Abstract
Hydroxyurea (HU) is an effective therapeutic agent for patients with myeloproliferative disorders (MPDs) or sickle cell disease (SCD). Short-term HU toxicities primarily include transient myelosuppression, but long-term HU risks have not been defined. The mutagenic and carcinogenic potential of HU is not established, although HU has been associated with an increased risk of leukemia in some patients with MPD. In this study, 2 assays were used to quantitate acquired somatic DNA mutations in peripheral blood mononuclear cells (PBMCs) after in vivo HU exposure. The HPRT assay measures hypoxanthine phosphoribosyl transferase (hprt) mutations, while the VDJ assay identifies “illegitimate” T-cell receptor Vγ-Jβ interlocus recombination events. PBMCs were analyzed from patients with MPD, adults and children with SCD, and normal controls. MPD patients with prolonged HU exposure had numbers of DNA mutations equivalent to patients with low HU exposure or controls. Similarly, adults with SCD had equivalent numbers of DNA mutations regardless of HU exposure. Children with SCD and 30-month HU exposure had equivalenthprt− mutations but significantly more VDJ mutations (1.82 ± 1.20 events per μg DNA) than children with 7-month HU exposure (1.58 ± 0.87 events) or no HU exposure (1.06 ± 0.45 events), P = .04 by analysis of variance. Taken together, these data suggest that the mutagenic and carcinogenic potential of in vivo HU therapy is low. Although increased numbers of illegitimate VDJ recombination events do not directly portend leukemia, young patients with SCD and HU exposure should be monitored serially for increases in DNA mutations.
Hydroxyurea (HU) is a relatively simple chemical compound, NH2CONHOH, that is an effective chemotherapeutic agent for a variety of hematological diseases. HU is commonly used for patients with clonal myeloproliferative disorders (MPDs), such as polycythemia vera, essential thrombocythemia, and chronic myelogenous leukemia,1,2 as well as for malignant solid tumors.3,4 HU inhibits ribonucleoside diphosphate reductase,5,6 the enzyme that converts ribonucleotides into deoxyribonucleotides (dNTPs), which are the building blocks for DNA synthesis and repair. By depleting intracellular dNTP pools, HU acts as a cytotoxic and anti-neoplastic S-phase–specific agent that inhibits DNA synthesis with less effect on RNA and protein synthesis.7 The most common short-term HU toxicity is transient and reversible myelosuppression, especially of granulocytes.8
Oral hydroxyurea is also a prototypic therapeutic agent for the treatment of patients with sickle cell disease (SCD), since it increases the amount of fetal hemoglobin within circulating erythrocytes.9-11 The clinical efficacy of HU in adults with sickle cell anemia was proven in a randomized placebo-controlled trial.12 A recent phase I/II trial in school-aged patients concluded that short-term HU therapy is safe in children and causes similar hematological changes as in adults with SCD.13 Preliminary data from a pilot trial of HU therapy for infants with SCD are also encouraging.14 HU may soon become a standard treatment option for SCD and could be offered as a lifelong therapeutic agent even for young children with SCD.
Although short-term HU toxicities are typically well tolerated, the risks associated with long-term HU therapy are unclear. Specifically, the risk of developing leukemia or other malignancies following HU exposure has not been determined. Hydroxyurea has been experimentally shown to have clastogenic,15,16teratogenic,17,18 and, in some settings, mutagenic effects,19 but its potential as a carcinogen at therapeutic doses has not been established. Several reports have described patients with MPDs, which are known preleukemic conditions, who underwent leukemic transformation on HU therapy.20-25 Larger studies have documented an increased risk of leukemia for patients with MPD on HU therapy.26-29 In other clinical settings, these long-term risks of HU therapy have not been reported. Sixty-four patients with congenital heart disease treated with HU (mean, 5.6 years) had no cases of secondary malignancy.30 Similarly, no cases of malignancy have occurred in adults with SCD enrolled in the Multicenter Study of Hydroxyurea trial.31 However, higher cumulative doses of HU, larger numbers of patients, and a younger population could lead to an increased cancer risk. It is important to investigate the carcinogenic risks of HU at this time, before it becomes widely used for young patients with SCD.
To quantify the long-term mutagenic effects of HU therapy, we measured acquired DNA mutations associated with in vivo HU exposure. We used 2 established in vitro assays that quantitate acquired somatic DNA mutations: the HPRT assay measures the frequency of mutations at the selectable hypoxanthine phosphoribosyl transferase (hprt) gene locus,32,33 while the VDJ assay detects “illegitimate” interlocus recombination events between the T-cell receptor Vγ and Jβ gene loci.34 35 In vivo HU exposure was associated with significantly higher numbers of VDJ events in children with SCD, although the values remained within the normal range. These results suggest that the mutagenic potential of in vivo HU exposure is low, but long-term serial studies should be performed to determine the leukemic potential of HU therapy for young patients with SCD.
Patients and methods
Patient population
A total of 68 patients with sickle cell anemia (30 adults, 38 children), 27 patients with MPD (23 with polycythemia vera, 4 with essential thrombocythemia), and 32 healthy adult controls were studied. No patient had evidence of clonal lymphocyte expansion at the time of analysis. Approximately one third (32%) of the patients with sickle cell anemia were female, as compared with 30% of those with MPD and 47% of the controls. A 9-year-old African-American female with ataxia telangiectasia, a premalignant condition with known genetic instability, served as a positive control for the HPRT and VDJ assays.36
Isolation and purification of peripheral blood mononuclear cells
Peripheral venous blood was collected from the patients and normal controls in accordance with a protocol approved by the Institutional Review Boards at Duke University Medical Center, the Durham Veterans Administration Hospital, and Mt. Sinai Medical Center. The peripheral blood mononuclear cell (PBMC) fraction was isolated by density centrifugation as previously described.37 All cell numbers were determined with the use of a hemacytometer. Genomic DNA was purified from PBMCs by means of a standard kit (Gentra, Research Triangle Park, NC) according to the manufacturer's recommendations.
HPRT mutation assay
Acquired somatic mutations at the hprt gene locus were identified by negative selection, ashprt− T-cell mutants can be grown in the presence of 6-thioguanine (6-TG). The HPRT assay was performed as originally reported32,33 with only minor modifications.36 Between 20 and 30 × 106 PBMCs were incubated at 2 × 106 cells/mL in RPMI 1640 (Gibco BRL, Grand Island, NY) supplemented with 20% HL-1 nutrient medium (Biowhittaker, Walkersville, MD), 1.0 μg/mL phytohemagglutinin (PHA) (Murex, Dartford, England), and 5% fetal bovine serum (FBS) (Gibco) in T-25 culture flasks (Costar, Cambridge, MA) at 37°C under 5% CO2. After overnight (20 hours) mitogenic stimulation, the cells were centrifuged, washed twice, and counted. Cells were then plated in 96-well round-bottom microtiter plates (Costar) at 1 or 2 cells per well (2 plates at each concentration) in nonselection growth medium and 2 × 104 cells per well (6 plates) in selection medium. Nonselection growth medium consisted of RPMI 1640 supplemented with 20% HL-1, 5% FBS, 0.125 μg/mL PHA, T-stim culture supplement (Collaborative Biomedical Products, Bedford, MA) as a source of interleukin-2 (10 U/mL), and irradiated feeder cells (104/well). Selection growth medium included the addition of 5 μmol/L 6-TG (Sigma, St Louis, MO). Feeder cells were a mycoplasma-free hprt− derivative of WI-L2 lymphoblastoid cells designated TK-6, the kind gift of R. J. Albertini (University of Vermont), grown in RPMI with 10% FBS and exposed to 9000 cGy137Cs γ-irradiation.
Determination of cloning efficiency and hprt−mutant frequency
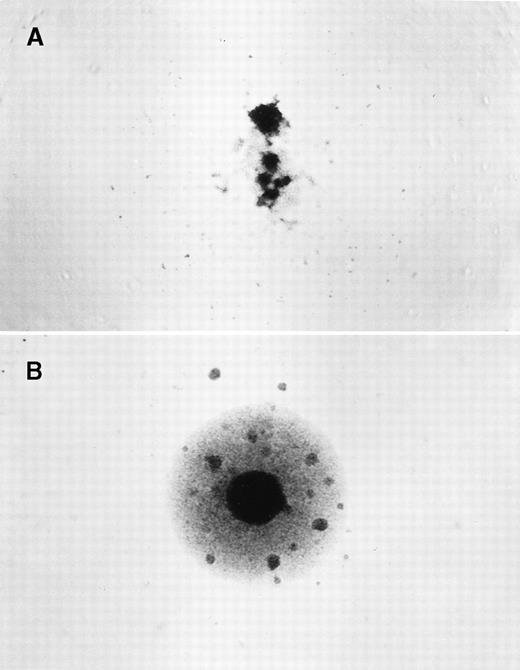
After 14 to 16 days of incubation, plates were scored for colony growth with the use of an inverted phase contrast microscope (Figure1). The cloning efficiency (CE) was calculated by means of the Poisson relationship, CE = (−lnPo)/x, in whichPo = the fraction of negative wells andx = the average number of cells per well.32 33 A mean CE was determined from the CE of the nonselection plates and then used to calculate the mutant frequency (Mf), defined as the ratio of the CE in the presence and absence of 6-TG.
Identification of hprt−T-lymphocyte clones.
PBMCs were isolated and cloned at 1 to 2 × 104cells/well in the presence of 6-thioguanine (6-TG). Individual wells were scored after 14 to 16 days. (A) A negative clone with no growth in the presence of 6-TG, and only residual feeder cells observed. (B) A positive hprt− mutant clone that grew in the presence of 6-TG, with clusters of growing cells.
Identification of hprt−T-lymphocyte clones.
PBMCs were isolated and cloned at 1 to 2 × 104cells/well in the presence of 6-thioguanine (6-TG). Individual wells were scored after 14 to 16 days. (A) A negative clone with no growth in the presence of 6-TG, and only residual feeder cells observed. (B) A positive hprt− mutant clone that grew in the presence of 6-TG, with clusters of growing cells.
Analysis of “illegitimate” interlocus recombination events
The measurement of interlocus VDJ recombination events was performed as originally described35,38 with only minor modifications,36 by means of a 2-step nested polymerase chain reaction (PCR) protocol to amplify rare rearrangements between T-cell receptor (TCR) Vγ and Jβ1 segments. In the first round of the PCR, 0.1 μg of the outer 5′ Vγ and 3′ Jβ1 primers were added to serial dilutions of PBMC DNA. The template and primers were heated for 3 minutes at 94°C and then diluted in a 50 μL reaction containing 200 μmol/L dNTPs (Gibco), 1.5 mmol/L MgCl2, 20 mmol/L Tris (pH 8.4), 50 mmol/L KCl, and 2.5 U Taqpolymerase (Gibco). This reaction was heated at 94°C for 1 minute followed by 25 cycles of 30 seconds at 95°C, 30 seconds at 50°C, and 6 minutes at 72°C, followed by a 10 minute extension at 72°C after the last cycle. In the second round of the PCR, a 5 μL aliquot of the first reaction was preheated with 0.5 μg of each nested primer, Vγb and Jβ1b, as described.35 38 The Jβ1b primer was end-labeled with γ-[32P]dATP with the use of a forward T4 Kinase (Gibco) reaction. The mixture was then diluted into a 50 μL reaction identical to that outlined above, and the same thermal cycle sequence was followed. PCR products were resolved on a 6% polyacrylamide gel and exposed at −70°C overnight to x-ray film (Kodak X-omat) (Kodak, Rochester, NY).
Statistical analysis
Descriptive statistics were performed with the use of Primer of Biostatistics (McGraw-Hill, New York, NY). Comparison of means between 2 groups used the Student t test, while comparison among several groups used analysis of variance (ANOVA) (Statview, SAS Institute, Cary NC). Comparison of values for patients at 2 different timepoints was also performed by Wilcoxon signed rank sum (Statview).
Results
Characteristics of patients with in vivo hydroxyurea exposure
The 27 patients with MPD had an average age (mean ± 1 SD) of 59 ± 16 years and a range of 19 to 87 years. These MPD patients included 15 with a low exposure to HU (median, 0 months; range, 0 to 21 months) and 12 with prolonged HU exposure (median, 11 years; range, 4 to 18 years). PBMCs were also analyzed from 30 adult patients with SCD (mean age 28 ± 10 years), including 15 with short (median, 24 months) HU exposure and 15 age- and disease-matched adult patients with no HU exposure. A total of 38 children with SCD were tested, consisting of 21 with no HU exposure and 17 who were tested serially after a short HU exposure (median, 7 months) and then approximately 2 years later after a longer HU exposure (median, 30 months). A total of 32 normal adult controls were studied with an average age of 43 ± 15 years and a range of 22 to 87 years.
Quantitation of acquired somatic DNA mutations
Table 1 summarizes the results of the HPRT and VDJ assays for patients with in vivo HU exposure and controls. The mean T-lymphocyte cloning efficiency (CE) in the HPRT assay was similar for patients with MPD (12.5 ± 8.5%) and for normal adult controls (16.0 ± 8.7%, P = not significant [NS]). Similarly, there were no statistically significant differences in the CE among adults with SCD (13.8 ± 10.3%), children with SCD (16.2 ± 8.8%), and adult controls.
Quantitation of acquired DNA mutations in association with in vivo hydroxyurea exposure
| Patient population . | No.patients . | Mean age (years) . | Median HU exposure . | HPRT cloning efficiency (%) . | HPRT mutational frequency (×10−6) . | VDJ events (per μg DNA) . |
|---|---|---|---|---|---|---|
| Adults with MPD | 27 | |||||
| Low HU exposure | 15 | 57 ± 17 | 0 months | 12.8 ± 8.9 | 37.3 ± 37.6 | 1.06 ± 0.73 |
| Prolonged HU exposure | 12 | 62 ± 16 | 11 years | 12.2 ± 8.4 | 41.1 ± 29.3 | 0.64 ± 0.29 |
| Adults with SCD | 30 | |||||
| No HU exposure | 15 | 27 ± 12 | 0 months | 15.1 ± 12.3 | 19.1 ± 19.1 | 1.07 ± 0.38 |
| Short HU exposure | 15 | 29 ± 9 | 24 months | 12.4 ± 8.2 | 16.7 ± 10.9 | 1.14 ± 0.38 |
| Children with SCD | 38 | |||||
| No HU exposure | 21 | 11 ± 3 | 0 months | 14.9 ± 8.3 | 11.5 ± 18.7 | 1.06 ± 0.45 |
| With HU exposure | 17 | |||||
| After short HU exposure | 11 ± 3 | 7 months | 13.2 ± 6.1 | 11.2 ± 6.7 | 1.58 ± 0.87 | |
| After longer HU exposure | 13 ± 3 | 30 months | 20.9 ± 10.2 | 9.2 ± 7.8 | 1.82 ± 1.20 | |
| Normal controls | 32 | 43 ± 15 | 0 months | 16.0 ± 8.7 | 25.8 ± 24.8 | 1.04 ± 0.38 |
| Ataxia telangiectasia | 1 | 9 | 0 months | 2.3 | 240.7 | 12.99 |
| Patient population . | No.patients . | Mean age (years) . | Median HU exposure . | HPRT cloning efficiency (%) . | HPRT mutational frequency (×10−6) . | VDJ events (per μg DNA) . |
|---|---|---|---|---|---|---|
| Adults with MPD | 27 | |||||
| Low HU exposure | 15 | 57 ± 17 | 0 months | 12.8 ± 8.9 | 37.3 ± 37.6 | 1.06 ± 0.73 |
| Prolonged HU exposure | 12 | 62 ± 16 | 11 years | 12.2 ± 8.4 | 41.1 ± 29.3 | 0.64 ± 0.29 |
| Adults with SCD | 30 | |||||
| No HU exposure | 15 | 27 ± 12 | 0 months | 15.1 ± 12.3 | 19.1 ± 19.1 | 1.07 ± 0.38 |
| Short HU exposure | 15 | 29 ± 9 | 24 months | 12.4 ± 8.2 | 16.7 ± 10.9 | 1.14 ± 0.38 |
| Children with SCD | 38 | |||||
| No HU exposure | 21 | 11 ± 3 | 0 months | 14.9 ± 8.3 | 11.5 ± 18.7 | 1.06 ± 0.45 |
| With HU exposure | 17 | |||||
| After short HU exposure | 11 ± 3 | 7 months | 13.2 ± 6.1 | 11.2 ± 6.7 | 1.58 ± 0.87 | |
| After longer HU exposure | 13 ± 3 | 30 months | 20.9 ± 10.2 | 9.2 ± 7.8 | 1.82 ± 1.20 | |
| Normal controls | 32 | 43 ± 15 | 0 months | 16.0 ± 8.7 | 25.8 ± 24.8 | 1.04 ± 0.38 |
| Ataxia telangiectasia | 1 | 9 | 0 months | 2.3 | 240.7 | 12.99 |
HU indicates hydroxyurea; MPD, myeloproliferative disorder; SCD, sickle cell disease; VDJ events, T-cell receptor V-J interlocus recombination events.
Note: Patient and control PBMCs were tested for acquired DNA mutations in both the HPRT and VDJ assays. The adults with MPD or SCD had no significant differences in hprt cloning efficiency, mutational frequency, or VDJ recombination events, according to HU exposure. Children with SCD and HU exposure had a trend for more VDJ events compared with those with no HU exposure, P = .04 by ANOVA (with no adjustment for multiple comparisons). The child with ataxia telangiectasia had increased numbers of acquired DNA mutations in both assays.36
The calculated frequency of hprt− mutants (Mf) for patients with MPD was not statistically different for those with prolonged HU exposure (41.1 ± 29.3 × 10−6), compared with those with low HU exposure (37.3 ± 37.6 × 10−6) and normal controls (25.8 ± 24.8 × 10−6),P = NS by ANOVA and t tests (Table 1). The averagehprt− Mf for adults with SCD and short HU exposure was similar to those with no HU exposure and controls, P = NS by ANOVA and t tests (Table 1). For children with SCD, the average hprt−Mf for patients with no HU exposure (11.5 ± 18.7 × 10−6) was equivalent to those with short HU exposure (11.2 ± 6.7 × 10−6) or longer HU exposure (9.2 ± 7.8 × 10−6),P = NS. The child with ataxia telangiectasia had an elevatedhprt− Mf of 240.7 × 10−6 as previously described.36
The number of illegitimate VDJ recombination events was lower for MPD patients with prolonged HU exposure (0.64 ± 0.29 events per μg of DNA), compared with those with low HU exposure 1.06 ± 0.73,P = .085 or normal controls (1.04 ± 0.38,P = .003). Figure 2A illustrates a representative gel from a VDJ interlocus assay using PBMCs from patients with MPD and normal controls. Adults with SCD had no significant differences in the number of VDJ events according to HU exposure. However, children with SCD and no HU exposure had a significantly lower number of VDJ mutations (1.06 ± 0.45 events per μg DNA) compared with those with short HU exposure (1.58 ± 0.87 events, P = .036 by t test) or longer HU exposure (1.82 ± 1.20 events, P = .017 byt test), P = .04 by ANOVA (with no adjustment for multiple comparisons). Figure 2B shows a representative VDJ gel for adults and children with SCD. The child with ataxia telangiectasia had 12.99 events per μg of DNA as previously described.36
VDJ recombination events in patients with in vivo hydroxyurea exposure.
A 2-step nested PCR protocol amplifies rare recombination events between TCRγ variable and TCRβ junctional genes on chromosome 7. Dilutions of PBMC DNA ranging from 2.0 to 0.3 μg are listed for each sample. (A) Results for 2 patients with MPD and 2 normal controls. There are positive bands at 2.0 μg DNA for all 4 samples and at 1.0 μg DNA for 1 control. (B) Results for 4 patients with SCD, including 2 adults and serial samples from a child. The gel shows bands at 2.0 and 1.0 μg DNA for both adult patients. The child had a positive signal at 2.0 μg DNA after short-term HU therapy (7 months), but 2 years later had bands at 2.0 and 1.0 μg DNA. (−) is a negative control and (+) is a positive control.
VDJ recombination events in patients with in vivo hydroxyurea exposure.
A 2-step nested PCR protocol amplifies rare recombination events between TCRγ variable and TCRβ junctional genes on chromosome 7. Dilutions of PBMC DNA ranging from 2.0 to 0.3 μg are listed for each sample. (A) Results for 2 patients with MPD and 2 normal controls. There are positive bands at 2.0 μg DNA for all 4 samples and at 1.0 μg DNA for 1 control. (B) Results for 4 patients with SCD, including 2 adults and serial samples from a child. The gel shows bands at 2.0 and 1.0 μg DNA for both adult patients. The child had a positive signal at 2.0 μg DNA after short-term HU therapy (7 months), but 2 years later had bands at 2.0 and 1.0 μg DNA. (−) is a negative control and (+) is a positive control.
Discussion
The striking clinical efficacy of HU therapy, coupled with its modest toxicity profile and ease of oral administration, makes HU an attractive therapeutic option for patients with MPD and SCD. The mutagenic and carcinogenic potential of HU, however, is a serious risk associated with long-term therapy. Since HU is a potent inhibitor of ribonucleotidase reductase (RR), which reduces intracellular dNTP pools, HU interferes in vitro not only with DNA synthesis but also with DNA repair mechanisms.39 In vitro, DNA damage that develops either spontaneously or from environmental mutagens cannot be fully repaired in the presence of HU, leading to the accumulation of somatic mutations and chromosomal damage.40 These laboratory observations provide a plausible biochemical mechanism by which in vivo HU therapy could lead to acquired somatic DNA mutations and eventual carcinogenesis. The inhibitory effect of HU on RR reduces dNTP levels and impairs DNA repair mechanisms, which leads to an accumulation of acquired DNA mutations and eventual leukemic transformation.
Somatic mutations are acquired randomly throughout the genome from unrepaired errors of replication and the effects of mutagenic stimuli, but are difficult to quantitate owing to their rarity. Laboratory assays that identify gross chromosomal breaks or sister chromatid exchanges are relatively insensitive, since they require a large amount of DNA damage to be detected. More sensitive and accurate assays for acquired DNA mutations identify individual cells with acquired mutations at a single locus, such as hprt,41 glycophorin A,42 or the T-cell receptor.35,43 The HPRT assay is a sensitive biomarker for DNA damage that occurs after exposure to mutagenic chemotherapy44,45 and may identify cancer patients at risk for second malignancy.46 Similarly, the VDJ interlocus assay identifies increased numbers of “illegitimate” recombination events in patients with ataxia telangiectasia35,36 and persons exposed to mutagenic stimuli,38 47 suggesting that this assay also provides a sensitive measurement of DNA damage.
To study the mutagenic effects of in vivo HU exposure, we analyzed PBMCs from patients receiving HU therapy. Thehprt− Mf was highest for patients with MPD, owing either to their older age or their underlying disease.48 49 Regardless of the duration of HU therapy, however, the patients with MPD did not have increased numbers of acquired DNA mutations compared with normal controls (Table 1, Figure2A). In fact, the number of illegitimate VDJ recombination events was significantly lower for MPD patients with prolonged HU exposure, compared with those with low HU exposure or controls. These results indicate that HU therapy for MPD does not invariably lead to increased numbers of acquired somatic mutations and suggest that HU is not leukemogenic for all patients with MPD. Perhaps only a subset of patients with MPD, such as those who are especially susceptible to the effects of HU therapy on DNA repair, have an increased risk of leukemic transformation.
The children with SCD on HU therapy had a significantly higher number of illegitimate VDJ recombination events compared with children without HU exposure (Table 1) although the increased number of VDJ mutations was still within the normal range. This observation causes concern since normal lymphoid expansion with development of the T-cell repertoire and the incidence of acute leukemia is highest during childhood.50 It should be noted that the VDJ assay tests circulating lymphocytes, yet most cases of leukemia associated with HU therapy have been myeloid in origin. An increased number of illegitimate VDJ interlocus T-cell receptor recombination events does not directly portend leukemia, as the hybrid Vγ-Jβ T-cell receptors do not have intrinsic transforming ability.38 However, aberrant VDJ recombination can affect other genes by DNA deletion51 or chromosomal translocation.52 The VDJ assay simply provides a sensitive biomarker for mutagenic effects in an accessible tissue.47
Taken together, these data suggest that the mutagenic and carcinogenic potential of HU therapy is low. For patients with MPD, there may be certain persons who have exaggerated DNA toxicity from HU therapy and an increased risk of leukemic transformation. Patients with SCD on HU therapy, especially children, should be monitored carefully by clinicians experienced with the use of chemotherapeutic agents. Long-term serial measurements of acquired DNA mutations in young patients with SCD on HU therapy may be warranted.
Acknowledgments
The authors thank Dr J. Brice Weinberg and Dr Paul Shami for contributing patients with MPD for this study, and Dr Pat Gerber for providing blood samples from the child with ataxia telangiectasia. Dr Denise Adams, Dr David Purow, and Simone Heumayer performed early experiments that led to the design of this study.
Supported by the Duke Children's Miracle Network Telethon and Leukemia Society of America Translational Research Award 6121-98 (R.E.W.).
Reprints:Russell E. Ware, PO Box 2916, Duke University Medical Center, Durham, NC 27710; e-mail: ware0005@mc.duke.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.