Abstract
Platelet activation is associated with an increase of cytosolic Ca++ levels. The 1,4,5IP3receptors [1,4,5IP3R] are known to mediate Ca++ release from intracellular stores of many cell types. Currently there are at least 3 distinct subtypes of1,4,5IP3R—type I, type II, and type III—with suggestions of distinct roles in Ca++ elevation. Specific receptors for 1,3,4,5IP4 belonging to the GAP1 family have also been described though their involvement with Ca++ regulation is controversial. In this study we report that platelets contain all 3 subtypes of1,4,5IP3R but in different amounts. Type I and type II receptors are predominant. In studies using highly purified platelet plasma (PM) and intracellular membranes (IM) we report a distinct localization of these receptors. The PM fractions were found to contain the type III 1,4,5IP3R and GAP1IP4BP in contrast to IM, which contained type I1,4,5IP3R. The type II receptor exhibited a dual distribution. In studies examining the labeling of surface proteins with biotin in intact platelets only the type III1,4,5IP3R was significantly labeled. Immunogold studies of ultracryosections of human platelets showed significantly more labeling of the PM with the type III receptor antibodies than with type I receptor antibodies. Ca++ flux studies were carried out with the PM to demonstrate in vitro function of inositol phosphate receptors. Ca++ release activities were present with both 1,4,5IP3 and1,3,4,5IP4 (EC50 = 1.3 and 0.8 μmol/L, respectively). Discrimination of the Ca++-releasing activities was demonstrated with cyclic adenosine monophosphate (cAMP)-dependent protein kinase (cAMP-PK) specifically inhibiting 1,4,5IP3 but not1,3,4,5IP4-induced Ca++ flux. In experiments with both PM and intact platelets, the1,4,5IP3Rs but not GAP1IP4BP were found to be substrates of cAMP-PK and cGMP-PK. Thus the Ca++ flux property of1,3,4,5IP4 is insensitive to cAMP-PK. These studies suggest distinct roles for the1,4,5IP3R subtypes in Ca++movements, with the type III receptor and GAP1IP4BPassociated with cation entry in human platelets and the type I receptor involved with Ca++ release from intracellular stores.
Platelet activation represents an important event in hemostasis and thrombosis. The activation of platelets by all stimulatory agonists involves the elevation of cytosolic Ca++ levels (for a review, see Authi1). Stimulatory agonists acting on their surface receptors lead, through a number of steps, to the hydrolysis of phosphatidylinositol 4,5-bisphosphate, resulting in the formation of inositol 1,4,5-trisphosphate (1,4,5IP3) and 1,2-diacylglycerol. Elevated cytosolic Ca++ levels arise as a consequence of release of the cation from intracellular stores and entry from the extracellular medium. Although Ca++ release from intracellular stores by the action of1,4,5IP3 is relatively well understood, Ca++ entry mechanisms are not.2,31,4,5IP3 can be further metabolized to inositol tetrakisphosphate (1,3,4,5IP4) before being degraded to 1,3,4IP3 and lower inositol phosphates. Considerable evidence suggests that Ca++ entry can occur as a result of Ca++ release from intracellular stores (termed store-regulated Ca++ entry or capacitative Ca++ entry2), but the link between the stores and the plasma membrane (PM) Ca++ entry channel is poorly defined. Possible mechanisms include the involvement of a soluble factor,4 small G proteins,5 tyrosine phosphorylation mechanisms,6 or a coupling action possibly involving an association of the 1,4,5IP3receptor (1,4,5IP3R) with the PM channel.7 In platelets, alternative routes of Ca++ entry include activation of a receptor-operated Ca++ entry channel as described for the platelet agonist ADP8; a second-messenger–operated mechanism such as an action of 1,4,5IP3 on an1,4,5IP3R at the PM9 that could be mediated in a similar manner to that described in endothelial cells by1,3,4,5IP410; and a possible action of 1,3,4,5IP4 at the level of the PM.11 Specific receptors for1,3,4,5IP4, such as GAP1IP4BP, GAP1m, and centaurin,12-14 have been described. GAP1IP4BP and GAP1m are closely related GTPase-activating proteins that exhibit activities toward the small G-protein Ras and have been implicated in Ca++movements.15
There are at least 3 distinct genes that code for1,4,5IP3Rs, namely type I, type II, and type III, and at the protein level further heterogeneity arises because of alternative splicing events.16,17 The 3 subtypes share approximately 60% to 75% sequence similarity, which is highest at the ligand-binding domain and the putative channel domain. The possibility that subtypes may serve distinct functions involved with Ca++ movements arises from differing properties, modes of regulation, and distinct localization within cells. Binding affinities for 1,4,5IP3 differ between the subtypes, with the highest expressed by the type II receptor, then by type I, and then by type III.18 Modes of regulation of1,4,5IP3R include interactions with Ca++, calmodulin, adenine nucleotides, FK binding proteins, and protein kinases.17,19 Consensus sites for phosphorylation by cAMP- and cGMP-dependent protein kinases (cAMP-PK, cGMP-PK) are present for all 3 receptor subtypes and by tyrosine kinases on types I and II,18 though not all the functional consequences of phosphorylation are understood. In addition, with platelets there is still debate as to whether1,4,5IP3R is phosphorylated at all.1,20 21
Platelets have been shown to contain the type I and type II, and possibly the type III,1,4,5IP3Rs.20,22,23 The type I receptor appears to be present predominantly in the intracellular membranes (IM). There is an uncertain location for the type II receptor, and a distinct 1,4,5IP3R-like protein is present exclusively in the PM fraction prepared using free-flow electrophoresis (FFE).22 23 The purpose of this study was to determine the localization and functional regulation of1,4,5IP3R isoforms and GAP1IP4BPusing a range of isoform-specific antibodies, a combination of highly purified PM and IM preparations, and whole-cell techniques. We report the expression of all 3 types of 1,4,5IP3R isoforms in different amounts at distinct locations, in vitro Ca++ flux activities for both the1,4,5IP3R and the GAP1IP4BP that co-localize at the pm, and the discrimination of their activities in vitro by cAMP-PK. These results imply involvement of a specific population of 1,4,5IP3R (type III and type II) and GAP1IP4BP with cation influx that localize to the PM and another population (type I and type II)1,4,5IP3R that localizes in the IM to be involved in Ca++ release.
Materials and methods
Materials
All chemical reagents were obtained from Sigma Chemical (Dorset, UK) unless otherwise stated. Electrophoresis reagents were obtained from National Diagnostics (Hull, UK) and nitrocellulose membranes were from Schleicher and Schuell (Dassel, Germany). Sp-5,6-DCl-cBiMPS (BiMPS) and 8pCPT-cGMP (8pCPT) were obtained from Biolog Life Science Institute (Bremen, Germany). 2,4,5IP3 was a gift from Prof Robin Irvine (Cambridge, UK). Regarding antibodies, CT1 was raised to the C-terminal 19-amino acid sequence of the rat type I1,4,5IP3R, CT2 to the C-terminal peptide GFLGSNTPHENHHMPPH of the rat type II receptor, and CT3 raised to the C-terminal peptide RLGFVDVGNCMSR of the rat type III1,4,5IP3R. CT1, CT2, and CT3 were raised in the laboratory of R.J.H.W. and have been previously characterized (for full details, see Wojcikiewicz24). Two additional polyclonal antibodies were raised to 1,4,5IP3R in the laboratory of KSA and were affinity purified as were the CT antibodies. R26 was raised to the last 12 C-terminal amino acids of the human type I receptor and is thus similar to CT1, but R45 was raised to the sequence TASPLGMPHGAA, which resides between the predicted transmembrane region M5 and M6 of the human type III receptor and is thus unique. These are characterized in this study and are used interchangeably. The monoclonal antibody 18A10 recognizes the C-terminal part of the type I 1,4,5IP3R and was a kind gift from K. Mikoshiba (Japan). An antibody to the C-terminus of GAP1IP4BP was also raised using the sequence (C)VQSYIRQQSETSTHSI and affinity purified. This reagent was found not to significantly recognize GAP1M (R. F. Irvine, personal communication).
Purification of platelet plasma and intracellular membranes by free-flow electrophoresis
Platelet PM and IM were prepared as described in detail in previous publications.23,25 26 Briefly, platelets were separated from human blood and treated with neuraminidase (0.05 U/mL) for 20 minutes at 37°C. After further washing, platelets were sonicated in 0.34 mol/L sorbitol and 10 mmol/L HEPES, pH 7.4, with a cocktail of inhibitors including aprotinin (0.3 U/mL), pepstatin A (5 μg/mL), phenylmethylsulfonyl fluoride (PMSF; 0.2 mmol/L), dithiothreitol (DTT; 1 mmol/L), soybean trypsin inhibitor (1 mg/mL), and E64 (2 μmol/L). Platelet sonicates were centrifuged at 40 000g for 90 minutes on a linear (1 to 3.5 mol/L) sorbitol density gradient to obtain a mixed membrane fraction (free of granular contamination). After centrifugation of the mixed membrane (100 000g for 60 minutes), they were separated into PM and IM by FFE using an Octopus apparatus (Dr Weber GmbH, Ismaning, Germany) running at 750 V 100 mA. Two discrete peaks comprising PM (less electronegative) and IM (more electronegative) were obtained. Tops of peaks were pooled, centrifuged (100 000g for 60 minutes), and resuspended in 0.34 mol/L sorbitol, 10 mmol/L HEPES, pH 7.2, for studies involving Western blotting and protein phosphorylation.
Western blotting
Samples of purified membranes (50 μg PM and 50 μg IM) were applied to SDS-PAGE gradient gels (5% to 15%) following the method of Laemmli27 and were subjected to electrophoresis. Separated proteins were transferred to nitrocellulose paper by semi-dry blotting using a current density of 0.8 mA/cm2 for 1 hour, and the nitrocellulose was then blocked for 3 hours (or overnight) in blocking medium (5% dried milk, 1% normal goat serum, 20 mmol/L Tris, pH 7.4, 500 mmol/L NaCl, 0.1% Tween). Filters were washed 3 times in the above buffer without dried milk and normal goat serum, followed by incubation with the corresponding primary antibody (CT1, CT2, CT3, R26, R45, etc) in blocking medium for 1 hour. After washing, the membranes were incubated with an appropriate second antibody (eg, goat antirabbit) conjugated to horseradish peroxidase for 1 hour, followed by detection using ECL reagents (Amersham, Little Chalfont, UK). The blocking medium for biotinylation studies (see below) contained 5% bovine serum albumin (BSA), 20 mmol/L NaHPO4, 130 mmol/L NaCl, and 2 mmol/L EDTA, pH 7.5.
Phosphorylation of purified membranes by cyclic nucleotide analogues and kinases and immunoprecipitation of receptors
Phosphorylation of purified PM was carried out as described previously.23 The reaction mixture consisted of 50 mmol/L HEPES buffer (pH 7.4), 10 mmol/L MgCl2, 1 mmol/L DTT, 0.2 mmol/L EGTA, 500 μg plasma membrane protein, 10 μmol/L adenosine triphosphate (ATP) containing 1 μCi (γ-32P ATP), and, where indicated, cAMP-PK CAT 2 μg protein (70 U)/reaction (see lanes) and ± 1 μmol/L cAMP-PK CAT inhibitor peptide (see El-Daher et al23 and Cheng et al28; also legend to Table 2 for peptide composition). The reaction was initiated by the addition of ATP and cAMP-PK CAT, mixing, and incubation at 30°C for 5 minutes and was terminated by the addition of cold solubilization buffer (1% NP40 [or 1% Triton X100 where stated], 0.2% LDS, 10 mmol/L NaF, 20 mmol/L sodium pyrophosphate, 5 mmol/L EDTA, 75 mmol/L NaCl, 1 mmol/L PMSF, 100 μmol/L sodium vanadate, 0.2 mmol/L leupeptin, 20 mmol/L Tris, pH 7.8). After 30 minutes at 4°C, the samples were centrifuged at 13 000gfor 5 minutes. The supernatant was treated with one-tenth volume 20% Protein-A Sepharose (PAS; Pharmacia, St Albans, UK) for 30 minutes at 4°C to preclear the sample and centrifuged, and the supernatant was added to 200 μL appropriate antibody covalently attached to PAS and incubated for 4 hours at 4°C. (In some experiments, antibodies alone were added for 2 hours followed by PAS and incubated with mixing overnight at 4°C). The immunoadsorbent was spun down, washed 4 times with washing buffer (0.2% NP40 [or 0.2% Triton], 0.1% BSA, 0.01% sodium azide in phosphate-buffered saline [PBS]) and once in washing buffer without BSA. SDS–Laemmli buffer was then added to the immunoadsorbent, and this was followed by SDS–PAGE and autoradiography to visualize the protein bands.
Labeling of intact platelets with [32Pi] and activation of cyclic nucleotide kinases
Freshly prepared platelets were resuspended in a wash buffer consisting of 36 mmol/L citric acid, 103 mmol/L NaCl, 5 mmol/L KCl, 5 mmol/L glucose, pH 6.5, and 60 nmol/L prostacyclin (PGI2). They were then incubated for 90 minutes at 37°C with 0.25 mCi carrier-free [32P] Pi/mL and washed twice before resuspension in a HEPES tyrode medium containing 1 mmol/L EGTA (and no Ca++) at 3 × 109 cells/mL. Incubations with either BiMPS or 8pCPT were carried out using 300-μL suspensions at 37°C with mixing for 5 minutes, and reactions were stopped by the addition of Triton solubilization buffer. After 30 minutes at 4°C, the mixtures were centrifuged at 14 000g for 5 minutes at 4°C, and the supernatants were used for immunoprecipitation with respective antibodies as described earlier, followed by analysis of phosphorylated proteins by SDS–PAGE and transfer to nitrocellulose and autoradiography.
Labeling of surface proteins in intact platelets with biotin
Labeling of surface proteins with biotin was carried out as described by Clemetson.29 Platelets were resuspended at 5 × 109 cells/mL in 20 mmol/L NaHPO4, 130 mmol/L NaCl, 2 mmol/L EDTA, pH 8.0.N-hydroxysuccinimidobiotin was added at 0.02 mg/mL and left for 1 hour at room temperature with gentle rocking. Platelets were then washed 5 times in the same buffer at pH 6.5. The washed platelets were solubilized, and proteins were immunoprecipitated with the appropriate antibody and then probed to detect for biotin on Western blots using streptavidin-HRP (1:2000 dilution, 1 hour) and ECL reagents.
Immunogold labeling on ultracryosections
Ultracryosectioning was carried out using the methods of Tokuyasu30 and van Genderen et al.31 Briefly, washed platelets were fixed by the addition of equal volume 4% formaldehyde solution in PBS. After 30 minutes, the cells were centrifuged at 1200g for 15 minutes, and the pellet placed in 5% sucrose solution and then onto small specimen pins and snap frozen in liquid nitrogen. Thereafter, the samples were quickly transferred to a Dupont MT6000 ultramicrotome (Stevenage, UK) adapted for ultracryomicrotomy and sectioned using a dry glass knife. The sections were retrieved from the knife using a small drop of 2.3 mol/L sucrose solution in PBS and transferred onto formar/carbon-coated specimen grids. Immunolabeling with polyclonal antibodies was carried out by floating grids section-side down on small drops as follows: (1) blocking of nonspecific sites with 10% fetal calf serum in PBS for 30 minutes; (2) incubation with primary antibody for 1 hour; (3) wash with PBS 3 × 5 minutes; (4) incubation with Protein A–gold in 1% BSA/PBS; (5) wash with PBS 3 × 5 minutes and with water 5 × 1 minute. For monoclonal antibodies, an additional step involving the use of a rabbit antimouse antibody as a bridge (1 mg/mL, 1:200 dilution, 1 hour) and washing is included in step 4 before Protein A–gold treatment. The sections were then treated with 0.3% uranyl acetate in methylcellulose for 10 minutes, blotted with Whatman filter paper (Maidstone, UK), air dried, and examined under a Philips 201 transmission electron microscope. Illustrations shown are typical of 2 separate preparations with at least 30 cells examined with each antibody.
Ca++ flux determinations in purified platelet PM
Platelet PM were prepared as above using protease inhibitors in the sonication medium as follows: PMSF, 0.25 mmol/L; pepstatin, 5 μg/mL; aprotinin, 0.3 U/mL; DTT, 0.5 mmol/L; soybean trypsin inhibitor, 0.5 mg/mL. PM (40 μg protein in 4μL) resuspended in NaCl–Tris buffer (150 mmol/L NaCl, 10 mmol/L Tris-HCl pH 7.4) was incubated at 37°C for 30 minutes for Na+ loading and then diluted 60-fold into exchange buffer containing 150 mmol/L KCl, 10 mmol/L Mopes-Tris and 45Ca++ (2 μCi/300 μL) and further incubated for up to 15 minutes to allow Na+/Ca++ exchange. Reactions were stopped by the addition of 200 μL ice-cold stop buffer (containing 5 mmol/L Mopes-Tris, pH 7.4, 150 mmol/L KCl, and 5 mmol/L LaCl3) and rapid filtration through a 0.45-μm filter under vacuum. The filter was washed twice with ice-cold stop buffer, and, after semi-drying, the radioactivity was counted by liquid scintillation spectrometry. Addition of various concentrations of inositol phosphates or ionomycin was made at 10 minutes incubation of membranes in exchange buffer and reactions stopped at 15 minutes (or as indicated).
All Western blots and autoradiographs shown represent typical results obtained with at least 3 different platelet or membrane preparations.
Results
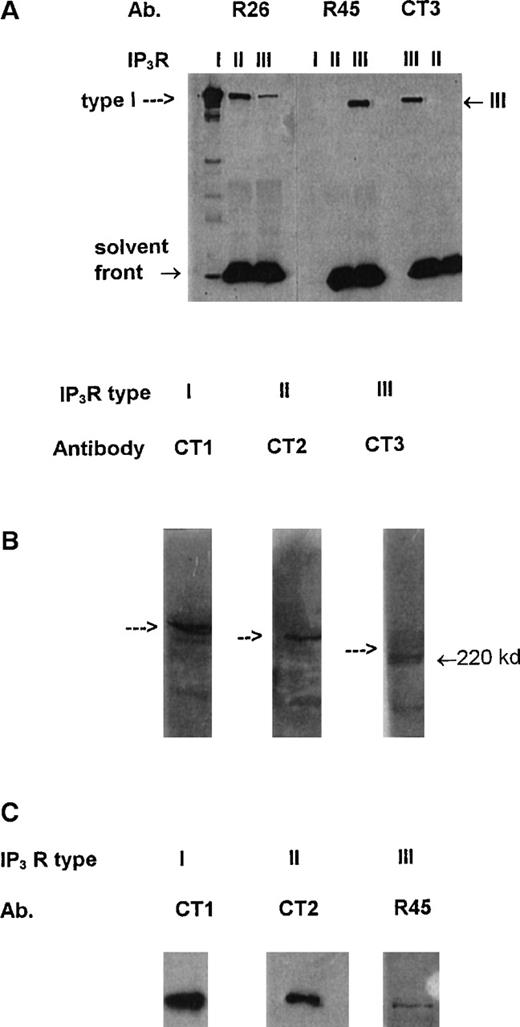
Studies initially examined the expression of1,4,5IP3R in platelet-mixed membranes using specific antibodies. CT1, CT2, and CT3 represent affinity-purified antisera specific for the type I, type II, and type III1,4,5IP3R, respectively, and have been previously well characterized.24 The specificities of the newly described antibodies R26 (to the type I receptor) and R45 (to the type III receptor) are demonstrated in Figure1A, where immunoprecipitated type I (from SHSY-5Y cells), mainly type II (from AR4-2J cells), andmainly type III (from RINm5F cells)1,4,5IP3R24 are examined with these antibodies. R26 shows strong recognition of the type I receptor with identical characteristics as previously described for CT1,24 and R45 shows specific recognition of the immunoprecipitated type III receptor. R26 does recognize a band in the immunoprecipitated type II and type III receptor lanes that reflect the presence of contaminating type I receptor in the preparations of the type II and type III from AR4-2J and RINm5F cells, respectively,24 and, we believe, not a recognition of the type II and type III receptors by R26. Further, R26 recognized very well the 1,4,5IP3 receptor from rat cerebellum but very poorly, if at all, membrane preparations from AR4-2J cells (rich in the type II receptor) or RINm5F cells (rich in the type III receptors; results not shown). Figure 1B shows the relative expression of the type I, type II, and type III receptors using CT1, CT2, and CT3 antibodies at equal antibody dilutions and with equal loading of platelet-mixed membranes. There is good expression of the type I and type II receptors and much reduced levels of the type III receptor. Immunodetection by each antibody was specific as co-incubation with the respective peptide to which the antibody was generated resulted in loss of the immune signal (results not shown). Figure 1C shows typical immunoprecipitation analysis of the 1,4,5IP3R types (repeated at least 6 times) using equal quantities of membranes and antibodies. There is good immunoprecipitation of the type I and type II receptors and significantly reduced amounts of the type III receptor, suggesting much lower expression of this subtype. The reduced immunoprecipitation of the type III receptor from platelets was not caused by a reduced ability of the antibodies to immunoprecipitate the protein because both CT3 and R45 were effective at immunoprecipitating the type III receptor from RINm5F cells that are rich in the type III receptor (lane III in Figure 1A reflects this). In cross-checking experiments, each antibody did not co-immunoprecipitate any other subtype from platelet membranes. For example, CT1 (or R26) only immunoprecipitated the type I receptor, and other types were not detected; the same was true for the CT2, CT3 and R45 (results not shown). The post-antibody lysates were found to be devoid of the immunoprecipitated receptor, suggesting the antibody amounts used totally extracted the appropriate receptor. These results suggest that in platelets these receptors most probably exist as homotetramers.
Specificity of antibodies and presence of1,4,5IP3R in platelets.
(A) Approximately 18 ng immunopurified type I, mainly type II, and mainly type III 1,4,5IP3R (see “Results” and ref. 24) were run in the lanes indicated and probed with antibodies R26, R45, and CT3 using Western blotting. Arrow indicates position of IP3R. R45 and CT3 are highly specific for the type III receptor and R26 for the type I receptor. Cross- reactivity by R26 in lanes for the types II and III receptors reflects co-immunopurification of the type I receptor from AR4-2J and RINm5F cells. (B) Western blot analysis of platelet-mixed membranes with the antibodies CT1 (to type I), CT2 (to type II), and CT3 (to type III receptor). Arrows reflect position of IP3 receptors. (C) Equal amounts of platelet lysates were immunoprecipitated with equal amounts of either CT1, CT2, or R45 antibodies. Immunoprecipitates were reprobed with the respective antibodies on Western blots. Each blot is typical of 3 separate experiments.
Specificity of antibodies and presence of1,4,5IP3R in platelets.
(A) Approximately 18 ng immunopurified type I, mainly type II, and mainly type III 1,4,5IP3R (see “Results” and ref. 24) were run in the lanes indicated and probed with antibodies R26, R45, and CT3 using Western blotting. Arrow indicates position of IP3R. R45 and CT3 are highly specific for the type III receptor and R26 for the type I receptor. Cross- reactivity by R26 in lanes for the types II and III receptors reflects co-immunopurification of the type I receptor from AR4-2J and RINm5F cells. (B) Western blot analysis of platelet-mixed membranes with the antibodies CT1 (to type I), CT2 (to type II), and CT3 (to type III receptor). Arrows reflect position of IP3 receptors. (C) Equal amounts of platelet lysates were immunoprecipitated with equal amounts of either CT1, CT2, or R45 antibodies. Immunoprecipitates were reprobed with the respective antibodies on Western blots. Each blot is typical of 3 separate experiments.
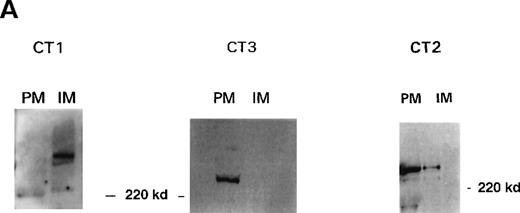
High-voltage FFE enables human platelet PM and IM to be obtained with high purity. These membrane fractions have been extensively characterized since their first description (see Menashi et al,32 Crawford et al,33 and Hack and Crawford34). Full documentation of the analytical and enzymatic characterization including protein, lipid, and marker enzymes (eg, NADH cytochrome C reductase for IM, actin and myosin for PM) are reported.33 We have recently shown the IM to be the site of the sarco-endoplasmic reticulum Ca++ATPase (SERCA) 2b and SERCA 3.26 The PM preparations have been shown to be rich in the surface glycoproteins GPIIb-IIIa and GP1b and the proteins VASP and Rap1B, which are associated with the inner surface of the PM.23,34 Western blot analysis of purified platelet membranes using the 1,4,5IP3R antibodies is shown in Figure 2A. Immune recognition by the antibodies CT1 and CT3 is striking. The IM fractions contain the type I receptor, and the PM fractions contain the type III receptor, with little or no cross-contamination. This clear separation of cellular localization may suggest a differentiation of function for the1,4,5IP3R in platelets. The antibody CT2 recognized a protein in the 250-kd range in both PM and IM fractions (Figure 2B; some blots showed more intensity in the IM than PM fractions). This suggests a dual distribution for the type II receptor. Immunodetection of 1,4,5IP3R with R26 (to the type I receptor) and R45 (to the type III receptor) showed a similar distribution to that observed with CT1 and CT3, respectively (results not shown). With a number of membrane preparations, we have observed that the type III receptor is more prone to degradation by endogenous proteases during the lengthy procedure than either the type I or type II receptors. An example of this phenomenon is illustrated in Figure2B, which shows a disappearance of the type III receptor parent protein and an increase of smaller products (at approximately 200 and 180 kd) as the procedure progresses, whereas this effect was observed to a lesser extent with the type II and type I receptors (the latter not shown). Preparation of platelet extracts in the presence of the protease inhibitor cocktail (see “Materials and methods”) plus a 10-fold excess of aprotinin (but not PMSF, soybean trypsin inhibitor, leupeptin, or pepstatin) resulted in greater protection against proteolysis of the type III receptor, suggesting that perhaps a kallikrein-type protease (rather than trypsin) was probably responsible. Coupled with the reduced expression, this may explain the lack of detection of the type III receptor with platelet membranes in the earlier studies (eg, Quinton and Dean22).
Localization of 1,4,5IP3R types in platelets.
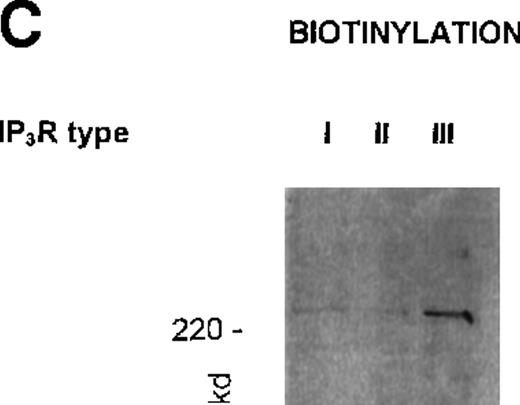
(A) Western blot analysis of platelet 1,4,5IP3R isoform distribution in plasma (PM) and intracellular membranes (IM) prepared using high-voltage FFE. Numbers reflect molecular size markers in kilodaltons. Marker enzyme NADH cytochrome C reductase activity IM = 1.5 μmol/min per milligram protein; PM = 0.005 μmol/min per milligram protein. Actin was only detected in pm, not in IM. Ref. 33 shows full characterization of membranes. (B) Degradation of type III 1,4,5IP3R by endogenous proteases during membrane purification. PM were prepared using FFE, as outlined in “Materials and methods.” Samples loaded; lane 1, platelet lysate; lane 2, mixed membranes (MM) before FFE; lane 3, PM. Antibodies used in Western blot analysis reflect CT2 for type II and CT3 for the type III receptor. Numbers reflect positions of molecular size markers. (C) Intact platelets were surface labeled with biotin (see “Materials and methods”) and1,4,5IP3R were immunoprecipitated with R26, CT2, and R45 antibodies. Biotinylated proteins were detected using streptavidin–HRP.
Localization of 1,4,5IP3R types in platelets.
(A) Western blot analysis of platelet 1,4,5IP3R isoform distribution in plasma (PM) and intracellular membranes (IM) prepared using high-voltage FFE. Numbers reflect molecular size markers in kilodaltons. Marker enzyme NADH cytochrome C reductase activity IM = 1.5 μmol/min per milligram protein; PM = 0.005 μmol/min per milligram protein. Actin was only detected in pm, not in IM. Ref. 33 shows full characterization of membranes. (B) Degradation of type III 1,4,5IP3R by endogenous proteases during membrane purification. PM were prepared using FFE, as outlined in “Materials and methods.” Samples loaded; lane 1, platelet lysate; lane 2, mixed membranes (MM) before FFE; lane 3, PM. Antibodies used in Western blot analysis reflect CT2 for type II and CT3 for the type III receptor. Numbers reflect positions of molecular size markers. (C) Intact platelets were surface labeled with biotin (see “Materials and methods”) and1,4,5IP3R were immunoprecipitated with R26, CT2, and R45 antibodies. Biotinylated proteins were detected using streptavidin–HRP.
The near exclusive localization of the type III receptor to the PM fraction prompted us to examine this property further; particularly, was this receptor surface exposed? Intact platelets were labeled with N-hydrosuccinimidobiotin (see “Materials and methods”). After washing, platelets were solubilized and receptors were immunoprecipitated with the respective antibodies. A typical experiment (repeated 3 times) is shown in Figure 2C. Immunoprecipitates of the type III 1,4,5IP3R (using the antibody R45) showed avid labeling with biotin. Immunoprecipitates of the type I and type II receptors (with R26 and CT2) showed little, if any, biotin associated with these proteins, as was to be expected for IM proteins. In parallel experiments, all 3 antibodies immunoprecipitated their respective receptors as in Figure 1B. Thus, although the type III receptor was present in the least amount, it was the only one with significant surface exposure. The PM association of the type II receptor may thus reflect the presence of a distinct membrane fraction closely associated with the PM through protein or other linkages.
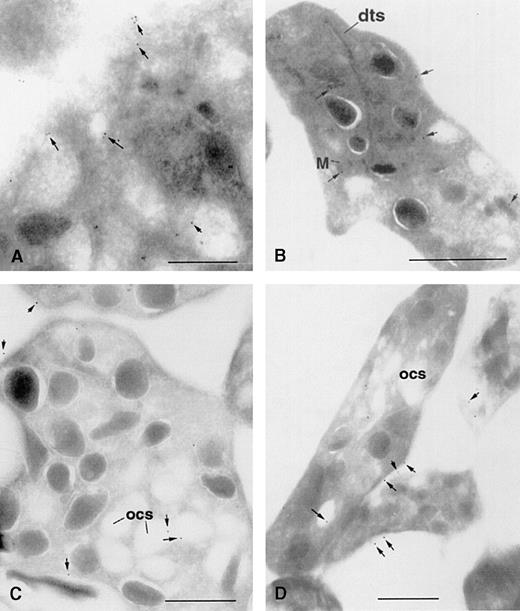
The cellular distribution of 1,4,5IP3R was also examined using immunogold electron microscopy. All the antibodies to the 1,4,5IP3R were tested; however, only antibodies to the type I and type III receptors showed positive immunogold labeling. It is not known why CT2 was not successful in this regard. Figure 3 shows representative labeling patterns obtained for the type I and type III receptors using 2 distinct antibodies for each receptor type—R26 and a monoclonal antibody 18A10 [23] for the type I receptor, R45 and CT3 for the type III receptor. The type I receptor is observed to have a widespread distribution among membrane structures throughout the cytoplasm, including the dense tubular system and granular membranes, with some staining near the PM and in some cells with mitochondrial membranes (Figures 3A and 3B). The type III receptor also showed some staining with intracellular structures, but noticeably significant staining was seen in the PM and the open canalicular system that represents invaginated portions of the PM (Figures 3C and 3D). A statistical analysis of the distribution of immunogold label obtained for each polyclonal antibody for either 15 or 16 cells is shown in Table1. Gold particles associated with PM versus IM for the type I receptor were statistically significant (P = .0001). Further comparison of the PM association of the type I receptor versus the PM association of the type III receptor obtained with either CT3 or R45 was significant (P = .0013 and P = .047, respectively).
Immunogold labeling of type I and type III1,4,5IP3R in human platelets.
Ultracryosections were prepared and immunostained for type I1,4,5IP3R using the monoclonal antibody 18A10 (A) and R26 (B) and for the type III receptors using CT3 (C) and R45 (D). Bar represents 1 μm; arrows point to selected gold particles. dts, dense tubular system; M, mitochondria; ocs, open canalicular system.
Immunogold labeling of type I and type III1,4,5IP3R in human platelets.
Ultracryosections were prepared and immunostained for type I1,4,5IP3R using the monoclonal antibody 18A10 (A) and R26 (B) and for the type III receptors using CT3 (C) and R45 (D). Bar represents 1 μm; arrows point to selected gold particles. dts, dense tubular system; M, mitochondria; ocs, open canalicular system.
Distribution of type I and type III IP3R as determined by immunogold labeling
| Antibody . | Gold particles/cell means ± SE . | Paired t test (Pvalues) . | |
|---|---|---|---|
| IM vs PM . | R26 PM vs . | ||
| R26 (type I) | |||
| IM | 6.33 ± 0.76 (15) | ||
| PM | 1.4 ± 0.32 (15) | .001 | |
| R45 (type III) | |||
| IM | 3.06 ± 0.36 (16) | ||
| PM | 2.58 ± 0.36 (16) | .283 | .047 |
| CT3 (type III) | |||
| IM | 4.53 ± 0.63 (15) | ||
| PM | 4.07 ± 0.58 (15) | .41 | .0013 |
| Antibody . | Gold particles/cell means ± SE . | Paired t test (Pvalues) . | |
|---|---|---|---|
| IM vs PM . | R26 PM vs . | ||
| R26 (type I) | |||
| IM | 6.33 ± 0.76 (15) | ||
| PM | 1.4 ± 0.32 (15) | .001 | |
| R45 (type III) | |||
| IM | 3.06 ± 0.36 (16) | ||
| PM | 2.58 ± 0.36 (16) | .283 | .047 |
| CT3 (type III) | |||
| IM | 4.53 ± 0.63 (15) | ||
| PM | 4.07 ± 0.58 (15) | .41 | .0013 |
Gold particles were counted for 15 (R26, CT3) or 16 (R45) cells and assigned to either PM (including open canalicular system) or IM structures. Means ± SE are noted, and paired t tests were analyzed using Stat-View software for IM vs PM for each antibody and R26 PM vs R45 PM or CT3 PM. P < .05 indicates statistical significance.
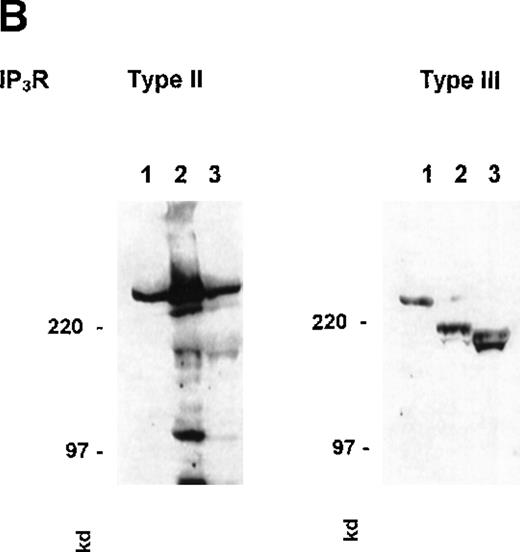
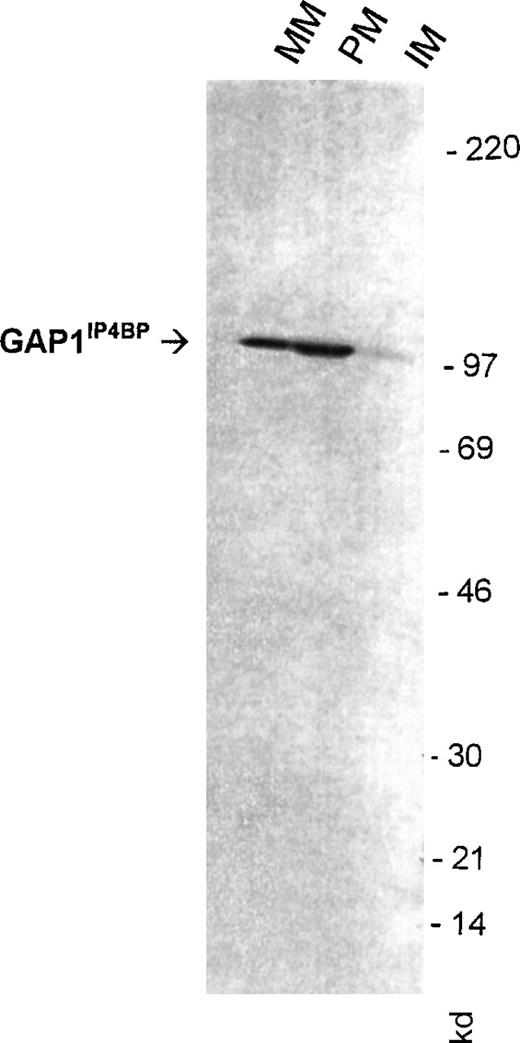
We have also examined the distribution of the1,3,4,5IP4R GAP1IP4BP using an antibody raised to a sequence in the C-terminal portion of the protein. Previously we had reported a binding site for [3H]1,3,4,5IP4 in the PM fractions.35Figure 4 shows that the antibody recognized GAP1IP4BP as a 97-kd protein present in mixed membranes, with enrichment in the PM but little staining is seen in the IM fraction. Immunoprecipitation of GAP1IP4BP with this antibody from platelet lysates prepared from biotinylated cells did not reveal the protein to be biotinylated, confirming the protein to be associated with the inner surface of the PM (results not shown).
Detection of GAP1IP4BP in purified human platelet membranes.
50 μg platelet-mixed (MM), plasma (PM), and intracellular membranes (IM) were probed for Western blotting detection with an antibody raised to a C-terminal peptide of GAP1IP4BP. Numbers reflect molecular size markers.
Detection of GAP1IP4BP in purified human platelet membranes.
50 μg platelet-mixed (MM), plasma (PM), and intracellular membranes (IM) were probed for Western blotting detection with an antibody raised to a C-terminal peptide of GAP1IP4BP. Numbers reflect molecular size markers.
Demonstration of an involvement of inositol phosphate receptors in Ca++ flux in purified PM in vitro
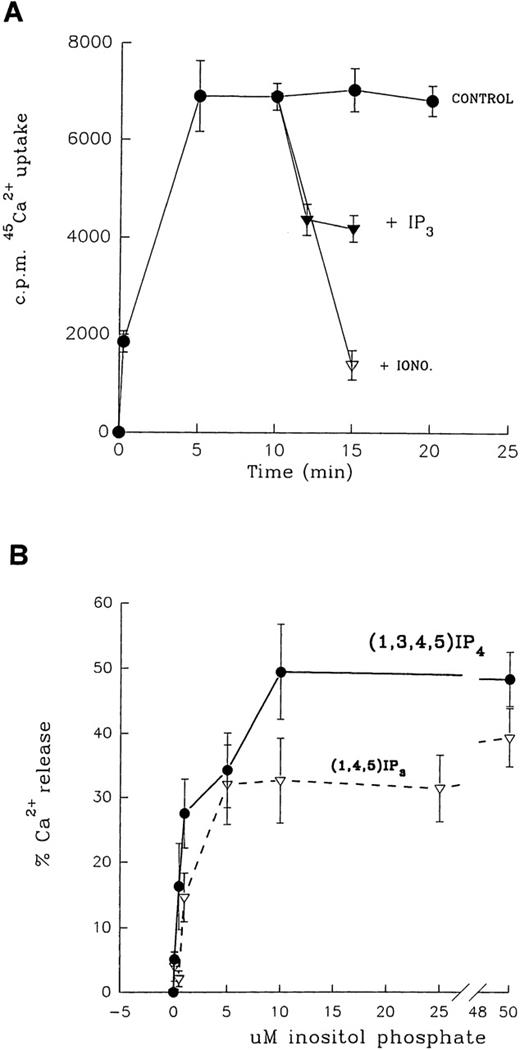
Studies were carried out to determine the possible involvement of the 1,4,5IP3R and the GAP1IP4BPwith cation influx by examining 45Ca++ flux in the purified PM. Membrane vesicles were loaded with45Ca++ using a Na+/Ca++exchange activity,9 and the functionality of the receptors was measured using inositol phosphate-mediated Ca++ flux. Figure 5A shows the characteristics of 45Ca++ loading into PM. The steady-state 45Ca++ was retained within the vesicle, as measured up to 30 minutes of incubation. This level of trapped 45Ca++ at 10 minutes represented 0.63 nmol/mg protein. The addition of 10 μmol/L ionomycin at 10 minutes of incubation led to the rapid release of approximately 80%45Ca++ retained by the vesicle, indicating that it represented Ca++ that was trapped and not membrane bound. Inclusion of ionomycin at the start of the incubation resulted in no loading of 45Ca++ into the vesicles. Na+/Ca++ exchange activity was predominantly associated with PM; IM vesicles contained approximately one tenth the exchange activity of PM. Additionally, the Ca++ exchange mechanism was not ATP dependent.
Inositol phosphate-mediated Ca++ flux activity in platelet PM.
(A) Na+/Ca++ exchange activity of PM and inositol phosphate-mediated Ca++ flux. The illustration shows kinetics of Na+/Ca++ exchange and addition of either 10 μmol/L ionomycin (iono) or 50 μmol/L1,4,5IP3 (IP3) at 10 minutes. All points are mean ± SEM of triplicate determinations, with similar results obtained in at least 2 other membrane preparations. (B) Dose–response relationship of 1,3,4,5IP4 (•) and 1,4,5IP3 (▿) induced Ca++flux in plasma membranes.
Inositol phosphate-mediated Ca++ flux activity in platelet PM.
(A) Na+/Ca++ exchange activity of PM and inositol phosphate-mediated Ca++ flux. The illustration shows kinetics of Na+/Ca++ exchange and addition of either 10 μmol/L ionomycin (iono) or 50 μmol/L1,4,5IP3 (IP3) at 10 minutes. All points are mean ± SEM of triplicate determinations, with similar results obtained in at least 2 other membrane preparations. (B) Dose–response relationship of 1,3,4,5IP4 (•) and 1,4,5IP3 (▿) induced Ca++flux in plasma membranes.
The effect of inositol phosphates on PM Ca++ flux was tested by addition at equilibrium conditions (10 minutes of incubation). The addition of 20 μmol/L1,4,5IP3 led to the release of approximately 40% trapped 45Ca++ within 5 minutes; a similar response was elicited with 20 μmol/L1,3,4,5IP4. The time course of1,3,4,5IP4- or1,4,5IP3-induced Ca++ release was found to be maximal at 5 minutes, and there was no further Ca++ release at 10 or 15 minutes of incubation. Figure 5B shows the dose–response relationship of inositol phosphate-induced Ca++ release from PM, with an EC50 = 0.8 μmol/L and 1.3 μmol/L for 1,3,4,5IP4 and1,4,5IP3, respectively, with concentrations of 10 μmol/L and above giving maximal response. Calcium flux determinations were carried out on the day of electrophoresis because we detected a decrease in the sensitivity of the response the next day. Concentrations of 100 μmol/L of either inositol phosphate did not cause more than a maximal 50% Ca++ release (variation from 25% to 50% between preparations) under conditions in which ionomycin released 75% to 85% trapped 45Ca++. This suggested that approximately half the PM vesicles were of inside-out orientation. Such vesicles would not be expected to exhibit inositol phosphate-stimulated Ca++ flux because the ligand would only have access to its receptor on the cytoplasmic face of the membrane. The effects of other inositol phosphate isomers, namely2,4,5IP3, 1,3,4IP3,1,4IP2, and IP6, were tested in this model of PM Ca++ flux. With all isomers, addition was made after 10 minutes, and reactions were stopped after a further 5 minutes. 45Ca++ release activity equipotent with 1,4,5IP3 was observed with2,4,5IP3 but not with1,3,4IP3 (EC50 = 20 μmol/L), and 1,2IP2 or IP6 was found to be ineffective tested up to 100 μmol/L maximum.
Contamination of PM with a Ca++ flux activity associated with IM was checked with the use of the Ca++ATPase inhibitor thapsigargin (Tg). At 3 μmol/L, Tg is sufficient for total inhibition of SERCA Ca++ ATPases in platelets26and to release Ca++ by 70% within 5 minutes from platelet IM loaded with 45Ca++ (results not shown). The addition of 3μmol/L Tg at 10 minutes of incubation with45Ca++-loaded pm, followed by stopping 5 minutes later, resulted in no release of45Ca++ (101% of controls), indicating little or no contamination of the PM by SERCA Ca++ pumps. Further inclusion of Tg with 1,4,5IP3 did not significantly alter Ca++ flux mediated by1,4,5IP3 (results not shown).
We have attempted to differentiate the Ca++-releasing actions of 1,4,5IP3 from those by1,3,4,5IP4. First, heparin (at 1 mg/mL) was considered unsuitable for this purpose because it inhibited the Na+/Ca++ exchange activity by approximately 50%, added at either the start of the incubation or at equilibrium (10 minutes). We have recently shown that PM are rich in both cAMP- and cGMP-dependent protein kinases.23 Thus, the effects of protein phosphorylation on Ca++ fluxes in PM was investigated using exogenous addition of the catalytic subunit of cAMP-PK (cAMP-PK CAT). Addition of 50 U cAMP-PK CAT to PM at the start of Na+/Ca++ exchange resulted in a stimulation of 45Ca++ uptake by at least 3-fold (maximum up to 7-fold). The kinetics of 45Ca++ loading was similar, with equilibrium reached within 10 minutes of incubation and maintained for up to 30 minutes (results not shown). (This increased45Ca++-exchange activity was probably mediated by the presence of phosphate ions and DTT used to stabilize the kinase preparation because it was evident in the absence of added ATP or in the presence of an inhibitor peptide specific for cAMP-PK; results not shown.) The effect of cAMP-PK CAT on 45Ca++flux induced by 1,4,5IP3 and1,3,4,5IP4 was investigated in the presence or absence (reflecting controls) of 100 μmol/L ATP. Table2 shows 2 typical experiments with similar results obtained in 3 other membrane preparations. Stimulation of Ca++ flux by 1,3,4,5IP4 was found to be unaffected by cAMP-PK CAT in the presence of ATP, but45Ca++ flux stimulated by1,4,5IP3 was markedly inhibited. In 3 other experiments, 50 U cAMP-PK CAT in the presence of ATP inhibited1,4,5IP3 induced Ca++ flux by 65%, 78%, and 100% while having no effect or, in some cases, causing a slight stimulation (18%) of 1,3,4,5IP4stimulated Ca++ flux. The effect of cAMP-PK CAT was reversed by a peptide inhibitor specific for this kinase (Table 2). The possibility that the action of cAMP-PK CAT may be to hasten the metabolism of 1,4,5IP3 and thus contribute to its inhibitory effect on Ca++ release was considered unlikely because the nonmetabolizable analogue,2,4,5IP3 (50 μmol/L) also caused Ca++ release from PM that was similarly inhibited by cAMP-PK CAT (results not shown). In a further series of experiments, 100 μmol/L ATP added in the absence of cAMP-PK CAT did not affect the dose–response relationship of 1,4,5IP3-induced Ca++ release, suggesting that the nucleotide alone, which has been suggested to have a modulatory role on the1,4,5IP3 receptor, did not account for the observed effects. The results suggest that in platelets, the Ca++-releasing function of1,4,5IP3, but not that of1,3,4,5IP4, is sensitive to the action of cAMP-PK.
Effect of cAMP-PK CAT on (1,4,5)IP3 and (1,3,4,5)IP4 induced Ca2+ flux in highly purified plasma membranes
| Incubation conditions . | 45Ca2+ Uptake . | % Release . |
|---|---|---|
| Experiment 1 | ||
| 1. Control uptake (+CAT, no ATP) (15-min incubation) | 59 500 ± 1600 | 0 |
| 2. Same as 1 + (1,3,4,5)IP4 | 43 900 ± 300* | 27 |
| 3. Same as 1 + ATP, + (1,3,4,5)IP4 | 41 600 ± 5000* | 30 |
| 4. Same as 1 + (1,4,5)IP3 | 5900 ± 1400* | 40 |
| 5. Same as 1 + ATP + (1,4,5)IP3 | 2300 ± 700 | 12 |
| Experiment 2 | ||
| 6. Control same as 1 (+CAT, no ATP) | 22 300 ± 1990 | 0 |
| 7. Same as 6 + (1,4,5)IP3 | 16 700 ± 527* | 25 |
| 8. Same as 6 + ATP + (1,4,5)IP3 | 21 600 ± 996 | 3 |
| 9. Same as 6 + ATP + inh. pep. + (1,4,5)IP3 | 18 800 ± 1920 | 16 |
| Incubation conditions . | 45Ca2+ Uptake . | % Release . |
|---|---|---|
| Experiment 1 | ||
| 1. Control uptake (+CAT, no ATP) (15-min incubation) | 59 500 ± 1600 | 0 |
| 2. Same as 1 + (1,3,4,5)IP4 | 43 900 ± 300* | 27 |
| 3. Same as 1 + ATP, + (1,3,4,5)IP4 | 41 600 ± 5000* | 30 |
| 4. Same as 1 + (1,4,5)IP3 | 5900 ± 1400* | 40 |
| 5. Same as 1 + ATP + (1,4,5)IP3 | 2300 ± 700 | 12 |
| Experiment 2 | ||
| 6. Control same as 1 (+CAT, no ATP) | 22 300 ± 1990 | 0 |
| 7. Same as 6 + (1,4,5)IP3 | 16 700 ± 527* | 25 |
| 8. Same as 6 + ATP + (1,4,5)IP3 | 21 600 ± 996 | 3 |
| 9. Same as 6 + ATP + inh. pep. + (1,4,5)IP3 | 18 800 ± 1920 | 16 |
Incubations as in Figure 5. Control incubations contained no ATP (which is required for both phosphorylation and inhibition of Ca2+ effect). cAMP-PK catalytic subunit (CAT) was added to all experiments at 50 U/incubation; ATP, where used, 100 μmol/L final concentration, inhibitor peptide (inh. pep.) at 10 μmol/L. Composition of inh. pep. is H-Thr-Thr-Tyr-Ala-Asp-Phe-Ile-Ala-Ser-Gly-Arg-Thr-Gly-Arg-Arg-Asn- Ala-Ile-His- Asp-OH. 50 μmol/L (1,4,5)IP3 or (1,3,4,5)IP4was added to the mixtures after 10-minute incubation (equilibrium), and reactions were stopped after a further 5 minutes by rapid filtration through 0.45-μm filters. Values shown are means ± SEM of triplicate observations, with similar data obtained in 3 other membrane preparations.
P < .05 compared with respective controls using Mann-WhitneyU Confidence Interval and Test using the Minitab Data Analysis Software (Coventry, UK).
Phosphorylation of 1,4,5IP3R and1,3,4,5IP4R by cyclic nucleotide kinases in platelet PM fractions and whole cells
The above studies with cAMP-PK CAT provided a means to discriminate the actions of 1,4,5IP3 from that of1,3,4,5IP4 and also implied that the1,4,5IP3R (but probably not GAP1IP4BP) were substrates for cAMP-PK. Studies were carried out to verify this in PM fractions by examining possible phosphorylation of the receptors using [32P]ATP and either cAMP-PK CAT or BiMPS, which directly activates endogenous cAMP-PK (23 and references therein), and with intact platelets labeled with [32Pi] and incubated with either prostacyclin (PGI2) or BiMPS. The results obtained with PM preparations and intact cell systems were similar in the extent of phosphorylation for each 1,4,5IP3R isoform and GAP1IP4BP. The intact cell experiments reflect information that is more physiologically important, and, to avoid duplication, only these are presented here. Figure 6A shows that incubation of platelets labeled with [32Pi] with either PGI2 or BiMPS resulted in increased phosphorylation of the type III receptor, as observed after immunoprecipitation with CT3. Both treatments proved effective to phosphorylate the receptor with BiMPS marginally better probably because it directly activates cAMP-PK. Using BiMPS the relative extents of phosphorylation of all 31,4,5IP3R isoforms was examined (Figure 6B). BiMPS treatment led to the stimulated phosphorylation of the type I and type III receptors avidly, but phosphorylation of the type II receptor was considerably less; parallel experiments showed comparable immunoprecipitation of each receptor in control and BiMPS-treated cells (results not shown). An essentially similar finding was obtained with PM fractions stimulated with either BiMPS or cAMP-PK CAT in the presence of [32P]ATP (ie, good phosphorylation of the type III receptor that was also blocked by inclusion of the cAMP-PK inhibitor peptide and with markedly poor effect on the type II receptor; results not shown). Figure 6C shows stimulation of platelet cGMP-PK using 8pCPT23 and effects on phosphorylation of the types I and III 1,4,5IP3 receptors and on GAP1IP4BP. Both cyclic nucleotide kinases showed nearly equal ability to phosphorylate each of the IP3R examined, but neither kinase was an effective stimulus to phosphorylate GAP1IP4BP. In each set of experiments, there was equal immunoprecipitation of the respective receptor in control and treated incubations (shown only for GAP1IP4BP). GAP1IP4BP was also not phosphorylated in PM preparations when stimulated by cAMP-PK CAT in the presence of [32P]ATP (results not shown). Failure of GAP1IP4BP to be phosphorylated by either cyclic nucleotide kinase in whole cells or PM preparations supports the lack of effect by cAMP-PK CAT on 1,3,4,5IP4-mediated Ca++ flux in the PM preparations (Table 2). Verification that both cyclic nucleotide kinases were activated in the negative experiment involving GAP1IP4BP was carried out by assessing the phosphorylation status of the phosphoprotein VASP in reaction lysates after GAP1IP4BP immunoprecipitation. In its nonphosphorylated state, VASP migrates on SDS-PAGE as a 46-kd band. On phosphorylation in intact platelets by either cAMP-PK or cGMP-PK, the protein migrates as a 50-kd species.36 Figure 6D shows that both BiMPS and 8pCPT induced the phosphorylation of VASP as measured by the appearance of the upper 50-kd species, suggesting that both cAMP-PK and cGMP-PK had been activated to similar extents.
Phosphorylation of 1,4,5IP3R and GAP1IP4BP in intact platelets by cAMP-PK and cGMP-PK.
(A) [32P] labeled platelets were incubated with either vehicle (CON) or PGI2 (0.5 μmol/L) or BiMPS (500 μmol/L), and type III 1,4,5IP3R immunoprecipitated with CT3. (B) Experiments were carried out as in (A), and immunoprecipitations were carried out using CT1, CT2, and CT3. (C) Experiments were carried out as above with additional stimulation of cGMP-PK using 8pCPT. Immunoprecipitations were carried out using R26 (for type I receptor), R45 (for type III receptor), and anti-GAP1IP4BP antibody. Western blotting analysis of immunoprecipitates of GAP1IP4BP is shown. (D) Lysates from experiments involving GAP1IP4BP were subjected to analysis of the phosphorylation status of VASP using Western blot analysis. Activation of the kinases is associated with an increase of the upper 50-kd form of VASP. In control cells, nonphosphorylated VASP migrates as a 46-kd protein.
Phosphorylation of 1,4,5IP3R and GAP1IP4BP in intact platelets by cAMP-PK and cGMP-PK.
(A) [32P] labeled platelets were incubated with either vehicle (CON) or PGI2 (0.5 μmol/L) or BiMPS (500 μmol/L), and type III 1,4,5IP3R immunoprecipitated with CT3. (B) Experiments were carried out as in (A), and immunoprecipitations were carried out using CT1, CT2, and CT3. (C) Experiments were carried out as above with additional stimulation of cGMP-PK using 8pCPT. Immunoprecipitations were carried out using R26 (for type I receptor), R45 (for type III receptor), and anti-GAP1IP4BP antibody. Western blotting analysis of immunoprecipitates of GAP1IP4BP is shown. (D) Lysates from experiments involving GAP1IP4BP were subjected to analysis of the phosphorylation status of VASP using Western blot analysis. Activation of the kinases is associated with an increase of the upper 50-kd form of VASP. In control cells, nonphosphorylated VASP migrates as a 46-kd protein.
Discussion
In this study, we report the distinct localization of inositol phosphate receptors in human platelets using a number of techniques. We demonstrate functional activity in vitro for both1,4,5IP3R and GAP1IP4BP related to Ca++ flux in a highly purified PM preparation. We also describe a mechanism to discriminate the Ca++ flux activities of 1,4,5IP3 and1,3,4,5IP4 in platelets using cAMP-PK under conditions in which phosphorylation of1,4,5IP3R but not of GAP1IP4BP is demonstrated in both PM and intact cells.
The distinct distribution of 1,4,5IP3R types described in this study supports earlier observations of the distribution of the type I and type II receptors reported by Quinton and Dean,22 our own report of the presence of the type I receptor in the IM,23 and suggestions of distinct roles of1,4,5IP3R subtypes in specialized functions related to Ca++ signals.17-19 Our studies imply, in platelets, that the main role for the type I receptor is involvement with Ca++ release from intracellular stores and for the type III receptor a role in cation influx. The type II receptor, depending on its location, may be involved in both processes. A specialized role for the type III receptor is indicated because it appears to be the only member of this family to have surface exposure, as measured by biotin labeling of surface proteins in intact cells and an almost exclusive elution of the protein with PM prepared using FFE. The PM location of the type III receptor is further supported by studies using immunogold labeling of platelet ultracryosections in which R45 and CT3 both show significant labeling to the PM and the open canalicular system, whereas the type I receptor shows a widespread distribution among membrane structures in the cytoplasm. The type II receptor that partially elutes with the PM, but is not surface exposed, may reside on a membrane system that is tightly linked to the PM, but this membrane system is substantially free of SERCA contamination. Since the initial suggestions of a possible function for1,4,5IP3 at the PM,37,38 a number of studies have provided evidence for the possible presence of1,4,5IP3R in the PM (eg, Fujimoto et al39 and Cunningham et al40) with one study41 suggesting that between 10% and 20% of the receptors in MDCK cells may be accessible to biotin labeling. It is only recently that a specific role for the type III receptor in Ca++ entry has been suggested, namely in lymphocyte cell lines undergoing apoptosis42 and in Xenopus where overexpression of the type III receptor led to increases of Ca++ influx. Overexpression of the type I receptor, however, led to an increase of the rate of Ca++elevation.43 The ability of the type III receptor to remain in the open state at higher Ca++concentrations44 is compatible with this function. Putney45 has even hypothesized that in some cells the type III receptor may serve as a capacitative Ca++ entry channel.
The Ca++ flux measurements suggest that the1,4,5IP3R and 1,3,4,5IP4R in the PM are functional in stimulating cation movements. We have previously demonstrated1,4,5IP3 to release Ca++ from intracellular membranes with high potency (EC50 = 0.25 μmol/L46). We now show these membranes to contain mainly the type I and type II 1,4,5IP3Rs. A Ca++ flux activity for1,4,5IP3, along with the same for1,3,4,5IP4, is demonstrated in PM. An important property of this PM preparation is its insensitivity to thapsigargin in Ca++ATPase activity26 and also to Ca++ flux (this study). The system may thus be useful to provide a better dissection of the inositol phosphate receptors involved with cation entry from those involved in Ca++release. We show that the Ca++ flux activity stimulated by1,3,4,5IP4 in the PM can be distinguished well by the use of cAMP-PK. This kinase has no observable effect on the cation flux activity of 1,3,4,5IP4 but markedly inhibits that by 1,4,5IP3. cAMP-PK is also found to be ineffective at stimulating the phosphorylation of GAP1IP4BP, even though the protein has a number of consensus sequences suitable for this kinase (eg, Ser90 in the sequence ARDS that is suitable also for cGMP-PK)12 and may reflect an inaccessibility of this site by the kinase. This observation supports a Ca++ flux action of1,3,4,5IP4 by its specific receptor, GAP1IP4BP15,47, rather than by a possible effect on1,4,5IP3R, as indicated by Wilcox et al.48 A function for 1,3,4,5IP4related to Ca++ movements is still controversial49 but has been found in a number of preparations, including those from lachrymal cells,50-52platelets,11 NIH/3T3 cells,53 and many others. The exogenous addition of phosphatidylinositol 3,4,5-trisphosphate (PIP3), which has the same head group as1,3,4,5IP4, was found to induce Ca++ entry and to activate rabbit platelets. It has been suggested that it also acts through GAP1IP4BP.54 There is no unified mechanism to explain 1,3-5IP4-stimulated Ca++flux, and GAP1IP4BP is not an ion channel. Therefore, future studies must probe its possible association with cation channels. It is of particular interest that in overexpression studies using COS 7 or HeLa cells, GAP1IP4BP appears to locate solely at the plasma membranes (as with our studies), whereas GAP1m exhibits a perinuclear localization.55The relative expression and location of these 2 receptors may thus be important determinants that stipulate whether1,3,4,5IP4 exerts an effect on Ca++at the level of intracellular stores (and be exerted by GAP1m) or be related to cation entry (and be mediated by GAP1IP4BP).
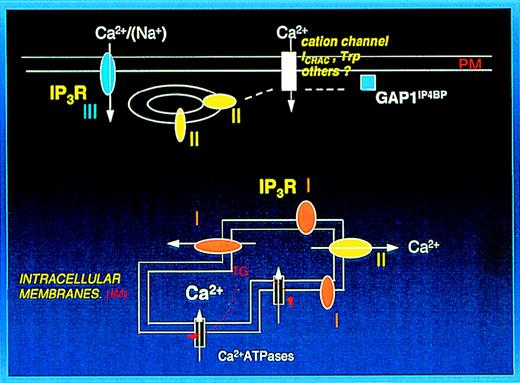
The distribution of 1,4,5IP3R in the PM and IM fractions is of particular interest. Recently, Parekh et al,56 from studies of 1,4,5IP3perfusion in RBL-1 cells, have suggested that there are at least 2 types of Ca++ stores; 1 involved in Ca++release with a high sensitivity to 1,4,5IP3 and another responsible for Ca++ influx wherein higher levels of 1,4,5IP3 are required. These reflect well the properties of our PM and IM system. In addition,1,4,5IP3 bound to its receptor has been shown to activate Htrp3 channels overexpressed in HEK 293 cells.57 The latter study provides long-awaited support for the conformational coupling mechanism for Ca++ entry first suggested by Irvine.7 The coupling mechanism in HEK 293 cells did not require channel activity by the1,4,5IP3R because it was equally shown by1,4,5IP3 bound to a truncated type I receptor and appeared to be shown by all the 1,4,5IP3R isoforms. Thus, the localization of isoforms may be particularly important. Although that study suggested no direct contact between the1,4,5IP3R and Htrp3,57 more recent data from the same group suggest that direct contact has been demonstrated (S. Muallem, personal communication). We suggest that the type II receptor eluting with the platelet PM may serve to link a PM cation channel to intracellular stores. As far as we are aware, the presence of trp isoforms in platelets is yet to be demonstrated. However, as we have reported, a number of megakaryocytic cell lines express mRNA for Htrp1 and Htrp3,58 and it is likely that they are present in platelets. The presence of1,4,5IP3 receptors and GAP1IP4BP at the PM may shed new light on a number of previous studies on platelets that suggested a (more) direct role for1,4,5IP3 in cation entry than mediated by store depletion. Stopped flow studies of thrombin-stimulated platelets suggested a component of Ca++ entry that precededCa++ release from intracellular stores.591,4,5IP3 infusion in rat megakaryocytes has been reported to activate Na+ entry by a mechanism independent of store depletion.60 Exogenous PIP3 added to rabbit platelets stimulates Ca++entry.54 All these could be accommodated as occurring through the type III receptor that is surface exposed or through a possible interaction of the cation channel with the type II receptor or GAP1IP4BP. Figure 7 depicts the possible organization of inositol phosphate receptors in human platelets encompassing these suggestions.
Localization of IP3 receptors (IP3R) and GAP1IP4BP in platelets.
The plasma membrane preparation (PM) contains the type III receptor, which is surface exposed and is likely to be involved in either Ca++ or Na+ entry (see text). The type II receptor and GAP1IP4BP eluting with the plasma membrane preparation are suggested to link with a plasma membrane cation channel. The intracellular membranes contain the type I and type II receptors involved with Ca++ release from intracellular stores and thapsigargin (TG) sensitive SERCA Ca++ATPases.
Localization of IP3 receptors (IP3R) and GAP1IP4BP in platelets.
The plasma membrane preparation (PM) contains the type III receptor, which is surface exposed and is likely to be involved in either Ca++ or Na+ entry (see text). The type II receptor and GAP1IP4BP eluting with the plasma membrane preparation are suggested to link with a plasma membrane cation channel. The intracellular membranes contain the type I and type II receptors involved with Ca++ release from intracellular stores and thapsigargin (TG) sensitive SERCA Ca++ATPases.
Our studies also clarify an important but controversial aspect of1,4,5IP3R phosphorylation by cAMP-PK and its effects on Ca++ fluxes. Depending on the cell type, elevation of cAMP has been suggested to potentiate or to inhibit agonist-induced Ca++ elevation1,19 with the effect of cAMP thought to be mediated by cAMP-PK. However, the effects of phosphorylation of the 1,4,5IP3R by cAMP-PK are unclear, with conflicting results presented from studies on isolated membranes61 and immunopurified receptors.62 In platelets, though it is well established that cAMP (and cGMP) elevation leads to inhibition of cytosolic Ca++ elevation,63, 64 the site of action for cAMP-PK or cGMP-PK, whether this be at the level of phospholipase C,65 the 1,4,5IP3R21 or Ca++ pumps,66,67 and to what extent an effect at each of these sites is important, is unclear. Recently studies on intact platelets68 and on rat megakaryocytes69implied an effect at the level of the1,4,5IP3R, though no direct examination of the phosphorylation status of the 1,4,5IP3R subtypes was carried out. The need for studies on each receptor subtype was further emphasized by the finding that purified type I1,4,5IP3R from platelets was found not to be a substrate for cAMP-PK.20 Rather, in isolated membranes, it was reported to be phosphorylated by an endogenous kinase and then further phosphorylated by cAMP-PK.21 Our studies demonstrate for the first time in intact platelets that all 3 types of1,4,5IP3Rs are substrates of cAMP-PK to differing extents. The type I and type III receptors are highly favored substrates for both kinases, and the type II receptor is less favored. Our results support the work of Quinton et al21 regarding cAMP-PK dependent phosphorylation of the1,4,5IP3R and the inhibition of Ca++ release. Similar findings were recently reported for the 3 subtypes of 1,4,5IP3R isolated from 3 distinct cell types, each expressing high levels of 1 subtype but with the kinase enhancing Ca++ release.70 Our finding that cGMP-PK acts on platelet1,4,5IP3Rs extends the work of Komalavilas and Lincoln71 in which phosphorylation of the type I1,4,5IP3 receptor by cGMP-PK in smooth muscle cells was demonstrated. It is unclear why in some cell types the effect of cAMP-PK phosphorylation on 1,4,5IP3R is stimulatory to function but in others (such as platelets) the effect is inhibitory and if this effect requires the participation of another protein. But clearly these receptors represent highly potent targets of cross-talk regulation with cyclic nucleotides and 1,4,5IP3 representing second messengers with strongly opposing actions on platelet Ca++ movements. In conclusion, our studies report a distinct location for1,4,5IP3R isoforms and GAP1IP4BPthat most probably relate to their specific roles in the promotion of Ca++ release and in the propagation of cation entry.
Acknowledgments
We thank Prof R. F. Irvine (Cambridge, UK) and Dr P. Cullen (Bristol, UK) for many discussions during the course of this work.
Supported by grants from the Wellcome Trust, the British Heart Foundation, and the Thrombosis Research Trust.
Reprints:Kalwant S. Authi, Platelet Section, Thrombosis Research Institute, Manresa Rd, Chelsea, London SW3 6LR UK; e-mail;ksauthi@tri-london.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 6. Phosphorylation of 1,4,5IP3R and GAP1IP4BP in intact platelets by cAMP-PK and cGMP-PK. / (A) [32P] labeled platelets were incubated with either vehicle (CON) or PGI2 (0.5 μmol/L) or BiMPS (500 μmol/L), and type III 1,4,5IP3R immunoprecipitated with CT3. (B) Experiments were carried out as in (A), and immunoprecipitations were carried out using CT1, CT2, and CT3. (C) Experiments were carried out as above with additional stimulation of cGMP-PK using 8pCPT. Immunoprecipitations were carried out using R26 (for type I receptor), R45 (for type III receptor), and anti-GAP1IP4BP antibody. Western blotting analysis of immunoprecipitates of GAP1IP4BP is shown. (D) Lysates from experiments involving GAP1IP4BP were subjected to analysis of the phosphorylation status of VASP using Western blot analysis. Activation of the kinases is associated with an increase of the upper 50-kd form of VASP. In control cells, nonphosphorylated VASP migrates as a 46-kd protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3412/6/m_bloo01103006aw.jpeg?Expires=1769097565&Signature=T812yE0Mk4HBTSf045Pn3G-TZqA6-A4AbbK6O8zszJSqzt-Hhsm5Ql3BX9xxmgA1kIJd5i~-4kMsb6j8M7MOEANdaGs4rLQ7bPs8NK-3g7B~FbiHnw9g-qVncTEli4YOMpYvfB2MxAT8QQWFjbCl8gIRcc2C6igd5aHd~P6dKX4TyfuK7cjyirMP3p3mlWKPI~ExmU72ykxAKpWTOePOVqy1tlqGOWaGrJgaBGgnRMWshMIl6Lu8JYBmoQcf~07KjGcThatuCy0bvm~SUJe-l9oT7T~6YakIcglrxuolsKf1IhODiwdBxl7YqOXhjwDQKFLb5jdcAjinj3nOlYSEOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Phosphorylation of 1,4,5IP3R and GAP1IP4BP in intact platelets by cAMP-PK and cGMP-PK. / (A) [32P] labeled platelets were incubated with either vehicle (CON) or PGI2 (0.5 μmol/L) or BiMPS (500 μmol/L), and type III 1,4,5IP3R immunoprecipitated with CT3. (B) Experiments were carried out as in (A), and immunoprecipitations were carried out using CT1, CT2, and CT3. (C) Experiments were carried out as above with additional stimulation of cGMP-PK using 8pCPT. Immunoprecipitations were carried out using R26 (for type I receptor), R45 (for type III receptor), and anti-GAP1IP4BP antibody. Western blotting analysis of immunoprecipitates of GAP1IP4BP is shown. (D) Lysates from experiments involving GAP1IP4BP were subjected to analysis of the phosphorylation status of VASP using Western blot analysis. Activation of the kinases is associated with an increase of the upper 50-kd form of VASP. In control cells, nonphosphorylated VASP migrates as a 46-kd protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3412/6/m_bloo01103006bw.jpeg?Expires=1769097565&Signature=Yqsi2KtP7H3cRg5VWtt1dCkqFykgQQq5EiRAy9P~-v56BoPO1DBdim7uNW4U3BWpE2DF-ZbM-TfRjlcKo~6XK7R2bpx1mb1dYyDm0rzijgdr4b51mcI-AkQls3AltOBM5Sc8fCvNfV87rhZjeh~HFcweGure8LG13S1gxjqad3GvO2VzNHW5G9q5NdwRMNgbEA1vmqkOzLzQELpI3xpj1R4kist12iwMm-536Jx7oB5t9hGxOGpta34W9lPG74Cyx4dCwMP28JDZEcJOZ7uJ4goiPPKlOyXyUcHLtLdULDd0SJjjq0gp70~7yjU7dJX5tx8cdOelndbRxlUKqGX7bw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Phosphorylation of 1,4,5IP3R and GAP1IP4BP in intact platelets by cAMP-PK and cGMP-PK. / (A) [32P] labeled platelets were incubated with either vehicle (CON) or PGI2 (0.5 μmol/L) or BiMPS (500 μmol/L), and type III 1,4,5IP3R immunoprecipitated with CT3. (B) Experiments were carried out as in (A), and immunoprecipitations were carried out using CT1, CT2, and CT3. (C) Experiments were carried out as above with additional stimulation of cGMP-PK using 8pCPT. Immunoprecipitations were carried out using R26 (for type I receptor), R45 (for type III receptor), and anti-GAP1IP4BP antibody. Western blotting analysis of immunoprecipitates of GAP1IP4BP is shown. (D) Lysates from experiments involving GAP1IP4BP were subjected to analysis of the phosphorylation status of VASP using Western blot analysis. Activation of the kinases is associated with an increase of the upper 50-kd form of VASP. In control cells, nonphosphorylated VASP migrates as a 46-kd protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3412/6/m_bloo01103006cw.jpeg?Expires=1769097565&Signature=cREwzhaADjHfkLPZOGmMeYZ6GADF6qUvUo0ATb7frcMLm0V3zuLkjHJO~8d5CqFK33b4Qw9GvDnxHlPdJxr9Cp~-RtYD5-DR3h5FJyOpLcNtrPwtEsqWvb3kNh4OYTShDRzZPhwy1fdoJwOHhm8Wxb115RzFtXeT7CEvwNIwAgM3gO27PZKJszlYOsliL7mwpls7tLedeVh-NtOjsDmneJRhFVcI-LdTYsDca6yHEkDjo2YfuTKU6og-dM2dYTVrGNzLduxNG7~WjTnappD8dNzCmAgcqHFjeIc-2Hr53yV3Zn3VqdcnrOl63~8egOjTYyfB5mczOz0IxIMMv9wSXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Phosphorylation of 1,4,5IP3R and GAP1IP4BP in intact platelets by cAMP-PK and cGMP-PK. / (A) [32P] labeled platelets were incubated with either vehicle (CON) or PGI2 (0.5 μmol/L) or BiMPS (500 μmol/L), and type III 1,4,5IP3R immunoprecipitated with CT3. (B) Experiments were carried out as in (A), and immunoprecipitations were carried out using CT1, CT2, and CT3. (C) Experiments were carried out as above with additional stimulation of cGMP-PK using 8pCPT. Immunoprecipitations were carried out using R26 (for type I receptor), R45 (for type III receptor), and anti-GAP1IP4BP antibody. Western blotting analysis of immunoprecipitates of GAP1IP4BP is shown. (D) Lysates from experiments involving GAP1IP4BP were subjected to analysis of the phosphorylation status of VASP using Western blot analysis. Activation of the kinases is associated with an increase of the upper 50-kd form of VASP. In control cells, nonphosphorylated VASP migrates as a 46-kd protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3412/6/m_bloo01103006dw.jpeg?Expires=1769097565&Signature=jQMuphuK6CSCRaURSRH8Y2M4wjSVyl1RMQWA~zFKNrxj1hpXXFujHAe8pbEHeMnMsY47DeIxUhMLDHtoL8l6ZsnLcvM~zusntmjnphdtJdt7TLjCEpjbmrQHid17NbOuYDZUb2ek5nvBVYd7JNh5ZXBY21kPO-HC7ggYb5xwRpmGyZ-3GMJvIr2bakMcbwZsIQXTltcrhoZJ0zvYhLMVNDA~d0OUttM2L1PWNEe4rQFYxHT~K~ZfIXFGjqbRGiLqL5Vqtm99AtP4hcK2zKclJdZGmTCf1Vh5Q4Uy-dQdghU3nI6mTtzADqjDFqblu9nvZqwNr4Ld~rDAsEcPnJth3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal