL-7 is necessary for prothymocyte survival or expansion. In contrast, the NK-cell differentiation block results principally from defects in IL-15 signaling pathways, because this cytokine is required to promote NK-cell differentiation from bone marrow (BM) precursors.8Concerning human B-cell development, it appears that lymphoid precursors progress through γc-dependent stages, but γc-independent pathways can compensate in SCIDX1.
Without treatment, patients with SCIDX1 suffer from severe, recurrent infections, failure to thrive, and die within the first year of life. The recent results of Buckley et al9 clearly demonstrate that BM transplantation is the treatment of choice for SCIDX1, which is curative for those patients who have an HLA-identical donor. Haplo-identical transplants have also been performed, but they have a lower success rate and are plagued by poor B-cell reconstitution, thereby often necessitating long-term immunoglobulin replacement therapy.9 10
Gene therapy remains an attractive alternative therapy for SCIDX1. In principle, γc-transduced hematopoietic precursors should demonstrate a marked selective advantage for lymphoid differentiation. This hypothesis has been supported by several in vitro studies, demonstrating that human γc gene transfer into Epstein-Barr virus–immortalized SCIDX1 B-cells lines could reconstitute IL-2R signaling.11-13 Retroviral transduction of SCIDX1 CD34+ BM precursor cells can permit NK- or T-cell differentiation,14 although these results were obtained in vitro under conditions that might not be attainable in vivo.
The existence of canine15 and murine16-18models of γc deficiency offer the possibility to test gene therapy as an alternative treatment for SCIDX1. γc-Deficient mice are characterized by severe reductions in B cells, NK cells, and gut-associated intraepithelial lymphocytes (IEL). In contrast, mature activated T cells develop and accumulate in γc- mice, provoking inflammatory bowel disease and splenomegaly.19,20 Despite this abnormal T-cell development, γc- mice are functionally immunodeficient: (1) γc- lymphocytes fail to proliferate to mitogens or in mixed lymphocyte culture16,17; (2) γc- mice fail to reject tumors21; and (3) γc-mice fail to clear intracellular pathogens, such as Listeria monocytogenes and Toxoplasma gondii.22 23γc- Mice, therefore, recapitulate many features of patients with SCIDX1. In this study, we have used γc- mice to examine the feasibility of in vivo retroviral gene transfer to correct the immune system defects in this SCIDX1 mouse model.
Methods and materials
Mice
Mice with a targeted γc deletion16 were maintained in specific pathogen-free conditions in an isolator barrier facility (CNRS, Orleans, France) and were from the fourth generation backcross to the C57Bl/6 background. A novel alymphoid (T-, B-, NK-) mouse strain was generated by intercrossing recombinase activating gene (RAG)-2 deficient mice, and γc-deficient mice (RAG2/γcmice21) were used as recipients for the transplants. All mice were used at 4 to 12 weeks of age.
Cells
All cell lines were cultivated in DMEM supplemented with 10% fetal calf serum (FCS) and penicillin/streptomycin. The derivation of the retroviral packing cell line BOSC 23 and the transfection protocol used to generate infectious retrovirus has been described in detail.24 Transfectants expressing a soluble form of the murine stem cell factor and a hybridoma producing murine IL-6 were kindly provided by Genetics Institute and Dr. Van Snick, respectively. Cytokine-containing supernatants were used at optimal concentrations after titration on appropriate cytokine-dependent cell lines.
Production of retroviral particles and infection of BM precursors
Production of infectious, replication-defective ecotrophic retrovirus using BOSC 23 cells was performed according to an established protocol.24 A retroviral expression plasmid (pMX25) using long terminal repeat (LTR) sequences from the Moloney murine leukemia virus was engineered to express the murine γc chain. A full-length γc complementary DNA (cDNA) was amplified with the use of reverse transcription-polymerase chain reaction (RT-PCR) and normal splenocyte RNA, subcloned into the EcoRI site of pMX, and fully sequenced. Subconfluent BOSC 23 cells were transfected with 10 μg of pMX-γc by the calcium phosphate method.24Retrovirus-containing supernatants were recovered 48 hours posttransfection, filtered through 0.45 μm filters, and used directly for infection of BM cells. Viral titer was determined using NIH3T3 cells as described.12
Bone marrow hematopoietic precursors were isolated following intraperitoneal injection of 5-fluorouracil (5-FU at 150 mg/kg; 3 days prior to harvest). Cells flushed from femora and tibias of 5-FU-treated γc- donor mice were cultured at 106 cells/mL in X-Vivo-10 medium (BioWhittaker) supplemented with 5% FCS (Gibco BRL), murine stem cell factor (1/100 supernatant dilution), IL-6 (1/100 supernatant dilution), and Flt-3 ligand 100 ng/mL (kindly provided by Immunex Corp) with an equal volume of retroviral supernatant in the presence of 10 μg/mL of Polybrene (Sigma) on fibronectin-coated tissues culture plates at 37°C in 5% CO2 fully humidified incubators. Purified whole fibronectin (Sigma; dissolved at 100 μg/mL in phosphate-buffered saline) was used to coat plates for 2 hours at room temperature. This infection protocol was repeated daily for 2 more days; supernatants were removed, and nonadherent cells recovered and replated in fresh virus-containing medium. A mock transduction protocol was performed in exactly the same fashion, except that BOSC 23 cells received the parental pMX vector. Recovered nonadherent BM cells (2 × 106) were grafted into the tail vein of irradiated (0.3 Gy) RAG2/γc double mutant recipient animals. Total BM cells from primary recipient mice were used for secondary transfers.
Cell isolation, in vitro proliferation analysis, and immunofluorescence
Lymphoid organs were removed, and single-cell suspensions were prepared using a mesh filter. Hepatic lymphocytes are a rich source of NK cells and were analyzed as described.21 Splenocytes were cultured at 2 × 105 cells/well in flat-bottom 96 well plates in DMEM supplemented with 10% FCS with or without concanavalin A (Con A; 2.5 μg/mL) and γc-dependent cytokines (IL-2 or IL-7 at 20 ng/mL) for 72 hours. Thymocytes were similarly cultured in U-bottom 96 well plates with or without phorbol myristate acetate (PMA) (10 ng/mL) and IL-4 (100 ng/mL). Cells were pulsed with 0.5 μCi of 3H-thymidine during the final 16 hours of culture.
Immunofluorescence analysis was performed as described.16 21 Antibodies against the following cell surface antigens were used for immunofluorescence analysis (all from Pharmingen) as FITC-, PE-, or TRICOLOR conjugates: CD4, CD8, TCRαβ, TCRγδ, B220, immunoglobulin M (IgM), IgD, and DX5. A combination of anti-TCRαβ and anti-B220 antibodies were used to sort splenocytes, using a FACStar+ cytometer prior to DNA isolation.
PCR detection of the γc gene, transgene, and transcripts
For detection of the retrovirally transduced γctransgene, total splenocyte or sorted lymphocyte DNA was extracted, using proteinase K in polymerase chain reaction (PCR) buffer containing 0.1% Tween 20. Following enzyme inactivation, a transgene specific PCR was performed, using exon 6 (5′-CTTCCTTGTTTGCACTGG-3′) and exon 8 (5′-GGGGAGGTTAGCGTCACTTAGGAC-3′) primers amplify a 400-base pair (bp) γc cDNA fragment. For RT-PCR, total peripheral blood lymphocyte RNA was converted into first strand cDNA using standard procedures, and amplification was performed using the primers described above.
T-dependent antigen-specific immunoglobulin responses and total immunoglobulin levels
Animals were immunized intraperitoneally with 100 μg of alum-precipitated nitrophenyl (NP)-conjugated bovine serum albumin (NP-BSA) and 109 inactivated Bordatella pertussis. Pre-immune, day 7, and day 14 sera were collected. Levels of NP-specific IgM or IgG were determined by enzyme-linked immunosorbent assay (ELISA) as described.26 Briefly, ELISA plates were coated with 5 μg/mL NP-BSA overnight at 4°C and blocked with 5% BSA. Sera and standards were serially diluted and incubated for 1 hour at 37°C. Specific bound antibodies were revealed using isotype-specific anti-mouse antibodies directly conjugated to alkaline phosphatase followed by conversion of the chromogenic substrate p-NPP (Sigma). Total serum immunoglobulin isotype levels were determined using an ELISA kit (Pharmingen) according to the manufacturer's instructions. Statistical analysis was performed using the Studentt test.
Histological analysis
Tissues (1-cm piece of small bowel) were fixed in Carnoy solution and embedded in paraffin. Sections were stained with methyl/pyronin or by the periodic acid-Schiff reaction. Intraepithelial lymphocytes were enumerated as described.16
Results
Study design and peripheral lymphoid reconstitution in γc-transduced mice
Bone marrow cells were harvested from 5-FU-treated γc- mice and infected with retroviral particles (titer: 106/mL) harboring the murine γc chain driven by the MoMLV LTRs.25 Three 1-day infection cycles were performed before transfer into alymphoid recipient animals (a new strain deficient in both the RAG2 and γc genes: RAG2/γc mutant mice). RAG2/γc rather than γc- mice were used as hosts because the latter demonstrate abnormal T-cell development that has been shown to perturb steady-state hematopoiesis and provoke splenomegaly.19 RAG2/γc mice have no mature T, B, or NK cells but have otherwise normal hematopoietic parameters,21 thereby permitting donor lymphopoeisis to be determined in an unambiguous fashion. RAG2/γc mice (n = 7) that had been transplanted with retrovirally infected γc- BM cells will be referred to as “γc-transduced” mice. Lymphoid reconstitution in an equivalent number of RAG2/γc mice that had been transplanted with mock infected γc- BM cells gave an immunophenotype and function identical to nonmanipulated γc- mice (data not shown). We have previously demonstrated lymphoid reconstitution in the RAG2/γc strain, using normal BM-derived hematopoietic precursors.21
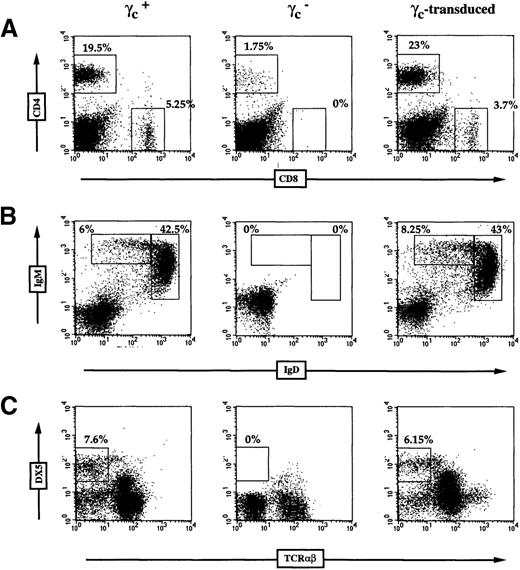
Peripheral lymphoid cells were analyzed in γc-transduced mice as well as γc, RAG2/γc, and control C57Bl/6 (γc+) mice. As previously reported,16-18 γc- mice developed some mature T cells but very few B cells (Figure1A), whereas circulating lymphoid cells were not detected in the RAG2/γc mutants. In γc-transduced mice, mature T and B cells could be detected as soon as 4 weeks after the transplant, and, by 12 weeks postgraft, steady-state levels had been reached (Figure 1A). The kinetics and magnitude of lymphoid reconstitution observed in γc-transduced mice were comparable to that observed following transplantation of unmanipulated or mock-infected normal BM into RAG2/γc mice21 (Table1 and data not shown). Among individual γc-transduced mice, some variation between relative proportions of T and B cells could be documented, yet all animals showed peripheral lymphoid reconstitution (Table 1). These results contrast sharply with the immune phenotype in γc-deficient mice, whereby B lymphocyte numbers decline with age.19
Lymphoid reconstitution in γc-transduced mice.
(A) Flow cytometric analysis of peripheral blood T and B cells, using FITC-conjugated anti-TCRαβ and PE-conjugated anti-B220 antibodies. In these experiments, lymphocytes expressing T- or B-cell markers are calculated as a percentage of total nucleated cells to emphasize the kinetics of lymphoid reconstitution. (B-E) Analysis of γctransgene integration and expression in γc-transduced animals. Polymerase chain reaction (PCR) was performed using exon 6- and exon 8-specific primers (B). The endogenous γc locus (0.8 kilobase [kb]) is amplified in wild-type mice but not in γc- mice in which exon 6 has been deleted.16 γc-Transduced mice show a 0.4-kb product derived from the integrated γc transgene at 23 weeks postgraft. (C) Expression of the γc transgene was detected by RT-PCR from peripheral blood cells of control and γc-transduced animals at 7 weeks postgraft. Contaminating genomic DNA in the γc+ sample gives rise to a PCR product in the absence of reverse transcription. (D) Schematic of the retroviral construct used with the location of transmembrane (exon 6) and intracytoplasmic (exon 8) primers. (E) Expression of γc on γc+, γc-, and γc-transduced cells. Staining of total thymocytes with isotype control monoclonal antibody (dotted line) and γc-specific monoclonal antibody (solid line) are shown.
Lymphoid reconstitution in γc-transduced mice.
(A) Flow cytometric analysis of peripheral blood T and B cells, using FITC-conjugated anti-TCRαβ and PE-conjugated anti-B220 antibodies. In these experiments, lymphocytes expressing T- or B-cell markers are calculated as a percentage of total nucleated cells to emphasize the kinetics of lymphoid reconstitution. (B-E) Analysis of γctransgene integration and expression in γc-transduced animals. Polymerase chain reaction (PCR) was performed using exon 6- and exon 8-specific primers (B). The endogenous γc locus (0.8 kilobase [kb]) is amplified in wild-type mice but not in γc- mice in which exon 6 has been deleted.16 γc-Transduced mice show a 0.4-kb product derived from the integrated γc transgene at 23 weeks postgraft. (C) Expression of the γc transgene was detected by RT-PCR from peripheral blood cells of control and γc-transduced animals at 7 weeks postgraft. Contaminating genomic DNA in the γc+ sample gives rise to a PCR product in the absence of reverse transcription. (D) Schematic of the retroviral construct used with the location of transmembrane (exon 6) and intracytoplasmic (exon 8) primers. (E) Expression of γc on γc+, γc-, and γc-transduced cells. Staining of total thymocytes with isotype control monoclonal antibody (dotted line) and γc-specific monoclonal antibody (solid line) are shown.
Lymphoid reconstitution in γc-transduced mice
| Mouse Strain . | Peripheral Blood Percentages . | Spleen Cells (×106) . | Gut Cells % IEL . | ||
|---|---|---|---|---|---|
| Lymphocytes . | B Cells . | T Cells . | |||
| C57B1/6 (γc+) | 80 ± 12 | 39 ± 13 | 55 ± 12 | 60 ± 9 | 13 ± 4 |
| γc− | 8 ± 2 | 0.5 ± 0.1 | 10 ± 3.0 | 15 ± 11 | 0.3 ± 0.1 |
| RAG2/γc− | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.5 ± 0.2 | 0 ± 0 |
| γc-transduced | 48 ± 17 | 56 ± 33 | 40 ± 15 | 45 ± 17 | 4.3 ± 0.5 |
| γc+BM → RAG2/γc− | 79 ± 5.5 | 47 ± 3 | 47 ± 3 | 59 ± 15 | 15 ± 9 |
| Mouse Strain . | Peripheral Blood Percentages . | Spleen Cells (×106) . | Gut Cells % IEL . | ||
|---|---|---|---|---|---|
| Lymphocytes . | B Cells . | T Cells . | |||
| C57B1/6 (γc+) | 80 ± 12 | 39 ± 13 | 55 ± 12 | 60 ± 9 | 13 ± 4 |
| γc− | 8 ± 2 | 0.5 ± 0.1 | 10 ± 3.0 | 15 ± 11 | 0.3 ± 0.1 |
| RAG2/γc− | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.5 ± 0.2 | 0 ± 0 |
| γc-transduced | 48 ± 17 | 56 ± 33 | 40 ± 15 | 45 ± 17 | 4.3 ± 0.5 |
| γc+BM → RAG2/γc− | 79 ± 5.5 | 47 ± 3 | 47 ± 3 | 59 ± 15 | 15 ± 9 |
Results are the mean of 7 animals of each genotype. Peripheral blood cells were analyzed by staining with antibodies specific for B (anti-B220) and T (anti-CD3) cell antigens. Gut intraepithelial lymphocytes (IEL) were enumerated on histological sections and expressed per 100 epithelial cells. BM = bone marrow; RAG = recombinase activating gene.
Integration and expression of retrovirally transduced γc transgene
To verify the presence and expression of the retroviral γc transgene in γc-transduced animals, genomic DNA and RNA were prepared from either total peripheral blood cells, total splenocytes, or from sorted splenic T and B cells. A PCR was then performed, using primers specific for exons 6 and 8 of the γc gene (Figure 1B-D). These primer pairs amplify a 0.8-kilobase (kb) fragment from the wild-type γc locus and a 0.4-kb fragment from the γc cDNA transgene or endogenous transcript, but they do not amplify the targeted γc locus lacking exon 6.16
A PCR product (0.4 kb) corresponding to the size expected for γc cDNA transgene was detected, using DNA derived from peripheral blood of all γc-transduced mice (data not shown). Genomic retroviral integration could be demonstrated in total splenocyte DNA from 7 of 7 γc-transduced animals at 7 to 47 weeks postgraft (Figure 1B; data not shown) and was also detected in sorted splenic lymphocytes from 2 of 2 animals tested at 23 weeks postgraft. No amplification products were found in mock-transduced animals, and, in C57Bl/6 mice, only the expected fragment corresponding to the wild-type γc locus was detected (Figure 1B; data not shown). Moreover, γc-transduced animals (5 of 5 tested) expressed transgene-specific γc transcripts of the expected size in peripheral blood cells (Figure 1C). Finally, γc expression could be detected on the surface of γc-transduced cells (Figure 1E). We conclude that retroviral infection of γc- BM precursors can generate peripheral lymphoid cells with stable integration and expression of the transduced γc transgene.
Phenotype and function of peripheral lymphocytes from γc-transduced mice
Splenic-cell populations were further characterized in γc-transduced animals. The total number of splenocytes was significantly increased in γc-transduced animals compared with RAG2/γc recipients or to the γc- donor mice (Table 1). A normal ratio of CD4- to CD8-expressing cells was observed in 7 of 7 γc-transduced animals (Figure2A); these cells expressed normal levels of TCRαβ (data not shown). Splenocytes from these mice demonstrated a normal pattern of IgM and IgD expression, indicating the presence of both newly generated and recirculating mature B cells (Figure 2B; these cells co-expressed CD19, data not shown). In contrast, CD3+TCRγδ T cells were detected in 3 of 7 γc-transduced animals (data not shown), while DX5+ TCRαβ-NK cells were present in 3 of 7 γc-transduced mice (Figure 2C). One γc-transduced mouse harbored both TCRγδ cells and NK cells (data not shown). Why γδ T cells and NK cells were found in only a fraction of γc-transduced mice remains unclear but may be related to the level or timing of transgene expression by the retroviral promoter.
Characterization of splenic and gut lymphocytes from γc-transduced mice.
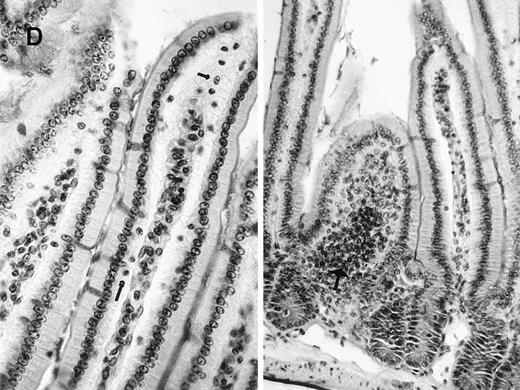
Flow cytometric analysis of splenocytes using (A) FITC-conjugated anti-CD8 and PE-conjugated anti-CD4 antibodies or (B) FITC-conjugated anti-immunoglobulin D (IgD) and PE-conjugated anti-IgM antibodies. (C) Analysis of hepatic lymphocytes using FITC-conjugated anti-DX5 (natural killer cell specific) and PE-conjugated anti-TCRαβ antibodies. Positive cells are expressed as percentage of gated lymphocytes. (D) Representative sections of small bowel epithelium in a γc-transduced mouse (Periodic Acid Shiff staining, × 250). Intraepithelial lymphocytes (arrows), normal cellularity of the lamina propria, and lymphoid cryptopatches (large arrow) were identified that were indistinguishable from control γc+ animals16 (and data not shown).
Characterization of splenic and gut lymphocytes from γc-transduced mice.
Flow cytometric analysis of splenocytes using (A) FITC-conjugated anti-CD8 and PE-conjugated anti-CD4 antibodies or (B) FITC-conjugated anti-immunoglobulin D (IgD) and PE-conjugated anti-IgM antibodies. (C) Analysis of hepatic lymphocytes using FITC-conjugated anti-DX5 (natural killer cell specific) and PE-conjugated anti-TCRαβ antibodies. Positive cells are expressed as percentage of gated lymphocytes. (D) Representative sections of small bowel epithelium in a γc-transduced mouse (Periodic Acid Shiff staining, × 250). Intraepithelial lymphocytes (arrows), normal cellularity of the lamina propria, and lymphoid cryptopatches (large arrow) were identified that were indistinguishable from control γc+ animals16 (and data not shown).
To determine whether peripheral T lymphocytes present in γc-transduced animals expressed functional γc-containing receptor complexes, splenocytes were stimulated with Con A alone or in the presence of the γc-dependent cytokines IL-2 or IL-7 (Table2). We have previously demonstrated that IL-2 and IL-7 do not promote the proliferation of γc-deficient cells.16 In contrast, IL-2 or IL-7 stimulated the Con A mitogenic response of splenocytes from 3 of 3 γc-transduced animals (Table 2). Thymocytes from γc-transduced mice also responded to the combination of IL-4 plus PMA, unlike γc- thymocytes (Table2). These results demonstrate that γc gene transfer can restore expression of functional IL-2, IL-4, and IL-7 receptors on T cells from γc-transduced animals.
Lymphoid cell proliferation in γc-transduced mice
| Mouse . | Splenocytes . | Thymocytes PMA + IL-4 . | ||
|---|---|---|---|---|
| Con A . | Con A + IL-2 . | Con A + IL-7 . | ||
| C57B1/6 (γc+) | 3802 ± 575 | 33 440 ± 3720 | 55 354 ± 2738 | 12 261 ± 559 |
| γc− | 1432 ± 15 | 1494 ± 273 | 1567 ± 131 | 215 ± 70 |
| γc-transduced | 2411 ± 636 | 11 024 ± 1415 | 18 236 ± 2038 | 13 313 ± 747 |
| Mouse . | Splenocytes . | Thymocytes PMA + IL-4 . | ||
|---|---|---|---|---|
| Con A . | Con A + IL-2 . | Con A + IL-7 . | ||
| C57B1/6 (γc+) | 3802 ± 575 | 33 440 ± 3720 | 55 354 ± 2738 | 12 261 ± 559 |
| γc− | 1432 ± 15 | 1494 ± 273 | 1567 ± 131 | 215 ± 70 |
| γc-transduced | 2411 ± 636 | 11 024 ± 1415 | 18 236 ± 2038 | 13 313 ± 747 |
Results are the mean of triplicate cultures. Representative of two independent experiments. Con A = concanavalin A; IL = interleukin; PMA = .
B-cell responses in γc-transduced animals
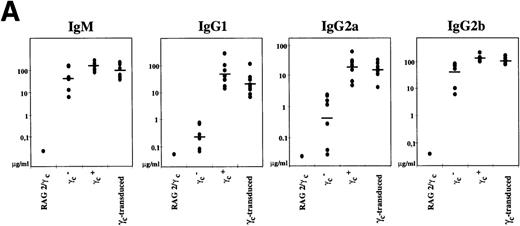
To assess B-cell function in γc-transduced mice, we first analyzed steady-state circulating plasma immunoglobulin levels in animals >8 weeks posttransplant. γc- mice exhibit abnormal serum immunoglobulin levels, reflecting defective B-cell differentiation.16 17 In particular, γc- mice have extremely reduced levels of IgG1 and a 2 log reduction in the concentrations of serum IgG2a (Figure3A). Following γc gene transfer, we found a normal concentration of all serum immunoglobulin levels tested in γc-transduced animals (Figure 3A). Levels of IgG1 and IgG2a were not significantly different from control C57Bl/6 mice (P = .21 for IgG1 and P = .35 for IgG2a). Sera from RAG2/γc mice were negative for all immunoglobulin subclasses.
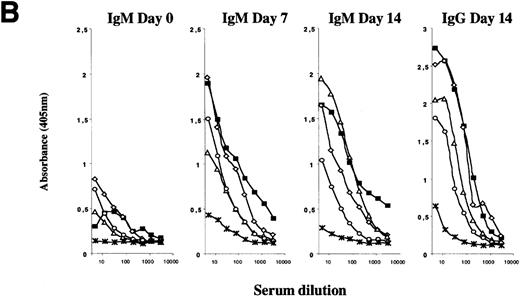
B-cell responses in γc-transduced animals.
(A) Serum immunoglobulin isotype concentrations were established by enzyme-linked immunosorbent assay (ELISA) using purified immunoglobulin standards. Each dot represents 1 mouse (7 animals for each strain listed, except for RAG2/γc mice). (B) Normal T-dependent antigen immunoglobulin responses in γc-transduced animals. Following immunization with nitrophenyl-conjugated bovine serum albumin, circulating NP14-specific immunoglobulin M (IgM) and IgG antibodies were determined by ELISA for control (black square), 3 independent γc-transferred animals (open symbols), and γc- mice (-X-). Only 1 each of control and γc- mice is shown for simplicity; 3 additional mice of each genotype gave similar results.
B-cell responses in γc-transduced animals.
(A) Serum immunoglobulin isotype concentrations were established by enzyme-linked immunosorbent assay (ELISA) using purified immunoglobulin standards. Each dot represents 1 mouse (7 animals for each strain listed, except for RAG2/γc mice). (B) Normal T-dependent antigen immunoglobulin responses in γc-transduced animals. Following immunization with nitrophenyl-conjugated bovine serum albumin, circulating NP14-specific immunoglobulin M (IgM) and IgG antibodies were determined by ELISA for control (black square), 3 independent γc-transferred animals (open symbols), and γc- mice (-X-). Only 1 each of control and γc- mice is shown for simplicity; 3 additional mice of each genotype gave similar results.
B-cell immunoglobulin responses were evaluated, following immunization with the T cell-dependent antigen NP-BSA. As shown in Figure 3B, normal titers of circulating anti-NP-BSA-specific IgM antibodies could be detected in γc-transduced animals by day 7 postimmunization and persisted at day 14. Moreover, switched IgG antibodies could be detected in these mice at day 14. In contrast, immunization of γc- mice, using either T-dependent or T-independent antigens, fails to elicit any antigen-specific immunoglobulin (Figure 3B; Vosshenrich et al, submitted). Taken together, these data demonstrate that γc-transduced animals harbor mature B cells and T cells capable of functional immune responses.
Restoration of the gut-associated lymphoid cells following γc gene transfer
Distinct morphological structures located in between the intestinal crypts (cryptopatches) contain cells with an immature lymphoid phenotype (c-kit+, IL-7Rα+) that appear to play a role in the generation of some IEL T-cell subsets.27,28 Cryptopatch generation requires the IL-7Rα chain28 or the γc chain (our unpublished observations). The development of all IEL subsets is γcdependent as well.16
Gut-associated lymphoid cells developed in γc-transduced mice. Cryptopatches were detected in 7 of 7 γc-transduced animals (Figure 2D). Their morphological structure was comparable to that of control C57Bl/6 mice (data not shown). In addition, IELs could be detected in the γc-transduced animals (Figure 2D, Table 1), whereas IELs have never been observed in γc- mice.29 Together, these observations suggest that γc gene transfer into hematopoietic precursors capable of generating intestinal lymphoid cells had occurred.
Stability of retroviral gene expression in γc-transduced animals
Two γc-transduced animals were analyzed at 40 or 47 weeks after the initial transplant. In these animals, we were able to detect normal percentages of circulating mature B and T cells (Figure 4). The retrovirally transduced γc transgene was detected in splenocyte DNA from these mice, and development of gut-associated lymphoid cells had occurred (data not shown). No pathological effects of gene transfer were observed in γc-transduced animals analyzed during the course of this study.
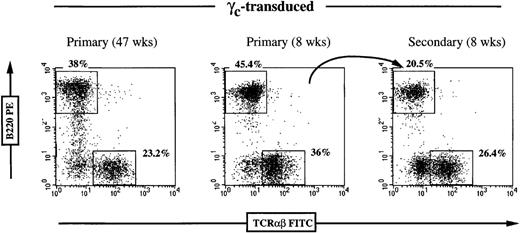
Stability of lymphoid reconstitution in γc-transduced mice.
Flow cytometric analysis of peripheral blood T and B cells using FITC-conjugated anti-TCRαβ and PE-conjugated anti-B220 antibodies. Positive cells are expressed as percentage of gated lymphocytes. The left panel demonstrates long-term peripheral reconstitution 47 weeks posttransfer with normal percentage of T and B cells. The center panel shows a γc-transduced animal at 8 weeks postgraft. Bone marrow cells from this mouse were transferred into a secondary irradiated recipient, which, after 8 weeks, demonstrated normal peripheral reconstitution (right panel).
Stability of lymphoid reconstitution in γc-transduced mice.
Flow cytometric analysis of peripheral blood T and B cells using FITC-conjugated anti-TCRαβ and PE-conjugated anti-B220 antibodies. Positive cells are expressed as percentage of gated lymphocytes. The left panel demonstrates long-term peripheral reconstitution 47 weeks posttransfer with normal percentage of T and B cells. The center panel shows a γc-transduced animal at 8 weeks postgraft. Bone marrow cells from this mouse were transferred into a secondary irradiated recipient, which, after 8 weeks, demonstrated normal peripheral reconstitution (right panel).
To determine whether stable integration of the γctransgene could be achieved in γc-hematopoietic stem cells, we transplanted 106 BM cells from 3 different γc-transduced mice (at 8 to 12 weeks postgraft) into secondary RAG2/γc recipient mice. The primary γc-transduced mice demonstrated normal circulating B and T cells at this time, and, on secondary transfer, the γc-transduced BM inoculum could again give rise to normal percentages of mature B and T cells (a representative example is shown in Figure 4). A total of 8 mice were analyzed up to 23 weeks following secondary transfers, and the kinetics and stability of peripheral lymphoid reconstitution were similar between primary and secondary recipients (data not shown).
Discussion
In this study we have shown that ex vivo γc gene transfer into hematopoietic precursor cells from γc-deficient mice can lead to a correction of the immune deficiency without obvious side effects. Following transfer of the retrovirally transduced γc- BM cells to alymphoid recipients, we found that engrafted animals generated peripheral lymphoid cells that stably integrated and expressed the γc transgene. Both mature B cells and T cells were present in the recipients: γc-transduced lymphocytes responded to γc-dependent cytokines and were capable of generating a cooperative (B-T cell) immune response following antigen immunization. Finally, γc-transduced mice showed development of gut-associated lymphoid cells, the presence of which is entirely dependent on expression of the γcchain.16 Taken together, these results demonstrate the feasibility of γc gene transfer ex vivo and fulfill an important prerequisite for further clinical studies of γcgene therapy for patients with SCIDX1.
The retroviral infection protocol used in our model was not aimed at optimizing gene transfer conditions. Nevertheless, cytokine stimulation of BM progenitor cells followed by 3 cycles of infection (using viral supernatants and fibronectin-coated plates) was found to work efficiently as previously documented.30-32 Importantly, this protocol closely matches current conditions established for treatment of patients with SCIDX1 by BM transplantation or ex vivo gene transfer, including noncytoablative host conditioning.9 The observations that distinct T-cell subsets, B cells, and IEL-cell populations were stably reconstituted in γc-transduced mice up to 47 weeks postprimary transplant as well as on secondary marrow transplantation (up to 23 weeks after transfer) strongly suggest that early hematopoietic progenitor cells with self-renewal capacity were successfully infected. It should be noted that both primary and secondary RAG2/γc recipients were not subjected to lethal irradiation, so that competition between endogenous hematopoietic cells and transduced donor cells could well have occurred.
A human autosomal SCID syndrome due to JAK-3 deficiency has been described that is immunologically identical to SCIDX1,33,34thereby highlighting the requirement for JAK-3 activation following γc-receptor signaling. Recently, a mouse model of JAK-3 deficiency was successfully treated by ex vivo gene transfer with the generation of peripheral T and B cells that were functionally responsive to γc-dependent cytokines.35 Thus, ex vivo retroviral gene transfer appears capable of correcting immune deficiencies secondary to γc or JAK-3 defects.
It is important to note that, in these experiments, the transduced genes were constitutively expressed under the control of the viral LTR. Dysregulated γc or JAK-3 signaling could in theory lead to autonomous cell activation caused by receptor or kinase overexpression. However, in the case of γc-transduced mice, no evidence of myelo- or lymphoproliferation or autoimmune manifestations was observed in the long-term reconstituted animals. The absence of side effects could reflect the low level of γc expression detected in the treated animals. Still, the lymphoid system of γc-transduced mice appeared functional by a number of criteria (in vitro proliferative responses, specific immunoglobulin production following immunization), suggesting that high-level γc expression may not be a requisite for immune reconstitution. Because γc is constitutively expressed in multiple hematopoeitic lineages (reviewed in 36), a potential protein excess might not have negative consequences. These results suggest that (1) regulated transgene expression may not be required for the generation of functional lymphoid cells from γc-hematopoietic precursors and that (2) retroviral gene transfer to correct deficiencies in the γc-signaling pathways may have low-potential toxicities, which is an important consideration for any new therapeutic approaches.
Although secondary extinction of retroviral transgenes has been reported,37-39 extinction of the γc transgene was not observed in this study, including mice receiving secondary transplants and analyzed 23 weeks later. Whether extinction is a randomly occurring event or requires a particular chromatin conformation around the proviral integration site is not known. It is, therefore, difficult to predict from this animal model of SCIDX1 what the outcome of gene therapy in patients with SCIDX1 will be with regard to long-term expression from retroviral vectors. Natural selection of γc-expressing cells may be an important factor in preserving transgene expression in vivo following gene transfer. It is worthwhile noting that γc-transduced SCIDX1 Epstein-Barr virus–transformed B-cell lines were maintained for more than 1 year in culture without evidence of loss of the γc transgene expression.12
Spontaneous in vivo reversion of inherited mutations has been observed in patients with ADA and SCIDX1 (reviewed in 1). In the latter case, the γc reversion resulted in a significant and long-lasting correction of the T-cell deficiency. This result strongly suggests that a γc+ lymphoid precursor carries a major survival and/or growth advantage over γc- cells in vivo and are in agreement with the original observations of skewed X-inactivation patterns in SCIDX1 obligate female carriers made by Puck et al.40 These observations provide a logical basis to hypothesize that a selective advantage would be conferred to corrected progenitor cells in patients with SCIDX1. The results obtained in γc- and JAK3-deficient mice support the notion that human SCID syndromes represent a rather favorable model for treatment by ex vivo gene transfer.
Acknowledgments
We are grateful to Dr. Francois Huetz for providing immunization reagents, Fabian Gross for fibronectin, Françoise Selz for cell sorting, and Michele Malassis for technical help.
Supported by grants from the Institut National de la Santè et de la Recherche Medicale (INSERM), Ligue Contre le Cancer, and Association Francaise contre les Myopathies.
Reprints:James P. Di Santo, Unité des Cytokines et Développement Lymphoide, Institut Pasteur, 25 rue du Dr Roux, F-75 724 Paris, France; e-mail: disanto@pasteur.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Lymphoid reconstitution in γc-transduced mice. / (A) Flow cytometric analysis of peripheral blood T and B cells, using FITC-conjugated anti-TCRαβ and PE-conjugated anti-B220 antibodies. In these experiments, lymphocytes expressing T- or B-cell markers are calculated as a percentage of total nucleated cells to emphasize the kinetics of lymphoid reconstitution. (B-E) Analysis of γctransgene integration and expression in γc-transduced animals. Polymerase chain reaction (PCR) was performed using exon 6- and exon 8-specific primers (B). The endogenous γc locus (0.8 kilobase [kb]) is amplified in wild-type mice but not in γc- mice in which exon 6 has been deleted.16 γc-Transduced mice show a 0.4-kb product derived from the integrated γc transgene at 23 weeks postgraft. (C) Expression of the γc transgene was detected by RT-PCR from peripheral blood cells of control and γc-transduced animals at 7 weeks postgraft. Contaminating genomic DNA in the γc+ sample gives rise to a PCR product in the absence of reverse transcription. (D) Schematic of the retroviral construct used with the location of transmembrane (exon 6) and intracytoplasmic (exon 8) primers. (E) Expression of γc on γc+, γc-, and γc-transduced cells. Staining of total thymocytes with isotype control monoclonal antibody (dotted line) and γc-specific monoclonal antibody (solid line) are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/10/10.1182_blood.v95.10.3071/5/m_bloo01006001w.jpeg?Expires=1765896324&Signature=qIKgJ8Im~zN6asUIKTrkeG0UV7-4Ag0ugn18~qboRULs0Pp3Sm7tA3TQLMKJYQ-ShtOavvtN49Vyedc6ka02Xh4BKvB~9~l2ABIVPYWYY~wnZd7~07jfiPT~1hGu8Nm3lPz4M6kgX9x-KulIFcEKqwyG3Bd3cRg43fbBvHP2GdUr-cgImlneOFuJ4uNGm-mO9nW3VZc6ub853vTozJ6phCGCaZCMHAQ7oM8P-ns8ay2-nn6rF3WyYQum3aTiWbO-D3vLol54wRI4Lb9xs3aqSr9j3qLoW3YJTTzj2DiCfRFS3bw1bpHQZg2jOtRC4tUC71up7VB150aSLA34bYbgOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal