Abstract
The authors have examined the role of the src-family of protein tyrosine kinases in leukotriene B4(LTB4)–induced activation of guinea-pig eosinophils. Western blot analysis identified the src-like protein tyrosine kinases p53lyn, p56lyn, p56/59hck, p55fgr, and p56lck whereas p60src, p62yes, p55blk, and p59fyn were not detected. LTB4 promoted a rapid increase in p53/56lyn activity in eosinophils, which peaked at 5 seconds and remained elevated at 60 seconds; hck, fgr, and lck were not activated. A role for p53/56lyn in eosinophil activation was investigated with the use of the src-selective inhibitor PP1 (1 μmol/L to 10 μmol/L), which attenuated LTB4-stimulated p53/56lyn activity and the phosphorylation of extracellular signal-regulated kinase–2 in intact cells. At comparable concentrations, PP1 was also shown to attenuate LTB4-induced nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidase activation, chemotaxis, and Ca++-dependent [3H]arachidonic acid (AA) release. Moreover, an inhibitor of mitogen-activated protein kinase kinase-1, PD 098059, significantly inhibited LTB4-induced chemotaxis but had no effect on oxidant production or [3H]AA release. Collectively, these results implicate lyn kinase in LTB4-induced eosinophil activation through the recruitment of divergent cell-signaling pathways.
Eosinophils are thought to play an important role in host defense in response to parasite infestation, but have also been implicated in the etiology of certain inflammatory and allergic diseases, such as asthma. In this respect, it is believed that following migration into tissue, the inflammatory actions of eosinophils are mediated by the release of highly basic granule proteins and a host of lipid and protein inflammatory mediators together with toxic, oxygen-derived free radicals.1 Central to the production of lipid mediators is the activation of phospholipase A2 (PLA2), which catalyzes hydrolysis of thesn-2 fatty acyl bond of phospholipids to liberate arachidonic acid (AA) and lysophospholipids, which can be metabolized to produce eicosanoids and platelet-activating factor, respectively. Similarly, the activity of the nicotinamide adenine dinucleotide phosphate (reduced form) NADPH oxidase is thought to be the rate-limiting step in the production of a range of cytotoxic, oxygen-derived free radicals. In response to appropriate stimuli, the NADPH oxidase catalyzes the single electron reduction of molecular O2 to superoxide (.O2−), a powerful oxidizing and reducing agent, which can be converted into a range of toxic radicals, including H2O2, by the action of superoxide dismutase.2
Leukotriene B4 (LTB4) is a potent chemoattractant for guinea-pig eosinophils3,4 and also induces a rapid release of incorporated [3H]AA by PLA2 and the generation of H2O2 by the NADPH oxidase.5-7 However, the intracellular mechanisms that mediate LTB4-induced [3H]AA release and H2O2 generation are unclear, and nothing, to the authors' knowledge, is known of the pathways that govern chemotaxis. Previous studies have established that activation of the NADPH oxidase can be partially suppressed by Ro-31 8220, a nonselective inhibitor of protein kinase C (PKC),5 and mepacrine,6 an inhibitor of PLA2 but is Ca++-independent and unaffected by wortmannin, an inhibitor of phosphatidylinositol 3-kinase (PtdIns 3-kinase).5 With respect to LTB4-induced [3H]AA generation, both Ca++-dependent and Ca++-independent processes have been implicated that are regulated independently of signal molecules derived from the hydrolysis of PtdIns (4-5)P2 by PLC.6 In guinea-pig eosinophils, LTB4 has been shown to promote the phosphorylation (activation) of extracellular signal-regulated kinase (ERK)–1 and ERK-2, but not c-jun N-terminal kinase-46/54 or p38 mitogen-activated protein (MAP) kinase.7 However, the significance of this finding is unclear since inhibition of ERK-1 and ERK-2 phosphorylation with the selective MAP kinase kinase-1 (MEK-1) inhibitor, PD098059, has no significant effect upon either [3H]AA generation or oxidant production.7 In contrast, the nonselective protein tyrosine kinase inhibitors herbimycin A and lavendustin A caused a concentration-dependent inhibition of oxidant production and [3H]AA release, implicating tyrosine phosphorylation in LTB4-induced eosinophil responses.7
Tyrosine kinases represent a divergent class of enzymes that have been categorized into those that are receptor-linked and those that are cytosolic or nonreceptor associated. The tyrosine kinases involved in signaling from G-protein–linked receptors are thought to be primarily cytosolic in origin and include the src-related tyrosine kinase family, which comprises p60src, p53lyn, p56lyn, p56hck, p59hck, p62yes, p55blk, p55fgr, p59fyn, p56lck, and p60yrk. In neutrophils, hck and lyn are thought to be involved in the activation of the NADPH oxidase8 and the formation of PtdIns (3,4,5)P3 via stimulation of PtdIns 3-kinase.9,10 In addition to these cellular responses, src-related tyrosine kinases are implicated in G-protein–mediated ERK-1 and ERK-2 kinase activation.11-13In this report, experiments are described in which we have extended our previous studies with herbimycin A and lavendustin A by determining whether the src-family of protein tyrosine kinases are implicated in LTB4-induced eosinophil activation, with emphasis on chemotaxis, [3H]AA release, and activation of the NADPH oxidase.
Materials and methods
Drugs and analytical reagents
The following drugs and analytical reagents were used: LTB4(Cascade Biochemicals, Norwich, England); [γ-32P]ATP (adenosine triphosphate) (more than 5000 Ci/mmol) and [5,6,8,9,11,12,14,15(n)-3H] arachidonic acid (74 Ci/mmol) (Amersham International, Buckinghamshire, UK); cdc2(6-20)-NH2 and agarose-conjugated antibody to phosphotyrosine, 4G10, (TCS Biologicals, Bucks, UK); protein G plus agarose, horse-radish-peroxidase–conjugated antirabbit, antigoat antibodies and rabbit and goat antibodies to lyn, src, hck, yes, blk, fgr, fyn, lck, and cPLA2 (Santa Cruz Biotechnology/Autogen Bioclear, Wiltshire, UK); PD 089059 and methyl arachidonyl fluorophosphonate (Calbiochem, Nottingham, UK); superoxide dismutase, aprotinin, pepstatin A, leupeptin, lucigenin, mouse immunoglobulin (Ig) G2α, and all other chemicals (AnalaR grade) (Sigma, Poole, Dorset, UK). PP1 (CP-118556; 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) was kindly donated by Dr J. H. Hanke (Pfizer Inc, Groton, MA).
Induction and purification of eosinophils
Eosinophils were purified from the peritoneum of male Dunkin-Hartley guinea pigs. Briefly, a macrophage- and eosinophil-rich exudate was produced by weekly intraperitoneal injection of animals with human serum (1 mL per animal) for a period of 2 to 4 weeks. Three to 6 days following injection, guinea pigs were anesthetized, and the peritoneal cavity was lavaged with 50 mL sterile glucose (5% wt/vol). The lavage fluid was washed in Hanks' balanced salt solution (HBSS) and was pooled, and eosinophils were separated by centrifugation at 1600g for 20 minutes at 18°C through discontinuous Percoll density gradients (1.080, 1.085, 1.090, and 1.100 g/mL) according to Gartner.14 With this procedure, eosinophils were recovered from the 1.085/1.090 g/mL, and 1.090/1.100 g/mL Percoll interfaces and were greater than 97% pure and greater than 95% viable as assessed by trypan blue exclusion. Eosinophils were washed and resuspended in Hepes buffer (10 mmol/L Hepes, pH 7.4, 0.1% wt/vol bovine serum albumin in HBSS).
Measurement of [3H] arachidonic acid release
Agonist-induced release of [3H]AA from eosinophils was performed with the use of a modification of the method detailed in Cockcroft and Stutchfield.15 Eosinophils (107/mL) were prelabeled with [3H]AA (1 μCi/mL) for 60 minutes at 37°C in Hepes buffer, washed 3 times, and resuspended at a concentration of 3 × 107/mL. Aliquots (100 μL) of eosinophils were transferred to Eppendorf microfuge tubes containing 80 μL Hepes buffer with/without 1 mmol/L CaCl2/1 mmol/L MgCl2 and incubated at 37°C prior to the addition of LTB4 (20 μL). Reactions were terminated at specified times by the addition of 500 μL ice-cold NaCl (0.9% wt/vol). Eosinophils were sedimented by centrifugation (12 000g for 1 minute), and the pellet and supernatant were counted in 4 mL ACS II.
Measurement of respiratory burst
Eosinophil superoxide generation was determined by superoxide dismutase–inhibitable lucigenin-enhanced chemiluminescence16 with a plate-shaking luminometer (Lucy II, Labtech Ltd, Uckfield, UK). The reaction mixture (180 μL) contained 25 μmol/L lucigenin in Hepes buffer (plus 1 mmol/L CaCl2/1 mmol/L MgCl2), the indicated inhibitor and/or vehicle and/or superoxide dismutase and eosinophils at a final concentration of 106/mL. Following preincubation at 37°C for 5 minutes, the reaction was initiated by the addition of LTB4 (20 μL) to a final concentration of 100 nmol/L. Duplicate samples were monitored for 3 minutes, and the peak chemiluminescence response (approximately 30 seconds) was recorded.
Measurement of chemotaxis
Eosinophil chemotaxis was measured in a 48-well micro-chemotaxis chamber with the use of a modification of the method described by Wilkinson.17 The lower wells of the chamber were loaded with 25μL of Hepes buffer containing 100 nmol/L LTB4, and the upper well contained eosinophils (105suspended in 50μL Hepes buffer) and the indicated concentration of inhibitor. The chamber was then incubated for 3 hours at 37°C, after which the filter was removed and the cells were fixed in 70% (wt/vol) ethanol. The eosinophils were stained with hematoxylin and counted.
Measurement of intracellular Ca++ concentration
Eosinophils (107/mL) were suspended at 37°C in Hepes buffer and incubated for 30 minutes with fura-2/am (1 μmol/L). After 3 washes, cells were resuspended at 4 × 106cells/mL and stored on ice. LTB4-induced changes in [Ca++]i were monitored spectrofluorimetrically (λex = 340/380 nm; λem = 510 nm; slit width = 4 nm) in the presence or absence of 1 mmol/L CaCl2 /1 mmol/L MgCl2 as described previously.5
Immunoprecipitation
Eosinophils (3 × 106 in 180 μL) were suspended in Hepes buffer containing 1 mmol/L CaCl2/1 mmol/L MgCl2 and the indicated concentration of PP1 when required, then incubated for 5 minutes at 37°C. Cells were exposed to LTB4 (0.1 pmol/L to 100 nmol/L in 20 μL), and the reaction was terminated at various times by the addition of 800 μL ice-cold immunoprecipitation buffer (final concentration: 10 mmol/L Tris base, pH 7.4, 1% Triton X-100, 0.5% Na-deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 150 mmol/L NaCl, 1 mmol/L EDTA, 0.5 mmol/L PMSF, 2 mmol/L Na-orthovanadate, 10 μg/mL leupeptin, 25 μg/mL aprotinin, 10 μg/mL pepstatin A, 1.25 mmol/L NaF, and 1 mmol/L Na-pyrophosphate). Samples were vortex-mixed, incubated on ice for 30 minutes, and centrifuged (16 000g for 10 minutes) to yield a supernatant that was used for immunoprecipitation. In some experiments, agarose-conjugated antiphosphotyrosine antibody (4G10) (10 μL) was added to the supernatant, which was incubated overnight at 4°C. Cells were subsequently washed 4 times by centrifugation (16 000g for 3 minutes) in immunoprecipitation buffer, and the final pellet was boiled in Laemmli buffer for immunoblotting. In other studies, members of the src family of protein tyrosine kinases were immunoprecipitated. Initially samples were clarified by incubation for 60 minutes with 20 μL protein G plus agarose and pelleted by centrifugation (16 000gfor 3 minutes). The resultant supernatant was mixed for 120 minutes at 4°C with 5 μg of the relevant anti-src antibody and incubated overnight following the addition of 20μL protein G plus agarose. Cells were washed twice, by centrifugation (16 000g for 3 minutes), in immunoprecipitation buffer and twice in kinase assay buffer (100 mmol/L Tris.HCl, pH 7.2, 125 mmol/L MgCl2, 25 mmol/L MnCl2, 2 mmol/L EGTA, 0.25 mmol/L sodium orthovanadate, 2 mmol/L dithiothreitol [DTT]) before resuspending the final pellet in 40 μL kinase assay buffer. Twenty microlitres was removed and boiled in Laemmli buffer for Western blot analysis, and the remainder was employed for measurement of src-like protein tyrosine kinase activity as described below.
Western blot analysis
Protein samples were subjected to electrophoresis on 10% SDS polyacrylamide gels and transferred to nitrocellulose (Hybond-ECL, Amersham) for 2 hours at 1000 mA in transblotting buffer (183 mmol/L glycine-HCl, 25 mmol/L Tris-base, and 20% methanol). The nitrocellulose was incubated for 1 hour in TBS-T (25 mmol/L Tris-base, 150 mmol/L NaCl, 0.05% Tween 20, pH 7.4) containing 5% [wt/vol] nonfat dry milk to block nonspecific antibody binding, and incubated overnight in TBS-T containing 5% bovine serum albumin and the relevant antibody. Membranes were washed with TBS-T (5 × 5 minutes) and incubated with either horseradish peroxidase (HRPO)–linked antirabbit IgG (diluted 1:2000) or HRPO-linked antigoat IgG (diluted 1:1000) in TBS-T/5% nonfat dry milk for 1 hour at room temperature. The nitrocellulose was then washed in TBS-T (5 × 5 minutes), and antibody-labeled proteins were detected by enhanced chemiluminescence (ECL) on Kodak X-OMAT-S film. The intensity of the relevant bands was quantified by laser-scanning densitometry.
Measurement of src-tyrosine kinase activity
The activity of immunoprecipitated lyn was assessed by measuring the incorporation of 32P from [γ-32P]ATP into cdc2(6-20)-NH2. Samples were incubated for 10 minutes at 30°C in 40 μL of kinase assay buffer (final: 100 mmol/L Tris.HCl, pH 7.2, 125 mmol/L MgCl2, 25 mmol/L MnCl2, 2 mmol/L EGTA, 0.25 mmol/L sodium orthovanadate, 2 mmol/L DTT, 150 μmol/L cdc2[6-20]-NH2, 25 μmol/L ATP [10 μCi], and, when appropriate, the lyn kinase inhibitor PP1), and the reaction was stopped by spotting aliquots (25 μL) of each sample onto P81 phosphocellulose paper squares (Whatman), which were washed 4 times (5 minutes) in 0.75% vol/vol phosphoric acid and once (3 minutes) in acetone. The radioactivity retained by the paper (reflecting phospho-cdc2[6-20]-NH2) was then measured by liquid scintillation counting in 4 mL ACS II (Amersham).
Statistical analysis
Data points and values represent the mean ± SEM of “n” independent determinations taken from different cell preparations. Concentration-response curves were analyzed by least-squares, nonlinear iterative regression with the PRISM curve-fitting program (GraphPad Software, San Diego, CA), and EC50/IC50 values were subsequently interpolated from curves of best-fit. When statistical evaluation was required, data were analyzed parametrically by Student t test for paired data or by 1-way analysis of variance (ANOVA)/Newman-Keuls multiple comparison test. The null hypothesis was rejected when P < .05.
Results
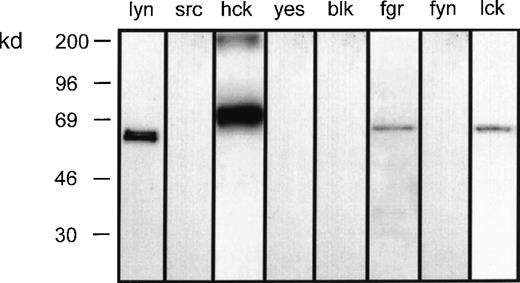
To investigate the role of the src-family of tyrosine kinases in LTB4-induced eosinophil responses, preliminary experiments were undertaken to determine the complement of these proteins expressed by eosinophils. Whole-cell lysates were subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE), and Western immunoblot analysis was performed with the use of antibodies specific for 8 members of this family. This procedure reproducibly detected p53lyn, p56lyn, p56/59hck, p55fgr, and p56lck but not p60src, p62yes, p55blk, or p59fyn (Figure1).
Complement of src-tyrosine kinases expressed in eosinophils.
Eosinophil homogenate was separated by SDS-PAGE and then immunoblotted with the indicated antibodies to the src-family tyrosine kinases. Representative blots obtained from 3 separate preparations are shown.
Complement of src-tyrosine kinases expressed in eosinophils.
Eosinophil homogenate was separated by SDS-PAGE and then immunoblotted with the indicated antibodies to the src-family tyrosine kinases. Representative blots obtained from 3 separate preparations are shown.
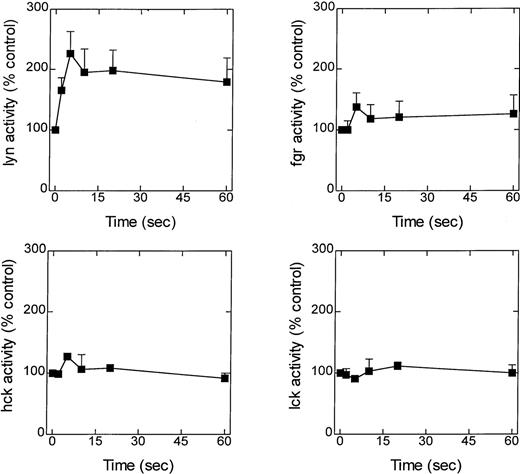
A possible role for the src-family of tyrosine kinases in LTB4-induced eosinophil responses was initially assessed by determining the time course of enzyme activation. Eosinophils were exposed to LTB4 (100 nmol/L), and at various times the reaction was stopped by the addition of lysis buffer, and lyn, hck, fgr, and lck were immunoprecipitated and their ability to phosphorylate cdc2(6-20)-NH2 was determined (Figure2A-D). Although basal activity could be detected with all 4 enzymes, only the activity of lyn was significantly elevated in response to LTB4 (Figure 2A). Thus, an increase in lyn kinase activity was detected 2 seconds after exposure to LTB4; the increase peaked at approximately 5 seconds and remained elevated over the duration of the experiment (60 seconds).
Effect of LTB4 upon lyn, fgr, hck, and lck activity.
Cells were preincubated at 37°C for 5 minutes and stimulated with LTB4 (100 nmol/L). At the indicated times, immunoprecipitation buffer was added, and lyn, fgr, hck, and lck were immunoprecipitated, and their activity was determined by immunocomplex assay. Activity is expressed as a percentage of the control (basal counts were lyn, 30 040 ± 7105 cpm; fgr, 8462 ± 1742 cpm; hck, 20 841 ± 1161; lck, 4635 ± 852 cpm) and is the mean ± SEM of 4 to 7 independent experiments.
Effect of LTB4 upon lyn, fgr, hck, and lck activity.
Cells were preincubated at 37°C for 5 minutes and stimulated with LTB4 (100 nmol/L). At the indicated times, immunoprecipitation buffer was added, and lyn, fgr, hck, and lck were immunoprecipitated, and their activity was determined by immunocomplex assay. Activity is expressed as a percentage of the control (basal counts were lyn, 30 040 ± 7105 cpm; fgr, 8462 ± 1742 cpm; hck, 20 841 ± 1161; lck, 4635 ± 852 cpm) and is the mean ± SEM of 4 to 7 independent experiments.
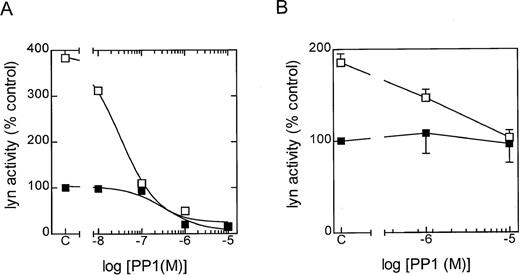
To further examine the role of lyn kinase in eosinophil activation, the selective src-tyrosine kinase inhibitor PP1 (CP-118556) was employed.18 In in vitro studies employing lyn that had been immunoprecipitated from control and LTB4-stimulated eosinophils, PP1 inhibited the phosphorylation of cdc2(6-20)-NH2 in a concentration-dependent manner, with IC50 values of approximately 300 and 20 nm, respectively (Figure3A). To determine the efficacy of PP1 in intact cells, we immunoprecipitated lyn at 5 seconds following exposure to LTB4 in the absence and presence of the inhibitor (Figure 3B). It can be seen that significantly higher concentrations of PP1 were required to attenuate LTB4-induced lyn activation in intact, giving an approximate IC50 of 1 mmol/L and total inhibition at 10 mmol/L. As with the cell-free studies, basal lyn activation was relatively insensitive to PP1, and we failed to observe inhibition even at a concentration of 10 mmol/L. The src family of protein tyrosine kinases has also been implicated as an upstream regulator of the ras/raf/MEK/ERK kinase cascade.11-13 Since we have previously demonstrated that LTB4 activates ERK-1/2 in guinea-pig eosinophils,7 we examined the effect of PP1 on ERK phosphorylation (activation) to provide an additional gauge of the efficacy of this inhibitor in intact cells. As shown in Figure4, PP1 (10 mmol/L) abolished basal ERK-2 phosphorylation and significantly inhibited (by > 80%) the increase in phophorylation effected by LTB4. In contrast, a lower concentration of PP1 (1 mmol/L) was inactive. Taken together, these results implicate lyn in basal and LTB4-induced ERK activation and suggest that concentrations of PP1 between 1 and 10 mmol/L are required to inhibit functional responses in intact eosinophils.
Effect of PP1 upon lyn activity in a cell-free system and intact cells.
The in vitro and in vivo efficacy of PP1 upon lyn kinase activity was determined in 3 independent experiments. The results are expressed as a percentage of the control (ie, unstimulated eosinophils). (A) In vitro results shown are representative graph. Eosinophils were incubated at 37°C for 5 minutes, and then lyn was immunoprecipitated from control (▪) and LTB4-stimulated (□) eosinophils at 20 seconds. Cell lysates were then preincubated with the indicated concentration of PP1 for 5 minutes at 4°C, and the lyn activity was determined by immunocomplex assay. (B) In vivo results shown as the means ± SEM. Eosinophils were preincubated at 37°C for 5 minutes with the indicated PP1 concentration. Lyn kinase was then immunoprecipitated from control (▪) and LTB4-stimulated (□) eosinophils at 5 seconds, and activity was determined by immunocomplex assay.
Effect of PP1 upon lyn activity in a cell-free system and intact cells.
The in vitro and in vivo efficacy of PP1 upon lyn kinase activity was determined in 3 independent experiments. The results are expressed as a percentage of the control (ie, unstimulated eosinophils). (A) In vitro results shown are representative graph. Eosinophils were incubated at 37°C for 5 minutes, and then lyn was immunoprecipitated from control (▪) and LTB4-stimulated (□) eosinophils at 20 seconds. Cell lysates were then preincubated with the indicated concentration of PP1 for 5 minutes at 4°C, and the lyn activity was determined by immunocomplex assay. (B) In vivo results shown as the means ± SEM. Eosinophils were preincubated at 37°C for 5 minutes with the indicated PP1 concentration. Lyn kinase was then immunoprecipitated from control (▪) and LTB4-stimulated (□) eosinophils at 5 seconds, and activity was determined by immunocomplex assay.
Effect of PP1 on LTB4-induced ERK-2 phosphorylation.
Cells were preincubated at 37°C for 5 minutes with the indicated concentration of PP1 and stimulated with LTB4 (100 nmol/L), and the reaction was stopped at the indicated times by the addition of immunoprecipitation buffer. Cell homogenates were separated by SDS-PAGE, and the presence of dual phosphorylated ERK-2 was detected by Western blot analysis. Representative blots obtained from 3 separate preparations are shown.
Effect of PP1 on LTB4-induced ERK-2 phosphorylation.
Cells were preincubated at 37°C for 5 minutes with the indicated concentration of PP1 and stimulated with LTB4 (100 nmol/L), and the reaction was stopped at the indicated times by the addition of immunoprecipitation buffer. Cell homogenates were separated by SDS-PAGE, and the presence of dual phosphorylated ERK-2 was detected by Western blot analysis. Representative blots obtained from 3 separate preparations are shown.
In subsequent experiments, PP1 was employed to investigate the role of lyn kinase in LTB4-induced eosinophil responses. In previous studies, we have shown that LTB4 (1 μmol/L) evokes rapid [3H]AA release by PLA2 (t1/2 = 4 seconds) that is essentially complete after approximately 10 seconds of exposure.6 Interestingly,this response could be separated into Ca++-dependent and Ca++-independent components (Figure5B). Thus, in Ca++/Mg2+-containing media,LTB4 produced a concentration-dependent increase in [Ca++]c and attendant elaboration of [3H]AA (Figure 5A/B, open circles). However, in Ca++-depleted media containing EGTA (100 μmol/L), which abolished the increase in the [Ca++]c in response to LTB4(Figure 5A, closed circles), [3H]AA was still released in a concentration-dependent manner albeit at a reduced magnitude (Figure5B, closed circles). An examination of the concentration-response curve that described [3H]AA release revealed that the Ca++-dependent and independent components were affected by low (< 10 nmol/L) and high (> 10 nmol/L) concentrations of LTB4, respectively (Figure 5B). A role for an src-like protein tyrosine kinase in [3H]AA release was indicated by the ability of PP1 to suppress (by 50% at 10 μmol/L) LTB4-induced, Ca++-dependent [3H]AA release without affecting the Ca++-independent response (Figure6A). This inhibition was not significantly affected by increasing the preincubation time to 15 minutes, at which time there was a steep increase in the level of basal [3H]AA release (data not shown). Furthermore, attenuation of [3H]AA release was not secondary to an inhibition of Ca++ mobilization since PP1 (10 μmol/L) did not affect the Ca++transient induced by 1 nmol/L or 100 nmol/L LTB4 (data not shown).
Identification of a Ca++-dependent and Ca++-independent pathway of [3H]AA release.
Cells were preincubated for 5 minutes at 37°C in the presence (○) or absence (•) of 1 mmol/L Ca++ and 1 mmol/L Mg++. Cells were then stimulated with the indicated concentration of LTB4. Data represent the mean ± SEM of 3 to 4 independent experiments and show (A) the maximal increase in [Ca++]c and (B) the release of [3H]AA release at 60 seconds, where an asterisk indicates that the stimulation of the LTB4-induced Ca++-independent release was significant (P < .05) compared to controls.
Identification of a Ca++-dependent and Ca++-independent pathway of [3H]AA release.
Cells were preincubated for 5 minutes at 37°C in the presence (○) or absence (•) of 1 mmol/L Ca++ and 1 mmol/L Mg++. Cells were then stimulated with the indicated concentration of LTB4. Data represent the mean ± SEM of 3 to 4 independent experiments and show (A) the maximal increase in [Ca++]c and (B) the release of [3H]AA release at 60 seconds, where an asterisk indicates that the stimulation of the LTB4-induced Ca++-independent release was significant (P < .05) compared to controls.
Effect of PP1 on LTB4-induced [3H]AA release and superoxide generation.
Cells were preincubated for 5 minutes at 37°C in the presence (○) or absence (•) of 1 mmol/L Ca++/1 mmol/L Mg2+ and the indicated concentrations of PP1. Data represent the mean ± SEM of 3 to 4 independent experiments. (A) Following stimulation with LTB4 (100 nmol/L), the release of [3H]AA at 60 seconds was determined. (B) Following stimulation with LTB4(100 nmol/L), the maximal rate of superoxide generation measured as relative light units (RLU) was determined. An asterisk indicates significant inhibition of LTB4-induced response by the indicated concentration of inhibitor compared with controls. P < .05.
Effect of PP1 on LTB4-induced [3H]AA release and superoxide generation.
Cells were preincubated for 5 minutes at 37°C in the presence (○) or absence (•) of 1 mmol/L Ca++/1 mmol/L Mg2+ and the indicated concentrations of PP1. Data represent the mean ± SEM of 3 to 4 independent experiments. (A) Following stimulation with LTB4 (100 nmol/L), the release of [3H]AA at 60 seconds was determined. (B) Following stimulation with LTB4(100 nmol/L), the maximal rate of superoxide generation measured as relative light units (RLU) was determined. An asterisk indicates significant inhibition of LTB4-induced response by the indicated concentration of inhibitor compared with controls. P < .05.
Of the many PLA2 families that are now recognized, cytosolic PLA2 (cPLA2) is believed to be central to the release of cellular AA.19 To investigate the role of this enzyme in Ca++-dependent [3H]AA release, we assessed the ability of LTB4 to phosphorylate cPLA2 by exploiting the ability of phospho-cPLA2 to be retarded on SDS polyacrylamide gels when compared with the dephosphorylated enzyme, producing a characteristic gel-shift. However, although cPLA2 was retarded in eosinophils treated with the phorbol 12-myristate 13-acetate PMA, no similar gel-shift of cPLA2 was seen in cells treated for up to 60 seconds with LTB4 (Figure 7A). The possibility that lyn directly catalyzes the tyrosine phosphorylation and activation of cPLA2 was analyzed by immunoprecipitating the total phosphotyrosine protein fraction using the antibody 4G10 and probing for cPLA2 by Western blotting. However, although LTB4 promoted the tyrosine phosphorylation of lyn kinase (Figure 7C), cPLA2 was unaffected (Figure 7B). The role of cPLA2 in [3H]AA release was also assessed pharmacologically by making use of the selective cPLA2inhibitor methyl arachidonyl fluorophosphonate (MAPF).20Consistently with our earlier results, MAPF failed to attenuate Ca++-dependent [3H]AA release (Figure 7D), implying that cPLA2 is not central to this LTB4-induced response.
Role of cPLA2 in LTB4-induced Ca++-dependent [3H]AA release.
Cells were preincubated for 5 minutes at 37°C and then stimulated for the indicated time with 100 nmol/L LTB4. Panels A-C show representative blots obtained from 3 separate experiments. (A) Whole-cell lysates were probed, by Western blotting, with antibodies to cPLA2. (B and C) Samples immunoprecipitated with the antiphosphotyrosine antibody 4G10 were then probed, by Western blotting, with antibodies to cPLA2 (B) or lyn (C). (D) cells were preincubated in the presence (°) or absence (l) of 1 mmol/L Ca++/1 mmol/L Mg2+ and the indicated concentrations of MAPF. Following stimulation with LTB4 (100 nmol/L), the release of [3H]AA at 60 seconds was determined. Data represent the mean ± SEM of 3 to 4 independent experiments.
Role of cPLA2 in LTB4-induced Ca++-dependent [3H]AA release.
Cells were preincubated for 5 minutes at 37°C and then stimulated for the indicated time with 100 nmol/L LTB4. Panels A-C show representative blots obtained from 3 separate experiments. (A) Whole-cell lysates were probed, by Western blotting, with antibodies to cPLA2. (B and C) Samples immunoprecipitated with the antiphosphotyrosine antibody 4G10 were then probed, by Western blotting, with antibodies to cPLA2 (B) or lyn (C). (D) cells were preincubated in the presence (°) or absence (l) of 1 mmol/L Ca++/1 mmol/L Mg2+ and the indicated concentrations of MAPF. Following stimulation with LTB4 (100 nmol/L), the release of [3H]AA at 60 seconds was determined. Data represent the mean ± SEM of 3 to 4 independent experiments.
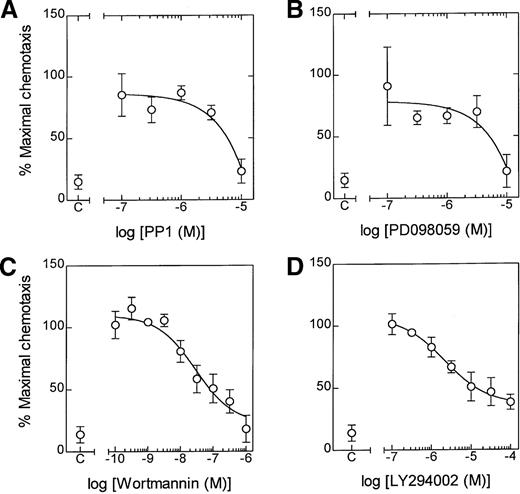
The role of lyn kinase in LTB4-induced NADPH oxidase activation was measured by lucigenin-enhanced chemiluminescence. Preincubation of eosinophils with PP1 (3 μmol/L and 10 μmol/L) for 5 minutes significantly inhibited LTB4-induced superoxide production (Figure 6B). However, in agreement with the [3H]AA release results, the effect of a maximally effective concentration of PP1 was partial, amounting to 55% inhibition at 10 μmol/L, and was unaffected when the preincubation was increased to 60 minutes (data not shown). In contrast, PP1 abolished LTB4-induced chemotaxis at the highest concentration studied (10 μmol/L), suggesting a central role for lyn kinase in the genesis of this response (Figure8A).
Effect of PP1, PD098059, wortmannin, and LY294002 on LTB4-induced chemotaxis.
Cells were preincubated for 5 minutes at 37°C in the presence of the indicated concentration of (A) PP1, (B) PD098059, (C) wortmannin, and (D) LY294002. Following stimulation of eosinophils with LTB4 (100 nmol/L), chemotaxis was determined at 3 hours. The effect of the inhibitor is expressed as a percentage of the maximal response. Data represent the mean ± SEM of 3 to 6 independent experiments.
Effect of PP1, PD098059, wortmannin, and LY294002 on LTB4-induced chemotaxis.
Cells were preincubated for 5 minutes at 37°C in the presence of the indicated concentration of (A) PP1, (B) PD098059, (C) wortmannin, and (D) LY294002. Following stimulation of eosinophils with LTB4 (100 nmol/L), chemotaxis was determined at 3 hours. The effect of the inhibitor is expressed as a percentage of the maximal response. Data represent the mean ± SEM of 3 to 6 independent experiments.
Since PP1 inhibits the phosphorylation of ERK-2, it is conceivable that the action of lyn kinase is mediated through the ras/raf/MEK/ERK protein kinase cascade. This hypothesis was strengthened by the finding that the selective MEK-1 inhibitor, PD 098059, abolished LTB4-induced chemotaxis (Figure 8B) at a concentration (10 μmol/L) that we have previously shown attenuates ERK phosphorylation.7 In addition, eosinophil chemotaxis appeared to require activation of PtdIns 3-kinase. Thus, the PI-3 kinase inhibitors, wortmannin (EC50 = 28 nmol/L) and LY294002 (EC50 = 2.1 μmol/L), caused a concentration-dependent attenuation of cell migration although they failed to abolish this response (Figure 8C and D).
Discussion
In the present study, the results of experiments designed to assess the role of src-related tyrosine kinases in the mechanism of LTB4-induced [3H]AA release, superoxide generation, and chemotaxis in guinea-pig peritoneal eosinophils are described. Initial studies reproducibly detected p53lyn, p56lyn, p56hck, p59hck, p55fgr, and p56lck but not p60src, p62yes, p55blk, or p59fyn. This expression profile is similar to that found in human neutrophils, which express lyn, hck, and fgr but not lck.21Immunoprecipitation of these protein tyrosine kinases from LTB4-stimulated eosinophils and an assessment of their ability to phosphorylate a peptide substrate, cdc2(6-20)-NH2, showed that only lyn was activated by LTB4.
Temporally, the time course of p53/p56lyn activation paralleled [3H]AA release and preceded superoxide generation and chemotaxis, suggesting that a causal relationship might exist between the biochemical and the functional responses.6,7 To test this hypothesis, the effect of a selective inhibitor of the src-related family of tyrosine kinases, PP1, was evaluated.18 In eosinophil lysates, PP1 potently inhibited basal (IC50 ∼ 300 nmol/L) and LTB4-stimulated (IC50 ∼ 20 nmol/L) lyn kinase activation at concentrations comparable to those reported previously (IC50 = 10 nmol/L) and for other src-related tyrosine kinases, including lck (IC50 = 95 nmol/L), fyn (IC50 = 6 nmol/L), src (IC50 = 170 nmol/L), and hck (IC50 = 20 nmol/L).18However, studies using intact cells showed that 50 to 100 times greater PP1 concentrations (1 μmol/L to 10 μmol/L) were required to suppress LTB4-mediated lyn kinase than those found necessary in a cell-free system. This potency of PP1 in intact eosinophils is comparable to that required to attenuate src-mediated responses in other cell types, including IgG- and IgA-induced adapter protein phosphorylation and respiratory burst in the U937 monocyte/macrophage derived cell line,22,23 the inhibition of CD3-induced lymphocyte proliferation,18 FcεRI- and Thy-1-mediated activation of rat basophilic leukemia cells,24 and 1,25-dihydroxyvitamin D stimulation of phospholipase C-γ.25 It is highly likely that the greater potency of PP1 against isolated lyn kinase relates to its mechanism of action. Indeed, it is thought that PP1 inhibits lyn kinase activity by acting at the ATP-binding site on the enzyme.18Thus, the IC50 of PP1 will be determined by the concentration of ATP in the vicinity of the enzyme, which was considerably less in the cell-free enzyme assay (25 μmol/L) than is present in intact cells (1 mmol/L to 10 mmol/L). This contention is supported by our demonstration that higher concentrations (10 μmol/L) of PP1 were required to inhibit LTB4-induced ERK phosphorylation in intact cells, which in previous investigations has been shown to be mediated by members of the src kinase family.11-13
PP1 selectively inhibited Ca++-dependent [3H]AA release by a mechanism that we have previously shown6 to be suppressed by mepacrine, implying that AA generation is mediated by an isoform of PLA2. It is now recognized that PLA2 is a generic term that refers to an expanding number of proteins that can be divided into specific families. In hemopoietic cells, 4 types of PLA2 have been identified, the intracellular isoforms cPLA2 and iPLA2 and the secreted isoforms sPLA2-IIA and sPLA-V. Previous studies have suggested that the major catalyst of cellular AA release is cPLA2, which translocates to the membrane fraction in response to increases in intracellular [Ca++]c whereupon it is activated by phosphorylation.19 However, experiments presented in this study suggest that cPLA2 is not central toLTB4-induced Ca++-dependent [3H]AA release; this suggestion is based on the observations that phospho-cPLA2 was not detected at any time point studied and MAPF, a selective inhibitor of cPLA2, was inactive. The enzyme(s) that catalyzes Ca++-dependent [3H]AA release is presently under investigation although the Ca++-sensitivity of the response suggests that it is probably mediated by either sPLA2-II or sPLA2-V or by both of these. This contention is supported by studies in human eosinophils in which sPLA2-II has been identified in specific granules and shown to be expressed at levels 20 to 100 times higher than in other leukocytes, such as neutrophils, basophils, monocytes, and lymphocytes.26 The idea that Ca++-dependent [3H]AA release is mediated by the extracellular release of sPLA2 would suggest that the action of PP1 occurs through the inhibition of degranulation. Indeed, degranulation of human eosinophils in response to IgG and secretory IgA has an absolute requirement for an increase in the [Ca++]cand tyrosine phosphorylation, possibly mediated by the src kinase, fgr.27 28 However, our inability to demonstrate an effect of PP1 upon LTB4-induced Ca++ mobilization suggests that lyn kinase mediates degranulation by phosphorylating and thereby activating an as-yet-unidentified protein or proteins.
PP1 also attenuated LTB4-induced superoxide anion generation in guinea-pig eosinophils, implicating lyn kinase in the activation of the NADPH oxidase. A similar conclusion has been derived from studies with fMLP-stimulated (single-letter amino acid code) human neutrophils. Thus, oxidant production in this cell is attenuated by the PI 3-kinase inhibitors, wortmannin,29 and LY29400230 whose activation, in turn, is thought to be mediated predominantly via lyn kinase. However, we have reported previously that wortmannin does not affect LTB4-induced activation of the NADPH oxidase in guinea-pig eosinophils, suggesting that the action of lyn is not mediated via PI 3-kinase.5 In fact, since AA release per se is implicated in oxidant generation,6 the ability of PP1 to inhibit the NADPH oxidase could be envisioned as secondary to attenuating Ca++-dependent [3H]AA release.
An examination of the action of PP1 upon chemotaxis indicated a central role for src-like protein tyrosine kinases. However, since LTB4-induced activation was monitored for only 60 seconds whereas chemotaxis was measured at 3 hours, it is not possible to definitely link the functional and biochemical responses in these studies. Nevertheless, neutrophils obtained from knockout mice deficient in hck and fgr fail to migrate in response to fMLP, unlike wild-type animals, indicating that src protein tyrosine kinases play a central role in chemotaxis.31Interestingly, unlike [3H]AA release and oxidant production,7 LTB4-induced chemotaxis was attenuated by the MEK-1 inhibitor PD 098059, implicating ERK-1 and/or ERK-2 in this response. Overall, these finding are in agreement with a recent study in human eosinophils where PD 098059 attenuated eotaxin-induced chemotaxis.32 In contrast, neutrophil migration induced by a range of agonists that act through G-protein–coupled receptors, including fMLP, PAF, and IL-8, is unaffected by PD 098059,31-33 which implies that significant differences may exist in the intracellular pathways mediating neutrophil and eosinophil chemotaxis. Although it is often difficult to interpret data obtained by densitometric quantification of immunoblots, we have demonstrated greater than 80% attenuation of ERK phosphorylation following eosinophil exposure to 10 μmol/L PP1. Since previous studies have shown that PD 098959 (10 μmol/L) was similarly unable to produce total inhibition of ERK activity at concentrations that, like PP1, abolish chemotaxis,7 this suggests that only partial inhibition of ERK activation is required for total attenuation of certain functional responses in eosinophils. However, attenuation of chemotaxis by wortmannin and LY294002 also suggested a role for PtdIns 3-kinase in eosinophil migration, although whether this lipid kinase is activated by lyn kinase, which has been reported in chemoattractant-stimulated neutrophils,9 or via an as-yet-undetermined mechanism, is unknown.
Although PP1 inhibited LTB4-induced [3H]AA release, superoxide anion generation, and chemotaxis, the magnitude of the effect varied. Thus, a concentration of PP1 that abolished chemotaxis suppressed Ca++-dependent [3H]AA release and oxidant production by only 50% to 60%. Since the latter 2 responses are completely inhibited by the nonselective tyrosine kinase inhibitor lavendustin A,7 the involvement of additional, as-yet-unidentified, tyrosine kinase(s) in these responses is suggested.
Taken together, the results of these studies demonstrate a role for lyn kinase in LTB4-induced induced AA release, NADPH oxidase activation, and chemotaxis. Moreover, the demonstration that chemotaxis, but not oxidant production and AA generation, is mediated via the ras/raf/MEK/ERK protein kinase cascade implies that these lyn kinase-mediated responses occur through divergent signaling pathways.
Supported by grants awarded by the Wellcome Trust (Grant 056814), GlaxoWellcome Research and Development, the British Lung Foundation, and the Trent Health Authority.
Reprints:Mark A. Lindsay, Thoracic Medicine, Imperial College of School of Medicine at the National Heart and Lung Institute, Dovehouse St, London SW3 6LY, England.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 5. Identification of a Ca++-dependent and Ca++-independent pathway of [3H]AA release. / Cells were preincubated for 5 minutes at 37°C in the presence (○) or absence (•) of 1 mmol/L Ca++ and 1 mmol/L Mg++. Cells were then stimulated with the indicated concentration of LTB4. Data represent the mean ± SEM of 3 to 4 independent experiments and show (A) the maximal increase in [Ca++]c and (B) the release of [3H]AA release at 60 seconds, where an asterisk indicates that the stimulation of the LTB4-induced Ca++-independent release was significant (P < .05) compared to controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3541/6/m_bloo01111005x.jpeg?Expires=1767775157&Signature=TT378G40n4Q-hHw7OXiCty5BCU8YQUZ3rh8097NrPA04P0Q6OcQYvirLB6nF2wbK8fYR1nD3stg64CPMbGDK1pGEN53CY51TYZQtQwNdPozze2MtXHuFofmkxfVi4CSvNl6pOTlEuxfxdwB~TVICFNzIsOqYjoCRj1SRxOyN1py~ksI7WpDLXQz0VwYXLwRDr5Bq8usivwbZnha4XqaXGuvaodD1hXe0GZWuBqrqJS1-gPehV6vCo13eylWkA2Pe0kmYMjdn8DGfyOlm-6xc5o3JU9qlYsicYldslmcbSvIwD0jHSNXP2ak~6f4~p7JG~w-0O9eDk1uIF3dJYegQoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Effect of PP1 on LTB4-induced [3H]AA release and superoxide generation. / Cells were preincubated for 5 minutes at 37°C in the presence (○) or absence (•) of 1 mmol/L Ca++/1 mmol/L Mg2+ and the indicated concentrations of PP1. Data represent the mean ± SEM of 3 to 4 independent experiments. (A) Following stimulation with LTB4 (100 nmol/L), the release of [3H]AA at 60 seconds was determined. (B) Following stimulation with LTB4(100 nmol/L), the maximal rate of superoxide generation measured as relative light units (RLU) was determined. An asterisk indicates significant inhibition of LTB4-induced response by the indicated concentration of inhibitor compared with controls. P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3541/6/m_bloo01111006x.jpeg?Expires=1767775157&Signature=rGQtsDuTwOE4J9N9aqbdUGFNvLe2GmmdwMKvjrcoug3EeUixbUfZhQuQhXTGPGXwEBrN33Mz-pC3bzaaNmLNFfXa0vzMdgsLExQTDqrDTLmRDAcojChclzS28C8yUsyGYbLkINeJPTiY9RXqndanJs0QVsKrHCimOP8SgulNY3FKLCzH4KW06gsIfe6iGZj2b-NDlvcQZq~G9Nl0OB8nZQUGS~IC5XtxDx-jZLMx8-tVcPQIMdHRn86f7uaf2mMQFXixHpy9Q4p-QvSynVgYx6RyU5p1-W7NHXWc9YbeAFgjEVYP6QpHc3j1qZNbR2UqiRnkP7fw3cT2FeMMDtRWLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Role of cPLA2 in LTB4-induced Ca++-dependent [3H]AA release. / Cells were preincubated for 5 minutes at 37°C and then stimulated for the indicated time with 100 nmol/L LTB4. Panels A-C show representative blots obtained from 3 separate experiments. (A) Whole-cell lysates were probed, by Western blotting, with antibodies to cPLA2. (B and C) Samples immunoprecipitated with the antiphosphotyrosine antibody 4G10 were then probed, by Western blotting, with antibodies to cPLA2 (B) or lyn (C). (D) cells were preincubated in the presence (°) or absence (l) of 1 mmol/L Ca++/1 mmol/L Mg2+ and the indicated concentrations of MAPF. Following stimulation with LTB4 (100 nmol/L), the release of [3H]AA at 60 seconds was determined. Data represent the mean ± SEM of 3 to 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3541/6/m_bloo01111007w.jpeg?Expires=1767775157&Signature=UwN2PODML5XTXSnBeqwKegELG0gHfTg1hXkHnH7qJof5gLlGiJfz3uG~mr3wl~AjtiRCwa8g1LcKheH9eLQHtLrP5okreZU7VUDIzlRN~brG~Zw4WwPZtiBdF4bDAjUvJwkCiJOkTce~C5tl940RwdpfZ2ZIV9PHkDGb7VoYwhB6M6gKWVIaulWR3sRa0sE-70Q501xsJVdfOMwvcZI-CIqIHqURG0D89Tr7E8C2tyN6dfhHV9vAITECkglIvRYh28E5ui95r2KlnLfXnEXQpTo6xxmVtYgK-WQ7uEn5G0HnjMli-k4Ec4VoLualIgS1LN~Oup8ebJdRiJQxIWDV3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal