Abstract
We examined the feasibility of a community blood bank granulocyte transfusion program utilizing community donors stimulated with a single-dose regimen of subcutaneous granulocyte colony-stimulating factor (G-CSF) plus oral dexamethasone. The recipients of these transfusions were neutropenic stem cell transplantation patients with severe bacterial or fungal infection. Nineteen patients received 165 transfusions (mean 8.6 transfusions/patient, range 1-25). Community donors provided 94% of the transfusions; relatives accounted for only 6% of the transfusions. Sixty percent of the community donors initially contacted agreed to participate, and 98% of these individuals indicated willingness to participate again. Transfusion of 81.9 ± 2.3 × 109 neutrophils (mean ± SD) resulted in a mean 1-hour posttransfusion neutrophil increment of 2.6 ± 2.6 × 103/μL and restored the peripheral neutrophil count to the normal range in 17 of the 19 patients. The buccal neutrophil response, a measure of the capacity of neutrophils to migrate to tissue sites in vivo, was restored to normal in most patients following the transfusion. Chills, fever, and arterial oxygen desaturation of ≥ 3% occurred in 7% of the transfusions, but these changes were not sufficient to limit therapy. Infection resolved in 8 of 11 patients with invasive bacterial infections or candidemia. These studies indicate that transfusion of neutrophils from donors stimulated with G-CSF plus dexamethasone can restore a severely neutropenic patient's blood neutrophil supply and neutrophil inflammation response. Further studies are needed to evaluate the clinical efficacy of this therapy.
Neutropenia is a major risk factor for the development of severe bacterial and fungal infections in patients undergoing hematopoietic stem cell transplantation and intensive chemotherapy of malignant diseases.1 Despite modern antibiotics and the use of hematopoietic growth factors to reduce the period of posttreatment neutropenia, infection, particularly fungal infection, remains a major cause of morbidity and mortality in these patients.2 3
Transfusion of normal neutrophils is a logical approach to the treatment of infections in neutropenic patients. Methods for collecting cells from normal donors have been available for more than 25 years.4,5 After a flurry of interest beginning in the 1970s, however, interest in neutrophil transfusion therapy declined rapidly for several reasons. First, significant improvements were made in antibiotic therapy for bacterial infections. Second, although clinical trials in aggregate suggested that neutrophil transfusion therapy is efficacious, the findings were not consistent, and the clinical effects in individual patients were rarely obvious.6 Third, conflicting reports were made of adverse effects attributable to neutrophil transfusion, including serious pulmonary reactions.4 6 All of these factors led clinicians to conclude that this approach to the management of infections in neutropenic patients was of little benefit, although the problem of bacterial and fungal infections in this setting had not been solved.
One possible explanation for the inconsistent results with neutrophil transfusion trials is that inadequate doses of functional neutrophils were provided.6 In the average adult, neutrophil production in the uninfected state is approximately 60 × 109cells/day, a figure that is probably increased several fold in the face of serious infection.7 Even with modern apheresis machines and corticosteroid stimulation of donors, collections yield at best approximately 20-30 × 109 neutrophils. Transfusion of these cells only transiently increases the blood neutrophil count above a few hundred cells/μL. This dose of cells is probably inadequate to treat an established infection according to evidence from several sources. Animal studies have shown that the survival of neutropenic animals with infections depends on the dose of neutrophils provided.8 The results of the early trials of neutrophil transfusion therapy indicated that the dose of normal cells delivered was one of the factors separating the positive trials from the negative trials.6 When neutrophil transfusions are given to neonates with sepsis, both clinical outcomes and posttransfusion neutrophil increments appear to be dose dependent.9
We have recently investigated the combined use of granulocyte colony-stimulating factor (G-CSF) plus dexamethasone to increase the yield and to maintain the function of neutrophils collected for transfusion therapy.10 This study showed that 80 × 109 neutrophils can be collected following this stimulus and that the functions of these cells are not compromised by the collection procedure. We now report on the use of neutrophils obtained from G-CSF/dexamethasone-stimulated community donors for transfusion support of neutropenic patients undergoing bone marrow or stem cell transplantation. The goals of the study were to examine (1) the feasibility of recruiting community apheresis donors for G-CSF administration, (2) the neutrophil yields that could be obtained by using such a strategy, (3) hematologic effects in transfusion recipients, (4) in vivo function of transfused neutrophils, and (5) donor and patient safety. Information on patient clinical outcome was also obtained.
Patients and methods
Study design
This study was designed primarily as a phase I trial to determine the feasibility and safety of a community blood bank–based neutrophil transfusion program employing community donors stimulated with a regimen consisting of single doses of subcutaneous G-CSF and oral dexamethasone administered simultaneously. This protocol was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center. Informed consent was obtained from all donors and recipients (patients) before enrollment in the study protocol.
Neutrophil donors and recipients
Donors were selected from the community pool of apheresis donors of the Puget Sound Blood Center (PSBC; Seattle, WA). In unusual circumstances, family members of a patient volunteered to serve as donors. All donors were selected to be ABO compatible with the recipient patients. All donors for cytomegalovirus seronegative patients were also cytomegalovirus seronegative. Regular donor recruiters employed by the PSBC contacted the donors by telephone and informed them that neutrophils were needed for a patient. The donors were invited to participate in a research study to substantially increase the quantity of cells that could be collected by centrifugation leukapheresis. The donors were also informed that participation would involve the administration of G-CSF subcutaneously, in addition to dexamethasone administered orally. For donors agreeing to participate in the protocol, the study was explained in further detail by the research study coordinator, and informed consent was obtained prior to entry. Each donor received dexamethasone 8 mg orally and G-CSF 600 μg subcutaneously approximately 12 hours before the scheduled collection. G-CSF was provided by Amgen Corporation (Thousand Oaks, CA).
Recipients of the neutrophil transfusions were hospitalized patients at the Fred Hutchinson Cancer Research Center (Seattle, WA). These patients had documented fungal or resistant bacterial infections and were either awaiting or had recently undergone hematopoietic stem cell (either marrow or peripheral blood stem cell) transplantation. In addition, all patients were severely neutropenic (< 100 neutrophils/μL of peripheral blood) and were expected to remain neutropenic for more than 5 days. As soon as the relevant infection was documented, an attempt was made to supply the patient with daily neutrophil transfusions for as long as deemed clinically necessary by the patient's attending physician. Transfusions were discontinued for one of the following reasons: (1) probable engraftment, (2) clearance of infection, (3) deterioration of the patient's condition to the point that further support was deemed futile by the patient's attending physician, or (4) death. Probable engraftment was defined as the point at which the patient's morning absolute neutrophil count exceeded 500 cells/μL of blood in the absence of neutrophil transfusion support. Neutrophils were given in addition to standard antimicrobial treatment (ie, amphotericin B products and combination antibiotic treatment), depending on the underlying conditions.
For bacteremia and fungemia (eg, candidemia), complete response was defined as clearance of infection documented by the conversion of blood cultures from positive to negative during neutrophil transfusion therapy. For invasive mold infections, complete response to therapy was defined as stabilization or improvement in infiltrates/masses by computed tomographic (CT) imaging of the site of infection. Partial response to therapy was defined as either (1) clearance of the original organism from the bloodstream but development of additional infection(s) during neutrophil transfusion therapy or (2) improvement or stabilization of original CT findings but development of additional infection(s) during neutrophil transfusion therapy. Failure of therapy was defined as (1) persistently positive blood cultures, (2) progressive worsening of CT findings, or (3) persistence of infection at the time of death.
Neutrophil collections
Neutrophils were collected by standard centrifugation leukapheresis (COBE Spectra, Lakewood, CO) utilizing hetastarch (ratio: 1 part 6% hetastarch [McGraw/Dupont Pharma, Wilmington, DE] to 13 parts blood) with processing of 10 L of blood in approximately 3 hours using peripheral venous access, as described previously.10 11 The collected granulocyte product was maintained at room temperature and irradiated with 2500 cGy from a cesium source (Gamma Cell, MDS Nordium, Canata, Ontario, Canada).
Neutrophil transfusions
Neutrophils were transfused to patients over a 1- to 2-hour period as soon as possible following collection. The patient's total leukocyte count and absolute neutrophil count were determined immediately before transfusion, at 1 and 4 hours following completion of the transfusion, and on the following morning. Vital signs (temperature, blood pressure, heart rate, respiratory rate) and pulse oximetry measures were determined immediately before transfusion and at 1 and 2 hours posttransfusion. During the transfusion, patients were continuously monitored by pulse oximetry, and vital signs were obtained every 15 minutes. In patients receiving intravenous amphotericin B for treatment of fungal infections, neutrophils were transfused at least 8 hours apart from the administration of amphotericin B.
Extravascular migration of transfused neutrophils
The ability of transfused neutrophils to migrate to an extravascular site was assessed by measuring the number of neutrophils that accumulated in the oral cavity on the morning following a transfusion, as previously described.12 Patients were given 25 mL of normal saline and asked to use it to rinse their mouth thoroughly. The mouthwash was collected, the volume measured, and the leukocyte content determined by counting the cells in a hemocytometer following staining with acridine orange. This procedure was performed for 14 of 19 patients receiving transfusions.
Leukocyte compatibility studies
Serum was obtained from each patient on study entry and weekly thereafter for the duration of the study. Prestudy and poststudy sera were assayed for leukocyte antibodies by the following four different methods: (1) the granulocyte agglutination test (GAT), (2) the granulocyte immunofluorescence test (GIFT), (3) a lymphocytotoxicity test, and (4) the lymphocyte immunofluorescence test (LIFT).
Granulocytes from 8 healthy donors were isolated from either heparin-anticoagulated or EDTA-anticoagulated blood for GIFT and GAT, respectively. After dextran sedimentation (Dextran T-500, Pharmacia LKB Biotechnology, Piscataway, NJ), granulocytes were isolated from the supernatant leukocyte-rich plasma by Ficoll-Hypaque (Histopaque-1077, Sigma Chemical, St Louis, MO) gradient centrifugation, followed by lysis of residual erythrocytes with dilute ammonium chloride solution. For GIFT, 2 × 105 granulocytes were fixed with 1% paraformaldehyde and incubated in microtiter plates with 20 μL of the sera of interest for 30 minutes at 37°C. After washing, the cells were incubated with FITC-labeled rabbit F(ab′)2-anti-human immunoglobulin G (light and heavy chains) (DAKO, Hamburg, Germany), rewashed, and evaluated with a fluorescence microscope.13-15 The strengths of the reactions are graded 0 to 3 as follows: 0 = negative, 1 (+) = weakly positive, 2 (++) = positive, and 3 (+++) = strongly positive. GAT was performed in Terasaki trays (Greiner, Nürtingen, Germany), in which a 2-μL volume of granulocytes was incubated under oil with a 2-μL volume of the serum of interest for 120 minutes at 37°C and 16-18 hours at 30°C, then read microscopically.15 The strength of the reaction is graded 0 to 4+ according to the proportion of cells participating in the reaction.
The microcytotoxicity assay with the addition of anti-human immunoglobulin was used to detect alloantibody to thymus-derived lymphocytes (T-cell cross-match). Lymphocytes were isolated from heparinized blood using density gradient separation and then frozen. After thawing, T cells were obtained by negative selection of adherent cells on nylon wool.
Each serum was cross-matched with T cells from 36 HLA-typed donors. Results were expressed as the percentage of donors with a positive cross-match (PRA). In addition, sera from each recipient were cross-matched with T cells from each of their neutrophil donors. All sera collected during the study were assayed with each of the donors. Results of each cross-match were reported. Data are summarized as the number of positive donors over the number tested.
LIFT was performed according to the techniques described above for GIFT, except lymphocytes were employed instead of granulocytes.
Donor adverse effects
Each donor was questioned at the time of donation regarding possible side effects attributable to either G-CSF or dexamethasone. Specific side effects addressed included bone pain, headache, and insomnia. Each donor was contacted approximately 1 week after donation to further assess any possible adverse effects, as well as to determine whether the donor would be willing to return at later dates for a G-CSF/dexamethasone-stimulated leukapheresis procedure. A previously developed, standardized symptom grading system was used to quantify the donor's responses to the questionnaire.16
Statistical analysis
Results are expressed as means ± SD unless otherwise specified. Mean values were compared with the use of the two-tailedt test analysis for independent means. Differences were considered statistically significant ifP < .05.
Results
Twenty patients were entered into the study, 19 of whom received neutrophil transfusions. Demographic and clinical characteristics of these patients are shown in Table 1. There were 13 men and 7 women, ranging in age from 7 to 58 years. The primary disease for which stem cell transplantation was being performed was acute leukemia in 10 patients, chronic myelogenous leukemia in 3, non-Hodgkin lymphoma in 3, aplastic anemia in 2, and myelodysplastic syndrome in 2. Sixteen of the patients were entered into the study after transplantation, and 4 were entered prior to transplantation. One of the latter (patient #15) eventually underwent transplantation with continued neutrophil transfusion support. Eleven patients received transplants from unrelated matched donors, 4 from related matched donors, 1 from a related mismatched donor, and 1 was autologous. All patients received G-CSF. The onset of infection ranged from pretransplantation to 35 days posttransplantation. Sixteen patients had fungal infections, and 7 had bacterial infections. Of the patients with fungal infections, 7 had fungemia with yeast and 9 had invasive tissue infections with molds (Table 1).
Demographic characteristics and infections of patients
| Patient . | Age/ sex . | Disease . | Infection site/ organism . | Infection diagnosis (transplant day) . |
|---|---|---|---|---|
| 1 | 12/M | ALL | Blood, skin/Fusarium sp. | 23 |
| 2 | 31/M | CML | Blood, bone marrow, Candida tropicalis, C. albicans, and C. lusitaniae | 35 |
| 3 | 58/M | CML | Blood/C. parapsilosis | 30 |
| 4 | 30/F | SAA | Lung/Aspergillus fumigatus | Pretransplant |
| Blood/Vancomycin-resistant Enterococcus faecium | ||||
| 5 | 37/M | NHL | Blood/C. glabrata | 18 |
| 6 | 24/F | SAA | Facial mass/A. fumigatusand A. flavus | 15 |
| 7 | 34/F | ALL | Lung/A. fumigatus | Pretransplant |
| 8 | 50/M | ALL | Blood/C. krusei | Pretransplant |
| 9 | 51/M | MDS | Blood/Enterobacter cloacae | 17 |
| 10 | 44/M | NHL | Blood/C. krusei | 18 |
| 11 | 45/F | AML | Blood/Stenotrophomonas maltophilia, C. krusei | 20 |
| 12 | 34/M | AML | Blood/C. krusei | 22 |
| 13 | 14/M | AML | Lung/A. fumigatus | 3 |
| 14 | 50/M | AML | Lung/A. flavus, multiple bacteria | 9 |
| 15 | 7/M | AML | Blood, gingiva/Pseudomonas aeruginosa | Pretransplant |
| 16 | 41/F | NHL | Neutropenic enterocolitis | 13 |
| 17 | 27/F | ALL | Lung/septate hyphae | 0 |
| 18 | 54/F | MDS | Lung/A. fumigatus | 13 |
| 19 | 34/M | CML | Blood/Vancomycin-resistant Enterococcus faecium | 12 |
| 20 | 14/M | AML | Blood/Fusarium sp. | 9 |
| Patient . | Age/ sex . | Disease . | Infection site/ organism . | Infection diagnosis (transplant day) . |
|---|---|---|---|---|
| 1 | 12/M | ALL | Blood, skin/Fusarium sp. | 23 |
| 2 | 31/M | CML | Blood, bone marrow, Candida tropicalis, C. albicans, and C. lusitaniae | 35 |
| 3 | 58/M | CML | Blood/C. parapsilosis | 30 |
| 4 | 30/F | SAA | Lung/Aspergillus fumigatus | Pretransplant |
| Blood/Vancomycin-resistant Enterococcus faecium | ||||
| 5 | 37/M | NHL | Blood/C. glabrata | 18 |
| 6 | 24/F | SAA | Facial mass/A. fumigatusand A. flavus | 15 |
| 7 | 34/F | ALL | Lung/A. fumigatus | Pretransplant |
| 8 | 50/M | ALL | Blood/C. krusei | Pretransplant |
| 9 | 51/M | MDS | Blood/Enterobacter cloacae | 17 |
| 10 | 44/M | NHL | Blood/C. krusei | 18 |
| 11 | 45/F | AML | Blood/Stenotrophomonas maltophilia, C. krusei | 20 |
| 12 | 34/M | AML | Blood/C. krusei | 22 |
| 13 | 14/M | AML | Lung/A. fumigatus | 3 |
| 14 | 50/M | AML | Lung/A. flavus, multiple bacteria | 9 |
| 15 | 7/M | AML | Blood, gingiva/Pseudomonas aeruginosa | Pretransplant |
| 16 | 41/F | NHL | Neutropenic enterocolitis | 13 |
| 17 | 27/F | ALL | Lung/septate hyphae | 0 |
| 18 | 54/F | MDS | Lung/A. fumigatus | 13 |
| 19 | 34/M | CML | Blood/Vancomycin-resistant Enterococcus faecium | 12 |
| 20 | 14/M | AML | Blood/Fusarium sp. | 9 |
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; SAA, severe aplastic anemia.
Donor recruitment
Six of the donors were relatives of the patient and accounted for 10 (6%) of the 175 granulocyte collections. The remaining 165 collections were obtained from donors recruited from the PSBC's pool of volunteer community apheresis donors. Approximately 60% of the community donors initially contacted agreed to participate in the study. For the 20 patients in the study, there were 233 days on which granulocyte collections were indicated by protocol. The 175 collections actually obtained represented 75% of this goal. The difference was not attributable primarily to difficulty in finding donors who were willing to participate, but rather to logistic problems in matching donor availability to the limited number of possible apheresis donation times. These limitations were due, in part, to the fact that the timing of the G-CSF/dexamethasone administration required that the collection be performed either early in the morning or in the late afternoon or early evening.
Donor stimulation and leukapheresis
Donor peripheral blood neutrophil counts were 3.7 ± 1.4 × 103/μL (mean ± SD) before G-CSF/dexamethasone administration and 30.9 ± 8.3 × 103/μL immediately before leukapheresis. The interval between stimulation and leukapheresis was 13.1 ± 2.9 hours. There were 175 leukapheresis procedures performed with an average yield of 81.9 ± 2.3 × 109 neutrophils (range 23.8-144.3 × 109). As demonstrated in Figure1, a positive correlation existed between the donor's neutrophil count at the time of the collection and the number of neutrophils obtained (r2 = 0.48).
Relationship between precollection absolute neutrophil count of donor and yield of PMN by centrifugation leukapheresis.
Each symbol represents the result from a single donor stimulated with single-dose administration of granulocyte colony-stimulating factor/dexamethasone as described in the “Materials and methods” section (n = 175).
Relationship between precollection absolute neutrophil count of donor and yield of PMN by centrifugation leukapheresis.
Each symbol represents the result from a single donor stimulated with single-dose administration of granulocyte colony-stimulating factor/dexamethasone as described in the “Materials and methods” section (n = 175).
Donor adverse effects
Adverse effects, such as bone pain and headache, likely attributable to G-CSF and/or dexamethasone, were relatively common but were usually no more than mild to moderate in degree. Bone pain, headache, and insomnia occurred in 41%, 30%, and 30% of the 175 donors, respectively. Insomnia was felt most likely to be primarily an effect of dexamethasone. Twenty-eight percent of donors reported no side effect. Ninety-eight percent of donors indicated that they would be willing to return for another donation.
Hematologic effects of transfusion
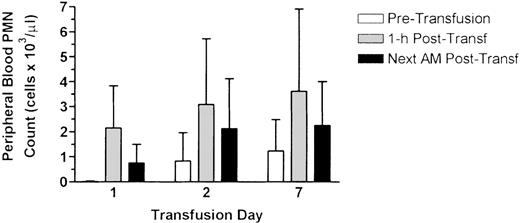
Nineteen (95%) of the 20 patients received at least 1 neutrophil transfusion, the 1 exception being patient #18 who died before the first transfusion could be given. One hundred sixty-five transfusions were administered for an average of 8.6 transfusions per patient (range 1-25). The hematologic effects of the transfusions for each patient are shown in Table 2. The average 1-hour posttransfusion peripheral blood neutrophil increment for all patients was 2.6 ± 2.6 × 103/μL, with the mean value for individual patients ranging from 0.2-5.3 × 103/μL. No change in the neutrophil count was seen in patients #3 and #12. Excluding these 2 patients, the mean 1-hour posttransfusion neutrophil increment was 2.7 ± 1.6 × 103/μL. No clinical features distinguished patients with large and small neutrophil increments. The relationship between the dose of neutrophils administered and the 1-hour increment is shown in Figure2. Although an overall poor correlation was found between the dose and the rise in the neutrophil count, those transfusions in which the dose was > 2.0 × 109/kg were associated with increments > 2 × 103/μL. In general, the transfused neutrophils persisted in the circulation, as reflected by a mean neutrophil count of 2.6 ± 2.8 × 103/μL the following morning. The cumulative effect of daily neutrophil transfusions on the neutrophil count is shown in Figure3. Although the baseline counts before the first transfusion were extremely low, pretransfusion counts for subsequent transfusions were considerably higher, resulting in posttransfusion counts averaging 3.1 × 103/μL and 3.6 ± 103/μL for the second and seventh transfusions, respectively.
Leukocyte antibody analysis and effects on PMN responses in transfused patients
| Patient . | No.transfusions . | PMN dose (cells ×109) . | Positive XM* . | PRA baseline/ max/end . | GIFT baseline/ end . | LIFT baseline/ end . | PMN 1-hr inc (cells ×103/μL) . | Next AM PMN (cells ×103/μL) . | Oral PMN (cells ×106) baseline/post-Tx . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 96.5 ± 46.6 | 1/1 | 83/83/3 | +/+ | +++/+++ | 5.3 ± 1.4 | 15 | 0/0.01 |
| 2 | 11 | 76.5 ± 26.5 | 11/11 | 7/93/93 | −/− | −/− | 2.7 ± 1.6 | 2.7 ± 1.7 | 0.01/0.22 |
| 3 | 11 | 91.2 ± 22.1 | 0/11 | 0/0/0 | −/− | −/− | 0.2 ± 0.1 | 0.0 ± 0.0 | 0/0 |
| 4 | 14 | 79.2 ± 14.7 | 0/14 | 0/0/0 | −/− | −/− | 3.1 ± 1.5 | 1.4 ± 1.0 | 0.02/0.07 |
| 5 | 3 | 79 ± 10.1 | 1/3 | 0/0/na | +/+ | −/− | 1.8 ± 1.0 | 1.5 ± 1.5 | 0.02/0.72 |
| 6 | 1 | 81.6 | 0/1 | 0/0/na | −/NA | −/NA | NA | 8.9 | NA |
| 7 | 13 | 80.3 ± 17.8 | 0/13 | 7/7/0 | −/− | −/− | 1.8 ± 1.4 | 0.9 ± 1.1 | 0.05/0.22 |
| 8 | 3 | 80.6 ± 16.2 | 0/3 | 0/0/0 | −/− | −/− | 1.3 ± 0.8 | 0.6 ± 0.8 | 0/0.02 |
| 9 | 7 | 85.5 ± 14.7 | 1/7 | 0/22/3 | −/+ | −/− | 1.4 ± 1.6 | 0.9 ± 0.4 | NA |
| 10 | 8 | 59.7 ± 30.1 | 0/8 | 0/0/0 | +/− | −/− | 4.9 ± 4.2 | 4.3 ± 2.5 | 0.05/1.04 |
| 11 | 7 | 105.0 ± 17.8 | 0/7 | 0/0/0 | −/− | −/− | 0.9 ± 1.0 | 1.5 ± 1.2 | NA |
| 12 | 9 | 82.9 ± 17.3 | 1/9 | 18/18/13 | +++/+++ | +++/+++ | 0.2 ± 0.4 | 0.3 ± 0.2 | 0.02/0.03 |
| 13 | 18 | 89.2 ± 26.3 | 3/18 | 0/24/24 | −/+ | −/+ | 5.1 ± 4.3 | 6.9 ± 3.3 | 0/0.3 |
| 14 | 2 | 62.0 ± 17.7 | 0/2 | 0/0/0 | −/NA | −/NA | NA | NA | NA |
| 15 | 25 | 78.2 ± 27.3 | 0/25 | 0/3/0 | −/− | −/− | 2.7 ± 1.3 | 3.3 ± 1.4 | 0/0.09 |
| 16 | 2 | 106.6 ± 32.5 | 1/2 | 0/95/95 | −/+ | −/− | 1.0 ± 0.1 | 3.7 ± 3.6 | NA/1.84 |
| 17 | 16 | 79.0 ± 21.1 | 6/16 | 6/29/6 | +/− | +/− | 4.5 ± 1.9 | 4.0 ± 2.2 | NA/4.26 |
| 19 | 12 | 69.7 ± 25.5 | 0/11 | 0/3/0 | −/− | −/− | 1.3 ± 0.9 | 0.8 ± 0.9 | 0/0.53 |
| 20 | 1 | 50.8 | 0/1 | 0/0/0 | +/NA | −/NA | NA | NA | NA |
| Group mean (±SD) | 8.6 | 2.58 ± 2.60 | 2.62 ± 2.83 | 0.01/0.52 | |||||
| Normal12 | 0.52 |
| Patient . | No.transfusions . | PMN dose (cells ×109) . | Positive XM* . | PRA baseline/ max/end . | GIFT baseline/ end . | LIFT baseline/ end . | PMN 1-hr inc (cells ×103/μL) . | Next AM PMN (cells ×103/μL) . | Oral PMN (cells ×106) baseline/post-Tx . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 96.5 ± 46.6 | 1/1 | 83/83/3 | +/+ | +++/+++ | 5.3 ± 1.4 | 15 | 0/0.01 |
| 2 | 11 | 76.5 ± 26.5 | 11/11 | 7/93/93 | −/− | −/− | 2.7 ± 1.6 | 2.7 ± 1.7 | 0.01/0.22 |
| 3 | 11 | 91.2 ± 22.1 | 0/11 | 0/0/0 | −/− | −/− | 0.2 ± 0.1 | 0.0 ± 0.0 | 0/0 |
| 4 | 14 | 79.2 ± 14.7 | 0/14 | 0/0/0 | −/− | −/− | 3.1 ± 1.5 | 1.4 ± 1.0 | 0.02/0.07 |
| 5 | 3 | 79 ± 10.1 | 1/3 | 0/0/na | +/+ | −/− | 1.8 ± 1.0 | 1.5 ± 1.5 | 0.02/0.72 |
| 6 | 1 | 81.6 | 0/1 | 0/0/na | −/NA | −/NA | NA | 8.9 | NA |
| 7 | 13 | 80.3 ± 17.8 | 0/13 | 7/7/0 | −/− | −/− | 1.8 ± 1.4 | 0.9 ± 1.1 | 0.05/0.22 |
| 8 | 3 | 80.6 ± 16.2 | 0/3 | 0/0/0 | −/− | −/− | 1.3 ± 0.8 | 0.6 ± 0.8 | 0/0.02 |
| 9 | 7 | 85.5 ± 14.7 | 1/7 | 0/22/3 | −/+ | −/− | 1.4 ± 1.6 | 0.9 ± 0.4 | NA |
| 10 | 8 | 59.7 ± 30.1 | 0/8 | 0/0/0 | +/− | −/− | 4.9 ± 4.2 | 4.3 ± 2.5 | 0.05/1.04 |
| 11 | 7 | 105.0 ± 17.8 | 0/7 | 0/0/0 | −/− | −/− | 0.9 ± 1.0 | 1.5 ± 1.2 | NA |
| 12 | 9 | 82.9 ± 17.3 | 1/9 | 18/18/13 | +++/+++ | +++/+++ | 0.2 ± 0.4 | 0.3 ± 0.2 | 0.02/0.03 |
| 13 | 18 | 89.2 ± 26.3 | 3/18 | 0/24/24 | −/+ | −/+ | 5.1 ± 4.3 | 6.9 ± 3.3 | 0/0.3 |
| 14 | 2 | 62.0 ± 17.7 | 0/2 | 0/0/0 | −/NA | −/NA | NA | NA | NA |
| 15 | 25 | 78.2 ± 27.3 | 0/25 | 0/3/0 | −/− | −/− | 2.7 ± 1.3 | 3.3 ± 1.4 | 0/0.09 |
| 16 | 2 | 106.6 ± 32.5 | 1/2 | 0/95/95 | −/+ | −/− | 1.0 ± 0.1 | 3.7 ± 3.6 | NA/1.84 |
| 17 | 16 | 79.0 ± 21.1 | 6/16 | 6/29/6 | +/− | +/− | 4.5 ± 1.9 | 4.0 ± 2.2 | NA/4.26 |
| 19 | 12 | 69.7 ± 25.5 | 0/11 | 0/3/0 | −/− | −/− | 1.3 ± 0.9 | 0.8 ± 0.9 | 0/0.53 |
| 20 | 1 | 50.8 | 0/1 | 0/0/0 | +/NA | −/NA | NA | NA | NA |
| Group mean (±SD) | 8.6 | 2.58 ± 2.60 | 2.62 ± 2.83 | 0.01/0.52 | |||||
| Normal12 | 0.52 |
PMN indicates polymorphonuclear leukocyte; PRA, positive cross-match; GIFT, granulocyte immunofluorescence test; LIFT, lymphocyte immunofluorescence test; inc, increments; Tx, treatment; NA, not available.
XM = T-cell cross-match (see text): positive cross-matches/total donors tested.
Relationship between the dose of PMN administered by transfusion and increment in recipient absolute neutrophil count determined 1 hour following transfusion.
Each symbol represents a single transfusion of granulocyte concentrate product obtained by centrifugation leukapheresis of a donor stimulated with single-dose administration of granulocyte colony-stimulating factor/dexamethasone as described in the “Materials and methods” section (n = 175).
Relationship between the dose of PMN administered by transfusion and increment in recipient absolute neutrophil count determined 1 hour following transfusion.
Each symbol represents a single transfusion of granulocyte concentrate product obtained by centrifugation leukapheresis of a donor stimulated with single-dose administration of granulocyte colony-stimulating factor/dexamethasone as described in the “Materials and methods” section (n = 175).
Effects of serial neutrophil transfusions on the absolute neutrophil count of the recipients.
The peripheral blood PMN count of the patients receiving transfusions was determined immediately before transfusion, 1 hour following transfusion, and the morning following transfusion (approximately 12 hours posttransfusion). Data represent values from patients receiving serial daily transfusions for at least 7 days (n = 10) and are expressed as mean ± SD.
Effects of serial neutrophil transfusions on the absolute neutrophil count of the recipients.
The peripheral blood PMN count of the patients receiving transfusions was determined immediately before transfusion, 1 hour following transfusion, and the morning following transfusion (approximately 12 hours posttransfusion). Data represent values from patients receiving serial daily transfusions for at least 7 days (n = 10) and are expressed as mean ± SD.
Extravascular migration of transfused neutrophils
The buccal neutrophil response was minimal in all patients prior to the first neutrophil transfusion (Table 2). The mean increase in this value as a result of transfusion varied from patient to patient, but the mean posttransfusion value observed for all transfusions (0.52 × 106 cells) was indistinguishable from that seen in normal non-neutropenic subjects.12 A general positive correlation was seen between the ability of the transfused cells to circulate and the buccal response results. Those patients with no appreciable posttransfusion neutrophil increment (patients #3 and #12) also had no detectable buccal neutrophil response after transfusion.
Leukocyte compatibility
Sera that were strongly and broadly reactive in LIFT or exhibited more than 10% PRA reactivity, even if weakly positive in GIFT, were considered to contain HLA class I antibodies. Several sera were broadly but weakly (score = 1(+)) reactive in GIFT and negative in all other tests; these sera were considered to contain neutrophil antibodies. No patient sample was positive in the GAT.
At study entry, very little evidence was seen of alloimmunization in the patients enrolled in this study (Table 2). Three patients (#5, #10, #20) demonstrated weakly reactive neutrophil antibodies at the onset of the study. No patient developed or increased the level of neutrophil antibody as a result of participation in the study. At the onset of the study, 17 patients had negative HLA antibody screens (PRA ≤ 10%). One patient (#12) had a PRA of 18% and showed strongly reactive HLA antibodies in GIFT and LIFT, the latter tests being positive against cells from only 1 of 8 donors. Patient #1 had a PRA of 83%, was weakly reactive in GIFT, and was strongly reactive in LIFT. Immunofluorescence (GIFT, LIFT) testing suggested that an additional patient (#17) had antibodies to HLA class I antigens at the onset of the study. Five patients (#2, #9, #13, #16, #17) showed substantial increase in PRA activity (> 10%) during the study period with values ranging from 22%-95%. Three of these patients also developed evidence of antibody by GIFT/LIFT. During the course of the study, a positive lymphocytotoxic cross-match with the neutrophil donor was detected in 8 (42%) of the 19 patients and for 25 (15%) of the 163 transfusions for which data were available. In 6 of these 8 patients, the maximum PRA observed was > 10%. In the remaining 2 patients, the prestudy and poststudy PRA was 0%.
The existence or development of leukocyte antibodies had minimal adverse effects on either the posttransfusion neutrophil increments observed or the buccal neutrophil responses. For patients with detectable leukocyte antibodies, the mean neutrophil increment in the peripheral blood 1 hour posttransfusion was 3.0 × 103/μL, and the mean neutrophil buccal response was 1.0 × 106 cells. In contrast, the mean neutrophil increment was 1.6 × 103/μL and the mean buccal neutrophil response was 0.2 × 106 cells in patients with undetectable leukocyte antibodies. For the 25 individual transfusions for which the donor/recipient lymphocytotoxic cross-match was positive, the mean 1-hour posttransfusion neutrophil increment was 3.9 × 103/μL, a value greater than the mean for all 165 transfusions (2.6 × 103/μL). One of the patients who exhibited no increase in neutrophil count as a result of transfusion (#3) had no evidence of leukocyte antibody. The other patient (#12), as previously stated, had a strongly reactive HLA antibody, but one which appeared to be directed toward a relatively uncommon HLA antigen. Only 1 of this patient's 9 transfusions was incompatible by T-cell cross-match, yet posttransfusion neutrophil increments were uniformly low for all of the transfusions.
Adverse effects in recipients
Adverse effects experienced by the transfusion recipients are shown in Table 3. Average temperature rise as the result of transfusion was minimal (0.2 ± 0.9°C), but chills and fever were seen in 7% of transfusions and, at some point in the study, in about one third of the patients. In all cases, these reactions were mild to moderate and were successfully prevented in subsequent transfusions by premedication with antipyretics or with corticosteroids. Baseline oxygen saturation varied from 61%-100%, depending on the patient's underlying pulmonary status. Decreases of more than 3% were seen in only 11 (7%) of 165 transfusions, and decreases of more than 6% in 3 of these 11 patients. In all cases, these changes were attributed to spontaneous variations in the patient's level of oxygenation and did not appear to be related to the transfusions. No cases of respiratory compromise could be attributed to neutrophil infusion.
Recipient adverse effects
| . | Patients . | Transfusions . |
|---|---|---|
| Chills | 7/19 (37%) | 12/165 (7%) |
| >1.5°C temp rise | 6/19 (32%) | 12/165 (7%) |
| Itching/hives | 2/19 (11%) | 3/165 (2%) |
| Temperature (C°) | O2 Saturation (%) | |
| Pretransfusion | 37.1 ± 0.9 (35.0-39.9) | 95.3 ± 3.8 (61-100) |
| Change 2° transfusion | 0.2 ± 0.9 ((−2.4)-3.8) | 0.5 ± 2.9 ((−16)-13) |
| . | Patients . | Transfusions . |
|---|---|---|
| Chills | 7/19 (37%) | 12/165 (7%) |
| >1.5°C temp rise | 6/19 (32%) | 12/165 (7%) |
| Itching/hives | 2/19 (11%) | 3/165 (2%) |
| Temperature (C°) | O2 Saturation (%) | |
| Pretransfusion | 37.1 ± 0.9 (35.0-39.9) | 95.3 ± 3.8 (61-100) |
| Change 2° transfusion | 0.2 ± 0.9 ((−2.4)-3.8) | 0.5 ± 2.9 ((−16)-13) |
Ranges in parentheses.
No clear relationship was seen between the occurrence of transfusion reactions and the existence or development of leukocyte antibodies. Chills and/or fever were observed following 9 (12%) of 76 transfusions to the 10 patients with any evidence of leukocyte antibodies and following 12 (13%) of 89 transfusions to patients with no evidence of antibody. For the 25 transfusions in which there was a positive donor/recipient HLA cross-match reaction, no transfusion-related adverse events were observed in the 23 transfusions for which there were data. In these same 8 patients, 3 episodes of chills occurred following transfusions that were not incompatible by lymphocytotoxic cross-match. Similarly, only 1 donor/recipient-incompatible transfusion was associated with a decrease in O2 saturation (≥ 6%), similar to the incidence seen in donor/recipient-compatible transfusions.
Clinical efficacy
The clinical course of the patients is summarized in Table4. Infection resolved in 8 patients. One patient (#4) cleared her bacterial infection but not her fungal infection. Eight of the 16 patients who received neutrophil transfusions following transplantation survived until engraftment. Reasons for discontinuing transfusion support included engraftment (n = 7), clearing of the infection (n = 3), realization that the clinical situation was hopeless (n = 6), and death (n = 3). None of the 5 patients with invasive aspergillosis cleared the infection, although 1 of 5 survived until engraftment. The 3 patients with bacterial infection only cleared the infection and survived until engraftment. Four of the 7 patients with fungemia cleared the infection, and 2 of the 7 survived until engraftment.
Clinical outcomes of patients receiving neutrophil transfusion therapy
| Infection . | N . | Clearance of infection . | Line removal . | Engraft. . | Alive day 304-150 . |
|---|---|---|---|---|---|
| Invasive aspergillosis | 3 | 0/3 | NA | 1/3 | 0/3 |
| Fusariosis | 2 | 0/2 | 0/2 | 1/2 | 0/2 |
| Disseminated candidiasis | 6 | 3/6 | 5/6 | 2/6 | 1/6 |
| Monomicrobial bacteremia | 2 | 2/24-151 | 0/2 | 2/2 | 1/2 |
| Mixed infections | 5 | ||||
| Aspergillosis/bacteremia (VRE) | 0/1 | 0/1 | NA | 0 | |
| Aspergillosis/bacterial pneumonia | 0/1 | NA | 0/1 | 0 | |
| Fungal pneumonia/CMV pneumonia | 0/1 | NA | 0/1 | 0 | |
| Candidemia (C. krusei )/bacteremia (S. maltophilia) | 1/1 | 0/1 | 0/1 | 0 | |
| Mixed bacteremia (S. maltophilia/VRE) | 1/1 | 0/1 | 1/1 | 1 | |
| Neutropenic enterocolitis (typhlitis) | 1 | 1/1 | NA | 1/1 | 1 |
| Any aspergillosis‡,4-153 | 6 | 0/6 | 0/14-155 | 1/6 | 0/6 |
| Any invasive mold infection‡,4-153 | 8 | 0/8 | 0/34-155 | 2/8 | 0/8 |
| Any candidiasis‡ | 7 | 4/7 | 5/7 | 2/7 | 1/7 |
| Bacteremia without concomitant fungal infection4-154 | 4 | 4/4 | 0/34-155 | 4/4 | 3/4 |
| Overall | 19 | 8/19 | 5/13 | 8/19 | 4/19 |
| Infection . | N . | Clearance of infection . | Line removal . | Engraft. . | Alive day 304-150 . |
|---|---|---|---|---|---|
| Invasive aspergillosis | 3 | 0/3 | NA | 1/3 | 0/3 |
| Fusariosis | 2 | 0/2 | 0/2 | 1/2 | 0/2 |
| Disseminated candidiasis | 6 | 3/6 | 5/6 | 2/6 | 1/6 |
| Monomicrobial bacteremia | 2 | 2/24-151 | 0/2 | 2/2 | 1/2 |
| Mixed infections | 5 | ||||
| Aspergillosis/bacteremia (VRE) | 0/1 | 0/1 | NA | 0 | |
| Aspergillosis/bacterial pneumonia | 0/1 | NA | 0/1 | 0 | |
| Fungal pneumonia/CMV pneumonia | 0/1 | NA | 0/1 | 0 | |
| Candidemia (C. krusei )/bacteremia (S. maltophilia) | 1/1 | 0/1 | 0/1 | 0 | |
| Mixed bacteremia (S. maltophilia/VRE) | 1/1 | 0/1 | 1/1 | 1 | |
| Neutropenic enterocolitis (typhlitis) | 1 | 1/1 | NA | 1/1 | 1 |
| Any aspergillosis‡,4-153 | 6 | 0/6 | 0/14-155 | 1/6 | 0/6 |
| Any invasive mold infection‡,4-153 | 8 | 0/8 | 0/34-155 | 2/8 | 0/8 |
| Any candidiasis‡ | 7 | 4/7 | 5/7 | 2/7 | 1/7 |
| Bacteremia without concomitant fungal infection4-154 | 4 | 4/4 | 0/34-155 | 4/4 | 3/4 |
| Overall | 19 | 8/19 | 5/13 | 8/19 | 4/19 |
NA indicates not applicable; VRE, vancomycin-resistant enterococci; CMV, cytomegalovirus.
After onset of infection.
One patient cleared infection before first transfusion.
With and without copathogens.
One patient with septate hyphae on lung biopsy included (patient #17).
Denominator does not include those cases for which line removal would not be applicable (eg, invasive aspergillosis, typhlitis).
One patient with neutropenic enterocolitis included (#16).
Discussion
Infection in severely neutropenic patients continues to be a major cause of morbidity and mortality following hematopoietic stem cell transplantation and cancer chemotherapy.2 Granulocyte transfusions are a logical approach to this problem, and several early trials5,6,17-19 suggested benefits of this adjunct to antibiotic therapy. Other trials,20-22 however, were inconclusive or showed no benefit. A major reason for the apparent lack of effectiveness was that the dose of neutrophils supplied was inadequate in several of the studies.23 Several other reviews24-26 have summarized these investigations.
A new era in transfusion medicine began with the availability of the recombinant hematopoietic growth factors to treat neutropenic patients and normal donors for granulocyte (neutrophil) transfusion therapy. G-CSF, with or without concomitant dexamethasone, has now been used in several centers to treat granulocyte donors.11,27-29 The use of G-CSF alone for donor stimulation permits collection of approximately 40 × 109 neutrophils,11and the combination of G-CSF (600 μg subcutaneously in a single dose) plus dexamethasone (8 mg orally in a single dose) allows collection of approximately 80 × 109 neutrophils.10Neutrophils collected from normal donors stimulated with this combination regimen have normal or near normal function in vitro (as measured by chemiluminescence and bacterial killing assays), prolonged intravascular life span, and the capacity to migrate to a site of inflammation.10111Indium-labeled cells collected from G-CSF-stimulated donors have also been shown to migrate to a site of inflammation in patients with severe neutropenia.30
The present study was conducted to examine the feasibility of providing G-CSF-stimulated granulocytes from unrelated volunteer blood donors from a community blood center to a neutropenic patient population undergoing hematopoietic stem cell transplantation. This study was undertaken assuming that short-term granulocyte transfusion support could be organized similar to volunteer apheresis programs for platelet support. This effort required the existence of a pool of volunteer apheresis donors, as well as an operational mechanism for contacting and scheduling donors 7 days per week. Such a system was already in place in Seattle at the PSBC. This study demonstrates that most community donors are quite willing to undergo G-CSF/dexamethasone stimulation prior to leukapheresis with the understanding that their cells may benefit patients with serious infections. It was possible to schedule and collect neutrophils from regular apheresis donors on approximately 75% of the days for which cells were requested. The major obstacles for providing cells on the other days were the availability of apheresis equipment and staffing at PSBC. The inconvenience and side effects of pretreatment of donors during the study period were similar to those previously reported11 27-30 and generally well tolerated. The majority of donors stated that they would be willing to undergo stimulated leukapheresis again.
As observed previously, G-CSF/dexamethasone stimulation caused marked neutrophilia in the donor and permitted the collection of large numbers of neutrophils by routine centrifugation leukapheresis techniques. The average yield (81.9 ± 2.3 × 109 cells) represents a threefold to fourfold larger collection than observed previously following stimulation of donors with corticosteroids alone.11 When these cells were transfused, substantial variation in posttransfusion increments was observed, but 17 (89%) of 19 patients consistently showed large and sustained increases in their circulating neutrophil counts. In the majority of patients, transfusion resulted in normal or near-normal circulating neutrophil levels for the duration of the study period. In addition, buccal neutrophil responses generally correlated with the number of circulating neutrophils, indicating that these transfused cells were capable of migrating from the blood stream to extravascular sites.
We investigated the relationship of HLA and neutrophil antibodies to the transfusion responses. At the outset of the study, little evidence was seen for alloimmunization in these patients. HLA antibodies were detected in 3 patients, and 3 patients had detectable neutrophil antibodies before receiving granulocyte transfusion. During the course of the study, 5 additional patients developed HLA antibodies. However, no patient developed neutrophil antibodies during transfusion therapy, and the level of neutrophil antibodies did not increase in the patients with evidence of antibodies at baseline. Lymphocytotoxic cross-matching data for each transfusion showed donor/recipient incompatibility in approximately 15% of the transfusions. However, no correlation was seen between the presence of antibody or the donor/recipient HLA compatibility of the transfusions and the posttransfusion neutrophil increments observed in the patients. In particular, of the 2 patients with uniformly poor neutrophil increments, 1 had no evidence of leukocyte antibodies; the other had a strongly reactive HLA antibody that was, however, only reactive against 1 of his 9 donors. The lack of correlation between the antibody findings and the neutrophil increments in the recipients is in contrast to previous reports,31 32particularly in patients with functional leukocyte abnormalities (eg, chronic granulomatous disease) who received repeated granulocyte transfusions. This difference may be because the transplantation patients described here were not alloimmunized at the onset of the study and were unable to mount a normal immune response secondary to their immunocompromised status.
Concern regarding serious pulmonary reactions is one of the reasons limiting widespread use of granulocyte transfusion therapy in clinical practice. In this study, much larger doses of neutrophils were administered than in any previous large study. Importantly, few adverse reactions were observed. In addition, no correlation was found between the occurrence of adverse reactions and the presence of leukocyte antibodies in the recipients. However, none of the detectable leukocyte antibodies showed neutrophil agglutination that seems to be a prerequisite for induction of transfusion-related acute lung injury.33 Previous reports34 35 have also suggested that concomitant treatment with amphotericin B increases the likelihood of severe pulmonary reactions. Such reactions were not observed in this patient population, in which the vast majority was receiving concomitant amphotericin B therapy. However, the interval between the administration of amphotericin B and infusion of granulocytes in these patients was at least 8 hours.
This study reports clinical outcomes, but these data must be interpreted carefully. All of the patients receiving granulocyte transfusion therapy were severely ill with a poor clinical prognosis. Therapeutic outcome was favorable in patients with bacterial infections, whereas patients with invasive fungal infections had a high mortality rate (Table 4). Of 7 patients with candidemia, 4 cleared their infection from the blood, but only 1 patient was alive 30 days after diagnosis. In these cases, death was attributed to progressive multiple organ failure, despite microbiologic clearance of candidal infection. Outcome was particularly poor in patients with invasive mold infections, since all 8 patients died within 30 days without clearing their infection by clinical criteria. We did observe, however, that 2 of 3 patients with candidemia and all 3 patients with invasive mold infection who died and had an autopsy performed had cleared their infection at autopsy. We hypothesize that intervention with neutrophil transfusion therapy may have been initiated too late during the course of the infection to affect outcomes. This factor may account for the apparent differences between our results and the more favorable results reported by other groups.36-38 Improvements in early diagnosis, such as with polymerase chain reaction based detection methods or by greater use of pretransplantation CT examinations in patients at high risk for invasive aspergillosis, might be useful in this regard.
An important concern for the development of granulocyte transfusion therapy is the long-term safety of the practice of treating normal donors with G-CSF.39 It should be noted that G-CSF is a naturally occurring cytokine, and transient increases in G-CSF levels undoubtedly occur as part of the normal cytokine response to infections.40-43 We assume that intermittent treatment of donors with G-CSF is similar to these natural events. To date, administration of G-CSF to normal donors has not been associated with serious or long-term side effects, except in rare instances.
From this study we conclude that it is feasible to use community donors, stimulated with G-CSF and dexamethasone, to provide neutrophils for patients with severe neutropenia and severe infections. In both the donors and the recipients, adverse events are very infrequent and alloimmunization does not develop rapidly. With this approach, neutropenia can be corrected and the capacity to deliver neutrophils to a site of inflammation can be restored. Controlled clinical trials will be required to determine the appropriate clinical applications of this therapy.
Acknowledgments
The authors would like to acknowledge Monica Cays, RN, Fariba Fuller, RN, Claire Llewellyn, RN, and Steve Koets, RN for their invaluable assistance in carrying out this study.
Supported in part by Amgen Corporation (Thousand Oaks, CA); COBE BCT (Lakewood, CO), NIH grants CA 18029 (MB) and HL53515 (DCD, WCL, THP), and a grant from the Deutsche Forschungsgemeinschaft DFG BU 770/3-4 (JB).
Reprints:Thomas H. Price, Medical Director, Puget Sound Blood Center, 921 Terry Ave, Seattle WA 98105-1256.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal