B-cell chronic lymphocytic leukemia (B-CLL) results from the clonal expansion of CD5+/CD23+ B-lymphocytes. The malignant transformation is assumed to occur at the level of mature B lymphocytes. Newer studies, however, have questioned this assumption. Estrov et al were able to generate CLL B-lymphoid colonies in vitro from CD34+ progenitor cells.1 Gahn et al have detected genetic abnormalities, such as trisomy 12 and Rb1 deletions, at the level of CD34+ progenitor cells,2 and Faguet et al have found granulocytes and monocytes expressing common CLL antigen in a large subset of B-CLL patients.3 In order to further explore this important issue, we studied different cell populations for the presence of certain genetic abnormalities, such as deletions on chromosome 13q14, commonly found in B-CLL.4Populations of progenitor cells were defined according to the positivity to CD34 and AC133 antigens. AC133 is a novel marker that is selectively expressed on a CD34bright subset of human hematopoietic stem and progenitor cells including the noncommitted, the majority of those committed to the granulocytic-monocytic pathway and the long-term repopulating stem cells.5
Peripheral blood samples from 22 patients with B-CLL were tested. Ten healthy individuals of the hospital staff served as controls. After preparation of mononuclear cell suspensions, AC133+ and CD34+ cells were positively selected by magnetic-activated cell sorting (MACS) according to the manufacturer's directions. Three different cell populations were thus separated by sequential selection: CD34+/AC133+, representing the more immature progenitor cell fraction; CD34+/AC133−, the less immature cell fraction; and CD34−/AC133−, the fraction of more mature cells. The purity of isolated progenitor cells was evaluated by fluorescence microscopy, using CD34-FITC and AC133-PE monoclonal antibodies, and was 90% to 98% in all instances. Each cell population was examined for the presence of deletions of D13S25 (13q14.3), D13S319 (13q14.3), and Rb1 (13q14.2) genetic loci by combining fluorescence immunophenotyping and dual color fluorescence in situ hybridization (FISH). The study was performed on cytospin preparations, and signals were enumerated in at least 300 interphase cells.
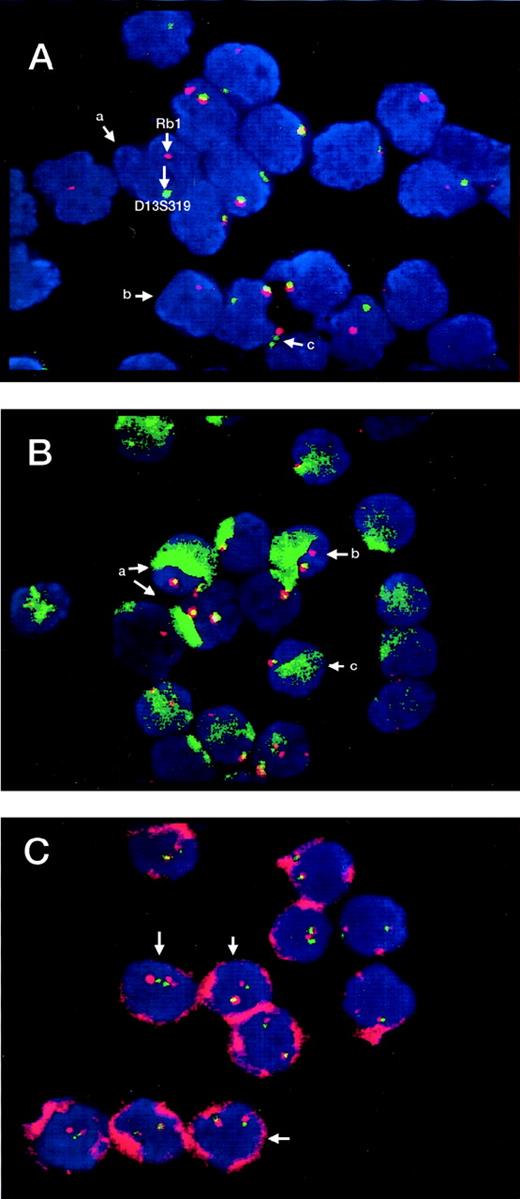
In the CD34−/AC133− cell population, heterozygous or homozygous deletions of D13S25 and D13S319 were found in 10 out of 22 patients (45.5%) (Figure, A) in a high proportion of cells (63% to 95.8%). Rb1 deletions (all heterozygous) were observed in 6 out of 22 cases (27.3%) in 23% to 83% of cells. All 6 patients also displayed deletions of D13S25 and D13S319. In the CD34+/AC133− population, heterozygous deletions of D13S25 and D13S319 loci were detected in 4 out of 22 patients (18.2%), in a relatively small proportion of cells (10%, 15%, 17%, and 18%, respectively) (Figure, B). Heterozygous Rb1 deletion was found in 1 patient, in 33% of cells. No cells with deletions in any of the examined loci were detected in the CD34+/AC133+ cell fraction (Figure, C). In controls, hetero- (1 signal) and homozygous (no signal) deletions of D13S25 and D13S319 could be detected in a mean ± 2 SD of only 1.5 ± 0.56 of cells and Rb1 deletions in a mean ± 2 SD of 0.5 ± 0.36 of cells.
Detection of genetic markers by FISH in different cell populations of patients with B-CLL.
(A) CD34−/AC133− cells displaying (a) heterozygous deletions of both Rb1 and D13S319, (b) heterozygous deletion of Rb1 and homozygous of D13S319, and (c) heterozygous deletion of only Rb1. Red dots (signals) correspond to the Rb1 gene and green ones to the D13S319 locus. Yellow signals are produced by the fusion of red and green signals from Rb1 and D13S319 lying close on the same chromosome (13q14). (B) CD34+/AC133−cells (green surface staining) with different genetic profile: (a) with presence of 2 red and 2 green signals (no deletions), (b) with heterozygous deletion of D13S319, and (c) with heterozygous deletion of both Rb1 and D13S319. (C) CD34+/AC133+ cells (red surface staining) with no marker deletions (all 4 signals present).
Detection of genetic markers by FISH in different cell populations of patients with B-CLL.
(A) CD34−/AC133− cells displaying (a) heterozygous deletions of both Rb1 and D13S319, (b) heterozygous deletion of Rb1 and homozygous of D13S319, and (c) heterozygous deletion of only Rb1. Red dots (signals) correspond to the Rb1 gene and green ones to the D13S319 locus. Yellow signals are produced by the fusion of red and green signals from Rb1 and D13S319 lying close on the same chromosome (13q14). (B) CD34+/AC133−cells (green surface staining) with different genetic profile: (a) with presence of 2 red and 2 green signals (no deletions), (b) with heterozygous deletion of D13S319, and (c) with heterozygous deletion of both Rb1 and D13S319. (C) CD34+/AC133+ cells (red surface staining) with no marker deletions (all 4 signals present).
In conclusion, we found in a subset of B-CLL patients genetic abnormalities in a small but appreciable proportion of moderately immature progenitor cells (CD34+/AC133−), similar to those found in the more mature fraction of cells (CD34−/AC133−). Our findings support the concept that malignant transformation in B-CLL may indeed involve a population of early hematopoietic cells, though less immature than the primitive progenitor cells. In accordance with that is our recent finding that, in a large subset of B-CLL patients, dendritic cells carry the same genetic markers as clonal lymphoid cells and are probably neoplastic in origin.6 All these results are significant for our understanding of B-CLL biology and for selection of the proper fraction of progenitor cells for autologous transplantation in this disease.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal