Abstract
In an attempt to transduce monocyte-derived dendritic cells (DCs) by a retroviral vector coding for a cell surface marker, we were confronted by the observation of high transfer of the surface molecule in the absence of vector proviral DNA in the treated cells. Indeed, DCs acquired the surface marker by a mechanism independent of the vector machinery, requiring cell-to-cell contact and involving transfer of lipids and a variety of intact membrane proteins. Most important, this property of DCs also includes acquisition of foreign human leukocyte antigen (HLA) molecules. Consequently, DCs become immunological hybrids as they display their own and foreign HLA molecules. The newly acquired HLA is fully functional because it allows recognition by allo-specific T lymphocytes and the binding and presentation of antigen peptides.
Dendritic cells (DCs) are central players in the immune system. They present autologous antigens (Ags) during T-cell development and foreign Ags during the induction of immune responses.1 To stimulate an efficient T-cell response, DCs located in the majority of tissues capture and process Ags displaying major histocompatability complex–peptide (MHC-peptide) complexes at their surface with high efficiency. This process allows maturation of DCs2 that up-regulate costimulatory molecules and migrate to lymphoid organs,3 4 where they activate Ag-specific T cells.
All these properties suggest the potential role of DCs as an adjuvant in immune approaches to cancer treatment. Indeed, in the last few years great attention has been given to the role of DCs in inducing an effective and long-lasting antitumor immunity in various murine tumor systems.5 This has been obtained by pulsing DCs with both synthetic and natural peptides6-8 or by genetically engineering DCs for constitutive expression of a given antigen.9,10 The relevance of these preclinical experiments to the treatment of human cancer has been recently confirmed.11 12
In addition to immune stimulation, DCs play a central role in the induction of T-cell tolerance to autologous Ags. In the thymic medulla, bone marrow–derived DCs present autologous Ags in the context of autologous MHC molecules, thereby allowing deletion of high-affinity autoreactive T cells by negative selection. Recently, an important role for DCs in the induction of peripheral tolerance has been suggested.13 Host antigen presenting cells (APCs) acquire Ags in a form that is able to gain access to MHC class I and II molecules. This process, which is fundamental for the induction of both immunity and tolerance, is referred to as cross-presentation14,15 and can be facilitated by apoptotic cell death of the Ag-expressing cells.16
A different mechanism involving direct cell-to-cell transfer of self-determinants has been hypothesized for endogenous super Ags and peptide fragments of self-proteins.17 Here, we provide evidence for a new property of monocyte-derived DCs that allows DC acquisition of intact foreign human leukocyte antigen (HLA) molecules. This phenomenon requires cell-to-cell contact and involves transfer of membrane lipids and a variety of surface molecules.
Materials and methods
Cell lines and antibodies
DET-mel and MZ2-G43 are melanoma lines expressing HLA-A2 and HLA-DR*0101 respectively. MSR3-B52 was derived by transfection of the MSR3-mel tumor line18 with HLA-B52 (Bw4). The vector-producing line SFCMM2 has been previously described.19 The 3T3-ΔLNGFr was derived by transduction of NIH/3T3 cells with the SFCMM2 vector. The cell lines were maintained in Roswell Park Memorial Institute medium (RPMI 1640) supplemented with 10% fetal calf serum (FCS). Monoclonal antibodies (mAbs) used in the study included mAb 20.4 (HB8737; American Type Culture Collection, Rockville, MD), which recognizes a truncated form of the low-affinity nerve growth factor receptor (ΔLNGFr); anti–HLA-DR, anti-CD3, and anti-CD1a (Becton Dickinson, Mountain View, CA); anti-CD83 (Immunotech, Marseilles, France); HLA-A2/A28–specific 4B and 2A.1 (anti-Bw4) mAbs (Dr Soo Young Yang, Memorial Sloan Kettering Cancer Center, New York, NY); and HLA-DR*0101–specific mAb (gift from Dr G. B. Ferrara, Advanced Biotechnology Center, Genoa, Italy).
We performed phenotypic analyses using either mAbs directly labeled with phycoerythrin/fluorescein isothiocyanate (PE/FITC) or uncoupled mAb revealed by antimouse immunoglobulin (Southern Biotechnology Associates, Birmingham, AL) conjugated by PE, FITC, Texas red, or Cyanin-5. Negative controls were performed by staining with unrelated murine mAbs. Fluorescence analysis was determined by a fluorescence-activated cell sorter (FACS) (Coulter Elite; Coulter Electronics, Miami, FL), and 5000 to 10 000 events were collected for each staining.
Retroviral vector-mediated transduction of monocyte-derived DCs
Peripheral blood mononuclear cells (5-6 × 107cells) were allowed to adhere 1 hour at 37°C. The adherent cells were then cultured in RPMI 1640 with 10% FCS supplemented with 10 ng/mL lipopolysaccharide (LPS); 800 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Schering-Plough, Innishannon, County Cork, Ireland); 100 U/mL interleukin-4 (IL-4) (gift from Dr P. Coulie, Ludwig Institute for Cancer Research, Brussels, Belgium); 2 mmol/L L-glutamine; and 50 mmol/L 2β-ME. At day 2 of culture, differentiating DCs were transduced by cocultivation with a monolayer of irradiated (100 Gy) vector-producing cells in the presence of 4μg/mL polybrene. The vector-encoded surface marker was the ΔLNGFr.19 After 72 hours, DCs were harvested and seeded in fresh medium. DCs were analyzed 48 hours later for ΔLNGFr expression by flow cytometry with the specific mAb 20.4.
Transfer of cell surface molecules to DCs
DCs at day 5 of culture (CD14−, CD1a+ with 40%-80% CD83 expression) were seeded on a monolayer of nonirradiated donor cells (ie, 3T3-ΔLNGFr or human melanoma cells) or cocultured with phytohemagglutinin-activated (PHA-activated) lymphocytes at a 2:1 (DC:lymphocyte) ratio. DCs were harvested 20 hours later and stained for the transferred molecule using appropriate mAbs.
Confocal microscopy experiments were performed as above, seeding DCs on adherent donor cells grown on poly-L-lysine–coated glass. After 20 hours of cocultivation, cells were fixed in cold ethanol for 1 minute, permeabilized in PBS/0.05% saponin/0.02% BSA and incubated with FITC-labeled primary mAbs. Indirect staining was performed by incubation with different primary mAbs, followed by isotype Texas red– or FITC-labeled secondary mAbs. The analysis was performed with a confocal laser scanning microscope system (MRC-1024; BioRad Laboratories, Hercules, CA) attached to a microscope (Axioplan; Carl Zeiss, Inc, Thornwood, NY) equipped with a krypton/argon laser. Appropriate controls were always included.
Transfer of plasma membrane lipids to DCs
3T3-ΔLNGFr cells were labeled according to manufacturer's protocol with the membrane red dye PKH26 (Sigma, St Louis, MO). The labeled cells were cocultured with DCs for 20 hours as described above, and further processed for confocal analysis using an HLA-DR-FITC mAb and a Cy-5–labeled secondary mAb to detect ΔLNGFr expression. In Figure 1C, the Cy-5–labeled secondary mAb is shown as a light blue color.
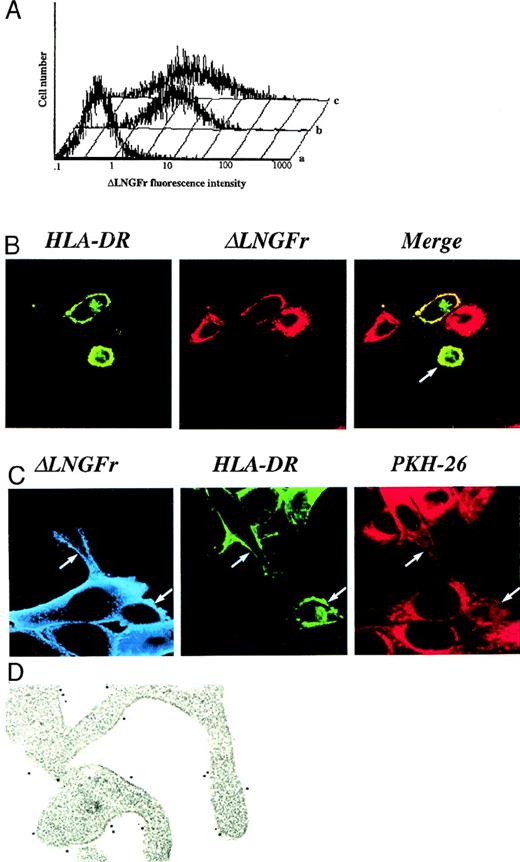
DC acquisition of intact cell surface molecules displayed on the plasma membrane of donor cells.
(A) DC acquisition of the cell surface marker ΔLNGFr is vector-independent. Monocyte-derived DCs were differentiated and cocultured with (b) vector-producing cells19 or with (c) the 3T3-ΔLNGFr cell line expressing the ΔLNGFr on its cell surface, but unable to produce any vector particle. Transduction efficiency was evaluated by immunofluorescence for the expression of the cell surface marker ΔLNGFr encoded by the retroviral vector. ΔLNGFr expression on (a) untreated and (b, c) treated DC populations is shown. (B) DC acquisition of the ΔLNGFr cell surface marker appears to require a cell-to-cell interaction. DCs were cultured on glass cover slips on a monolayer of nonirradiated 3T3-ΔLNGFr. Cocultures were processed for immunofluorescence and confocal microscopy 20 hours later. HLA-DR molecules expressed by DCs were visualized using an HLA-DR-FITC mAb and displayed as green staining. ΔLNGFr surface marker was visualized by the 20.4 specific primary mAb followed by a Texas red–conjugated second antibody and displayed as red staining. Optically merged confocal images showed the colocalization, displayed as yellow staining, of the HLA-DR marker with ΔLNGFr. The dependence of the ΔLNGFr transfer by intercellular contact between cell membranes of DCs and donor cells is suggested by the observation that positive DCs were always in close contact with donor cells, while negative DCs were distant (arrow). (C) DC acquisition of ΔLNGFr cell surface marker is associated with the transfer of plasma membrane lipids. DCs were exposed to 3T3-ΔLNGFr cells previously labeled with PKH26, a stable membrane-soluble red dye that does not exchange spontaneously between membranes for prolonged periods. Cocultures were processed, 20 hours later, for immunofluorescence and confocal microscopy by using an HLA-DR-FITC mAb and a Cy-5-labeled secondary mAb to detect ΔLNGFr expression. The Cy-5–labeled secondary mAb is shown as a light blue color. All DCs that acquired the cell surface marker from ΔLNGFr-expressing cells (arrows) also became positive for the PKH26 red dye. (D) Cell surface distribution of the acquired ΔLNGFr molecules. Cocultures of DCs and 3T3-ΔLNGFr cells were analyzed by double immunolabeling for ΔLNGFr and HLA-DR expression. ΔLNGFr molecules (5-nm gold particles) are interspersed between HLA-DR molecules (15-nm gold particles) on the cell surface of DCs.
DC acquisition of intact cell surface molecules displayed on the plasma membrane of donor cells.
(A) DC acquisition of the cell surface marker ΔLNGFr is vector-independent. Monocyte-derived DCs were differentiated and cocultured with (b) vector-producing cells19 or with (c) the 3T3-ΔLNGFr cell line expressing the ΔLNGFr on its cell surface, but unable to produce any vector particle. Transduction efficiency was evaluated by immunofluorescence for the expression of the cell surface marker ΔLNGFr encoded by the retroviral vector. ΔLNGFr expression on (a) untreated and (b, c) treated DC populations is shown. (B) DC acquisition of the ΔLNGFr cell surface marker appears to require a cell-to-cell interaction. DCs were cultured on glass cover slips on a monolayer of nonirradiated 3T3-ΔLNGFr. Cocultures were processed for immunofluorescence and confocal microscopy 20 hours later. HLA-DR molecules expressed by DCs were visualized using an HLA-DR-FITC mAb and displayed as green staining. ΔLNGFr surface marker was visualized by the 20.4 specific primary mAb followed by a Texas red–conjugated second antibody and displayed as red staining. Optically merged confocal images showed the colocalization, displayed as yellow staining, of the HLA-DR marker with ΔLNGFr. The dependence of the ΔLNGFr transfer by intercellular contact between cell membranes of DCs and donor cells is suggested by the observation that positive DCs were always in close contact with donor cells, while negative DCs were distant (arrow). (C) DC acquisition of ΔLNGFr cell surface marker is associated with the transfer of plasma membrane lipids. DCs were exposed to 3T3-ΔLNGFr cells previously labeled with PKH26, a stable membrane-soluble red dye that does not exchange spontaneously between membranes for prolonged periods. Cocultures were processed, 20 hours later, for immunofluorescence and confocal microscopy by using an HLA-DR-FITC mAb and a Cy-5-labeled secondary mAb to detect ΔLNGFr expression. The Cy-5–labeled secondary mAb is shown as a light blue color. All DCs that acquired the cell surface marker from ΔLNGFr-expressing cells (arrows) also became positive for the PKH26 red dye. (D) Cell surface distribution of the acquired ΔLNGFr molecules. Cocultures of DCs and 3T3-ΔLNGFr cells were analyzed by double immunolabeling for ΔLNGFr and HLA-DR expression. ΔLNGFr molecules (5-nm gold particles) are interspersed between HLA-DR molecules (15-nm gold particles) on the cell surface of DCs.
Electron microscopy analysis
DCs were cocultured with 3T3-ΔLNGFr cells as described above and further processed for electron microscopy analysis. The cells were incubated at 4°C for 1 hour with anti-ΔLNGFr and biotinylated HLA-DR–specific mAbs. After extensive washing, cells were incubated with 5-nm gold particle conjugated antimouse immunoglobulin G (IgG) and 15-nm gold particle conjugated antibiotin mAbs for 1 hour. After the incubation, cells were washed, fixed in 2% glutaraldehyde, and then harvested by scratching and centrifugation. The pellet was washed with 0.1 mol/L cacodylate buffer, postfixed with 2% osmium tetroxide (OsO4), dehydrated, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and examined in a Hitachi H7000 electron microscope (Hitachi, Japan).
Results
Transfer of vector-encoded surface marker into monocyte-derived DCs
To improve the current technology of retroviral vector–mediated gene transfer9,20 into human monocyte-derived DCs,21,22 we were confronted by the evident dissociation between transfer of the vector-encoded surface marker, ΔLNGFr,19 and the absence of integrated viral genome into the treated cells. In several independent experiments, upon cocultivation with irradiated vector-producing cells, a high proportion of DCs (50%-90%) expressed the ΔLNGFr surface marker (Figure 1A). However, the apparent high efficiency of gene transfer was in sharp contrast to the inherited inefficiency of retroviral vectors in transducing nondividing cells such as monocyte-derived DCs.23 This discrepancy was confirmed by the failure to detect any significant levels of integrated proviral vector into the ΔLNGFr-positive population of DCs (data not shown). Further experiments clearly demonstrated that the mechanism involved in the acquisition of the cell surface marker is independent of the retroviral vector machinery.
DC acquisition of the cell surface marker
To demonstrate that DCs acquire the ΔLNGFr surface molecules, even in the absence of retroviral particles, we performed coculture experiments using as donor cells the 3T3-ΔLNGFr cell line, which express the ΔLNGFr on the cell surface but is unable to produce any vector particles. Indeed, nonirradiated 3T3-ΔLNGFr cells were able to transfer the cell surface marker to DCs at frequencies similar to those observed by the use of vector-producing cell lines (Figure1A). These observations suggest that acquisition of the cell surface marker by DCs is dependent upon membrane contact and transfer. Indeed, serial confocal microscopy analysis showed that only DCs in close and direct contact with the ΔLNGFr-expressing cells acquire and display the cell surface marker (Figure 1B).
To understand whether the surface marker acquisition by DCs was associated with membrane transfer, DCs were exposed to ΔLNGFr-expressing cells previously labeled with the lipophilic red dye PKH26. As shown in Figure 1C, all DCs that acquired the cell surface marker from ΔLNGFr-expressing cells (arrows) also became positive for PKH26 red dye, demonstrating that ΔLNGFr is acquired by DCs as a component of the cell surface membrane. Moreover, exposure of DCs to culture supernatant of PKH26-labeled cells did not allow red dye transfer, thus excluding the presence of an artifact due to dye leakage in the medium (data not shown).
Once the exogenous plasma membranes were acquired by DCs, remodeling and redistribution of the transmembrane proteins occurred, as demonstrated by electron microscopy analysis (Figure 1D). This analysis showed the acquired ΔLNGFr molecules (5-nm gold particles) interspersed within the endogenous HLA-DR molecules (15-nm gold particles).
DC acquisition of foreign HLA molecules
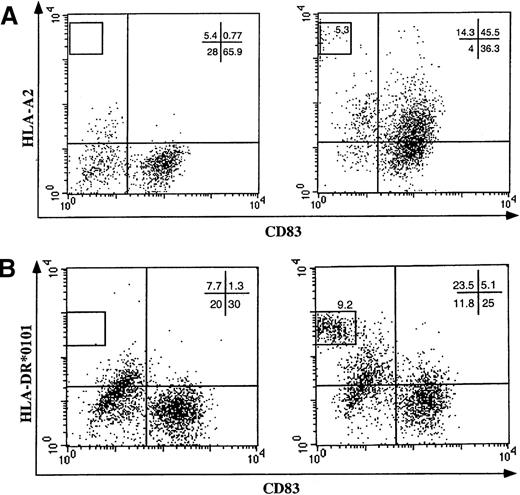
To define whether the observed acquisition of cell surface molecules is part of a more general property of DCs and whether this acquisition may play a role in cross-presentation to immune effectors, we investigated whether HLA molecules were involved in this intercellular transfer. DCs obtained from an HLA-A2–negative individual were exposed to human melanoma cells expressing HLA-A2 (Figure 2A, right panel) or not expressing HLA-A2 (Figure 2A, left panel). Following coculture, DCs were analyzed by flow cytometry for CD83 and HLA-A2 expression and were found to have acquired HLA-A2 molecules (Figure 2A). The amount of foreign HLA molecules acquired by DCs was about 2 logs lower, in terms of fluorescence intensity, compared with that naturally expressed by HLA-A2 DCs and DET-mel melanoma cells (gated in Figure 2A, upper-left quadrant).
DC acquisition of allogeneic HLA class I molecules.
DCs from (A) HLA-A2–negative or (B) HLA-DR*0101–negative individuals were cultivated on a monolayer of nonirradiated melanoma cells expressing the HLA-A2 or the HLA-DR*0101 allele, respectively (right panels). As a control, DCs were cocultured with HLA-unrelated melanoma cells (left panels). After 20 hours of cocultivation, the expression of the allogeneic HLA molecules on the cell surface of DC populations was analyzed by flow cytometry using mAbs specific for CD83 and (A) HLA-A2 or (B) DR0101 molecules.
DC acquisition of allogeneic HLA class I molecules.
DCs from (A) HLA-A2–negative or (B) HLA-DR*0101–negative individuals were cultivated on a monolayer of nonirradiated melanoma cells expressing the HLA-A2 or the HLA-DR*0101 allele, respectively (right panels). As a control, DCs were cocultured with HLA-unrelated melanoma cells (left panels). After 20 hours of cocultivation, the expression of the allogeneic HLA molecules on the cell surface of DC populations was analyzed by flow cytometry using mAbs specific for CD83 and (A) HLA-A2 or (B) DR0101 molecules.
Similar results were reproduced for HLA class II molecules (Figure 2B) by using a melanoma expressing HLA-DR*0101 (Figure 2B, right panel) as donor cells. The efficiency of transfer seems to be higher for HLA class I molecules compared with HLA class II. This could be related to the molecular structure of the 2 HLA molecules or to their concentration on donor cells. Indeed, transfer of ΔLNGFr molecules from highly expressing cells consistently allowed high levels of transfer to DCs, while lower levels of transfer were achieved using donor cells expressing intermediate amounts of ΔLNGFr (data not shown). However, the low efficiency of the class II transfer could be in part dependent on a different sensitivity of the detection system used (eg, different affinities of the 2 mAbs for their ligands).
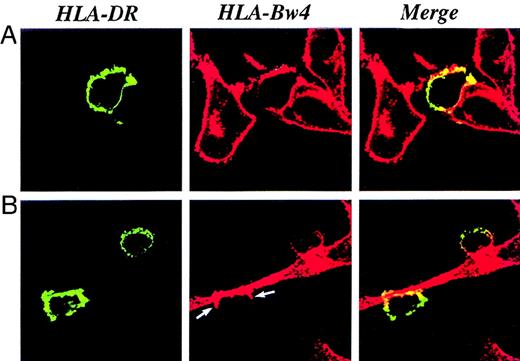
Acquisition of the foreign HLA molecules is independent of the maturation stage of the DC population because CD83+ and CD83− DCs acquire HLA-A2 with the same efficiency (Figure 2). CD83 is a surface marker specific for mature DCs expressed by 40%-80% of DCs at day 5 in our culture system. Dependence of the HLA transfer upon intercellular contact between cell membranes of DCs and donor cells was further confirmed by the confocal analysis. Transfer of HLA-Bw4+ melanoma cells to DCs occurs in a 1-way direction, starting from the point of contact and progressively diffusing to the entire DC surface (Figure 3; highlighted, arrow in lower panel). Transfer in the opposite direction (from DCs to tumor cells) never occurred.
DC acquisition of HLA-Bw4 molecules.
DCs were cultured on glass cover slips on a monolayer of nonirradiated melanoma cells. The cocultures were processed for immunofluorescence and confocal microscopy 20 hours later. (A, B) HLA-DR molecules expressed by DCs were visualized using an HLA-DR-FITC mAb and displayed as green staining. (A, B) HLA-Bw4 molecules were visualized by specific primary mAbs followed by a Texas red–conjugated second antibody and displayed as red staining. (A, B) Optically merged confocal images showed the colocalization, displayed as yellow staining, of the HLA-DR marker with HLA-Bw4 molecules on the cell surface of DCs. The transfer of cell membrane molecules starts from the point of contact between DCs and melanoma cells (B, indicated by arrows).
DC acquisition of HLA-Bw4 molecules.
DCs were cultured on glass cover slips on a monolayer of nonirradiated melanoma cells. The cocultures were processed for immunofluorescence and confocal microscopy 20 hours later. (A, B) HLA-DR molecules expressed by DCs were visualized using an HLA-DR-FITC mAb and displayed as green staining. (A, B) HLA-Bw4 molecules were visualized by specific primary mAbs followed by a Texas red–conjugated second antibody and displayed as red staining. (A, B) Optically merged confocal images showed the colocalization, displayed as yellow staining, of the HLA-DR marker with HLA-Bw4 molecules on the cell surface of DCs. The transfer of cell membrane molecules starts from the point of contact between DCs and melanoma cells (B, indicated by arrows).
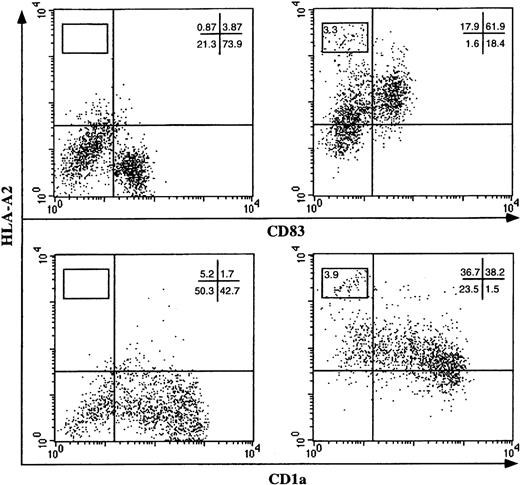
To evaluate the existence of such phenomenon in DC interaction with nontumor cells, we analyzed the ability of DCs to acquire HLA molecules from activated human T lymphocytes. The experiment was performed under the conditions already described, using PHA-activated lymphocytes grown in the presence of IL-2 as donor cells. Upon coculture, the mixture of the 2 populations was analyzed with flow cytometry by gating the DC population and using either CD83 or CD1a, which are not expressed by activated T lymphocytes, as specific markers. Only DCs cocultured with HLA-A2–expressing lymphocytes were found to have acquired HLA-A2 molecules (Figure 4, right panels). Contaminating T cells, expressing high levels of HLA-A2, are shown in the upper-left quadrants of Figure 4. Even in this system, transfer of surface HLA class I molecules is independent of the maturation stage of DCs.
DC acquisition of allogeneic HLA class I from activated human lymphocytes.
DCs from an HLA-A2–negative individual were cocultivated with PHA-activated human lymphocytes expressing the HLA-A2 allele (right panels). As a control, DCs were cocultured with HLA-unrelated lymphocytes (left panels). After 20 hours, the expression of the allogeneic HLA molecules on the cell surface of the DC populations was analyzed by flow cytometry using mAbs specific for HLA-A2 and CD83 (upper panels) or CD1a (lower panels).
DC acquisition of allogeneic HLA class I from activated human lymphocytes.
DCs from an HLA-A2–negative individual were cocultivated with PHA-activated human lymphocytes expressing the HLA-A2 allele (right panels). As a control, DCs were cocultured with HLA-unrelated lymphocytes (left panels). After 20 hours, the expression of the allogeneic HLA molecules on the cell surface of the DC populations was analyzed by flow cytometry using mAbs specific for HLA-A2 and CD83 (upper panels) or CD1a (lower panels).
Acquired foreign HLA molecules are targets of a specific T-cell response
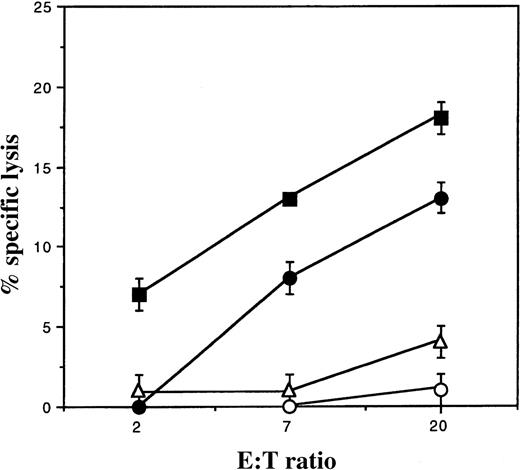
To confirm that foreign HLA class I molecules acquired by DCs are fully functional, hybrid DCs, displaying both their own molecules and the foreign HLA-A2 molecules, were used as targets in a cytotoxic assay. HLA-A2–specific cytotoxic T cells killed DCs exposed to an HLA-A2–positive melanoma line, while DCs untreated or cocultured with HLA-A2–negative melanoma were not recognized (Figure5). Analogous results were obtained by loading A*6801-hybrid-DCs with an exogenous peptide (TRP-2-INT2.222-231)24 and using a peptide-specific T-cell clone as an effector (data not shown). These results strongly suggest that cell surface molecules are acquired by DCs as intact proteins properly integrated into the cell membrane rather than as proteolyzed fragments released by the donor cells and bound by nonspecific or receptor-mediated mechanisms. This hypothesis is further supported by the inability of culture supernatant from ΔLNGFr and HLA expressing cells to transfer the relevant surface molecule (data not shown). Additionally, the half-life of the transferred molecules was monitored to be in the same range as that reported for endogenous HLA class I (ie, 10-20 hours).25 We observed that after 24 hours, only 20% of the acquired HLA-A2 molecules were expressed on the DC cell surface, while 70% of ΔLNGFr molecules were still present.
T-cell recognition of foreign HLA class I molecules displayed by DCs.
DCs from an HLA-A2–negative individual were cultivated on a monolayer of nonirradiated melanoma cells expressing the HLA-A2 allele (•). As a control, DCs were either untreated (○) or cocultured with an HLA-unrelated melanoma cell line (▵). After 20 hours, DCs were harvested, purified from the potential melanoma contamination by CD45RO microbead separation, and used as targets in a standard chromium release assay. Only DCs exposed to the HLA-A2 melanoma are recognized by anti–HLA-A2 cytotoxic T-cell effectors. Recognition of the HLA-A2–positive melanoma cells is shown (▪).
T-cell recognition of foreign HLA class I molecules displayed by DCs.
DCs from an HLA-A2–negative individual were cultivated on a monolayer of nonirradiated melanoma cells expressing the HLA-A2 allele (•). As a control, DCs were either untreated (○) or cocultured with an HLA-unrelated melanoma cell line (▵). After 20 hours, DCs were harvested, purified from the potential melanoma contamination by CD45RO microbead separation, and used as targets in a standard chromium release assay. Only DCs exposed to the HLA-A2 melanoma are recognized by anti–HLA-A2 cytotoxic T-cell effectors. Recognition of the HLA-A2–positive melanoma cells is shown (▪).
Discussion
Our studies provide evidence of a new mechanism involving intercellular transfer of intact proteins to DCs. The ability to acquire surface proteins by direct cell-to-cell contact is a unique feature of DCs. Other cell lines of hematopoietic origin (ie, CD8-activated T lymphocytes and Epstein-Barr virus–induced (EBV-induced) lymphoblastoid cell lines) did not show this property (data not shown). Acquisition of intact surface proteins by CD4+ lymphocytes has been recently described to occur in an in vitro transendothelial migration model,26 and it has been strongly correlated to the activation stage of the lymphocytic population.
Several lines of evidence obtained in this study suggest that the acquisition of the surface molecules is mediated by a close interaction between DCs and donor cells. In addition, this phenomenon seems not to be the consequence of the classical pathway of phagocytosis of vesicles or apoptotic bodies and recycling to the plasma membrane. First, the cell surface molecules are transferred to DCs with the same efficiency by both apoptotic and nonapoptotic donor cells (data not shown) and are also acquired in the presence of the saturating concentration of D-mannose (data not shown), suggesting that mannose receptor–mediated endocytosis27 is not involved in the phenomenon. Moreover, we never observed localization of the surface molecules within any intracellular compartment (Figure 1B, 1C, and Figure 3). Additionally, DCs exposed to culture supernatant of ΔLNGFr expressing cells do not acquire the surface marker (data not shown). Altogether these observations suggest that if ectocytosis28 by donor cells and up-take by DCs play a role in the transfer of surface molecules, this process may occur locally in the context of a close DC–donor cell interaction.
We could speculate that DCs at certain stages of differentiation may allow cell fusion with transfer of plasma membrane components by the use of fusogenic proteins, as described to occur during viral infection.29 The DCs may also induce locally triggered shedding of membrane vesicles from the tissues they interact with during their trafficking.
We believe that this mechanism, if confirmed in vivo, may provide an additional pathway of cross-presentation that is also active in the absence of tissue damage and apoptosis. Indeed, acquisition of preformed MHC-peptide complexes from normal tissues and neoplastic cells may allow efficacious cross-presentation by DCs in the absence of inflammation and other signals of tissue damage. A unique feature of this phenomenon may be occurring during allogeneic organ transplantation. Here, DCs from the recipient could seed the transplanted organ, up-take and display the intact HLA of the donor, thus representing a continuous source of foreign HLA presentation.
Acknowledgments
We thank M.G. Roncarolo, A. Manfredi, and P. Panina for useful discussions.
Supported in part by grants from the Italian Association for Cancer Research, Milan, Italy, and the Ministry of Health (RF 98.51), Italy.
Submitted September 23, 1999; accepted January 28, 2000.
Reprints:Claudio Bordignon, TIGET, Istituto Scientifico H.S. Raffaele, via Olgettina 58, 20132 Milano, Italy; e-mail:claudio.bordignon@hsr.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal