In recent years, the prognosis of chronic myeloid leukemia (CML) has been greatly improved either with interferon- (IFN-) therapy or allogeneic bone marrow transplantation (BMT). In the present study, minimal residual disease was evaluated in 21 patients in complete cytogenetic response (CCR) after such treatments. Samples from bone marrow aspirates or peripheral blood or both were analyzed by conventional cytogenetics, Southern blot, interphase fluorescent in situ hybridization (FISH), and quantitative reverse transcription-polymerase chain reaction (Q-RT-PCR). In all patients, FISH detected 1% to 12% nuclei with a BCR-ABL fusion gene, whereas Q-RT-PCR experiments were negative or weakly positive. Based on these results, we hypothesize that the BCR-ABL genomic rearrangement persists unexpressed in nonproliferating cells whatever the treatment (IFN- or BMT). These data point to the need for follow-up of CML patients in CCR over an extensive period at the DNA level (FISH) to evaluate the residual disease and at the RNA level (Q-RT-PCR) to estimate the risk of relapse.
Chronic myeloid leukemia (CML) is characterized in more than 90% of patients by a reciprocal translocation between chromosomes 9 and 22: translocation t(9;22)(q34;q11), which is referred to as the Philadelphia chromosome (Ph).1,2 At the molecular level, the Ph chromosome results from a rearrangement between the ABL gene located on chromosome 9 and the BCR gene on chromosome 22 forming a BCR-ABL hybrid gene, which is a specific marker of the disease.3,4 In recent years, the prognosis of CML has been improved by allogeneic bone marrow transplantation (BMT) or interferon-α (IFN-α) therapy. It has been shown that CML can be cured by allografting and that IFN-α (with or without cytarabine) is able to induce a complete cytogenetic response in up to 26% of patients treated at diagnosis.5 In such cases, the question of maintenance therapy (dose and duration) remains unresolved. Hence, the definition of complete remission must be as accurate as possible.
In a preliminary study,6 we used cytogenetics, interphase fluorescent in situ hybridization (FISH), and quantitative reverse transcription-polymerase chain reaction (Q-RT-PCR) methods to study minimal residual disease in 3 patients with CML in sustained complete cytogenetic remission after treatment with IFN-α. The BCR-ABL fusion gene was still detectable by FISH, whereas RT-PCR remained negative or weakly positive. However, the precision of the percentage of BCR-ABL-positive nuclei was hampered by the rather high percentage of false-positive nuclei due to the use of BCR-ABL DNA probes that detected only a single BCR-ABL fusion signal. New FISH probes (D-FISH probes) are now available, and they detect BCR-ABL fusion in interphase nuclei with a false-positive signal rate close to zero.7 8Such probes were used in the current study, which was performed in 21 CML patients in sustained complete cytogenetic remission, either after IFN-α therapy (11 cases) or allogeneic BMT (10 cases). To monitor the minimal residual disease, conventional cytogenetics, Southern blot, FISH, and Q-RT-PCR were performed. Persistence of the BCR-ABL genomic rearrangement was evidenced by FISH in all cases, whereas most Q-RT-PCR experiments remained negative or weakly positive.
Patients and methods
Patients
Cells from 21 consecutive CML patients (10 men and 11 women) were studied. At presentation, 17 patients presented the classical t(9;22)(q34;q11) translocation (additional abnormalities were detected in a single case) and 4 patients had a complex chromosomal rearrangement (Tables 1 and2). The molecular rearrangement in M-BCR (major-breakpoint cluster region) was attested by Southern blot analysis. The type of chimeric transcript was assessed by qualitative RT-PCR experiments; all patients had the typical b2a2 or b3a2 transcript. After treatment with IFN-α or allogeneic BMT, the monitoring of the residual disease mainly involved cytogenetic analyses. When the patient had reached a partial or complete cytogenetic response, FISH, Southern blot (in the majority of cases), and Q-RT-PCR were performed together with further cytogenetic analyses. Informed consent was obtained as required by the Declaration of Helsinki.
Clinical data in 11 CML patients treated with IFN-
| Patient No. . | Sex/Age at Diagnosis . | Sokal's Score . | Karyotype at Presentation [% Ph+] . | Treatment . | Duration of Follow-Up (mos) . | Duration of CCR (mos) . |
|---|---|---|---|---|---|---|
| 1 | M/27 | 0.83 | t(9;22)(q34;q11) [100%] | HU, IFN-α2a, Ara-c | 150 | 120 |
| 2 | M/46 | 0.61 | t(9;22)(q34;q11) [100%] | HU, BU, IFN-α2a, Ara-c | 183 | 108 |
| 3 | M/68 | 0.79 | t(9;22)(q34;q11) [91%] | HU, IFN-α2b, Ara-c | 51 | 36 |
| 4 | M/70 | 0.83 | t(9;22)(q34;q11) [94%] | HU, IFN-α2b, Ara-c | 127 | 36 |
| 5 | F/53 | 0.72 | t(1;9;22)(q41;q34;q11) [95%] | HU, IFN-α2a, Ara-c | 144 | 12 |
| 6 | F/43 | 0.83 | t(9;22)(q34;q11) [100%] | HU, BU, IFN-α2a | 146 | 72 |
| 7 | M/56 | 0.71 | t(9;13;22)(q34;q12;q11) [74%] | HU, IFN-α2b | 57 | 36 |
| 8 | M/52 | 0.82 | t(2;9;22)(p13;q34;q11) [12%] | HU, IFN-α2b | 59 | 36 |
| 9 | F/35 | 0.61 | t(9;22)(q34;q11) [100%] | HU, BU, IFN-α2a | 170 | 36 |
| 10 | F/57 | 1.5 | t(9;11;18;22)(q34;q13;p11;q11) [100%] | HU, IFN-α2b | 47 | 24 |
| 11 | M/60 | 0.79 | t(9;22)(q34;q11) [17%] | HU, IFN-α2b, Ara-c | 41 | 36 |
| Patient No. . | Sex/Age at Diagnosis . | Sokal's Score . | Karyotype at Presentation [% Ph+] . | Treatment . | Duration of Follow-Up (mos) . | Duration of CCR (mos) . |
|---|---|---|---|---|---|---|
| 1 | M/27 | 0.83 | t(9;22)(q34;q11) [100%] | HU, IFN-α2a, Ara-c | 150 | 120 |
| 2 | M/46 | 0.61 | t(9;22)(q34;q11) [100%] | HU, BU, IFN-α2a, Ara-c | 183 | 108 |
| 3 | M/68 | 0.79 | t(9;22)(q34;q11) [91%] | HU, IFN-α2b, Ara-c | 51 | 36 |
| 4 | M/70 | 0.83 | t(9;22)(q34;q11) [94%] | HU, IFN-α2b, Ara-c | 127 | 36 |
| 5 | F/53 | 0.72 | t(1;9;22)(q41;q34;q11) [95%] | HU, IFN-α2a, Ara-c | 144 | 12 |
| 6 | F/43 | 0.83 | t(9;22)(q34;q11) [100%] | HU, BU, IFN-α2a | 146 | 72 |
| 7 | M/56 | 0.71 | t(9;13;22)(q34;q12;q11) [74%] | HU, IFN-α2b | 57 | 36 |
| 8 | M/52 | 0.82 | t(2;9;22)(p13;q34;q11) [12%] | HU, IFN-α2b | 59 | 36 |
| 9 | F/35 | 0.61 | t(9;22)(q34;q11) [100%] | HU, BU, IFN-α2a | 170 | 36 |
| 10 | F/57 | 1.5 | t(9;11;18;22)(q34;q13;p11;q11) [100%] | HU, IFN-α2b | 47 | 24 |
| 11 | M/60 | 0.79 | t(9;22)(q34;q11) [17%] | HU, IFN-α2b, Ara-c | 41 | 36 |
CML indicates chronic myeloid leukemia; HU, hydroxyurea; BU, busulfan; IFN, interferon; Ara-c, cytosine arabinoside; CCR, complete cytogenetic remission.
Clinical data in 10 CML patients treated with BMT
| Patient No. . | Sex/Age at Diagnosis . | Gratwohl's Score . | Karyotype at Presentation [% Cell Ph+] . | Therapy Before BMT . | Duration of Follow-Up (mos) . | Duration of CCR (mos) . |
|---|---|---|---|---|---|---|
| 12 | F/44 | 3 | t(9;22)(q34;q11) [95%] | HU, IFN-α2b, Ara-c | 32 | 4 |
| 13 | M/22 | 2 | t(9;22)(q34;q11) [100%] | HU, splenectomy | 56 | 65 |
| 14 | F/28 | 0 | t(9;22)(q34;q11) [54%] | HU, IFN-α2a | 95 | 36 |
| 15 | M/35 | 3 | t(9;22)(q34;q11)del(7)(p11p21) [100%] | HU, IFN-α2b, Ara-c | 37 | 12 |
| 16 | F/15 | 0 | t(9;22)(q34;q11) [81%] | HU, IFN-α2a | 40 | 24 |
| 17 | F/48 | 2 | t(9;22)(q34;q11) [100%] | HU, IFN-α2b | 47 | 24 |
| 18 | F/34 | 1 | t(9;22)(q34;q11) [100%] | HU | 76 | 60 |
| 19 | F/28 | 1 | t(9;22)(q34;q11) [100%] | HU | 29 | 18 |
| 20 | M/13 | 2 | t(9;22)(q34;q11) [100%] | HU, BU, IFN-α2b | 84 | 44 |
| 21 | F/41 | 3 | t(9;22)(q34;q11) [97%] | HU, IFN-α2b, Ara-c | 47 | 24 |
| Patient No. . | Sex/Age at Diagnosis . | Gratwohl's Score . | Karyotype at Presentation [% Cell Ph+] . | Therapy Before BMT . | Duration of Follow-Up (mos) . | Duration of CCR (mos) . |
|---|---|---|---|---|---|---|
| 12 | F/44 | 3 | t(9;22)(q34;q11) [95%] | HU, IFN-α2b, Ara-c | 32 | 4 |
| 13 | M/22 | 2 | t(9;22)(q34;q11) [100%] | HU, splenectomy | 56 | 65 |
| 14 | F/28 | 0 | t(9;22)(q34;q11) [54%] | HU, IFN-α2a | 95 | 36 |
| 15 | M/35 | 3 | t(9;22)(q34;q11)del(7)(p11p21) [100%] | HU, IFN-α2b, Ara-c | 37 | 12 |
| 16 | F/15 | 0 | t(9;22)(q34;q11) [81%] | HU, IFN-α2a | 40 | 24 |
| 17 | F/48 | 2 | t(9;22)(q34;q11) [100%] | HU, IFN-α2b | 47 | 24 |
| 18 | F/34 | 1 | t(9;22)(q34;q11) [100%] | HU | 76 | 60 |
| 19 | F/28 | 1 | t(9;22)(q34;q11) [100%] | HU | 29 | 18 |
| 20 | M/13 | 2 | t(9;22)(q34;q11) [100%] | HU, BU, IFN-α2b | 84 | 44 |
| 21 | F/41 | 3 | t(9;22)(q34;q11) [97%] | HU, IFN-α2b, Ara-c | 47 | 24 |
CML indicates chronic myeloid leukemia; BMT, bone marrow transplantation; HU, hydroxyurea; IFN, interferon; Ara-c, cytosine arabinoside; BU, busulfan; CCR, complete cytogenetic remission.
Eleven patients (7 men and 4 women, median age at diagnosis 53 years, range 27-70 years) were studied at the time of diagnosis and during IFN-α therapy (see Table 1). All were in chronic phase at presentation, most with a low (6 cases) or intermediate score (4 cases) according to Sokal's index.9 These patients were treated, using various doses of hydroxyurea, IFN-α, and cytarabine, based on data from 3 consecutive trials.10-12 A cytogenetic evaluation was performed every 3 or 4 months for the first 12 months and then at 4- or 6-month intervals. All patients achieved a complete cytogenetic response (CCR) after a median treatment period of 24 months (range, 5-40 months). The median follow-up of these patients is 127 months (range, 41-183 months). Currently, all patients are alive and in persistent CCR.
Ten other patients (3 men and 7 women, median age at diagnosis 31 years, range 13-48 years) received an allogeneic non–T-cell-depleted bone marrow transplant from an HLA-identical sibling donor (cases 12-14, 16-19, and 21) or matched unrelated donor (cases 15 and 20) after a median period of treatment with IFN-α or chemotherapy (mainly hydroxyurea) or both of 18 months since diagnosis (range, 3-57) (see Table 2). Patients 13 and 19 received a sex-mismatched allogeneic BMT. Most patients had at diagnosis a moderate Gratwohl's score.13 All patients were in first chronic phase, and most of them underwent transplantation because of treatment failure with no cytogenetic response. Patient 13, who had massive splenomegaly, underwent splenectomy before transplant. The conditioning regimen was either total body irradiation and cyclophosphamide or busulfan and cyclophosphamide. All patients received methotrexate (4 days) and cyclosporine as prophylaxis for graft-versus-host disease. The median follow-up time from diagnosis for the 10 BMT patients is 47 months (range, 29-95 months). All patients had normal karyotypes after grafting. Currently, patients 12, 13, 14, 17, 18, and 21 are well; patients 15, 19, and 20 are affected by a chronic graft-versus-host disease; and patient 16 is well after a donor lymphocyte infusion.
Cytogenetic and FISH analysis
Bone marrow aspirates or peripheral blood samples or both were obtained for simultaneous cytogenetic and molecular studies. Chromosome analysis was performed according to the R-banding method after short (24- or 48-hour) cultures without addition of mitogens. FISH analysis was performed on unseparated nucleated cells from cytogenetic preparations or fresh blood samples, using differently colored, directly labeled BCR and ABL probes developed by ONCOR (D-FISH, Gaithersburg, MD) according to manufacturer's instructions. A minimum of 200 cells in interphase were scored for each sample. Normal cells display 2 red signals and 2 green signals (2R2G), and abnormal cells 1 red, 1 green, and 2 yellow fusion signals (1R1G2F) or 2 red, 2 green, and 1 yellow fusion signals (2R2G1F) or 1 red, 1 green, and 3 yellow fusion signals (1R1G3F) indicating 2 Ph chromosomes.7 8 The positivity pattern for each specimen was recorded and final results were expressed as percentages of nuclei with fusion signals.
Negative FISH control studies were performed with blood or bone marrow smears from 5 patients with hematologic disorders other than CML. A total of 1000 interphase nuclei were scored and a single nucleus with a positive pattern (2R2G1F) was found. The cutoff limit for BCR-ABL positivity was hence set at the mean of normal result (0.1% + 3 SD, i.e., 0.3%). Positive controls were also performed with bone marrow cultures or uncultured blood smears from 6 patients in chronic phase of CML with a percentage of Ph+ metaphases ranging from 50% to 100%. By FISH, the percentage of positive interphase cells was similar to that of positive metaphases (range, 57-100%).
In the 2 sex-mismatched BMT cases (patients 13 and 19), the chimerism was studied by FISH using specific Y and X chromosome probes: LSI SRY probe (VYSIS Inc, Downers Grove, IL) and chromosome X alpha satellite probe (DXZ1, ONCOR, Gaithersburg, MD), respectively.
Southern blot
DNA for Southern blot analysis was available from patients 1 through 6, 8 through 11, 13, 14, and 20. DNA (15 μg) was digested with BglII restriction enzyme, electrophoresed on a 0.8% agarose gel, and transferred onto a nylon filter. After 32P-labeling, thephl/bcr-3 fragment (Oncogene Science, Uniondale, NY) was used as a probe. In the absence of the BCR-ABL translocation at the M-BCR locus, 3 DNA fragments were visualized. When a translocation had occurred, 1 or 2 additional fragments were present. To compare results from FISH analysis and Southern blot, mixtures of normal and 100% Ph+ cell samples at different ratios were prepared (Table3). Rearranged fragments observed by Southern blot were semiquantified by densitometry. In every dilution, the percentages of Ph+ metaphases and positive nuclei by FISH were similar. For the same dilutions, the percentage of rearranged fragments appreciated by densitometry after Southern blot was approximately half the percentage of Ph+ cells. A pure leukemic cell population has 1 normal chromosome 22 and 1 Ph+ chromosome. Every cell in such a population is positive by FISH (100% of positive nuclei), whereas the percentage of rearranged DNA fragments observed by Southern blot is theoretically close to 50%.14 Therefore, results from these 2 methods are not directly equivalent, the first yielding a percentage of nuclei with at least 1 BCR-ABL rearrangement, the other allowing an estimation of the rearrangement in M-BCR at the DNA level.
Correlation between FISH and Southern blot analyses
| Percentage of Ph+ Cells . | FISH Percentage of Positive Nuclei . | Southern Blot Percentage of Rearranged Fragments . |
|---|---|---|
| 100 | 94% | 47% |
| 50 | 47% | 31% |
| 10 | 10% | 6% |
| 7 | 4% | (a) |
| 4 | 2% | (a) |
| 2 | 2% | Negative |
| 0 | Negative | Negative |
| Percentage of Ph+ Cells . | FISH Percentage of Positive Nuclei . | Southern Blot Percentage of Rearranged Fragments . |
|---|---|---|
| 100 | 94% | 47% |
| 50 | 47% | 31% |
| 10 | 10% | 6% |
| 7 | 4% | (a) |
| 4 | 2% | (a) |
| 2 | 2% | Negative |
| 0 | Negative | Negative |
Dilutions of Ph+ cells in Ph− cells were analyzed by FISH and Southern blot. Results are shown as the percentage of positive nuclei for FISH and as an estimation of the relative intensity of the rearranged bands for Southern blot; (a) represents the presence of weak bands in Southern blot that could not be quantified.
Qualitative and quantitative RT-PCR
Total leukocyte RNA was extracted from the peripheral blood because Q-RT-PCR for the monitoring of residual disease in CML yields similar results on blood or bone marrow samples.15 RNA was extracted according to Chomczynski and Sacchi16 and controlled by electrophoresis on 1% agarose gel. Reverse transcription and qualitative and quantitative RT-PCR for ABL-mRNA and p210 BCR-ABL-mRNA were performed according to a standardized protocol using serial dilutions of a p210 competitor ranging from 1 to 107molecules.15 17 Results were expressed as the BCR-ABL/ABL mRNA ratio. In our hands, the method is able to detect as few as 3 BCR-ABL mRNA molecules in the assay. On average, 150,000 ABL transcript molecules per assay are found. Therefore, the sensitivity of the amplification can be estimated by a BCR-ABL/ABL ratio close to 0.002% (3/150,000). In this study, a RT-PCR is considered as negative when the BCR-ABL/ABL ratio is equal to or lower than 0.002%.
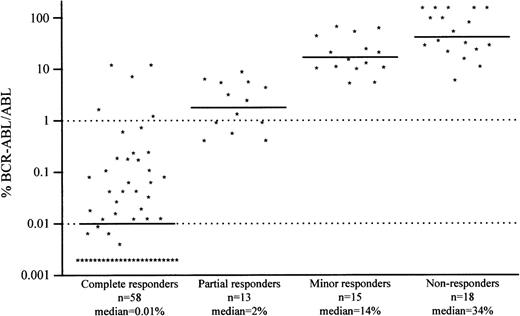
Quantitative RT-PCR was compared with cytogenetic findings in 104 CML cases treated with IFN-α in our institution (including the 11 patients in CCR of the present study). Cytogenetic responders were considered as complete responders (Ph+ = 0%), partial responders (0% < Ph+ ≤ 34%), minor responders (34% < Ph+ ≤ 94%), and nonresponders (Ph+ > 94%), with median BCR-ABL/ABL ratios of 0.01%, 2%, 14%, and 34%, respectively (Figure1). According to these results, we defined 2 arbitrary values (1% and 0.01%) for the BCR-ABL/ABL ratio. A ratio below 1% is generally associated with a CCR and a ratio below 0.01% with a long and stable CCR.
Comparison of the BCR-ABL/ABL ratio with the cytogenetic response status.
Results include 104 CML cases treated with IFN-α in our institution (including the 11 patients in CCR of the present study).
Comparison of the BCR-ABL/ABL ratio with the cytogenetic response status.
Results include 104 CML cases treated with IFN-α in our institution (including the 11 patients in CCR of the present study).
Results
Cytogenetic analysis
At the time of FISH study, 10 of the 11 patients treated with IFN-α had been in CCR for 2 to 10 years. The last patient (no. 5) achieved a complete response 1 year before FISH study, but very few positive metaphases (18/642 Ph+ metaphases in 7/22 samples) had been found during the 9 previous years of follow-up. At the time of study, karyotypic analysis failed to detect any Ph+ metaphases in the samples from the 10 patients who received BMT.
FISH and Southern blot experiments
In all 21 patients, FISH analyses detected a BCR-ABL fusion gene (Table 4). The percentage of fusion signals ranged from 1% to 12% nuclei (median 6-7%). Patients 1, 2, and 3, with 1% to 2% positive nuclei by FISH, were analyzed at 3 consecutive times with identical results. There was no significant relationship between percentages of positive nuclei and duration of CCR or BCR-ABL/ABL ratios. However, patients 1 and 2 (CCR > 9 years) showed the lowest percentages of both positive nuclei by FISH (2% and 1%, respectively) and BCR-ABL/ABL ratio (< 0.002%).
Results of FISH, Southern blot, Q-RT-PCR in 21 CML patients in CCR at the time of study
| Patient No. . | No. of Metaphases Analyzed During Remission . | FISH BCR-ABL Fusion Signal . | Southern Blot (Percentage of Rearranged DNA Fragments) . | Q-RT-PCR BCR-ABL/ABL Ratio . |
|---|---|---|---|---|
| 1 | 1047 | 2% (BM) | Negative | <0.002% |
| 2 | 882 | 1% (Blood) | Negative | <0.002% |
| 3 | 263 | 2% (BM) | Negative | <0.002% |
| 4 | 191 | 6% (BM) | Positive (<5%) | 0.12% |
| 5 | 62 | 7% (BM) | Positive (5-10%) | 0.2% |
| 6 | 508 | 2% (BM) | Positive (<5%) | 0.006% |
| 7 | 235 | 12% (Blood) | ND | <0.002% |
| 8 | 229 | 9% (BM) | Negative | 0.02% |
| 9 | 395 | 3% (Blood) | Positive (<5%) | 0.02% |
| 10 | 138 | 7% (BM) | Positive (<5%) | 0.17% |
| 11 | 255 | 9% (BM) | Negative | <0.002% |
| 12 | 94 | 5% (BM) | ND | <0.002% |
| 13 | 306 | 6% (Blood) | Negative | <0.002% |
| 14 | 276 | 8% (Blood) | Positive (<5%) | <0.002% |
| 15 | 100 | 6% (Blood) | ND | <0.002% |
| 16 | 74 | 6% (BM) | ND | <0.002% |
| 17 | 133 | 11% (Blood) | ND | <0.002% |
| 18 | 219 | 7% (BM) | ND | <0.002% |
| 19 | 111 | 3% (BM) | ND | <0.002% |
| 20 | 284 | 11% (BM) | Positive (<5%) | <0.002% |
| 21 | 175 | 7% (BM) | ND | <0.002% |
| Patient No. . | No. of Metaphases Analyzed During Remission . | FISH BCR-ABL Fusion Signal . | Southern Blot (Percentage of Rearranged DNA Fragments) . | Q-RT-PCR BCR-ABL/ABL Ratio . |
|---|---|---|---|---|
| 1 | 1047 | 2% (BM) | Negative | <0.002% |
| 2 | 882 | 1% (Blood) | Negative | <0.002% |
| 3 | 263 | 2% (BM) | Negative | <0.002% |
| 4 | 191 | 6% (BM) | Positive (<5%) | 0.12% |
| 5 | 62 | 7% (BM) | Positive (5-10%) | 0.2% |
| 6 | 508 | 2% (BM) | Positive (<5%) | 0.006% |
| 7 | 235 | 12% (Blood) | ND | <0.002% |
| 8 | 229 | 9% (BM) | Negative | 0.02% |
| 9 | 395 | 3% (Blood) | Positive (<5%) | 0.02% |
| 10 | 138 | 7% (BM) | Positive (<5%) | 0.17% |
| 11 | 255 | 9% (BM) | Negative | <0.002% |
| 12 | 94 | 5% (BM) | ND | <0.002% |
| 13 | 306 | 6% (Blood) | Negative | <0.002% |
| 14 | 276 | 8% (Blood) | Positive (<5%) | <0.002% |
| 15 | 100 | 6% (Blood) | ND | <0.002% |
| 16 | 74 | 6% (BM) | ND | <0.002% |
| 17 | 133 | 11% (Blood) | ND | <0.002% |
| 18 | 219 | 7% (BM) | ND | <0.002% |
| 19 | 111 | 3% (BM) | ND | <0.002% |
| 20 | 284 | 11% (BM) | Positive (<5%) | <0.002% |
| 21 | 175 | 7% (BM) | ND | <0.002% |
For each patient, FISH, Southern blot (when done), and Q-RT-PCR experiments were simultaneously performed. FISH was carried out on bone marrow (BM) or blood samples. Southern blot and Q-RT-PCR were performed on blood samples. Patients 1-11 were treated with IFN-α and patients 12-21 received allogeneic BMT. IFN indicates interferon; BMT, bone marrow transplantation; FISH, fluorescent in situ hybridization; Q-RT-PCR, quantitative reverse transcription-polymerase chain reaction; CML, chronic myeloid leukemia; CCR, complete cytogenetic remission; ND, not done.
In 2 BMT cases, donor and recipient were of different sex (patients 13 and 19). Because of the presence of a significant percentage of BCR-ABL rearranged cells in CML patients in CCR after BMT, the presence of residual host cells harboring the BCR-ABL rearrangement was suspected. To test this hypothesis, FISH with X and Y probes was performed from the same samples as those studied by FISH with BCR and ABL probes. In patient 19 (female recipient and male donor), 3% of XX host cells were detected as compared with 3% of cells with BCR-ABL fusion signals. In patient 13 (male recipient, female donor), 4% of XY-positive nuclei were detected as compared with 6% of cells with BCR-ABL fusion signals. Moreover, 2 years before the present study, Y chromosome sequences were detected by PCR18 in patient 13, and FISH analyses on the same samples revealed 6% of BCR-ABL rearranged cells and 5% of XY nuclei.
Southern blot analyses were negative in patients 1, 2, 3, 8, 11, and 13, but showed weak rearranged bands (< 5% of rearranged DNA fragments) in patients 4, 6, 9, 10, 14, and 20. In patient 5, in whom a CCR occurred only during the last year preceding FISH, rearranged bands reached a level of about 5% to 10%. Such discrepancies between FISH and Southern blot results can be explained by a number of rearranged cells of about 6% to 7%, which corresponds to a percentage of rearranged DNA fragments close to the detection limit of the Southern blot method.
Evolution of the BCR-ABL/ABL ratio
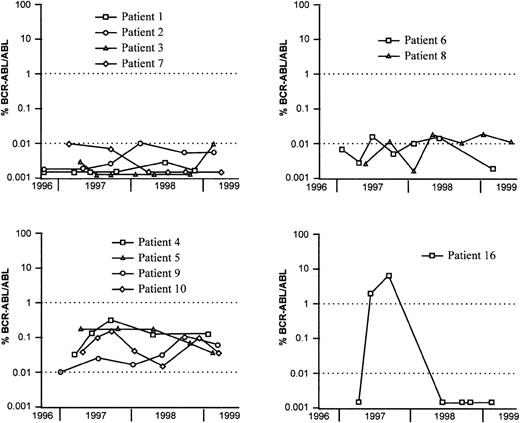
The kinetics of the BCR-ABL/ABL ratio was evaluated by Q-RT-PCR during the last 2 years of the study. In patients 1, 2, 3, and 7 (IFN-α therapy) and patients 12 through 15 and 17 through 21 (BMT), BCR-ABL/ABL ratios were stable and remained below 0.01% (Figure2). These ratios fluctuated close to 0.01% for patients 6 and 8 and varied between 0.01% and 1% for patients 4, 5, 9, 10, and 11. In summary, most patients who received BMT had persistent BCR-ABL/ABL ratios below 0.01% (FISH median 7%). Among the CML patients treated with IFN-α, 6 showed similar kinetics, with BCR-ABL/ABL ratios below or close to 0.01% (FISH median 2%) and 5 had ratios above this value (FISH median 7%). In patient 16, a molecular relapse occurred after 2 years of CCR and was followed by a cytogenetic relapse 2 months later. Infusion of the BMT donor's lymphocytes resulted in a second CCR with BCR-ABL/ABL ratio below 0.01% and FISH at 6% (see Figure 2).
Evolution of the BCR-ABL/ABL ratio in CML patients treated with IFN- (patients 1-10) or BMT (patient 16).
The BCR-ABL/ABL ratio kinetics for patient 16 is characteristic of a transient relapse. Other patients in CCR after BMT are not shown because they usually have BCR-ABL/ABL ratios close to 0.002%.
Evolution of the BCR-ABL/ABL ratio in CML patients treated with IFN- (patients 1-10) or BMT (patient 16).
The BCR-ABL/ABL ratio kinetics for patient 16 is characteristic of a transient relapse. Other patients in CCR after BMT are not shown because they usually have BCR-ABL/ABL ratios close to 0.002%.
Discussion
In a preliminary study of cells from 3 CML patients treated with IFN-α in long CCR,6 we observed the persistence of BCR-ABL rearranged cells detected by FISH, whereas conventional cytogenetics was negative and Q-RT-PCR negative or weakly positive. We concluded that the malignant cells harboring the genomic BCR-ABL rearrangement were nonproliferating neoplastic cells. These cells did not express significant levels of BCR-ABL transcript and a fortiori did not synthesize the chimeric p210BCR-ABLprotein. In the present study, we extended our preliminary data by using cytogenetics, Southern blot, FISH, and Q-RT-PCR methods to 21 patients in sustained CCR after treatment with IFN-α or BMT.
To accurately monitor the residual disease in CML, the methods must be well standardized, especially for FISH and Q-RT-PCR methods. In our preliminary study,6 the level of false-positive results by FISH reached 3% to 6%, whereas in the present study, using more specific BCR and ABL probes, they were close to 0.1%. Comparison between FISH and Southern blot showed that FISH is more appropriate for the quantification of small percentages (1-5%) of rearranged cells than is Southern blot, in accordance with previous data showing that a linear relationship between the percentages of rearranged fragments and of Ph+ cells is observed only in the range 20% to 100% of Ph+ cells.14 Moreover, weak bands on autoradiographs could not be correctly quantified. When the BCR-ABL/ABL ratio determined by Q-RT-PCR was compared with the cytogenetic response status of the patient, the results were similar to those published by Hochhaus et al.15 These results allowed us to define 2 cutoff points of 1% and 0.01%. Most patients in CCR have a BCR-ABL/ABL ratio below 1%, and most patients in long and stable CCR have a BCR-ABL/ABL ratio below 0.01%. It is thus important to underline the peculiar objective of cytogenetics, FISH, Southern blot, and Q-RT-PCR for the monitoring of patients with CML. (1) Conventional cytogenetics analyzes cycling cells, with results being expressed as the percentage of metaphases harboring the translocation t(9;22). (2) FISH methods test every cell with results in percentages of positive nuclei harboring the BCR-ABL genomic rearrangement. (3) Southern blot detects M-BCR rearrangements at the DNA level, and results are given in a boolean manner (presence or absence of rearranged DNA fragments) with a percentage of rearranged DNA estimated by densitometry. (4) Q-RT-PCR does not quantify the percentage of malignant cells, but BCR-ABL fusion mRNA and the results are expressed by the BCR-ABL/ABL ratio. Therefore, these methods are not equivalent but complementary.
In the patients in long CCR after IFN-α therapy, we confirm the presence of rearranged cells (median 6-7%) in the absence of significant levels of BCR-ABL transcripts. This result is not related to a lack of sensitivity of Q-RT-PCR experiments because, in our hands, this method can detect as few as 3 BCR-ABL mRNA molecules in the assay. It is noteworthy that, in our experience, we have never seen CML patients in CCR with D-FISH negative. However, the percentage of FISH-positive cells seems to be weaker in patients in very long CCR. Such apparent discrepancies between cytogenetics or FISH or Southern blot and RT-PCR have been already mentioned.19-26 In most of these reports, studies were performed after cultures of hematopoietic progenitor cells from untreated patients with CML,19-22,26 and it has been suggested that the BCR-ABL translocation was present in the most primitive hematopoietic progenitor cells, which might have been quiescent and transcriptionally silent. In our previous study, we postulated that this observation could be the consequence of the molecular action of IFN-α. Actually, IFN-α exerts various activities on cellular proliferation and immunoregulation.27,28 However, FISH analysis of the cells of the 10 patients in CCR after BMT showed similar results as after IFN-α therapy (6-7% of rearranged cells). In the 2 sex-mismatched BMT, we showed that a significant minority (3-6%) of hematopoietic cells are of host origin. These findings suggest that the persistence of nonproliferating neoplastic cells is a general feature of CML patients in CCR whatever the treatment. FISH has been performed from unseparated blood or bone marrow nucleated cells, and the nature of the FISH-positive cells could not be easily determined. One could speculate that some of the FISH-positive cells could represent residual lymphocytes in the IFN-α responders, but this explanation is improbable in the patients after BMT. The reasons for this persistence state and for the nonexpansion of the malignant clone are unclear. An early and transient relapse occurred in 1 of the 21 patients studied (patient 16 treated with BMT). It remains possible that the rearranged cells might re-enter the cell cycle, leading to the expansion of the malignant clone. In this situation, RT-PCR follow-up may be a precious help to predict a relapse.29 For example, in a patient not included in this study, we detected a molecular relapse by Q-RT-PCR in the absence of Ph+ metaphase and 7% positive nuclei by FISH. This patient was previously in CCR after allogeneic BMT; Q-RT-PCR was negative at 3 consecutive times during 7 months of follow-up, until the occurrence of a sudden and persistent increase of the BCR-ABL/ABL ratio (10%, then 50%) that preceded cytogenetic relapse and an acute myeloid transformation.
In summary, a significant number of nonproliferating neoplastic cells persist in patients with CML whatever the treatment (IFN-α or BMT). These data would provide a rational explanation for the relapses observed after allografting. However, a substantial number of patients treated by IFN-α and most patients who received BMT do not relapse over an extensive period. This suggests that the residual BCR-ABL-positive cells detected by FISH in CML patients in CCR may be dormant according to the definition of Uhr et al.30 Their oncogenic potential could be then controlled by various mechanisms such as specific immune response.31-33 Because the nature of the nonproliferating BCR-ABL cells attested by FISH is still unknown, a potential risk of relapse exists for patients with CML as long as they have these rearranged hematopoietic cells. These data indicate the need for a follow-up of patients with CML in CCR over an extensive period at the DNA level (FISH) to evaluate the residual disease and at the RNA level (Q-RT-PCR) to estimate the risk of relapse.
Acknowledgment
The authors are grateful to Pr Jean Louis Preud'homme for helpful comments.
Supported by the Association pour la Recherche contre le Cancer and the Ligue Nationale contre le Cancer (comité de Charente-Maritime).
Reprints:Alain Kitzis, Laboratoire de Génétique Cellulaire et Moléculaire, CHU de Poitiers, BP 577, 86021 Poitiers Cedex, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal