Abstract
Hepatocyte growth factor (HGF) is a pluripotent cytokine with mitogenic, motogenic, and morphogenic activity for mainly epithelial and endothelial target cells. We previously demonstrated that the specific HGF receptor, MET, is induced in stimulated peripheral blood monocytes. In this study, we analyzed the functional consequences of MET activation in primary cultures of peripheral blood monocytes from healthy donors. After stimulation of MET-expressing monocytes with recombinant HGF, the gene-expression profile of peripheral blood mononuclear cells and monocytes was significantly modulated, especially with regard to genes involved in cell movement. After stimulation of primary cultured monocytes with HGF, invasion assays showed a significantly increased matrigel invasion rate that was completely abolished by neutralizing antibodies to HGF. The HGF-activated invasiveness and the altered gene-expression profile suggest a proinflammatory role for HGF stimulation of monocytes and support the hypothesis that the HGF/MET signaling system plays an important part in the activation of the nonspecific cellular inflammatory response.
Hepatocyte growth factor (HGF) is a multifunctional cytokine with pleiotropic effects. It exerts mitogenic activity on a wide range of endothelial and epithelial cells,1,2Furthermore, it induces a motogenic and morphogenic program in a variety of cell types, such as hepatocytes and kidney epithelial cells.3-5 All the different effects of HGF are mediated through its specific receptor, MET, a heterodimeric transmembranous tyrosine kinase.6 After ligand-induced activation of MET, different intracellular signal-transduction pathways are activated through a C-terminal cytoplasmic multifunctional docking site.7
The broad activity of HGF and its impact on many physiologic and pathological processes is reflected by MET expression in a variety of organs and cell types. HGF/MET signaling is involved in organ regeneration, as has been shown in the liver and kidney,8,9and in embryogenesis.10,11 HGF is neurotrophic for central nervous system neurons,12,13 is also expressed in several hematopoietic cell lines,14,15 and costimulates proliferation and differentiation of CD34-positive hematopoietic precursor cells.16,17 In addition, HGF/MET signaling may contribute to tumor cell invasion.18-20
HGF is highly overexpressed during necroinflammatory liver disease and is elevated in the serum of patients with acute or chronic hepatitis, liver cirrhosis, or hepatocellular carcinoma.21 HGF is thought to have cytoprotective activity on hepatocytes,22to be antifibrogenic,23 and to stimulate transmigration of T-cell subsets from peripheral blood.24 Furthermore, HGF expression in necroinflammatory liver disease was demonstrated in infiltrating leukocytes by in situ hybridization (ISH) and immunohistologic studies.25,26 In addition, increased deposits of HGF were found along the basal lamina in inflammatory lesions of other organs, such as the lung and kidney.27 All these observations suggest that HGF/MET signaling is important in necroinflammation of various organs.
We previously showed that MET expression becomes selectively induced and functionally active in monocytes.28 In the study described here, we examined the functional role of MET expression, ie, the induced HGF responsiveness in monocytes specifically with regard to the inflammatory response. We found extensive modulation of the gene-expression profile, including effects on genes involved in directed cell movement, and a significantly increased invasiveness of MET-expressing monocytes after stimulation with HGF.
Materials and methods
Cell cultures and isolation of peripheral blood mononuclear cells (PBMNC) and monocytes from peripheral blood
PBMNC were separated by centrifugation over a Ficoll-Hypaque density gradient, recovered from the interface, and washed twice in phosphate-buffered saline. Monocytes were isolated from PBMNC preparations by negative immunomagnetic separation using antibody-coated magnetic beads (Deutsche Dynal, Hamburg, Germany) according to the manufacturer's instructions.29 Briefly, a highly monocyte-enriched fraction (purity > 90%) was obtained by negative selection, extracting T cells with anti-CD2 and B cells with anti-CD19 antibody–coated beads. Natural killer cells were bound to antibodies against CD16 (Immunotech, Marseille, France) and CD56 (Dako, Copenhagen, Denmark) and then extracted with antimouse IgG-coated beads (Deutsche Dynal). Cells were temporarily exposed to 0.5% fetal calf serum (FCS) to inhibit clumping. Isolated PBMNC and monocytes were then processed as described below.
To characterize the purity of the isolated cell fractions, aliquots were incubated with fluorescein isothiocyanate–labeled antibodies (Sigma, Deisenhofen, Germany) against monocyte antigen CD14, T-cell antigen CD3, and B-cell antigen CD19 and analyzed by using fluorescence-activated cell-sorter scanning (FACS; Becton Dickinson, Heidelberg, Germany).
Immunocytologic studies
Cytospin preparations containing 105 PBMNC or monocytes per slide were prepared. Immunocytologic studies were done by using the avidin-biotin–complex method with a rabbit polyclonal anti-human MET antibody (dilution: 1:100) (clone C-28; Santa Cruz, Santa Cruz, CA) and respective horseradish peroxidase/streptavidin–coupled sheep anti-rabbit IgG (dilution: 1:300; Dako) as secondary antibody. Detection was carried out with diaminobenzidine as the chromogen. As a negative control, the MET antibody was preincubated with a molar excess of a peptide specific for the binding site of the MET antibody (Santa Cruz). The cytospin preparations were counterstained with Mayer's hemalum and analyzed with light microscopy (Diaplan microscope; Leica, Bensheim, Germany).
Analysis of gene expression
To investigate the influence of HGF incubation on gene expression, 2 × 106 PBMNC/mL and monocytes were cultured in Teflon jars for 4 hours under standard conditions, with or without 100 ng/mL recombinant human HGF in RPMI 1640 medium supplemented with 1% FCS and 0.1% bovine serum albumin (BSA; Sigma). After the cells were harvested, total RNA was isolated with an RNeasy kit (Qiagen, Hilden, Germany). In the PBMNC, poly (A+) RNA was obtained from total RNA with an OligoTex kit (Qiagen).
The gene-expression analysis used a complementary DNA (cDNA) expression array that included nearly 600 different genes (Clontech, Palo Alto, CA). Briefly, total RNA or poly (A+) RNA was prepared for each assay simultaneously under identical conditions and reverse transcribed with Moloney murine leukemia virus reverse transcriptase. The resulting cDNA preparations were labeled with phosphorus 32 and hybridized to the array filters. Evaluation of the array filters was performed by using the Storm 840 PhosphoImager and ImageQuant software (Molecular Dynamics, Krefeld, Germany). To scale the hybridization signals of the 2 filters, values were adjusted to internal standards (housekeeping genes) present on both filters. In addition, all results were carefully controlled by autoradiographic assessment of the filters.
Matrix invasion assay
A monocyte matrix invasion assay was performed by using matrigel invasion chambers with 8-μm pores (Becton Dickinson). To reduce the influence of different cytokines, growth factor–reduced matrigel preparations were used. After matrigel rehydration, the wells were filled with 100 ng/mL recombinant human HGF (750 μL) and freshly isolated monocytes were seeded onto the inserts at a density of 1.2 × 106 cells/500 μL so that the matrigel separated the cells from the HGF solution and supported a gradient. Because it was necessary to use solutions free of protease inhibitors, FCS-free Diff 1000 medium (Biochrom, Berlin, Germany) supplemented with 0.1% BSA was used for the assay. For control experiments, the HGF polypeptides were preincubated with 10 μg/mL of a neutralizing anti-human HGF antibody (Sigma) in test medium at room temperature for 30 minutes and washed twice with medium before the assay was performed. At 2 different time points, after 18 hours (± 3 hours because of donor variability) and 48 hours of incubation at 37°C in 5% carbon dioxide, incubation was stopped. The first time point was selected when approximately 2% of the control cell population (ie, not the HGF-stimulated cell fraction) had transmigrated. The matrigel and the cells retained on the upper side of the membrane were removed, and the transmigrated cells at the bottom side were fixed with methanol, counterstained with Mayer's hemalum and eosin, mounted with oil, and covered with a glass coverslip. Optical evaluation of adherent cells on the bottom side of the membrane was done morphometrically. When the total number of cells was low, all cells on the membrane were counted. When cell numbers were higher, only cells along standardized centered lines were counted. Only 1 type of evaluation was used in each experiment, and relative cell numbers were determined. The number of cells in the cell fraction stimulated by HGF for 18 hours was arbitrarily set at 1.
Results
MET was moderately expressed in purified monocytes
Use of a polyclonal antibody binding to the intracellular domain of MET detected expression of MET protein in monocytes (Figure 1A-D). Although cytoplasmic and membranous expression was detected in a minority of freshly isolated monocytes, the signal was increased and found in more than 50% of the monocyte fraction after activation with phytohemagglutinin (PHA) and lipopolysaccharide (LPS). As in previous experiments with PBMNC, MET was detectable only in monocytes. FACS analysis revealed that the purity of the negatively isolated monocyte preparations was greater than 90% in all cases (Figure 1E).
Expression of MET receptor in peripheral blood mononuclear cells (PBMNC).
Immunocytologic detection of MET receptor in PBMNC (A) and monocytes (C) derived from a healthy donor by using a rabbit polyclonal MET antibody. The specificity of the signal was demonstrated by control experiments for PBMNC (B) and monocytes (D) using the MET antibody after preabsorption with a specific binding peptide. (E) Determination of monocyte concentrations by fluorescence-activated cell-sorter analysis using fluorescein isothiocyanate–labeled anti-CD14 (right) antibodies in the PBMNC fraction (above) and the negatively selected monocyte fraction (below). The respective unstained cell fractions are shown on the right side. The calculation resulted in a purity of the monocytic fraction of greater than 90%.
Expression of MET receptor in peripheral blood mononuclear cells (PBMNC).
Immunocytologic detection of MET receptor in PBMNC (A) and monocytes (C) derived from a healthy donor by using a rabbit polyclonal MET antibody. The specificity of the signal was demonstrated by control experiments for PBMNC (B) and monocytes (D) using the MET antibody after preabsorption with a specific binding peptide. (E) Determination of monocyte concentrations by fluorescence-activated cell-sorter analysis using fluorescein isothiocyanate–labeled anti-CD14 (right) antibodies in the PBMNC fraction (above) and the negatively selected monocyte fraction (below). The respective unstained cell fractions are shown on the right side. The calculation resulted in a purity of the monocytic fraction of greater than 90%.
HGF modulated the gene-expression pattern of monocytes
Freshly isolated PBMNC and monocytes derived from PBMNC were incubated with recombinant HGF [100 ng/mL] for 4 hours. After extraction of the RNA from the control and stimulated cell populations, gene expression in the 2 populations was analyzed and compared by using cDNA expression arrays. Expression analysis in monocytes revealed that 13% of the analyzed genes were modulated by HGF. Of those genes, 65% were activated and 35% were suppressed; differences in expression modulation ranged from about 1.5- to 4-fold (Figure 2, Table1). Most of the genes that were activated by HGF are known to be inducible in monocytes.
Hepatocyte growth factor-modulated gene expression in relation to monocytic function
| Gene . | Function in monocytes . | Modulation factor . | Gene bank accession no. . | |
|---|---|---|---|---|
| Monocytes . | PBMNC . | |||
| MAS proto-oncogene | 1, 7 | 1, 6 | M13150 | |
| Prohibitin | 1, 8 | 2, 1 | S85655 | |
| Protein ETA | 2, 0 | 3, 5 | L20422 | |
| Nitric oxide synthase* | 2, 4 | L09210 | ||
| Lymphotoxin α (TNF β) | 1, 7 | Increased | D12614 | |
| ICH-1 protease | Apoptosis | 2, 2 | Increased | U13021 |
| FAST kinase | 2, 0 | 2, 2 | X86779 | |
| Cysteine protease MCH4 | Apoptosis | 1, 7 | 2, 0 | U60519 |
| HDLC1 | 1, 8 | 3, 8 | U32944 | |
| Replication factor C | Replication | 1, 7 | 5, 2 | M87338 |
| Protein kinase (DNA related) | Transcription? | 1, 7 | Increased | U35835 |
| RAD50 | DNA repair | 2, 1 | Increased | U63139 |
| Calcitonin receptor | 1, 8 | 1, 5 | L00587 | |
| GM-CSF | Growth, proliferation | 2, 6 | M11220 | |
| FMLP-related receptor 1 | Chemokinesis, motility | 2, 3 | M76673 | |
| M-CSF | Proliferation | 1, 7 | M37435 | |
| MIP-1β* | Chemokinesis, motility, migration | 1, 9 | J04130 | |
| MCP-1* | Chemokinesis, motility, migration | 2, 5 | 2, 4 | M24545 |
| VEGF | Growth | 1, 8 | M32977 | |
| Stem cell factor | 1, 8 | 4, 1 | M59964 | |
| Calgranulin B (MIF related)* | Calcium binding in monocytes | 1, 4 | 2, 7 | X06233 |
| MIP-2α* | Chemokinesis, motility, migration | 2, 1 | X53799 | |
| Interleukin 8 | Chemokinesis, motility, migration | 1, 4 | 4, 6 | Y00787 |
| Interleukin 1β | Immune response | 2, 9 | K02770 | |
| Interleukin 4 | Immune response | 1, 9 | M13982 | |
| Interleukin 6 | B-cell differentiation | 1, 6 | 2, 9 | X04602 |
| Interleukin 11 | 1, 8 | M57765 | ||
| IGF-BP | Growth | 1, 7 | 3, 3 | M31159 |
| Proto-oncogene pim-1 | −2, 5 | Complete suppression | M54915 | |
| Tyrosine kinase Lyn | −1, 7 | −1, 8 | M16038 | |
| TNF receptor | −2, 7 | Decreased | M33294 | |
| Basic TF2 | Transcription | −2, 9 | M95809 | |
| SMUBP-2 | DNA binding protein | −2, 0 | L14754 | |
| Interferon-consensus sequence binding protein | DNA binding protein | −3, 8 | M91196 | |
| CNBP | DNA binding protein | −2, 2 | M28372 | |
| CRE-BP1 | DNA binding protein | −2, 0 | L05517 | |
| TSG-6 | TNF inducible protein | −1, 7 | −2, 6 | M31165 |
| Gene . | Function in monocytes . | Modulation factor . | Gene bank accession no. . | |
|---|---|---|---|---|
| Monocytes . | PBMNC . | |||
| MAS proto-oncogene | 1, 7 | 1, 6 | M13150 | |
| Prohibitin | 1, 8 | 2, 1 | S85655 | |
| Protein ETA | 2, 0 | 3, 5 | L20422 | |
| Nitric oxide synthase* | 2, 4 | L09210 | ||
| Lymphotoxin α (TNF β) | 1, 7 | Increased | D12614 | |
| ICH-1 protease | Apoptosis | 2, 2 | Increased | U13021 |
| FAST kinase | 2, 0 | 2, 2 | X86779 | |
| Cysteine protease MCH4 | Apoptosis | 1, 7 | 2, 0 | U60519 |
| HDLC1 | 1, 8 | 3, 8 | U32944 | |
| Replication factor C | Replication | 1, 7 | 5, 2 | M87338 |
| Protein kinase (DNA related) | Transcription? | 1, 7 | Increased | U35835 |
| RAD50 | DNA repair | 2, 1 | Increased | U63139 |
| Calcitonin receptor | 1, 8 | 1, 5 | L00587 | |
| GM-CSF | Growth, proliferation | 2, 6 | M11220 | |
| FMLP-related receptor 1 | Chemokinesis, motility | 2, 3 | M76673 | |
| M-CSF | Proliferation | 1, 7 | M37435 | |
| MIP-1β* | Chemokinesis, motility, migration | 1, 9 | J04130 | |
| MCP-1* | Chemokinesis, motility, migration | 2, 5 | 2, 4 | M24545 |
| VEGF | Growth | 1, 8 | M32977 | |
| Stem cell factor | 1, 8 | 4, 1 | M59964 | |
| Calgranulin B (MIF related)* | Calcium binding in monocytes | 1, 4 | 2, 7 | X06233 |
| MIP-2α* | Chemokinesis, motility, migration | 2, 1 | X53799 | |
| Interleukin 8 | Chemokinesis, motility, migration | 1, 4 | 4, 6 | Y00787 |
| Interleukin 1β | Immune response | 2, 9 | K02770 | |
| Interleukin 4 | Immune response | 1, 9 | M13982 | |
| Interleukin 6 | B-cell differentiation | 1, 6 | 2, 9 | X04602 |
| Interleukin 11 | 1, 8 | M57765 | ||
| IGF-BP | Growth | 1, 7 | 3, 3 | M31159 |
| Proto-oncogene pim-1 | −2, 5 | Complete suppression | M54915 | |
| Tyrosine kinase Lyn | −1, 7 | −1, 8 | M16038 | |
| TNF receptor | −2, 7 | Decreased | M33294 | |
| Basic TF2 | Transcription | −2, 9 | M95809 | |
| SMUBP-2 | DNA binding protein | −2, 0 | L14754 | |
| Interferon-consensus sequence binding protein | DNA binding protein | −3, 8 | M91196 | |
| CNBP | DNA binding protein | −2, 2 | M28372 | |
| CRE-BP1 | DNA binding protein | −2, 0 | L05517 | |
| TSG-6 | TNF inducible protein | −1, 7 | −2, 6 | M31165 |
PBMNC indicates peripheral blood mononuclear cells. “Increased” or “decreased” indicates the tendency of expression; values could not be specified because of a partly high background.
High basic expression (>10-fold the median expression value).
Importantly, the chemokine group of genes, especially MCP-1, MIP-1β, MIP-2α, and interleukin (IL) 8, were activated after HGF incubation. Other cytokines that were modulated by HGF included lymphotoxin α, IL-4, IL-1β, and the growth factors macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Furthermore, cell-surface receptors (CD11 and VCAM), genes involved in apoptosis (cysteine protease MCH4 and ICH1 protease), and some more ubiquitously expressed genes involved, for example, in DNA replication (replication factor C) and DNA repair (RAD50) were also activated by HGF. Genes that were suppressed by HGF belonged predominantly to the discriminative group of DNA binding proteins (Table 1).
Analysis of HGF-modulated gene expression of the unfractionated population of PBMNC revealed some differences with the monocyte fractions. Expression of the chemokines IL-8 and MCP-1 was up-regulated in PBMNC and in monocytes. Furthermore, some genes were additionally modulated in PBMNC by HGF in the same direction as in monocytes; examples are protein ETA, prohibitin, stem cell factor, replication factor C, IGF binding protein, and the proto-oncogenepim 1. For most of these genes, the modulation was more pronounced in PBMNC than in monocytes. On the other hand, some genes were modulated solely in PBMNC. For example, the gene for tumor necrosis factor α was up-regulated by HGF in every analyzed PBMNC fraction but not in monocytes.
HGF enhanced the migratory activity of MET-positive primary cultured monocytes
Because many genes involved in cell migration were significantly activated by incubation of monocytes or PBMNC with HGF, we analyzed the possible influence of HGF/MET signaling on monocytic invasiveness by using primary cultured monocytes, which were previously shown to express MET. In a chemotaxis assay, monocytes from different donors showed a broad variability in response, but overall a significant chemotactic reaction in response to HGF stimulation was not demonstrated (data not shown).
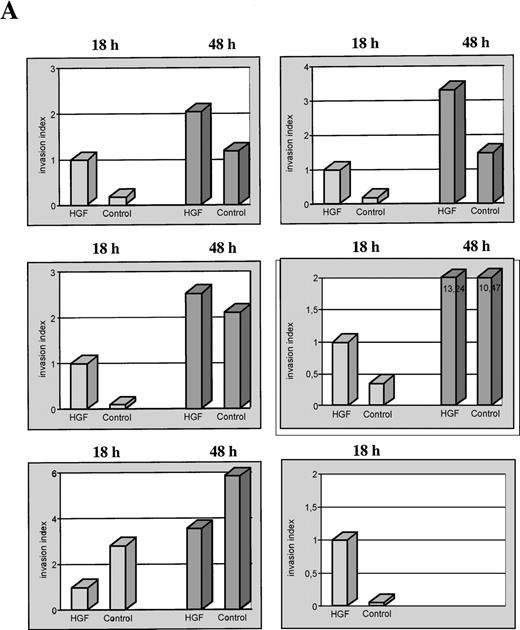
When an in vitro matrigel invasion assay was used, monocytic transmigration began after 15 to 20 hours of incubation with the selected cell numbers. Stimulation experiments revealed a significantly enhanced invasion of monocytes through matrigel in HGF-stimulated populations compared with the control populations (Figure 3). Particularly, invasion of primary cultured monocytes was stimulated with HGF (100 ng/mL) under serum-free conditions for 18 and 48 hours, and in 5 of 6 monocyte fractions obtained from different healthy donors, incubation with HGF led to a significantly enhanced transmigration through matrigel-coated membranes. When the assays were done for 48 hours, this HGF-induced effect on the migratory behavior disappeared (Figure4A): the number of transmigrating cells increased further, but the difference between invasion rates in the 2 fractions was no longer significant at 48 hours.
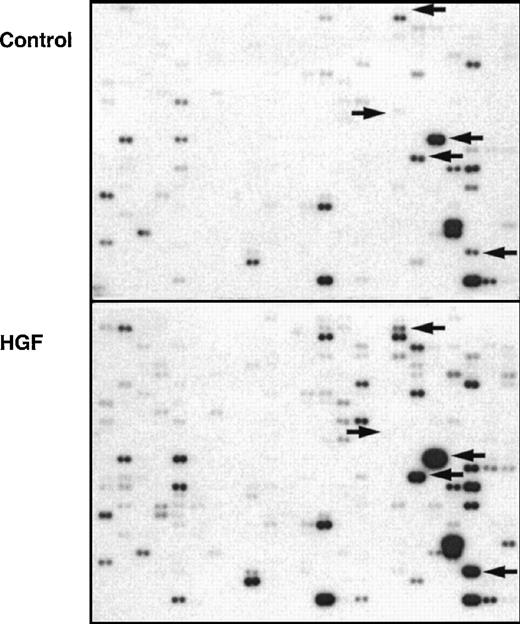
Hepatocyte growth factor (HGF)–stimulated gene-expression profile of isolated monocytes.
Autoradiograph of a complementary DNA (cDNA)-expression array, which consisted of 2 identical filters each spotted with duplicate cDNA samples of 588 genes hybridized with phosphorus 32–labeled total cellular cDNA obtained from unstimulated (control) and HGF-stimulated monocytes. Semiquantitative comparison of the 2 filters was done. Some examples of obvious changes in gene expression are indicated by arrows. The autoradiograph corresponds with the PhosphoImager data in Table 1(monocyte fraction).
Hepatocyte growth factor (HGF)–stimulated gene-expression profile of isolated monocytes.
Autoradiograph of a complementary DNA (cDNA)-expression array, which consisted of 2 identical filters each spotted with duplicate cDNA samples of 588 genes hybridized with phosphorus 32–labeled total cellular cDNA obtained from unstimulated (control) and HGF-stimulated monocytes. Semiquantitative comparison of the 2 filters was done. Some examples of obvious changes in gene expression are indicated by arrows. The autoradiograph corresponds with the PhosphoImager data in Table 1(monocyte fraction).
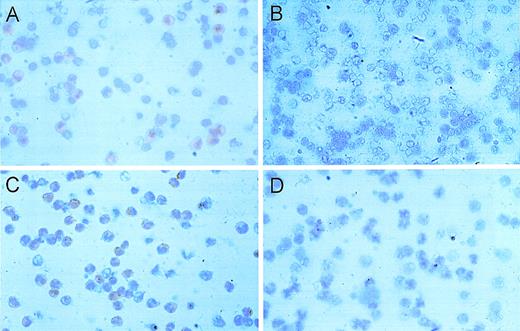
HGF-induced matrigel invasion of isolated monocytes.
Primary cultured monocytes migrate into matrigel, pass through a porous membrane filter, and adhere to the bottom side, where they are fixed and counterstained. (A) Several cells may simultaneously pass through a single pore (400 × magnification of the bottom side of a polycarbonate membrane filter). Invasions of unstimulated (B) and HGF-stimulated monocytes with diffuse (C) and irregular (D) cell distribution after 18 hours of incubation are shown (100-fold magnification of the bottom side of the membrane).
HGF-induced matrigel invasion of isolated monocytes.
Primary cultured monocytes migrate into matrigel, pass through a porous membrane filter, and adhere to the bottom side, where they are fixed and counterstained. (A) Several cells may simultaneously pass through a single pore (400 × magnification of the bottom side of a polycarbonate membrane filter). Invasions of unstimulated (B) and HGF-stimulated monocytes with diffuse (C) and irregular (D) cell distribution after 18 hours of incubation are shown (100-fold magnification of the bottom side of the membrane).
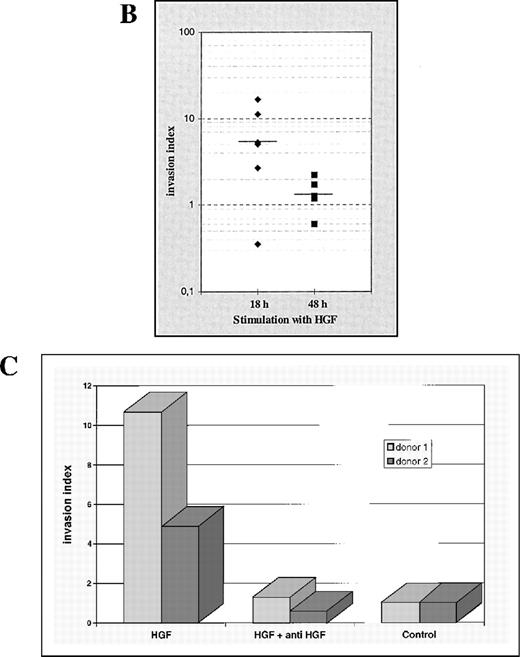
Extent and time course of induced monocytic matrigel invasion.
(A) Invasion of growth factor–depleted matrigel by monocytes derived from 6 different healthy donors, after incubation with HGF and without HGF (control) for 18 hours and 48 hours (separate representation for each donor). For relative comparisons of samples from the different donors, the invasion index of all HGF stimulations at 18 hours was arbitrarily set at 1. (B) Cumulative analysis of the monocytic invasion assays shown in Figure 4A. Each symbol represents a single experiment. The respective medians are indicated by a horizontal line. (C) Matrigel invasion of monocytes from 2 different donors, after incubation with HGF and without HGF (control). Specificity of the HGF-mediated biologic effect was controlled by preincubation of the HGF with a specific anti-human HGF neutralizing antibody for 30 minutes. The control incubation value for each donor sample was arbitrarily set at 1.
Extent and time course of induced monocytic matrigel invasion.
(A) Invasion of growth factor–depleted matrigel by monocytes derived from 6 different healthy donors, after incubation with HGF and without HGF (control) for 18 hours and 48 hours (separate representation for each donor). For relative comparisons of samples from the different donors, the invasion index of all HGF stimulations at 18 hours was arbitrarily set at 1. (B) Cumulative analysis of the monocytic invasion assays shown in Figure 4A. Each symbol represents a single experiment. The respective medians are indicated by a horizontal line. (C) Matrigel invasion of monocytes from 2 different donors, after incubation with HGF and without HGF (control). Specificity of the HGF-mediated biologic effect was controlled by preincubation of the HGF with a specific anti-human HGF neutralizing antibody for 30 minutes. The control incubation value for each donor sample was arbitrarily set at 1.
Freshly isolated monocytes from 2 different healthy donors were stimulated with recombinant HGF that was preincubated with an excess of an HGF neutralizing antibody. In both samples, the stimulative effect of HGF on monocyte invasiveness was completely abrogated (Figure 4B).
Discussion
In previous experiments, we showed by using ISH thatc-met transcription was induced in preactivated PBMNC treated with PHA and LPS and that among this heterogenous cell population, MET expression was derived only from monocytes. Here, we found that MET protein was detectable only in monocytes and especially in the enriched, activated monocyte fraction. In addition, the percentage of MET-expressing cells among PBMNC was comparable to that in the monocyte-enriched cell fraction. The higher percentage of MET-positive cells compared with results from previous ISH experiments28likely reflects the greater sensitivity of immunohistochemical analysis compared with ISH when the 2 assessments are used together.
In addition, a lower percentage of MET-expressing cells was found in monocyte-enriched cell fractions not preactivated by PHA and LPS. We cannot exclude the possibility that this expression was due to unspecific activation during the isolation procedure (preparation of buffy coats and monocyte isolation), although all methods available to minimize uncontrolled stimulation (eg, by adhesion) were used. Because strong preactivation (eg, by PHA and LPS) might have masked specific effects of HGF on monocytes, we have used isolated nonpreactivated monocytes and PBMNC fractions for all further functional experiments.
To delineate the pattern of HGF effects on monocytes, we analyzed the expression pattern of nearly 600 different genes by using a cDNA-expression array. Under the assumption that only those genes that were significantly altered in their expression during the period assayed would appear, we found that the expression of a marked number of genes (13%) was changed when isolated monocytes were stimulated with HGF. Most of the genes had increased expression after the HGF incubation, but we also observed suppressed expression of a limited number of genes.
The modulation of expression predominantly affected monocyte-related genes, especially genes involved in cell motility. Accordingly, expression of 4 chemokines (MIP-1β, MIP-2α, MCP-1, and IL-8) was stimulated. These results correlate well with those of previous studies that showed monocyte-derived up-regulation of MCP-1 and IL-8 in necroinflammatory liver disease and support the existence of an important contribution by those genes to the pathogenesis of the disease.30-34 However, other cytokines, such as IL-4, IL-1β, M-CSF, and GM-CSF, were also up-regulated, suggesting an important proinflammatory role for HGF stimulation of monocytes.
In relation to the expression analysis of RNA derived from isolated monocytes, the results from the analysis of unfractionated PBMNC were partly overlapping but also revealed some differences in the expression profile. More genes were modulated by HGF, and there was frequently a greater modulation of gene expression. These differences are likely to have been due to secondary effects on, or costimulatory signals exerted by, other cell types not present in substantial numbers in isolated monocyte populations.
Gene-expression analysis indicated a possible role for HGF in monocytic migration. In in vitro chemotaxis assays, we observed no significant effect of HGF on PBMNC preactivated with LPS and PHA or on monocytes during the assay time frame (2 hours; data not shown). Thus, significant direct chemotactic effects of the supplied HGF were excluded. However, the possibility remained that HGF acted as an indirect stimulator for chemotaxis through the up-regulation of chemokines. In the matrigel invasion assay, HGF markedly accelerated the invasion rate of freshly isolated monocytes. This was not due to the moderate improvement in monocytic survival we previously demonstrated after HGF stimulation,28 since this effect was observed after 72 hours and was not detectable at 24 hours. The activation of invasive properties had a relatively high variability and was not demonstrable in 1 of 6 monocyte preparations tested. However, the HGF neutralization of invasion in 2 independent samples clearly demonstrated the specificity of the effect. The variability was most likely due to the fact that isolated primary cells from different human donors may vary in the composition of their feedstock (buffy coats). In addition, a substantial variation in physiologic and immune variables and in the genetic background of the donor cell population cannot be excluded as contributing to this effect.
In the matrix invasion assay, monocytes must cleave extracellular-matrix proteins to migrate through the matrigel. The accelerated monocytic invasion rate was attenuated after prolonged incubation with HGF because of the mechanism by which monocytes invade matrigel. Although the initially invading monocytes actively digest the matrigel, the cells that follow can use the preformed channels in the matrigel. The data obtained with respect to the time frame of the monocytic invasion of tissue correlate well with in vivo findings. Investigations of murine and human wound-healing processes found that activated monocytes begin to infiltrate tissue at 18 hours after injury35 and reach maximal numbers at day 2.36
Thus far, all specific HGF effects have been shown to be mediated by the MET receptor that is present in monocytes. Therefore, it is reasonable to assume that HGF-induced monocytic invasiveness is also mediated by the MET-receptor molecules present on the cell surface. Correspondingly, transfected NIH 3T3 and C127 cells, which overexpress MET protein, also reacted to HGF stimulation with an increase in invasion rate.37-39
Interestingly, directed monocytic motility requires the presence of active urokinase,40 which may not only lead to extracellular matrix digestion but may also act as an HGF convertase, cleaving the inactive monomeric precursor molecule.41Therefore, urokinase knockout mice were found to have reduced monocyte motility,42 and HGF increased the expression of urokinase-type plasminogen activator (uPA) and the uPA receptor in a variety of cell types and stimulated their migration in vitro.43 Expression of uPA and uPA receptor was also induced in Madin-Darby canine kidney cells, which showed scattering and tubule formation after incubation with HGF.44These findings indicate that the HGF/MET signaling system may also exert influence on monocytic migration by means of direct or indirect activation of uPA, uPA receptor, or both. This hypothesis is further supported by our finding that the HGF effect on monocytes was abolished when they were cultured in conventional medium, presumably because of the high concentration of protease inhibitors in the FCS used for supplementation (data not shown).
Ligand-mediated MET activation leads to the induction of several intracellular signaling pathways through the interaction of various polypeptides containing src-homologous-2 domains by means of a multifunctional docking site.45 Specifically, the phosphoinositol-3-kinase intracellular signaling pathway was linked to HGF-induced increased cell motility,46 47 and it is therefore reasonable to assume that it may also contribute to HGF-induced enhanced monocytic invasiveness.
MET expression in monocytes is up-regulated in vitro by inflammatory mediators such as tumor necrosis factor α (TNF-α) in combination with IL-6 and TNF-α with interferon γ, as well as PHA, LPS, and 12-O-tetradecanoylphorbol-13-acetate.28,48Inaddition, HGF is increased in the tissue and serum of patients and animals with inflammatory diseases of the liver and other organs.27,49 50 Therefore, increased tissue concentrations of HGF in areas of active inflammation may induce a complex gene-expression pattern in monocytes in vivo and enable these cells to better penetrate the interstitial tissue. Future studies must determine whether HGF-stimulated monocytes induce further secondary cytokine-mediated effects on other inflammatory-cell compartments and elucidate the impact of the activated monocytic HGF/MET-signaling system in the complex network of cellular immune reactions.
Supported by grant Schi 273/3 from the Deutsche Forschungsgemeinschaft (P.S.) and the Stiftung Rheinland-Pfalz für Innovation (P.S. and H.P.D.) and Köln Fortune (P.S.).
Reprints:Peter Schirmacher, Institute of Pathology, University of Cologne, Joseph-Stelzmann-Str 9, 50931 Cologne, Germany; e-mail:peter.schirmacher@uni-koeln.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal