The purpose of our study was to investigate the efficacy of an acute lymphoblastic leukemia (ALL)-type treatment with moderate-dose, prophylactic cranial irradiation and without local radiotherapy for childhood T-cell lymphoblastic lymphoma (T-LBL). From April 1990 to March 1995, 105 evaluable patients, 1.1 to 16.4 years of age, with T-LBL were enrolled in study NHL-BFM 90 (non-Hodgkin's lymphoma–Berlin-Frankfurt-Munster 90). They received an 8-drug induction over 9 weeks followed by an 8-week consolidation including methotrexate (MTX) 5 g/m2 × 4. Patients with stage I (n = 2) and II (n = 2) continued with maintenance therapy (6-mercaptopurine daily and MTX weekly, both orally) until a total therapy duration of 24 months. Patients with stage III (n = 82) and IV (n = 19) received an 8-drug intensification over 7 weeks and cranial radiotherapy (12 Gy for prophylaxis) after consolidation, followed by maintenance. Residual tumor after induction had to be resected. Patients received intensified chemotherapy if tumor regression on day 33 of induction was less than 70% or when vital residual tumor was present after the complete induction phase. With a median follow-up of 4.5 years, the estimated event-free survival at 5 years is 90% (95% confidence interval, 82%-100%). Events were 1 early death, 8 tumor failures, and 1 secondary acute myeloid leukemia. A total of 101 patients were evaluable for the speed of tumor response. Two patients received intensified therapy due to less than 70% tumor regression on day 33. Of 19 patients with tumor residues after induction, 2 relapsed as compared to 4 of 80 patients with complete tumor regression. We conclude that, with intensive ALL-type chemotherapy including moderate cumulative doses of anthracyclines 240 mg/m2 and cyclophosphamide (3 g/m2) and moderate-dose prophylactic cranial irradiation but no local radiotherapy, an event-free survival rate of 90% can be achieved in childhood T-LBL.
T-cell lymphoblastic lymphoma (T-LBL) accounts for most cases of childhood non-Hodgkin's lymphoma (NHL) of T-cell lineage.1 Intensive combinations of chemotherapy with or without local radiotherapy (LRT) resulted in event-free survival rates of 60% to 70% for children suffering from lymphoblastic lymphoma.1-9 Important unsolved issues are the optimization of induction therapy, the value of maintenance therapy, presymptomatic central nervous system (CNS) therapy, and local tumor control. Although local manifestations are the most frequent site of failure,1,7,8 the value of local therapy modalities is not yet clear. LRT was mandatory for bulky disease in some studies3,9 or confined to patients with incomplete tumor resolution in others.1,7,8 LRT is certainly effective local therapy;10 however, mediastinal irradiation in particular carries serious late risks.11-13 The contribution of LRT to failure-free survival in addition to chemotherapy may depend on the efficacy of the chemotherapy applied.
In the Berlin-Frankfurt-Munster (BFM) trials on childhood NHL, patients with T-LBL were treated according to the strategy for acute lymphoblastic leukemia (ALL).1,3,14 15 Chemotherapy differed only slightly between the consecutive studies. Preventive CNS therapy consisted of cranial radiation therapy (CRT), intrathecal methotrexate (MTX), and intravenous MTX. The dose of prophylactic CRT was reduced from 18 Gy to 12 Gy, and high-dose MTX 5 g/m2 was introduced. LRT was mandatory in the first study, NHL-BFM 75, and was restricted to residual tumors after completion of induction in the subsequent trials. In study NHL-BFM 90, treatment was stratified according to tumor response to induction therapy. Local irradiation was omitted completely. Moreover, the maximal cumulative dose of anthracyclines was reduced from 280 mg/m2 in the preceding study, NHL-BFM 86, to 240 mg/m2. In compensation, the dose intensity over time was increased by condensation of induction therapy by 1 week. Here we report on treatment strategy and results in 105 evaluable patients with T-LBL treated in study NHL-BFM 90.
Patients and methods
Patients
Children and adolescents up to age 18 with NHL were eligible for trial NHL-BFM 90. Exclusion criteria were human immunodeficiency virus infection, severe immunodeficiency, posttransplantation lymphoma, NHL as a second malignancy, previous cytostatic treatment, and preexisting disease prohibiting chemotherapy. From April 1990 through March 1995, 682 eligible patients were enrolled from 90 centers in Austria, Germany, and Switzerland after informed consent of their guardians was obtained. The study population was subdivided according to NHL subtype into 3 groups with different therapy strategies: (1) therapy group non-B (patients with lymphoblastic lymphoma [precursor–B-cell, n = 24; T-cell, n = 106; immunophenotype not available, n = 7] or peripheral T-cell lymphoma, n = 13, or T-cell lymphoma not further specified, n = 12); (2) therapy group B-NHL/B-ALL (n = 431); (3) therapy group ALCL (patients with anaplastic large-cell lymphoma) (n = 89).
We report here on treatment and results in patients with T-LBL. Of the 106 patients with T-LBL, 1 was excluded from the evaluation of results. This patient had been initially misdiagnosed for Burkitt‘s lymphoma and had been treated according to the therapy strategy for B-NHL. After course number 5, he relapsed (bone marrow [BM] and CNS relapse) and died.
Diagnostic work-up
The diagnosis was established by lymph node or tumor biopsy and/or by cytologic16 and immunologic examination17 of cells from malignant effusions. Cases were classified according to the criteria of the updated Kiel Classification for NHLs18 and the Revised European American Lymphoma classification.19Subclassification of immunophenotypic subgroups was performed according to the European Group for the Immunological Characterization of Leukemias criteria.20
Staging and restaging studies included peripheral blood and BM aspiration smears, cerebrospinal fluid (CSF), serum lactate dehydrogenase (LDH) analyses, ultrasonography, X-ray, computed tomography (CT) or magnetic resonance imaging (MRI), and skeletal scintigraphy. The St. Jude staging system21 was used. Initial CNS disease was diagnosed in case of more than 5 cells/μL CSF and morphologically identifiable blasts in the CSF on cytospin preparations or, also, in case of cerebral infiltrates on cranial CT or MRI. Testicular involvement was diagnosed clinically as the presence of painless enlargement of 1 or both testicles, provided the diagnosis of NHL was otherwise established.
Therapy
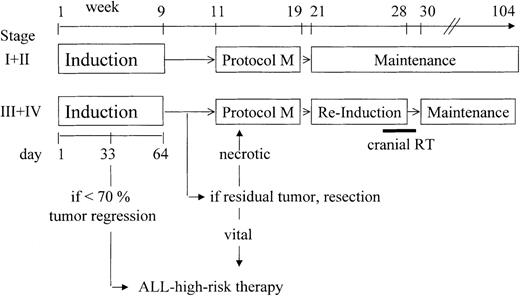
Patients were stratified in 2 therapy branches according to disease stage (Figure 1). Patients with stage I or II received induction protocol I, followed by the extracompartment-protocol M, and maintenance (6-mercaptopurine, 50 mg/m2 daily, and MTX, 20 mg/m2 once a week, both orally) up to a total therapy duration of 24 months. Patients with stage III or IV received an additional reinduction protocol II and CRT between protocol M and maintenance. The compositions of protocol I, M, and II are given in Table 1. The induction protocol I started with a 7-day prednisone phase to prevent acute cell lysis syndrome. In case of respiratory impairment due to a mediastinal mass, patients received 1 or 2 doses of cyclophosphamide, 200 mg/m2, and the lumbar puncture on day 1 was withheld until the patient was stabilized. In protocol M, 10% of the MTX dose (5 g/m2) was given intravenously in 30 minutes and 90% as a 23.5-hour continuous intravenous infusion. The serum levels of MTX should be less than 3 μmol/L at hour 36 after the start of the MTX infusion, ≤1 μmol/L at hour 42, and ≤0.4 μmol/h at hour 48. Leucovorin rescue was given intravenously, 30 mg/m2 at hour 42, 15 mg/m2 at hour 48, and 15 mg/m2 at hour 54 after the start of MTX infusion. In case of increased MTX serum concentrations at hour 42 or later, the dose of leucovorin was adjusted as follows: MTX level more than 1 to 2 μmol/L, leucovorin 30 mg/m2; MTX level more than 2 to 3 μmol/L, leucovorin 45 mg/m2; MTX level more than 3 to 4 μmol/L, leucovorin 60 mg/m2; MTX level more than 4 to 5 μmol/L, leucovorin 75 mg/m2. If the MTX-level exceeded 5 μmol/L, the leucovorin dose was calculated according to the formula: milligrams of leucovorin = MTX serum concentrations (in micromoles per liter) × body weight (in kilograms) and administered as an intravenous infusion to avoid hypercalcemia. In case of impaired MTX excretion, the leucovorin rescue was continued every 6 hours until the serum MTX concentration decreased below 0.25 μmol/L. Eleven doses of MTX (6 mg for patients aged under 1 year; 8 mg for age 1 year; 10 mg for age 2 years; and 12 mg for age 3 years and older) were admistered intrathecally for CNS prevention during induction (5 doses), protocol M (4 doses), and reinduction protocol II (2 doses) (Table 1). In protocol M, MTX was given intrathecally 1 to 2 hours after the start of the MTX intravenous infusion. CNS-positive patients received 2 additional doses of intrathecal MTX at days 8 and 22 of induction.
General treatment strategy.
RT indicates radiotherapy; the composition of treatment protocols are given in Table 1.
General treatment strategy.
RT indicates radiotherapy; the composition of treatment protocols are given in Table 1.
Treatment protocols
| Drug . | Dose . | Days of Administration . |
|---|---|---|
| Induction protocol I | ||
| Prednisone (orally) | 60 mg/m2 | 1-28, then taper over 3 × 3 days |
| Vincristine (iv) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (iv over 1 h) | 30 mg/m2 | 8, 15, 22, 29 |
| L-Asparaginase (iv over 1 h) | 10 000 IU/m2 | 12, 15, 18, 21, 24, 27, 30, 33 |
| Cyclophosphamide* (iv over 1 h) | 1000 mg/m2 | 36, 64 |
| Cytarabine (iv) | 75 mg/m2 | 38-41, 45-48, 52-55, 59-62 |
| 6-Mercaptopurine (orally) | 60 mg/m2 | 36-63 |
| Methotrexate (it)† | 12 mg† | 1, 15, 29, 45, 591-153 |
| Protocol M | ||
| 6-Mercaptopurine (orally) | 25 mg/m2 | 1-56 |
| Methotrexate‡ | 5 g/m2 | 8, 22, 36, 50 |
| Methotrexate (it)† | 12 mg† | 8, 22, 36, 50 |
| Reinduction protocol II | ||
| Dexamethasone (orally) | 10 mg/m2 | 1-21, then taper over 3 × 3 days |
| Vincristine (iv) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Doxorubicin (iv over 1 h) | 30 mg/m2 | 8, 15, 22, 29 |
| L-Asparaginase (iv over 1 h) | 10 000 IU/m2 | 8, 11, 15, 18 |
| Cyclophosphamide* (iv over 1 h) | 1000 mg/m2 | 36 |
| Cytarabine (iv) | 75 mg/m2 | 38-41, 45-48 |
| 6-Thioguanine (orally) | 60 mg/m2 | 36-49 |
| Methotrexate (it)† | 12 mg† | 38, 45 |
| Drug . | Dose . | Days of Administration . |
|---|---|---|
| Induction protocol I | ||
| Prednisone (orally) | 60 mg/m2 | 1-28, then taper over 3 × 3 days |
| Vincristine (iv) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (iv over 1 h) | 30 mg/m2 | 8, 15, 22, 29 |
| L-Asparaginase (iv over 1 h) | 10 000 IU/m2 | 12, 15, 18, 21, 24, 27, 30, 33 |
| Cyclophosphamide* (iv over 1 h) | 1000 mg/m2 | 36, 64 |
| Cytarabine (iv) | 75 mg/m2 | 38-41, 45-48, 52-55, 59-62 |
| 6-Mercaptopurine (orally) | 60 mg/m2 | 36-63 |
| Methotrexate (it)† | 12 mg† | 1, 15, 29, 45, 591-153 |
| Protocol M | ||
| 6-Mercaptopurine (orally) | 25 mg/m2 | 1-56 |
| Methotrexate‡ | 5 g/m2 | 8, 22, 36, 50 |
| Methotrexate (it)† | 12 mg† | 8, 22, 36, 50 |
| Reinduction protocol II | ||
| Dexamethasone (orally) | 10 mg/m2 | 1-21, then taper over 3 × 3 days |
| Vincristine (iv) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Doxorubicin (iv over 1 h) | 30 mg/m2 | 8, 15, 22, 29 |
| L-Asparaginase (iv over 1 h) | 10 000 IU/m2 | 8, 11, 15, 18 |
| Cyclophosphamide* (iv over 1 h) | 1000 mg/m2 | 36 |
| Cytarabine (iv) | 75 mg/m2 | 38-41, 45-48 |
| 6-Thioguanine (orally) | 60 mg/m2 | 36-49 |
| Methotrexate (it)† | 12 mg† | 38, 45 |
iv indicates intravenously; it, intrathecally.
With mesna.
Doses were adjusted for children under 3 years of age.
10% of the dose over 30 minutes and 90% as a 23.5-hour continuous iv infusion. Leucovorin rescue: 30 mg/m2 iv at hour 42; 15 mg/m2 iv at hour 48 and 54. Serum levels of MTX should be less than 3 μmol/L at hour 36 after the start of the MTX infusion, ≤1 μmol/L at hour 42, and ≤0.4 μmol/hour at hour 48.
Additional doses at days 8 and 22 for CNS-positive patients.
The response to treatment was evaluated on day 33 and at the end of induction therapy. Control punctures of BM and CSF were performed only for initial BM and meningeal involvement, respectively. In patients with a mediastinal mass, a complete normalization of chest X-ray on day 33 was considered a complete tumor response. In case of a residual mediastinal widening on chest x-ray, a CT or MRI should be performed. The tumor volume was calculated as centimeters of height × centimeters of width × centimeters of depth × 0.523. Percent tumor regression (TR) was calculated as initial tumor volume minus tumor volume on day 33: initial volume × 100. If there was less than 70% tumor regression on day 33 and/or more than 5% BM blasts and/or persistent blasts in the CSF, treatment was intensified according to the high risk branch of trial ALL-BFM 9022 (Figure 1). In case of an incomplete but at least 70% tumor regression on day 33, less than 5% BM blasts, and no blasts in the CSF, induction therapy was continued. If a residual tumor was present on CT/MRI after completion of induction, this residue was to be resected and examined histologically. If no vital lymphoma was found, the therapy was continued with protocol M. If vital lymphoma was found, treatment was continued with 8 therapy courses of ALL high-risk therapy.
Local therapy modalities
CRT for patients with stages III and IV was performed during the second phase of reinduction in protocol II. The dosage was 12 Gy for all CNS-negative patients (infants under 1 year of age were not irradiated). For CNS-positive patients, the dosage was 18 Gy in the second year of life and 24 Gy in older children. For boys with testicular involvement, orchiectomy was not foreseen, and irradiation (20 Gy) of testes should be confined to biopsy-proven persistent disease after protocol M. Irradiation of local manifestations was not included in the protocol. Three patients had protocol violations regarding radiotherapy: 1 patient had received LRT although not foreseen in the protocol; 1 stage III patient did not receive prophylactic CRT; and 1 patient (stage III) had received LRT but no prophylactic CRT. All 3 of these patients remained free of progression.
Statistical analysis
Analysis of event-free survival (EFS) was performed using the Kaplan-Meier method,23 with differences compared by the log-rank test.24 The 95% confidence bands for the Kaplan-Meier estimate of EFS were calculated using the bootstrap method.25 EFS was calculated from the date of diagnosis to the first event (death from any cause, tumor progression, or second malignancy) or to the date of last follow-up. Patients lost to follow-up were censored at the time of their last follow-up examination. Differences in the distribution of individual parameters among patient subsets were analyzed using the chi-square test or Fisher exact test. The statistical analysis was carried out using the SAS statistical program (SAS-PC, Version 6.04; SAS Institute Inc, Cary, NC). Follow-up data were actualized as of October 1, 1998.
Results
Patient characteristics
Of the 105 evaluable patients, 24 were girls and 81 were boys. The median age was 8.8 years (range, 1.1-16.4). The distribution of stages was 2, 2, 82, and 19 patients with stages I, II, III, and IV, respectively. Ninety-three patients had a mediastinal mass, 15 had BM disease, 3 had CNS disease, and 1 patient had BM and CNS disease. Two boys had testicular involvement. The median LDH value was 461 U/L (range, 119-3036). In 59 cases, subclassification of the immunophenotype could be performed. The immunophenotype was pro–/pre–T-cell, intermediate T-cell, and mature T-cell in 11, 42, and 6 cases, respectively. In 46 cases, the T-cell phenotype could not be classified further. Two stage III patients had not received prophylactic CRT; both remained free of progression.
Treatment results
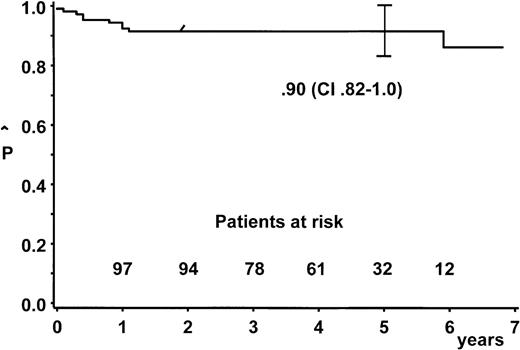
At a median follow-up of 4.5 years (range, 1.9-6.9), the estimate for a 5-year probability of EFS (pEFS) was 90% (95% confidence interval, 82%-100%) for all 105 patients (Figure2). Two patients were lost to follow-up after an event-free follow-up of 9 and 33 months, respectively.
Probability of duration of event-free survival and 95% confidence bands.
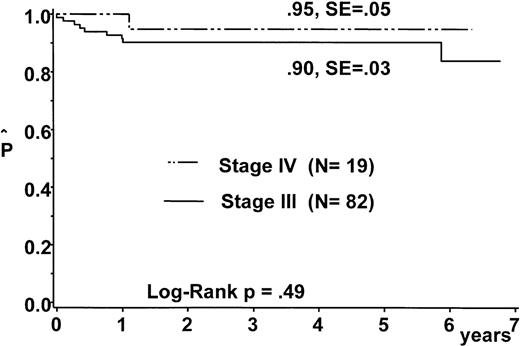
Eight patients (7 with stage III; 1 with stage IV, CNS-negative) relapsed and died. One patient with stage III disease died on day 3 due to vena cava–and–airway compression. No patient died of treatment-related toxicity. One patient developed acute myeloid leukemia 6 years after the diagnosis of T-LBL. The pEFS at 5 years was 90% ±3% and 95% ±5% for patients of stage III and stage IV, respectively (Figure 3).
Probability of duration of event-free survival for patients with stage III disease and stage IV disease.
Probability of duration of event-free survival for patients with stage III disease and stage IV disease.
Time and site of tumor failure
All therapy failures occurred within the first year of diagnosis. Five patients had local tumor failure only, 2 patients had combined local and BM relapse, and 1 patient had BM and CNS relapse combined with local tumor progression.
Speed of tumor response and outcome
Four patients were excluded from this analysis: 1 patient due to death on day 3; another 2 patients who initially received 1 course of B-NHL therapy, both due to diagnostic error (both free of progression); and 1 patient in whom follow-up studies at day 33 of induction and at the end of induction were not performed—this patient suffered from progression (local, BM, CNS) after reinduction protocol II.
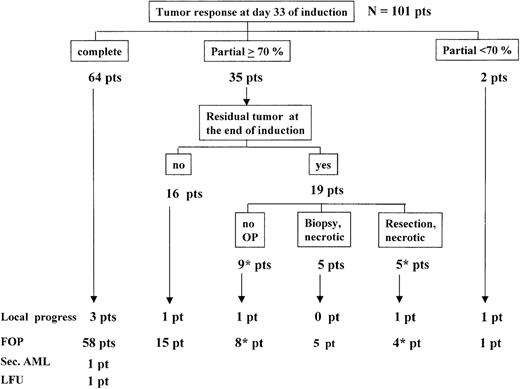
Of the remaining 101 patients on day 33 of induction, 64 patients had a complete tumor response, 35 patients had partial but 70% or more TR, and 2 patients had less than 70% TR (Figure4). pEFS at 5 years was 95% ±2% for the 64 patients with complete tumor response at day 33 and was 89% ±5% for the 37 patients with residual tumor on day 33 (P = .37) (Figure 5A). Three of 64 patients with complete tumor response on day 33 relapsed at the primary site. The 2 patients with less than 70% TR on day 33 received the ALL–high-risk therapy. One of them had local progression and died. The 35 patients who on day 33 had an incomplete but 70% or more TR continued with induction therapy. Sixteen of them had no residual tumor at the end of induction (1 suffered a BM/local relapse), and 19 others still had tumor remnants. Of these 19 patients, 9 had no second-look surgery, 1 died of progressive disease, and 8 remained free of progression (1 of them received LRT 30 Gy); 5 patients had a biopsy showing necrotic material, and all 5 remained free of progression. Five patients had a tumor resection; necrotic tissue was found in all 5 cases, and 4 of the 5 patients remained free of progression (1 of them received LRT 24 Gy), but 1 patient had local recurrence subsequently. pEFS at 5 years was 95% ±2% for the 80 patients with complete TR at the end of induction, and it was 89% ±5% for the 19 patients with tumor remnants after induction (P = .58) (the 2 patients with less than 70% TR on day 33 who continued with ALL–high-risk therapy were excluded) (Figure 5B).
Tumor response and outcome.
Pts indicates patients; FOP, freedom of progression; LFU, lost to follow-up. Four patients were excluded from this analysis. Indicates one patient from each group received local radiotherapy.
Tumor response and outcome.
Pts indicates patients; FOP, freedom of progression; LFU, lost to follow-up. Four patients were excluded from this analysis. Indicates one patient from each group received local radiotherapy.
Probabilities of duration of event-free survival according to tumor response.
(A) Probability of duration of event-free survival according to tumor response at day 33 of induction. Four patients were not evaluated for this analysis. (B) Probability of duration of event-free survival according to tumor regression at the end of induction. Two patients with less than 70% tumor regression on day 33 who continued with ALL–high-risk therapy are excluded.
Probabilities of duration of event-free survival according to tumor response.
(A) Probability of duration of event-free survival according to tumor response at day 33 of induction. Four patients were not evaluated for this analysis. (B) Probability of duration of event-free survival according to tumor regression at the end of induction. Two patients with less than 70% tumor regression on day 33 who continued with ALL–high-risk therapy are excluded.
Prognostic factors
None of the parameters listed in Table 2was found to define a subgroup of patients with significantly superior or inferior EFS.
Analysis of prognostic impact of clinical and biological characteristics
| Characteristics . | No. of Patients . | pEFS at 5 Years . | p Log-Rank . |
|---|---|---|---|
| Gender | |||
| Female | 24 | 83% ± 8% | |
| Male | 81 | 94% ± 3% | 0.15 |
| Age | |||
| Under 10 years | 57 | 89% ± 4% | |
| 10 years and older | 48 | 94% ± 3% | 0.72 |
| Stage of disease | |||
| Stage III | 82 | 90% ± 3% | |
| Stage IV | 19 | 95% ± 5% | 0.49 |
| LDH* | |||
| Less than 500 U/L | 57 | 89% ± 4% | |
| 500 U/L and above | 44 | 95% ± 2% | 0.41 |
| Immunophenotype | |||
| Pro-/pre-T-cell | 11 | 82% ± 9% | |
| Intermediate T-cell | 42 | 93% ± 4% | 2-153 |
| Mature T-cell | 6 | 100% | |
| T-cell, not further specified | 46 | 91% ± 4% | |
| Complete tumor response on day 33† | |||
| Yes | 64 | 95% ± 2% | |
| No | 37 | 89% ± 5% | 0.37 |
| Residual tumor on end of induction‡ | |||
| No | 80 | 95% ± 2% | |
| Yes | 19 | 89% ± 5% | 0.58 |
| Characteristics . | No. of Patients . | pEFS at 5 Years . | p Log-Rank . |
|---|---|---|---|
| Gender | |||
| Female | 24 | 83% ± 8% | |
| Male | 81 | 94% ± 3% | 0.15 |
| Age | |||
| Under 10 years | 57 | 89% ± 4% | |
| 10 years and older | 48 | 94% ± 3% | 0.72 |
| Stage of disease | |||
| Stage III | 82 | 90% ± 3% | |
| Stage IV | 19 | 95% ± 5% | 0.49 |
| LDH* | |||
| Less than 500 U/L | 57 | 89% ± 4% | |
| 500 U/L and above | 44 | 95% ± 2% | 0.41 |
| Immunophenotype | |||
| Pro-/pre-T-cell | 11 | 82% ± 9% | |
| Intermediate T-cell | 42 | 93% ± 4% | 2-153 |
| Mature T-cell | 6 | 100% | |
| T-cell, not further specified | 46 | 91% ± 4% | |
| Complete tumor response on day 33† | |||
| Yes | 64 | 95% ± 2% | |
| No | 37 | 89% ± 5% | 0.37 |
| Residual tumor on end of induction‡ | |||
| No | 80 | 95% ± 2% | |
| Yes | 19 | 89% ± 5% | 0.58 |
Data lacking from 4 patients.
Four patients excluded: 1 patient due to early death, 2 patients due to therapy deviation, 1 patient due to lack of required follow-up observation.
Two patients with less than 70% tumor regression at day 33 who continued on ALL-high-risk therapy excluded.
p log-rank >0.2 in all comparisons.
Discussion
Our data demonstrate that with intensive chemotherapy including high-dose MTX, moderate cumulative doses of anthracyclines (240 mg/m2) and cyclophosphamide (3 g/m2), and an efficacious CNS prevention, a favorable EFS in the range of 90% can be achieved in childhood T-LBL.
The treatment results of our study compare favorably with published data from other trials on childhood lymphoblastic lymphoma.2,4-9,26-30 Moreover, in other therapy studies the cumulative doses of critical drugs such as anthracyclines, cyclophosphamide, and epipodophyllotoxines were higher and accumulated during repetitive therapy cycles over periods of 18-24 months or longer.6-9,26,27 The main differences in our strategy compared with others were the concentration of intensive treatment, including cyclophosphamide and anthracyclines, within the first 7 months, the use of high-dose MTX, and the application of dexamethasone. In our series, no recurrences occurred later than 1 year from diagnosis while, in other studies, relapses out to 5 years were reported.9 30 This suggests that the prevention of late relapses with this kind of early intensive treatment may have contributed to the favorable results of our study. Moreover, this observation places the value of maintenance therapy into question.
Local recurrence was still the most frequent form of failure in our study. Our series did not provide evidence, however, that patients with incomplete tumor regression on day 33 and at the end of the 9-week induction phase have a significantly increased risk of subsequent progression. Local recurrences were observed with comparable frequency in patients with and without residual tumor by imaging at both time points. From our data, therefore, there is no evidence that incomplete tumor regression as such could be an indication for LRT. An exception may be patients with less than 70% tumor regression by day 33; this was observed in 2 patients in our series who had only a minor response at that time point. These findings contrast with observations of others who found in a retrospective analysis a significantly increased risk for failure in patients with incomplete tumor regression on day 60.31 This discrepancy may be due to the differences in the chemotherapy of both studies. In our series, all tumor remnants that were examined histologically at the end of induction consisted of necrotic material.
Preventive CNS therapy based upon steroids, intrathecal MTX, intravenous high-dose MTX, and moderate-dose prophylactic CRT (12 Gy) proved highly efficient. Only 1 patient had CNS involvement at relapse. Whether prophylactic CRT can be safely omitted from the treatment of patients with T-LBL remains to be studied.
Further improvement in the failure-free survival rate of front-line therapy is warranted because survival after relapse is poor. With our strategy, the remaining failures occurred early, within the first year of therapy. Attempts to avoid these early failures demand a change of treatment early in the induction phase. However, criteria currently are lacking for an identification of the minority of patients at risk of failure that is available early enough to expose them to a more intense or new therapy. In our series, the clinical and immunophenotypic features examined failed to identify a subgroup of patients with a significantly increased risk of treatment failure (Table 2). In childhood T-cell ALL, the decrease of blood blasts within the first days of therapy is the most important predictor of treatment outcome,32,33 but in T-LBL patients a quantifiable parameter of response to treatment is lacking. Monitoring of minimal residual disease is highly predictive of treatment outcome in childhood ALL.34 Whether this approach is also applicable and predictive in T-LBL patients remains to be determined.
Randomized trials are needed to examine the contribution of distinct treatment components to a cure. Although T-LBL is the second most frequent subtype of childhood NHL, the number of patients is still small. Therefore, international cooperation is necessary to investigate the optimal treatment for T-LBL patients.
Acknowledgments
We acknowledge the expert work of Edelgard Odenwald (cytomorphology), Ulrike Meyer, and U. Regelsberger (data management). We wish to thank Jennifer Meyers for proofreading the English text.
Study committee of trial NHL-BFM 90: W. Dörffel, Berlin, Germany; W. Ebell, Berlin; N. Graf, Homburg, Germany; H. Gadner, Vienna, Austria; G. Henze, Berlin; G. Janka-Schaub, Hamburg, Germany; T. Klingebiel, Tübingen; St. Müller-Weihrich, Munich, Germany; I. Mutz, Leoben; H. J. Plüss, Zürich, Switzerland; R. Parwaresch, Kiel; A. Reiter, Hannover, Germany; H. Riehm, Hannover; G. Schellong, Münster; M. Schrappe, Hannover; F. Zintl, Jena.
Contributing principal investigators and pathologists, respectively: R. Mertens and H. Mittermeyer (Aachen); R. Dickerhoff (St. Augustin); P. Imbach and H. Ohnacker (Basel, Switzerland); W. Dörffel and W. Schneider (Berlin-Buch, Germany); G. Henze and H. Stein (Berlin, Germany); U. Bode and H. J. Födisch (Bonn, Germany); W. Eberl and R. Donhuisen (Braunschweig); I. Krause and J. O. Habeck (Chemnitz); J. D. Möbius and P. Stosiek (Cottbus); W. Andler (Datteln); H. Breu and E. W. Schwarze (Dortmund); G. Weißbach and M. Müller (Dresden, University); G. Weinmann and D. Schreiber (Erfurt); J. D. Beck and V. Becker (Erlangen); W. Havers and L. D. Leder, (Essen); B. Kornhuber and S. Falk (Frankfurt, Germany); F. Lampert and W. Schultz (Gießen); M. Lakomek and E. Kunze (Göttingen); C. Urban and C. Schmid (Graz); H. Reddemann and G. Lorenz (Greifswald); P. Exadaktylos and F. W. Rath (Halle); H. Riehm and A. Georgii (Hannover); K.-M. Debatin and F. Otto (Heidelberg); N. Graf and K. Remberger (Homburg); G. Nessler and W. Gusek (Karlsruhe); R. Schneppenheim and R. Parwaresch (Kiel); F. Berthold and R. Fischer (Köln); W. Sternschulte (Köln); I. Mutz and G. Leitner (Leoben); K. Schmidt and M. Weber (Linz); H. Rütschle and K. Wegener (Ludwigshafen); U. Mittler and A. Roesser (Magde-burg); C. Eschenbach and C. Thomas (Marburg); S. Müller-Weihrich and K. Wurster (München, Technical University); C. Bender-Götze and U. Löhrs (München); H. Jürgens and M. Grundmann (Münster); H. Grienberger and O. Dietze (Salzburg); J. Treuner and B. Kraus-Hounder (Stuttgart); H. Rau and H. Mäusle (Trier); D. Niethammer and P. Kaiserling (Tübingen); G. Hartmann and O. Haferkamp (Ulm); H. Gadner and Th. Radasckiewicz (Wien); J. Kühl and H. K. Müller-Hermelink (Würzburg).
Reference laboratories: Pathology: Lymphnode Registry of the Society of German Pathologists, Institut of Hematopathology, University of Kiel; Institut of Pathology, University of Vienna. Immunophenotyping: W.-D. Ludwig, Berlin; W. Knapp, Vienna.
Supported by the Deutsche Krebshilfe, Bonn, grant M 109/91/Re1.
Reprints:Alfred Reiter, Justus-Liebig-University, Department of Pediatric Hematology and Oncology, Feulgenstr. 12, D-35385 Gießen, Germany; e-mail:alfred.reiter@paediat.med.uni-giessen.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal