Abstract
Vascular endothelial growth factor (VEGF) is highly expressed in vascular remodeling processes and accelerates reendothelialization after mechanical denudation. Two VEGF tyrosine kinase receptors have been reported—fms-like–tyrosine kinase-1 (Flt-1) and kinase domain region (KDR). Little is known about the regulation of the expression of these receptors after vascular injury. Herein, we have analyzed the expression of Flt-1 after mechanical denudation of primary cultures of endothelial cells, which has been considered a useful in vitro model to study endothelium responses to vascular injury. After denudation, the Flt-1 protein and mRNA levels are clearly up-regulated, and transient transfection experiments showed a strong induction of theflt-1 promoter-dependent transcription. Analysis of the flt-1 promoter sequence revealed the presence of a putative binding site for the early growth response factor-1 (Egr-1) at positions −24 to −16. Electrophoretic mobility shift and supershift assays showed that Egr-1 was able to bind to this DNA sequence, and cotransfection of the flt-1 promoter reporter plasmid with an Egr-1 expression vector resulted in enhancement of its transcriptional activity. Furthermore, the mutation of the Egr-1 binding site markedly reduced the denudation-induced flt-1promoter activity. These data demonstrate that Flt-1 is up-regulated after endothelial denudation and that Egr-1 plays a relevant role in this process.
Endothelial cells form an interface between blood and surrounding tissues. The integrity of this surface is essential for maintaining homeostasis and regulating vascular reactivity and blood flow. Vascular endothelial growth factor (VEGF) is a potent and specific mitogen for endothelial cells.1 It plays a major role in angiogenesis 1-4 and vasculogenesis; it has been reported that mice lacking 1 of the 2 VEGF alleles die before birth and show defects in the development of the cardiovascular system.5,6 VEGF also mediates vascular permeability,4 endothelial chemotaxis,7endothelium-derived relaxing factor-dependent vasodilatation,8 and thrombogenicity. 9
Two VEGF tyrosine–kinase receptors have been reported, Flt-1 (also named VEGFR-1)10 and KDR (also named VEGFR-2),11 and one KDR co-receptor, neuropilin-1,12 which only binds the 165-amino acid isoform of VEGF. Embryos lacking KDR have not differentiated endothelial cells, and blood vessels do not form.13 Embryos lacking the Flt-1 gene also die before birth; they have differentiated endothelial cells, but the development of functional blood vessels is severely impaired.14 When porcine aortic endothelial cells, which are reported to lack endogenous VEGF receptors, or nonendothelial cells are transfected with KDR, they proliferate in response to VEGF,7,15 whereas the function of Flt-1 remains unclear in endothelial cells because transfected cells do not show proliferation or other responses on exposure to VEGF.7,16 However, the migration of monocytes toward VEGF is mediated by Flt-1.17Mice lacking only the tyrosine–kinase domain of Flt-1 show normal development and angiogenesis, but monocytes do not migrate to VEGF,18 suggesting a different mechanism of action in endothelial cell organization and monocyte migration.
The mechanisms that regulate the expression of VEGF receptor genes remain poorly understood. In this regard, exposure of rats to acute or chronic hypoxia up-regulates these genes in the lung vasculature,19 and mRNA levels are also up-regulated throughout the heart after myocardial infarction in rats.20Hypoxia transcriptionally up-regulates Flt-1 in cultured cells,21 and, though it up-regulates KDR, this seems to be mediated by a posttranscriptional mechanism.22 Both receptors are reported to be down-regulated by tumor necrosis factor (TNF)-α,23 but recent studies have shown that KDR and neuropilin-1 are up-regulated by this factor24; hence, the effect of TNF-α remains unclear. Transforming growth factor-β has also been reported to down-regulate KDR,25 and VEGF itself is able to up-regulate both receptors.26-29
Vascular injury is followed by endothelial cell proliferation and migration to repair the vessel wall.30 Studies show that VEGF is highly expressed in smooth muscle cells after endothelial denudation and that it accelerates reendothelialization.31-35 However, little is known about the mechanisms of expression and action of VEGF receptors after endothelial cell monolayer disruption. Other genes implicated in the pathogenesis of vascular disease are PDGF-A,36,37PDGF-B,38 fibroblast growth factor (FGF)-2,39TNF-α,40 tissue factor,41 and intercellular adhesion molecule-142; some others43 have been shown to be highly dependent on the activation of the early growth response protein 1 (Egr-1) after vascular injury. Egr-1 is the prototype member of a zinc-finger family of transcription factors. It is induced in response to hormones, PMA (phorbol 12-myristate 13-acetate), and mitogens such as growth factors.44 Egr-1 is expressed in the nucleus after vascular injury or cell activation, and it regulates the transcription of the genes previously mentioned, all of which contain 1 or more Egr-1 consensus binding sites in their promoters.
In this article we show that the VEGF receptor Flt-1 is markedly up-regulated after endothelial denudation in vitro, accompanied by an increase of the mRNA level. We also show that flt-1promoter-dependent transcription is up-regulated in response to mechanical denudation, and we demonstrate that the transcription factor Egr-1 plays a principal role in this up-regulation after its binding to a consensus sequence at sites −24 to −16 of the human flt-1 promoter. Taken together, these findings suggest a role for this VEGF receptor in the cellular response of the endothelium to vascular injury.
Materials and methods
Cell culture
Human umbilical vein cells (HUVEC) were isolated from normal human umbilical cord veins. The umbilical vein was cannulated and incubated with 1% collagenase for 15 minutes at 37°C. After the removal of collagenase, cells were pooled and established as primary cultures in medium 199 (GIBCO, Gaithersburg, MD) containing 20% fetal bovine serum (FBS). HUVEC were serially passaged and maintained using medium 199 supplemented with 20% FBS, 50 μg/mL bovine brain extract, and 100 μg/mL heparin in tissue culture dishes precoated with 0.5% gelatin. Cells were used within the first 6 passages.
In vitro mechanical endothelial denudation
HUVEC were grown in culture dishes as described above until cells formed a confluent monolayer. Then 300- to 500-μm– wide wounds were systematically created with a sterile pipet tip (0.5- to 1-mm diameter) until only a 20% of the cells remained adhered to the culture dish. For immunofluorescence assays, one wound was created on the monolayer attached to gelatin on the coverslips.
Immunofluorescence microscopy
HUVEC on the coverslips were fixed with 4% formaldehyde in phosphate-buffered saline for 15 minutes at room temperature, then washed twice with Tris-buffered saline (TBS) and permeabilized with 0.5% Triton X-100 in TBS for 20 minutes at room temperature. After two washes with TBS, cells were preincubated with TNB (0.1 mol/L Tris.HCl/0.15 mol/L NaCl/0.5% blocking reagent [Boehringer Mannheim, Mannheim, Germany]) for 30 minutes at 37°C. Then cells were incubated with a rabbit polyclonal IgG antibody against Flt-1 (Flt-1 C-17; Santa Cruz Biotechnology, Santa Cruz, CA) dilution 1:50 in TNB, Egr-1 (C-19; Santa Cruz Biotechnology), or Sp1 (PEP2; Santa Cruz Biotechnology) dilution 1:100 for 45 minutes at 37°C, washed twice with TBS, and incubated with a goat antirabbit Cy3-labeled antibody (Amersham Life Science, Buckinghamshire, UK) 1:5000 in TNB for 25 minutes at 37°C. In Egr-1 and Sp-1 experiments, cells were incubated with 2 μg/mL fluorescein phalloidin (Molecular Probes, Eugene, OR) for 20 minutes at 37°C. Finally, cells were washed 3 times with TBS and once with distilled water. In Flt-1 assays, coverslips were mounted with 150 ng/mL DAPI (Serva, Heidelberg, Germany) in Mowiol (Calbiochem, La Jolla, CA) to stain cell nuclei. Cells were observed using a Nikon (Tokyo, Japan) Labophot-2 photomicroscope equipped with 40×, 60×, and 100× oil immersion objectives. Images were acquired with a Cohu (Tokyo, Japan) high-performance CCD camera coupled to the microscope and connected to a Leica Q550CW workstation (Leica Imaging Systems, Cambridge, UK). Images were visualized, processed, and stored by using Leica QFISH software version V1.01 and printed with a Tektronix Phaser 440 color printer (Tektronix, Wilsonville, OR).
RNA isolation and Northern blot analysis
HUVEC were grown to confluence in 150-mm culture dishes. Monolayers were mechanically denuded, and total cellular RNA was isolated according to the Ultraspect RNA Isolation System (Biotex Laboratories, Houston, TX) at the indicated times. Purified RNA from each sample (20 μg per lane) was denatured, electrophoresed through a formaldehyde 1% agarose gel, and blotted onto a nylon membrane (Hybond-N+; Amersham Life Science). Membranes were hybridized in ULTRAhyb buffer (Ambion, Austin, TX) with a 32P-labeledHindIII/BglII fragment of the Flt-1 cDNA10or a HindIII/BamHI fragment of β-actin cDNA for 20 hours at 42°C. Membranes were washed several times with SSC 0.2 × 0.1% SDS at 42°C and were exposed for 1 week at −80°C. Results were quantified by densitometry using the BioRad Multianalyst software (BioRad, Hercules, CA).
Plasmids
pFlt-1–Luc plasmid was generated from plasmid (−1195 to +284)–Luc.45 Sequences between −1195 and +284 of theflt-1 gene region were removed from the pGL2 luciferase reporter plasmid and cloned to the pXP2 luciferase reporter plasmid46 using BamHI and HindIII enzyme-restriction sites. For the construction of pmutFlt-1–Luc, polymerase chain reaction was performed over pFlt-1–Luc with 4 primers that generated 2 fragments with 2 appropriate enzyme-restriction sites—ApaI (site −229)/EcoRI (mutation, site −24) and EcoRI (mutation, site −24)/HindIII (pXP2 polylinker)—each of which could have been ligated after digestion with enzymes of the 2 fragments and the pFlt-1–Luc plasmid. Upper fragment primers were 5′TCCCGGGCCCGCGTCGCCAGCACCT3′ and 5′GAATTCTTTATAACCTTTCCCCA3′, and lower fragment primers were 5′AAAAGAATTCCCGCCCTCGGCTGCTCTTCATCG3′ and 5′GCCGGGCCTTTCTTTATGTTTTTG3′. To verify that the mutation and the ligated restriction sites were correct, the whole insert was sequenced. To generate p3EGR–Luc, we used the previously described construct Prolac−,47 which contains the firefly luciferase cDNA driven by the rat prolactin minimal promoter (−36 to +37), with a XhoI site for cloning purposes just upstream of this minimal promoter. The plasmid was linearized with XhoI and ligated in the presence of a XhoI–XhoI nucleotide sequence (5′ to 3′) containing 3 EGR DNA binding sites: GCGCCCCCGCAAGCTTCGCCCCCGCAAAACGCCCCCGCA. The 2XAP-1 luciferase reporter plasmid is described elsewhere.47 Empty expression vector pBX and pBXEGR1 containing the full-length Egr-1 cDNA driven by the SV40 promoter have been described. 48
Transfections and analysis of luciferase activity
HUVEC grown at confluence in culture dishes were transfected in Dulbecco's minimal essential medium/10% FBS with 6.5 μg DNA per million cells by using a standard calcium phosphate method.49 For cotransfection experiments, the total DNA level was raised to 24 μg per million cells using a 1:1 ratio of reporter plasmid to expression vector. The rate of renilla expression vector was always a quarter of the total DNA quantity. After 3 to 5 hours in the presence of DNA precipitates, HUVEC were washed with phosphate-buffered saline and cultured in fresh complete 199 medium for 16 hours. Transfected cell monolayers were mechanically denuded or treated with PMA (50 ng/mL) (Sigma, St Louis, MO) for 8 hours. Then cells were lysed, and luciferase activity was measured and normalized using the Dual–Luciferase Reporter Assay System (Promega, Madison, WI) with a Lumat LB9501 luminometer (Berthold, Germany).
Nuclear extracts
After different treatments, nuclear extracts were prepared according to a procedure described elsewhere50 with some modifications. Briefly, cell plates were washed once with ice-cold Hank's balanced salt solution and incubated with 1.5 mL buffer A (10 mmol/L HEPES [pH 7.3], 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.75 mmol/L spermidine, 0.15 mmol/L spermine, 1 mmol/L dithiothreitol (DTT), 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 mmol/L Na2MoO4, 1 μg/mL pepstatin, 6 μg/mL aprotinin, and 3 μg/mL leupeptin) on an orbital platform. Then 100 μL 10% NP-40 was added, and, after 10 minutes in the same shaking conditions, cell nuclei were harvested with a cell scraper and collected by centrifugation for 30 seconds at 14 000g. Nuclei pellets were washed twice with buffer A, and nuclear protein was extracted with 35 to 40 μL buffer C (20 mmol/L HEPES [pH 7.3], 400 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L PMSF, 10 mmol/L Na2MoO4, 1 μg/mL pepstatin, 6 μg/mL aprotinin, and 3 μg/mL leupeptin). Nuclear pellet volume was estimated to correct the final NaCl concentration to 400 mmol/L. After 30 minutes in a rocking platform and 10 minutes of centrifugation at 14 000g, supernatants containing the extracted nuclear proteins were collected and stored at −80°C. All steps were performed on ice or at 4°C. Nuclear extract protein concentrations were determined by Bradford assay.
Electrophoretic mobility shift assays
Nuclear extracts (3 μg in 2 μL buffer C per lane) were incubated with 0.5 μg poly(dI-dC), 100 ng calf thymus DNA, and 2.5 μL 5 × DNA binding buffer (50 mmol/L Tris buffer [pH 7.5], 130 mmol/L KCl, 5 mmol/L MgCl2, 2.5 mmol/L ZnCl2, 25 mmol/L DTT, 5 mmol/L EDTA, 25% vol/vol glycerol in a final volume of 11 μL for 10 minutes at room temperature. For supershift assays, 1 μL rabbit polyclonal IgG against Egr-1 (C-19) or Sp-1 (PEP2) (Santa Cruz Biotechnology) was added and incubated for another 10 minutes. Next, 0.5 to 1.5 ng (1.5 μL) 32P-labeled double-stranded oligonucleotide (4 to 20 × 107 cpm/μg) was added. After a 20-minute incubation at room temperature, DNA–protein complexes were resolved by electrophoresis on a 4% nondenaturing polyacrylamide gel in TBE 0.5× buffer. The sequences of complementary oligonucleotides (5′ to 3′) used as a probe in these assays were tcgaGTATAAATGCCCCCCGCCCTCGGCTc and tcgaGAGCCGAGGGCGGGGGCGATTTATAAc (capital letters indicate nucleotides −32 to −7 of human flt-1 5′ promoter sequence). For competition, the mutated oligonucleotides used were tcgaGTTATAAAGAATTCCCGCCCTCGGCTc and tcgaGAGCCGAGGGCGGGAATTCTTTATAAc (mutated bases are underlined).
Results
Mechanical endothelial denudation increases Flt-1 protein and mRNA levels
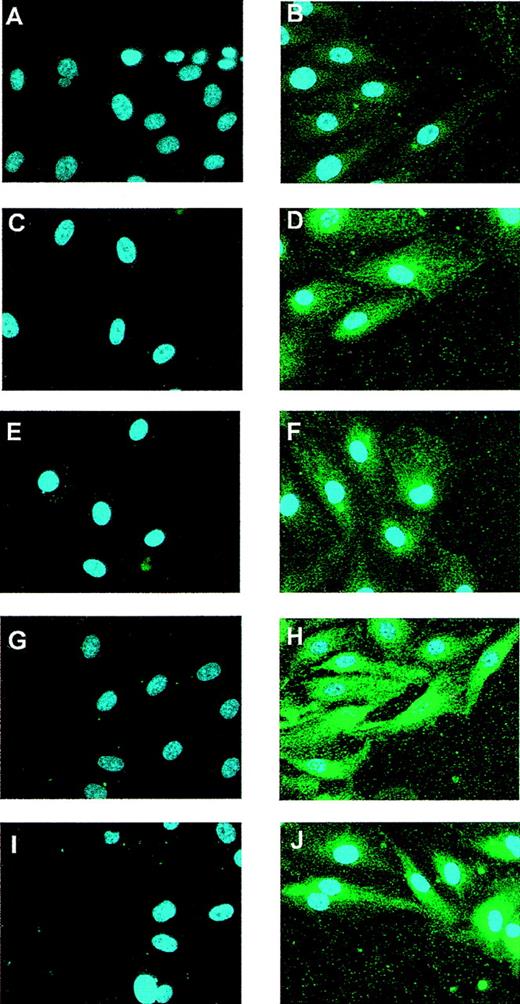
To investigate the Flt-1 expression pattern after in vitro mechanical denudation, we performed immunofluorescence experiments at different time points after wounding HUVEC monolayers using a specific antibody against Flt-1. The specificity of this antibody was verified in COS-7–transfected cells with an Flt-1 expression vector10 (data not shown). In control HUVEC, low Flt-1 protein levels were detected (Figure 1B). The expression of Flt-1 was clearly enhanced after 5 and 10 hours of stimulation (Figures 1D, 1F) and reached a maximum at 15 hours (Figure1H). The Flt-1 level was still higher than the basal level 20 hours after wounding (Figure 1J). This up-regulation was also observed, though to a lesser extent at 15 and 20 hours, in cells more distant from the wound edge (data not shown). Nonspecific staining was not observed when cells were incubated with the secondary antibody alone (Figures 1A, 1C, 1E, 1G, 1I).
Expression of Flt-1 in wounded monolayers of endothelial cells.
HUVEC monolayers were partially denuded and formaldehyde fixed at 0 (A, B), 5 (C, D), 10 (E, F), 15 (G, H), or 20 hours (I, J). (B, D, F, H, J) Cells were incubated with a rabbit-specific antibody against Flt-1 and a Cy3-conjugated goat antirabbit secondary antibody (green). Control cells (A, C, E, G, I) were incubated with the secondary antibody alone. All preparations were counterstained with DAPI to localize cell nuclei. Similar results were obtained in 2 independent experiments.
Expression of Flt-1 in wounded monolayers of endothelial cells.
HUVEC monolayers were partially denuded and formaldehyde fixed at 0 (A, B), 5 (C, D), 10 (E, F), 15 (G, H), or 20 hours (I, J). (B, D, F, H, J) Cells were incubated with a rabbit-specific antibody against Flt-1 and a Cy3-conjugated goat antirabbit secondary antibody (green). Control cells (A, C, E, G, I) were incubated with the secondary antibody alone. All preparations were counterstained with DAPI to localize cell nuclei. Similar results were obtained in 2 independent experiments.
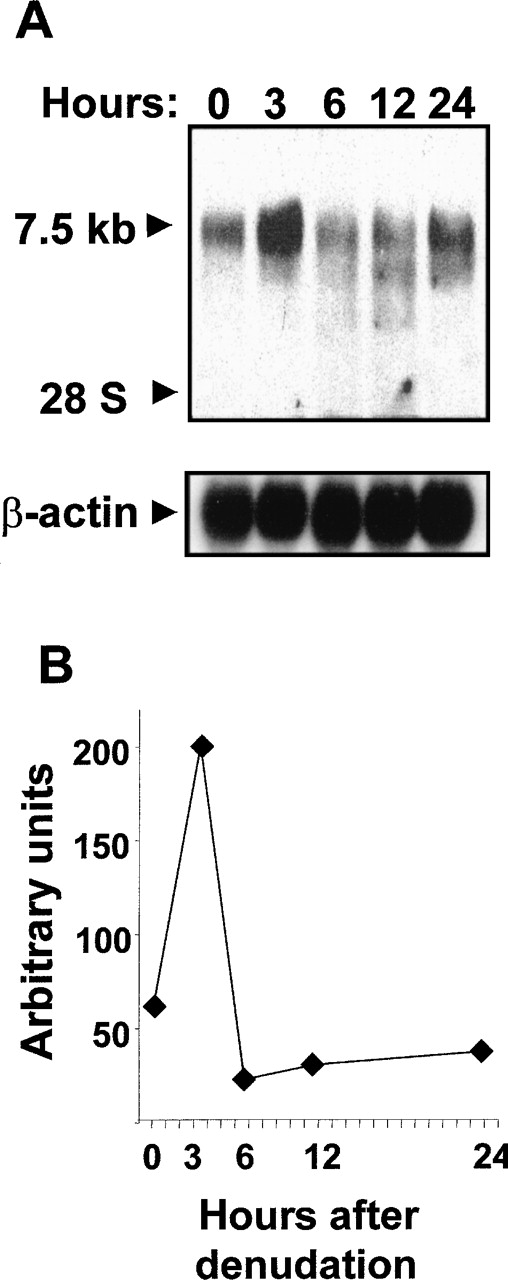
To investigate whether Flt-1 up-regulation was accompanied by increased mRNA level, Northern blot analysis using total RNA from denuded HUVEC monolayers was performed. A specific transcript of approximately 7.5 kb was detected after hybridization with an Flt-1 DNA probe (Figure2A). A 3-fold increase over basal level (Figure 2B) was observed at 3 hours after wounding; thereafter, the Flt-1 mRNA amount decreased.
Endothelial denudation increases Flt-1 mRNA level.
HUVEC monolayers were mechanically denuded, and total RNA was extracted at the indicated times. (A) Northern blot analysis was performed with 20 μg total RNA per lane. Nylon filters were hybridized to32P-labeled flt-1 or β-actin probe. (B) Values represented in the graphic are the data obtained from densitometry of the signals corresponding to the flt-1 mRNA normalizing to the corresponding β-actin levels. Similar results were obtained in an independent experiment.
Endothelial denudation increases Flt-1 mRNA level.
HUVEC monolayers were mechanically denuded, and total RNA was extracted at the indicated times. (A) Northern blot analysis was performed with 20 μg total RNA per lane. Nylon filters were hybridized to32P-labeled flt-1 or β-actin probe. (B) Values represented in the graphic are the data obtained from densitometry of the signals corresponding to the flt-1 mRNA normalizing to the corresponding β-actin levels. Similar results were obtained in an independent experiment.
Transcriptional activation of the flt-1 promoter by mechanical denudation
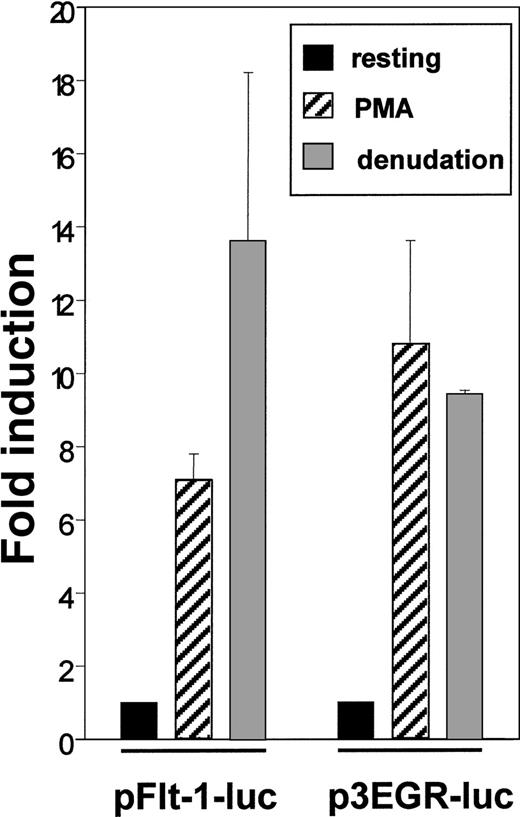
The enhanced level of Flt-1 mRNA led us to explore the possibility of a transcriptional regulation of this gene. For this purpose we transiently transfected HUVEC with the plasmid pFlt-1–Luc that contains the sequence −1195 to +284 of the flt-1 gene cloned to the pXP2 luciferase reporter plasmid. This promoter region has been reported to confer endothelial-specific expression to this gene.45 As a control, we used the p3EGR–Luc that contains 3 tandem repeats of an Egr-1 consensus binding site because the transcription factor Egr-1 has been described to be inducible by PMA and mechanical denudation.38 Transcriptional activity of pFlt-1–Luc was clearly enhanced by PMA treatment and by mechanical denudation (Figure 3), and similar results were obtained when transfecting the plasmid p3EGR–Luc. Transcriptional activity of the pXP2 promoterless plasmid was not modified by any stimuli tested in this study (data not shown). These experiments were also performed with the human microvasculature cell line HMEC-1, and similar results were obtained (data not shown).
Endothelial denudation and PMA enhance flt-1promoter-dependent transcription.
HUVEC were transiently transfected with the flt-1 (−1195 to +284) promoter luciferase construct pFlt-1–luc or the luciferase reporter plasmid containing 3 consensus binding sites for Egr-1 p3EGR–Luc. Cell monolayers were mechanically denuded or treated with PMA (50 ng/mL) for 8 hours. Luciferase activities were normalized for transfection efficiency to the renilla luciferase activities. Data shown are means ± SE of 3 independent experiments with 3 independent wells in each.
Endothelial denudation and PMA enhance flt-1promoter-dependent transcription.
HUVEC were transiently transfected with the flt-1 (−1195 to +284) promoter luciferase construct pFlt-1–luc or the luciferase reporter plasmid containing 3 consensus binding sites for Egr-1 p3EGR–Luc. Cell monolayers were mechanically denuded or treated with PMA (50 ng/mL) for 8 hours. Luciferase activities were normalized for transfection efficiency to the renilla luciferase activities. Data shown are means ± SE of 3 independent experiments with 3 independent wells in each.
Egr-1 binds to a putative binding site located at −24 to −16 bp of the flt-1 promoter region
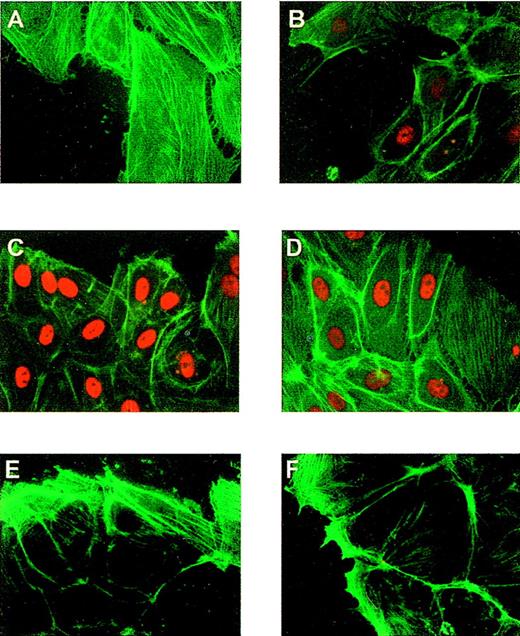
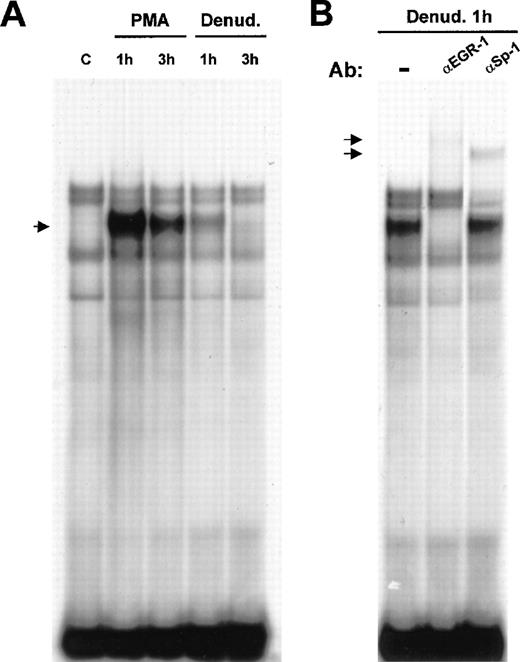
The up-regulation of the flt-1 promoter-dependent transcription led us to explore the possibility that the transcription factor Egr-1 was implicated in this process because its role in endothelium response to vascular injury was previously described.38 To ensure that the expression of endogenous Egr-1 was induced in our experimental protocol, we performed immunofluorescence assays in HUVEC at different times after denudation, using a specific antibody against Egr-1. Egr-1 protein level was clearly enhanced at 1 hour after denudation (Figures4A, 4B) in cells located at the edge of the denuded area, whereas Sp-1 level was not affected by denudation (Figures 4C, 4D). Nonspecific staining was not observed when incubating with the secondary antibody alone (Figures 4E, 4F). Three hours after denudation the amount of nuclear Egr-1 was low (data not shown).
Induction of Egr-1 expression in endothelial cells after mechanical denudation.
HUVEC monolayers were denuded and formaldehyde fixed after 0 (A, C, E) or 1 hour (B, D, F). (A, B) Cells were incubated with a rabbit-specific IgG against Egr-1, (C, D) with a rabbit specific IgG against Sp-1 (red), and (E, F) with the secondary antibody alone (same as Figure 1). All preparations were stained with fluorescein-conjugated phalloidin to localize the cells at the edge of the denuded area.
Induction of Egr-1 expression in endothelial cells after mechanical denudation.
HUVEC monolayers were denuded and formaldehyde fixed after 0 (A, C, E) or 1 hour (B, D, F). (A, B) Cells were incubated with a rabbit-specific IgG against Egr-1, (C, D) with a rabbit specific IgG against Sp-1 (red), and (E, F) with the secondary antibody alone (same as Figure 1). All preparations were stained with fluorescein-conjugated phalloidin to localize the cells at the edge of the denuded area.
Flt-1 promoter sequence analysis revealed a putative binding site for Egr-1 at −24 to −16 bp from the transcription start site. Therefore, we analyzed whether Egr-1 could bind this site by electrophoretic mobility shift assay. Nuclear extracts of HUVEC treated with PMA or after mechanical denudation were incubated with a DNA probe spanning from −32 to −7 of the flt-1promoter, containing the Egr-1 consensus sequence (see “Materials and methods”). An inducible complex appeared at 1 hour after stimulation, and its level clearly decreased after 3 hours in both cases (Figure 5). This was in good agreement with the published data about the induction of Egr-1 in response to mechanical denudation in endothelial cells51and with our results regarding Flt-1 mRNA levels.
Binding of Egr-1 to the −32 to −7 flt-1promoter sequence after endothelial denudation or PMA treatment.
(A) Electrophoretic mobility shift assays were performed with 3 μg nuclear extracts from HUVEC incubated with a 32P-labeled DNA probe of the (−32 to −7) flt-1 promoter region. Cell monolayers were mechanically denuded or treated with PMA (50 ng/mL) for the indicated times. C, control untreated cells. The arrow indicates the inducible complex. (B) Supershift assays were performed with polyclonal specific antibodies against Egr-1 and Sp-1 transcription factors using the same probe as in A. Ab, antibody; denud., mechanical denudation. The arrows indicate the supershifted complexes. These results are highly reproducible in more than 3 independent experiments.
Binding of Egr-1 to the −32 to −7 flt-1promoter sequence after endothelial denudation or PMA treatment.
(A) Electrophoretic mobility shift assays were performed with 3 μg nuclear extracts from HUVEC incubated with a 32P-labeled DNA probe of the (−32 to −7) flt-1 promoter region. Cell monolayers were mechanically denuded or treated with PMA (50 ng/mL) for the indicated times. C, control untreated cells. The arrow indicates the inducible complex. (B) Supershift assays were performed with polyclonal specific antibodies against Egr-1 and Sp-1 transcription factors using the same probe as in A. Ab, antibody; denud., mechanical denudation. The arrows indicate the supershifted complexes. These results are highly reproducible in more than 3 independent experiments.
To demonstrate that Egr-1 was present in the inducible complex, supershift experiments were carried out using nuclear extracts from HUVEC at 1 hour after denudation. When incubating the binding reaction with a purified IgG-specific antibody against Egr-1, the inducible complex was completely supershifted (Figure 5B). As a control for specificity, another purified IgG-specific antibody, against Sp-1, was added to the binding reaction without affecting the denudation-inducible complex. However, this antibody produced a supershift of one of the constitutive complexes because theflt-1 promoter contained an Sp-1 consensus binding sequence overlapping with the Egr-1 binding site. This is a recurrent motif in many Egr-1–regulated genes in which Sp-1 is constitutively bound to its site.51 These results demonstrate that Egr-1 binds to the consensus binding site contained within the proximal region (−32 to −7 bp) of the flt-1 promoter.
Exogenous Egr-1 transactivates flt-1 promoter
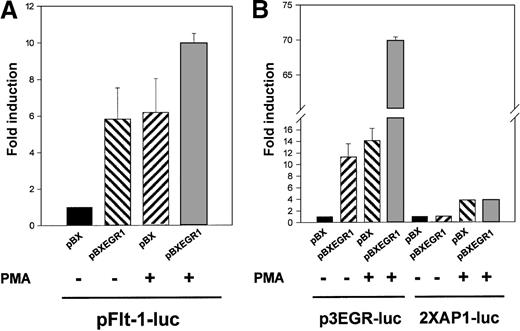
To elucidate whether the binding of Egr-1 was responsible for the observed transcriptional activation of the flt-1 promoter, the reporter plasmid pFlt-1–Luc was cotransfected with the Egr-1 expression vector pBXEGR-1 or the empty vector pBX. Overexpression of Egr-1 led to an increment of flt-1 promoter-dependent transcription similar to that obtained with PMA alone (Figure6A). When HUVEC transfected with the Egr-1 expression vector were also treated with PMA, clear cooperation was observed.
Cotransfection with an EGR-1 expression vector leads to a specific activation of the flt-1 promoter.
HUVEC were transiently cotransfected with pFlt-1–Luc in (A), p3EGR–Luc (both described in Figure 3) or the AP-1–dependent transcription reporter plasmid 2XAP-1–Luc in (B), together with an Egr-1 expression vector driven by the SV40 promoter (pBXEGR-1) or the empty vector (pBX). When indicated, cells were treated with PMA 50 ng/mL for 8 hours before measuring luciferase activity. The number of experiments, normalization, and statistics are similar to those in Figure 3.
Cotransfection with an EGR-1 expression vector leads to a specific activation of the flt-1 promoter.
HUVEC were transiently cotransfected with pFlt-1–Luc in (A), p3EGR–Luc (both described in Figure 3) or the AP-1–dependent transcription reporter plasmid 2XAP-1–Luc in (B), together with an Egr-1 expression vector driven by the SV40 promoter (pBXEGR-1) or the empty vector (pBX). When indicated, cells were treated with PMA 50 ng/mL for 8 hours before measuring luciferase activity. The number of experiments, normalization, and statistics are similar to those in Figure 3.
Similar experiments using the reporter plasmid p3EGR–Luc and the AP-1 dependent reporter plasmid 2XAP-1–Luc as controls were performed (Figure 6B). As expected, Egr-1 overexpression enhanced the p3EGR–Luc transcriptional activity and synergized with PMA treatment, whereas 2XAP-1–Luc basal activity or its inducibility by PMA treatment was not modified by the presence of exogenous Egr-1. This new evidence suggested a role for Egr-1 in the transactivation of the flt-1promoter.
Induction of the flt-1 promoter by mechanical denudation is dramatically impaired by the mutation of the Egr-1 binding site
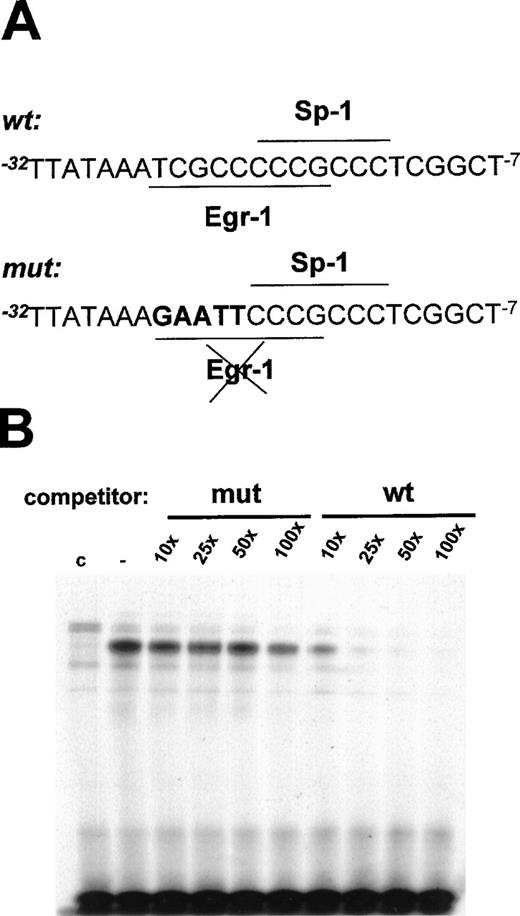
To demonstrate that the Egr-1 binding site at positions −24 to −16 bp in the flt-1 promoter mediated the transcriptional induction by mechanical denudation, site-directed mutagenesis of this motif was performed. Because Sp-1 is responsible for the basal transcriptional activity in many promoters,51 the mutation of its site could abrogate this activity. Thus, the mutation of the Egr-1 binding site was generated without affecting the overlapping Sp-1 binding site. To test the Egr-1 ability to bind the mutated sites, we carried out electrophoretic mobility shift assays using the wild-type probe previously described (see below) and competing with the indicated molar excess of mutated or wild-type oligonucleotide (shown in Figure7A). Competition with the mutated oligonucleotide did not significantly affect the binding of the inducible complex (Figure 7B), whereas the wild-type oligonucleotide completely competed Egr-1 binding to the probe.
Mutation of the EGR-1 binding site at −24 to −16 bp in the flt-1 promoter.
(A) Schematic representation of the mutation of the Egr-1 binding site; mutated base pairs are represented in bold characters. (B) Electrophoretic mobility shift assays were performed using conditions similar to those in Figure 5A but competing with the indicated molar excess of the mutated probe (mut) or the wild-type probe (wt), both represented in (A). C, nuclear extract from control cells. Similar results were obtained in 2 independent experiments.
Mutation of the EGR-1 binding site at −24 to −16 bp in the flt-1 promoter.
(A) Schematic representation of the mutation of the Egr-1 binding site; mutated base pairs are represented in bold characters. (B) Electrophoretic mobility shift assays were performed using conditions similar to those in Figure 5A but competing with the indicated molar excess of the mutated probe (mut) or the wild-type probe (wt), both represented in (A). C, nuclear extract from control cells. Similar results were obtained in 2 independent experiments.
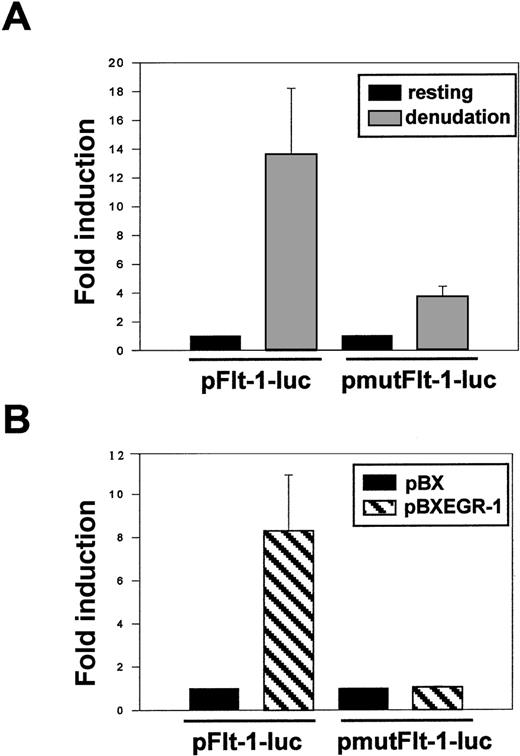
Transient transfection of HUVEC with the plasmid pFlt-1–Luc or its mutated version pmutFlt-1–Luc showed similar basal activities (data not shown). In contrast, after mechanical denudation, the mutated plasmid retained only 25% of the fold induction observed with the wild-type promoter plasmid (Figure 8A). In addition, cotransfection of the plasmid pmutFlt-1–Luc with the Egr-1 expression vector showed that the mutated plasmid no longer responded to Egr-1 (Figure 8B). These data clearly demonstrated that the Egr-1 binding to its site at −24 to −16 bp in the flt-1promoter played a major role in the flt-1–enhanced expression after endothelial denudation.
Mutation of the EGR-1 consensus sequence in theflt-1 promoter dramatically decreases its transcriptional activity after mechanical denudation.
(A) HUVEC were transiently transfected with the pFlt-1–Luc plasmid or the mutated version (see mutation in Figure 7A) pmutFlt-1–Luc. Cell monolayers were mechanically denuded, and luciferase activity was measured after 8 hours. (B) HUVEC were transiently cotransfected with the luciferase reporter plasmids pFlt-1–Luc or pmut-Flt-1–Luc and the EGR-1 expression vector pBXEGR-1 or the empty vector pBX. Luciferase activity was measured 24 hours after transfection. Number of experiments, normalization, and statistics are similar to those in Figure 3.
Mutation of the EGR-1 consensus sequence in theflt-1 promoter dramatically decreases its transcriptional activity after mechanical denudation.
(A) HUVEC were transiently transfected with the pFlt-1–Luc plasmid or the mutated version (see mutation in Figure 7A) pmutFlt-1–Luc. Cell monolayers were mechanically denuded, and luciferase activity was measured after 8 hours. (B) HUVEC were transiently cotransfected with the luciferase reporter plasmids pFlt-1–Luc or pmut-Flt-1–Luc and the EGR-1 expression vector pBXEGR-1 or the empty vector pBX. Luciferase activity was measured 24 hours after transfection. Number of experiments, normalization, and statistics are similar to those in Figure 3.
Discussion
Vascular endothelium provides a nonthrombogenic surface and a permeability barrier that modulates vascular reactivity and blood flow. Vascular injury and the subsequent inflammatory response are important events in diseases such as atherosclerosis and in postangioplasty restenosis lesions. Because of its angiogenic activity and therapeutic potential,52 it is of great interest to discern the possible role of VEGF and its receptors in these processes. Herein, we have studied the inducible expression of the VEGF receptor Flt-1 after vascular injury using an in vitro protocol of mechanical denudation of HUVEC monolayers. Our results show that Flt-1 protein and mRNA levels are clearly enhanced after mechanical denudation. The highest mRNA level is reached 3 hours after denudation, and the protein level is significantly increased at 5 hours after wounding. This rapid induction could be one of the earliest cellular events in the endothelium response to injury and is in agreement with the high expression in vivo of the Flt-1 mRNA described in denuded rat arteries.53 Given that the Flt-1 basal protein level is low,54 we could not detect the endogenous protein by Western blot. Therefore, we used immunofluorescence assays, which allowed us not only to detect the expression of Flt-1 in the HUVEC monolayers but also to study the spatial expression of Flt-1 with regard to the location of the wound. The highest expression was detected at the edge of the wound, but we also observed induction of Flt-1 in cells more distant from the leading edge of the wound. This could be explained by the release of soluble factors. In this regard, it was reported recently that FGF-2 is released after endothelial cell injury and is partially responsible for Egr-1 induction.55In vivo the blood flow could partially remove FGF-2 or other secreted soluble factors that would remain bound to matrix and cells at the edges of the wound. The role of other cytokines in this response to vascular injury, such as VEGF, which has been recently reported to induce Egr-1,56 remains to be completely elucidated. It would be interesting to study the possible interplay between the VEGF/KDR induction of Egr-1 and the up-regulation of Flt-1 because this pathway could play an important role in the regulation of the VEGF mitogenic signal.
The possibility that the early gene Egr-1 was implicated in the up-regulation of the flt-1 promoter-dependent transcription was explored. There is a putative binding site for this transcription factor in the promoter sequence; in addition, denudation and PMA are well-known inducers of Egr-1 and have similar time kinetics. The ability of Egr-1 to bind to the −24 to −16 bp sequence of the flt-1 promoter was demonstrated by electrophoretic mobility shift assays. The fact that exogenous Egr-1 was capable of transactivating the flt-1 promoter reporter plasmid gave evidence that Egr-1 was not only binding to this sequence in vitro but also mediating transcriptional activation. To demonstrate further that the activation of the flt-1 promoter was caused by the binding of Egr-1 to the −24 to −16 sequence, we constructed a mutated version of the flt-1 promoter that retained only 25% of the inducible activity of the wild-type version rendered after denudation. The remaining inducible activity could have resulted from other transcription factors that may have been induced after denudation. It has been reported that the transcription factor Ets-1 is expressed at the wound edge of endothelial monolayers,57and a functional Ets motif in the flt-1 promoter has been described58; therefore, this transcription factor is a good candidate for this transcriptional up-regulation.
These data are similar to the transcriptional mechanism of induction of other genes implicated in the response to vascular injury already described,53 and they place Flt-1 in the context of a complex cellular response with the aim of repairing the vessel wall. The role of Flt-1 in endothelium is not well understood. Mice lacking Flt-1 die before birth because of the abnormal formation of vascular channels,14 suggesting that an Flt-1 signaling pathway may regulate normal endothelial cell–cell or cell–matrix interactions. However, it was later described that mice lacking only the tyrosine kinase domain of Flt-1 show normal development and angiogenesis,18 arguing for a role of this receptor as a ligand-binding molecule and not as a first step of an intracellular signaling pathway in vascular endothelium. Fong et al59recently reported that flt-1 knock-out mice do not form functional vessels because of an excessive development of hemangioblasts. This could be explained by the fact that, in the absence of Flt-1, the amount of available VEGF is dysregulated, and therefore the development of the endothelial lineage is increased. Other evidence suggests a negative regulatory function of Flt-1 in VEGF-induced angiogenesis. That this receptor has more than 10-fold higher affinity for VEGF than Flk-1/KDR but only weak tyrosine kinase activity60 and that the extracellular domain of Flt-1 has a potent suppressor activity on VEGF both in vivo and in vitro61-66 strongly support this hypothesis. Silverman and Collins45 recently suggested a possible role for several Egr-1–regulated genes in growth retardation and cell survival after vascular injury. The induction of transforming growth factor-β could suppress the growth of damaged cells, hindering leukocyte recruitment, modulating vascular tone, and increasing the expression of growth factors such as PDGF.67 The Egr-1–dependent up-regulation of p53, or the down-regulation of bcl-2, could be implicated in the cell cycle arrest or apoptosis of the injured cells.68 The rapid up-regulation of Flt-1 reported in this article could be more evidence for this role of Egr-1–induced genes because Flt-1 could suppress VEGF mitogenic activity, whereas modestly damaged cells respond to injury and severely injured ones become apoptotic.
In conclusion, we report that Flt-1 protein and mRNA levels are enhanced in response to mechanical denudation using a primary culture of human endothelial cells. We demonstrate that the flt-1promoter–dependent transcription is clearly up-regulated and that Egr-1 is one of the main factors responsible for this up-regulation. These findings implicate Flt-1 in the first events of the endothelium response to vascular injury.
Acknowledgments
We thank J. G. Monroe for kindly providing the Egr-1 expression vector and L. T. Williams for his generous gift of the Flt-1 promoter constructs and expression vector, critical reagents that have made this work possible. We thank M. López–Cabrera, M. C. Castellanos, and M. D. Gutiérrez for critical reading of the manuscript. We thank the staff at Clı́nica del Rosario for generously providing human umbilical cords.
Supported by grants Fis 98/1382 and CAM 08./0015/97 (M.O.L.) from Fondo de Investigaciones Sanitarias and Comunidad Autónoma de Madrid. F.V. and A.A. were supported by fellowships from Ministerio de Educación y Cultura and J.A. by a fellowship from Fondo de Investigaciones Sanitarias.
Reprints:Manuel O. de Landázuri, Servicio de Inmunologı́a, Hospital de la Princesa, Diego de León 62, Madrid 28006, Spain; e-mail: mortiz@hlpr.insalud.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal