Abstract
High-dose therapy (HDT) has increased complete remission (CR) rates and survival in multiple myeloma (MM). We now report on continuous CR (CCR) and associated prognostic factors in 1000 consecutive patients receiving melphalan-based tandem HDT. Five-year CCR was 52% among 112 CR patients without chromosome 13 (▵13) abnormalities and with beta-2-microglobulin ≤ 2.5 mg/L, C-reactive protein ≤ 4 mg/L, and pre-HDT standard chemotherapy ≤ 12 months. Of all 390 CR patients without ▵13 abnormalities, 35% enjoyed 5-year CCR but none of 54 with ▵13 abnormalities. ▵13 abnormalities, present in overall 16%, reduced 5-year event-free survival from 20% to 0% and overall survival from 44% to 16% (both P < .0001). CR and a second HDT cycle applied within 6 months both extended event-free and overall survival significantly, justifying further pursuit of HDT, especially toward curing non-▵13 MM.
In newly diagnosed multiple myeloma (MM), melphalan (MEL) dose escalation has increased complete response (CR) rates to ∼50% and overall survival (OS) to > 5 years.1-5 After accruing 1000 consecutive patients into MEL-based tandem high-dose therapy (HDT) trials with autologous hematopoietic stem cell (AHSC) support, we now report on their long-term outcome with emphasis on 5-year continuous CR (CCR) and the associated prognostic factors.
Study design
Between September 1989 and June 1998, 1000 consecutive eligible patients with MM were enrolled in MEL HDT trials. Patients had to have adequate cardiopulmonary and hepatic functions, whereas renal insufficiency and advanced age (≥ 65 years) were not exclusion criteria. Comprehensive initial work-up included cytogenetic analysis of Giemsa-banded chromosomes6 and data on duration of and response to prior standard-dose therapy (SDT). Primary SDT resistance and resistant relapse were distinguished.7 AHSCs were mobilized prior to first HDT, either with granulocyte colony-stimulating factor alone (99 patients)8 or with high-dose cyclophosphamide (6 g/m2) plus granulocyte-macrophage colony-stimulating factor9 (462 patients) or granulocyte colony-stimulating factor (179 patients); the remaining 260 patients received other mobilization regimens.
All 1000 patients received MEL 200 mg/m2 as the first HDT cycle; 76% received a second HDT cycle (94% within 12 months). Major second HDT regimens were MEL 200 mg/m2 in 39%, MEL 140 mg/m2 + total body irradiation (850-1020 cGy) in 15%, MEL 200 mg/m2 + cyclophosphamide 120 mg/kg in 8%, BEAM10 (carmustine 300 mg/m2, etoposide 200 mg/m2 × 4 days, cytarabine 400 mg/m2 × 4 days, melphalan 140 mg/m2) in 2%, and other regimens in the remainder; 10% received allotransplants.
Serial laboratory parameters and clinical follow-up visits were scheduled at 3- to 6-month intervals during the initial 2 years and subsequently at least annually to document MM and clinical status. All patients provided written informed consent indicating their awareness of the investigational nature of treatment programs and alternative therapies available. All HDT trials were approved by the Institutional Review Board.
Study end points included treatment-related mortality (TRM) within 60 days of HDT, CR incidence and duration (from onset of CR) and event-free survival (EFS), and OS. Data were analyzed on an intent-to-treat basis. Response and relapse criteria were previously reported.5
Survival distributions (Kaplan and Meier)11 were compared, using the log rank test.12 Multivariate modeling of TRM and CR were performed, using stepwise selection methods and logistic regression. Similarly, multivariate modeling of CR duration, EFS, and OS employed stepwise selection in proportional hazard regression models.13 Of the many cytogenetic abnormalities affecting EFS and OS, those of chromosome 13 (Δ13) were the dominant adverse anomaly on multivariate analysis, hence considered as the sole cytogenetic variable evaluated along with standard prognostic factors. Time-dependent covariates (CR, second HDT) were used to model parameters measured after first HDT.14
Results and discussion
Patient characteristics included the following median values (ranges): age, 53 years (14-82 years); prior SDT, 10 months (0.5-222 months); beta-2-microglobulin (B2M), 2.3 mg/L (0.8-70 mg/L); and C-reactive protein (CRP), 4.1 mg/L (0.3-237 mg/L). Fifty-six percent had Durie-Salmon stage III at initiation of SDT; 38% had resistant MM (primary resistance, 24%; resistant relapse, 14%); and immunoglobulin isotypes were IgG in 53%; IgA in 21%; and light-chain only, IgD, or nonsecretory MM in the remainder. Cytogenetic abnormalities were present pre-HDT in 34%, including 16% with Δ13 abnormalities (monosomy 13, 109 patients; deletion 13q in 58, translocations involving 13q in 18; many had several abnormalities).
TRM was low (2.7% with first, 4.8% with second AHSCT-supported HDT); 44% achieved CR, which lasted a median of 2.4 years. Projected EFS and OS at 5 years were 25% (SE 2%) and 40% (SE 2%), respectively. According to multivariate analysis, CR rates were higher when SDT was still effective (sensitive MM) (OR: 3.2,P < .0001) and did not exceed 12 months (OR: 2.0,P < .0001); in the absence of Δ13 abnormalities (OR: 2.0,P < .0005) and with IgA isotype (OR: 1.8,P = .001). Both EFS and OS were significantly longer in the absence of Δ13 abnormalities; with low pre-HDT B2M and CRP levels; SDT ≤ 12 months; sensitive disease; and, in contrast to CR, in the absence of IgA isotype (Table1). Primary resistance versus resistant relapse did not independently affect EFS or OS. CR was more durable without Δ13 abnormalities (RR: 0.6, P = .01), SDT ≤ 12 months (RR: 0.6, P < .0001), CRP ≤ 4.0 mg/L (RR: 0.7, P = .02), and B2M ≤ 2.5 mg/L (RR: 0.8,P = .03).
Multivariate analysis of parameters predicting event-free survival and overall survival
| Event-free survival . | RR . | 95% CI . | P . | Overall survival . | RR . | 95% CI . | P . |
|---|---|---|---|---|---|---|---|
| No Δ13 | 0.5 | 0.4-0.5 | <.0001 | No Δ13 | 0.4 | 0.4-0.6 | <.0001 |
| B2M ≤2.5 mg/L | 0.7 | 0.6-0.8 | <.0001 | B2M ≤2.5 mg/L | 0.6 | 0.5-0.7 | <.0001 |
| ≤12 months SDT | 0.7 | 0.6-0.8 | <.0001 | ≤12 mo SDT | 0.7 | 0.6-0.9 | .0001 |
| Sensitive to SDT | 0.8 | 0.7-0.9 | <.0001 | CRP ≤4.0 mg/L | 0.7 | 0.6-0.9 | <.0002 |
| Any 2nd HDT* | 0.8 | 0.6-0.9 | .0004 | Sensitive to SDT | 0.8 | 0.6-0.9 | .0002 |
| Non-IgA isotype | 0.7 | 0.6-0.9 | .002 | (Days to 2nd HDT)−1 * | 0.5 | 0.4-0.6 | .001 |
| Any CR* | 0.8 | 0.6-0.9 | .002 | Any 2nd HDT* | 0.04 | 0.01-0.1 | <.0001 |
| CRP ≤4.0 mg/L | 0.8 | 0.7-0.9 | .03 | Non-IgA isotype | 0.7 | 0.6-0.9 | .002 |
| (Days to 2nd HDT)−1 * | .07 | Any CR* | 0.8 | 0.7-1.0 | .04 |
| Event-free survival . | RR . | 95% CI . | P . | Overall survival . | RR . | 95% CI . | P . |
|---|---|---|---|---|---|---|---|
| No Δ13 | 0.5 | 0.4-0.5 | <.0001 | No Δ13 | 0.4 | 0.4-0.6 | <.0001 |
| B2M ≤2.5 mg/L | 0.7 | 0.6-0.8 | <.0001 | B2M ≤2.5 mg/L | 0.6 | 0.5-0.7 | <.0001 |
| ≤12 months SDT | 0.7 | 0.6-0.8 | <.0001 | ≤12 mo SDT | 0.7 | 0.6-0.9 | .0001 |
| Sensitive to SDT | 0.8 | 0.7-0.9 | <.0001 | CRP ≤4.0 mg/L | 0.7 | 0.6-0.9 | <.0002 |
| Any 2nd HDT* | 0.8 | 0.6-0.9 | .0004 | Sensitive to SDT | 0.8 | 0.6-0.9 | .0002 |
| Non-IgA isotype | 0.7 | 0.6-0.9 | .002 | (Days to 2nd HDT)−1 * | 0.5 | 0.4-0.6 | .001 |
| Any CR* | 0.8 | 0.6-0.9 | .002 | Any 2nd HDT* | 0.04 | 0.01-0.1 | <.0001 |
| CRP ≤4.0 mg/L | 0.8 | 0.7-0.9 | .03 | Non-IgA isotype | 0.7 | 0.6-0.9 | .002 |
| (Days to 2nd HDT)−1 * | .07 | Any CR* | 0.8 | 0.7-1.0 | .04 |
Abbreviations: Δ13, chromosome 13 abnormalities; B2M, β-2-microglobulin; CR, complete remission; CRP, C-reactive protein; SDT, standard-dose therapy; HDT, high-dose therapy; RR, relative risk of experiencing event in favorable versus unfavorable categories; CI, confidence interval.
Time-dependent covariate.
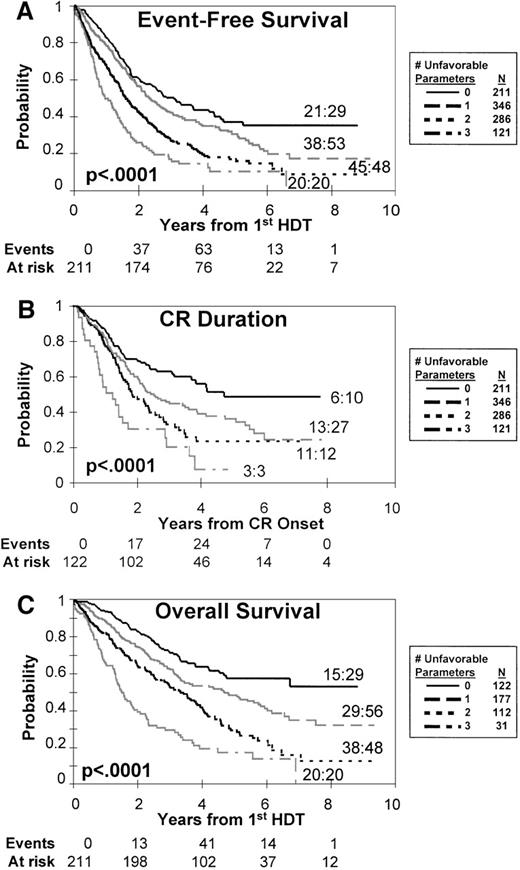
With the use of the 3 dominant standard prognostic factors readily available in most trials (B2M, CRP, SDT duration), 4 distinct risk groups were defined (Figure 1). Whereas plateaus of EFS, OS, and CR duration were noted at 5 years in the presence of only favorable factors, prognosis worsened progressively as the number of risk features increased. Within almost all standard prognostic factor-defined risk groups, Δ13 abnormalities identified a cohort with dismal prognosis with no 5-year CCR or EFS (Table 2; Figure1).
Clinical outcome according to risk.
Influence of the dominant standard prognostic factors (β-2-microglobulin, C-reactive protein, duration of standard therapy prior to high-dose therapy) on event-free survival (A); overall survival (B); and CR duration (C). Four distinct risk groups can be distinguished on the basis of the number of unfavorable features present (for details, see text). Ratios denote the fraction of patients experiencing an event among those presenting with chromosome 13 deletion. Events and at risk values are for curves with 0 unfavorable characteristics.
Clinical outcome according to risk.
Influence of the dominant standard prognostic factors (β-2-microglobulin, C-reactive protein, duration of standard therapy prior to high-dose therapy) on event-free survival (A); overall survival (B); and CR duration (C). Four distinct risk groups can be distinguished on the basis of the number of unfavorable features present (for details, see text). Ratios denote the fraction of patients experiencing an event among those presenting with chromosome 13 deletion. Events and at risk values are for curves with 0 unfavorable characteristics.
Adverse impact of chromosome 13 deletion by risk group
| Risk group . | Δ13 . | N . | %CR . | P . | Percent at 5 years ± standard error . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCR . | P* . | EFS . | P* . | OS . | P* . | |||||
| 0† | No | 182 | 62 | .006 | 52 ± 6.3 | .05 | 41 ± 4.6 | .001 | 62 ± 4.6 | .0005 |
| Yes | 29 | 34 | 0 | 0 | 22 ± 12.7 | |||||
| 1† | No | 288 | 52 | .6 | 37 ± 4.8 | .4 | 34 ± 3.3 | .01 | 52 ± 3.5 | .006 |
| Yes | 56 | 48 | 0 | 0 | 35 ± 8.2 | |||||
| 2† | No | 237 | 42 | .03 | 28 ± 5.7 | .09 | 20 ± 3.3 | <.0001 | 34 ± 4.1 | <.0001 |
| Yes | 48 | 25 | 0 | 0 | 13 ± 5.6 | |||||
| 3† | No | 101 | 28 | .2 | 0 | .5 | 12 ± 4.3 | .0002 | 20 ± 5.1 | <.0001 |
| Yes | 20 | 15 | 0 | 0 | 5 ± 4.9 | |||||
| Total | No | 830 | 47 | .0005 | 35 ± 3.2 | .01 | 28 ± 1.9 | <.0001 | 44 ± 2.2 | <.0001 |
| Yes | 163 | 33 | 0 | 0 | 16 ± 4.9 | |||||
| Risk group . | Δ13 . | N . | %CR . | P . | Percent at 5 years ± standard error . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCR . | P* . | EFS . | P* . | OS . | P* . | |||||
| 0† | No | 182 | 62 | .006 | 52 ± 6.3 | .05 | 41 ± 4.6 | .001 | 62 ± 4.6 | .0005 |
| Yes | 29 | 34 | 0 | 0 | 22 ± 12.7 | |||||
| 1† | No | 288 | 52 | .6 | 37 ± 4.8 | .4 | 34 ± 3.3 | .01 | 52 ± 3.5 | .006 |
| Yes | 56 | 48 | 0 | 0 | 35 ± 8.2 | |||||
| 2† | No | 237 | 42 | .03 | 28 ± 5.7 | .09 | 20 ± 3.3 | <.0001 | 34 ± 4.1 | <.0001 |
| Yes | 48 | 25 | 0 | 0 | 13 ± 5.6 | |||||
| 3† | No | 101 | 28 | .2 | 0 | .5 | 12 ± 4.3 | .0002 | 20 ± 5.1 | <.0001 |
| Yes | 20 | 15 | 0 | 0 | 5 ± 4.9 | |||||
| Total | No | 830 | 47 | .0005 | 35 ± 3.2 | .01 | 28 ± 1.9 | <.0001 | 44 ± 2.2 | <.0001 |
| Yes | 163 | 33 | 0 | 0 | 16 ± 4.9 | |||||
Abbreviations: Δ13, chromosome 13 abnormalities; CR, complete remission; CCR, continuous complete remission; EFS, event-free survival; OS, overall survival.
Log-rank test.
Number of adverse variables (B2M >2.5 mg/L, CRP >4 mg/L, SDT >12 months).
By using time-dependent covariate analysis, both CR and application of a second HDT cycle in a timely fashion were favorable variables in addition to pre-HDT features (see Table 1). Indeed, regardless of Δ13 abnormalities, EFS and OS were longer when a second HDT cycle was applied within 6 months (landmark analysis).15
These data indicate that (1) Δ13 MM should be considered as a separate incurable disease entity even after tandem HDT; (2) 5-year CCR of 52% in the best risk group (no Δ13 abnormalities; low B2M and CRP; SDT ≤ 12 months) is compatible with cure; and (3) dose intensity (timely administration of a second HDT cycle) contributes to extending EFS and OS.16-18 Consequently, programs aimed at curing MM should strive to increase CR rates and apply the key CR-inducing intervention (MEL 200) promptly and probably repeatedly to minimize further mutations and hence additional drug resistance during tumor regrowth. The shorter time-to-null effect (when benefit wears off)19 of a second MEL-based HDT cycle in Δ13 compared with non-Δ13 MM (≤ 3 months vs. 6-12 months, data not shown) indicates that Δ13 MM requires more frequent intensive chemotherapy as in acute leukemia, such as DT-PACE (dexamethasone 40 mg × 4 days, thalidomide 400 mg daily; 4-day continuous IV infusions of cisplatin 10 mg/m2, adrianycin [doxorubicin] 10 mg/m2, cyclophosphamide 400 mg/m2, etoposide 40 mg/m2),20that includes thalidomide as a new active agent in MM.21Thalidomide between HDT cycles and DCEP (dexamethasone 40 mg × 4 days, 4-day continuous IV infusions of cyclophosphamide 400 mg/m2, etoposide 40 mg/m2, cisplatin 10 mg/m2) after tandem HDT may prevent relapses.22 The improved tolerance of non-myeloablative MEL-based allotransplant regimens23 is currently being explored as an important adjunct to exploit a graft-versus-myeloma effect.24 The lack of adverse implications of advanced age and renal insufficiency after controlling for the key biological variables justifies inclusion of such patients in HDT trials.25
Supported in part by grants CA55819 and NCI 5 U10 CA38926-15 from the National Cancer Institute, Bethesda, Maryland.
Dedicated to all myeloma patients toward achieving cure.
Reprints:Bart Barlogie, Myeloma and Transplantation Research Center, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 623, Little Rock, AR 72205.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal