A novel glass needle–mediated microinjection method for delivery of macromolecules, including proteins and larger transgene DNAs, into the nuclei of blood stem/progenitor cells was developed. Temporary immobilization of cells to extracellular matrix–coated dishes has enabled rapid and consistent injection of macromolecules into nuclei of CD34+, CD34+/CD38−, and CD34+/CD38−/Thy-1lo human cord blood cells. Immobilization and detachment protocols were identified, which had no adverse effect on cell survival, progenitor cell function (colony forming ability), or stem cell function (NOD/SCID reconstituting ability). Delivery of fluorescent dextrans to stem/progenitor cells was achieved with 52% ± 8.4% of CD34+ cells and 42% ± 14% of CD34+/CD38−cells still fluorescent 48 hours after injection. Single-cell transfer and culture of injected cells has demonstrated long-term survival and proliferation of CD34+ and CD34+/CD38−cells, and retention of the ability of CD34+/CD38− cells to generate progenitor cells. Delivery of DNA constructs (currently ≤ 19.6 kb) and fluorescently labeled proteins into CD34+ and CD34+/CD38− cells was achieved with transient expression of green fluorescent protein observed in up to 75% of injected cells. These data indicate that glass needle–mediated delivery of macromolecules into primitive hematopoietic cells is a valuable method for studies of stem cell biology and a promising method for human blood stem cell gene therapy.
Hematopoietic stem cells are a major focus of investigation in both developmental biology and clinical medicine. The stem cell compartment consists of rare, long-lived, self-renewing, predominantly quiescent cells capable of long-term reconstitution of the complete hematopoietic system in transplanted hosts.1It is of significant biologic and clinical interest to elucidate the mechanisms controlling stem cell self-renewal, commitment to maturation, and selection of differentiation program. The potential to genetically correct the complete hematopoietic system by successfully modifying only the stem cell compartment has made these cells a primary target for gene therapy.
We have sought to develop a method that allows for the introduction of macromolecules into stem/progenitor cells. Current technologies (eg, standard retroviruses, adeno-associated viruses, liposomes) have demonstrated only limited success in efficiently transducing human stem cells.2-5 Glass needle–mediated microinjection of macromolecules into living mammalian cells, developed independently by Diacumakos6 and Graessman,7 has proven to be a powerful approach for analyzing the biologic activity of specific molecules (eg, peptide inhibitors, purified active proteins, neutralizing antibodies, RNA, and DNA) in adherent cells.8,9 However, this approach has rarely been used for primary hematopoietic cells10 because immobilization of hematopoietic cells has generally been ineffective and standard injection needles cause significant damage to these relatively small cells (∼ 6 μ diameter for stem cells11). Two developments have allowed us to apply glass needle–mediated microinjection technology to blood stem/progenitor cells. First, we have developed a method for attaching human blood stem/progenitor cells to extracellular matrix–coated dishes, which does not affect cell function. Second, we have developed injection needles with very small outer tip diameters (OTD; ∼ 0.2 μ), which have excellent flow properties and result in excellent postinjection viabilities.
We demonstrate that glass needle–mediated microinjection can be used effectively for the introduction of macromolecules (protein, DNA, and dextrans) into human CD34+, CD34+/CD38−, and CD34+/CD38−/Thy-1lo cord blood cells. Importantly, macromolecule delivery is accomplished with high postinjection cell viability, no discernible impact on stem/progenitor cell proliferation or biologic activity, and a high frequency of cells expressing injected transgenes.
Materials and methods
Isolation and further fractionation of human CD34+cells
CD34+ cells were immunomagnetically purified from human umbilical cord blood (University of Texas Medical Branch) using either the Progenitor or Multisort Kits (Miltenyi Biotec, Auburn, CA), then cultured in Iscoves' Modified Dulbecco's Medium without phenol red (IMDM) with 100 μg/mL glutamine/penicillin/streptomycin, 1 × BIT 9500 (StemCell Technologies, Vancouver, BC), 20 ng/mL each of human Interleukin (II)-3, flt-3 ligand, and stem cell factor (PeproTech, Inc, Rocky Hill, NJ), 40 μg/mL low-density lipoprotein (Sigma), and 50 μM 2-mercaptoethanol (Stem Cell Medium). Subpopulations of CD34+ cells were isolated by flow cytometry on a FACS Vantage (Becton Dickinson, San Jose, CA) after immunostaining with anti-CD34-FITC and anti-CD38-PE for CD34+/CD38− cells and anti-CD34-PerCP, anti-CD38-PE, and anti-Thy-1-FITC for CD34+/CD38−/Thy-1lo cells.
CD34+ cell matrix attachment and detachment
Dishes were coated overnight at 4°C with either Fibronectin (FN) or FN fragment CH-296 (Retronectin (RN); TaKaRa Biomedicals, Panvera, Madison, WI; 15 μg/mL) in phosphate-buffered saline (PBS) and washed with IMDM containing glutamine/penicillin/streptomycin. Cells were added to cloning rings and attached to the plates for 45 minutes at 37°C. When using FN, the integrin-activating monoclonal antibody (mAb) TS2/16.2.1 IgG purified from ascites (1 μg/mL; ATCC, HB-24312) was added to the cells. Cells were detached from the matrix-coated dishes by either: (1) incubating in a mixture of FN CS-1 fragment (0.42 mg/mL), H-Arg-Gly-Asp-Ser-OH (1.0 mg/mL) and Phenylac-Leu-Asp-Phe-D-Pro-NH2 (1.0 mg/mL; Bachem BioScience, Torrance, CA); or (2) pipetting under a stream of medium.
Injection of macromolecules into CD34+, CD34+/CD38−, and CD34+/CD38−/Thy-1lo cells
Injection needles were pulled from 10 cm borosilicate capillaries with a 1.2 mm outer/0.94 mm inner diameter using a Flaming/Brown Micropipette Puller Model P-97 (Sutter Instrument Co, Novato, CA) and had a range of 0.17 to 0.25 μ OTD, as determined by scanning electron microscopy. Cells were visualized using a Nikon Eclipse TE300 microscope equipped with a Fryer A-50 temperature-controlled stage (set at 37°C) and injected using the electronically interfaced Eppendorf Micromanipulator (Model 5171) and Transjector (Model 5246). Manual injections were performed, except where noted as semiautomatic injections. In some cases, manual injections were performed with a Narishige joystick-controlled micromanipulator. After injection, cells were maintained on matrix-coated plates and observed using fluorescent microscopy to determine the percentage viability (number of fluorescent cells/number of successfully injected cells × 100). A successfully injected cell was defined as a cell that was intact, was alive, and remained attached to the plate after injection. For the experiments presented in this report, the percentage of successful injections ranged from 50% to 95%. Alternatively, injected cells were transferred individually using a Quixell Automated Cell Selection and Transfer Unit (Stoelting, Wood Dale, IL) to wells of 96-well plates containing Stem Cell Medium with 50 mmol/L HEPES, pH 7.4.
Microinjection samples
Samples were dialyzed against 50 mmol/L HEPES, pH 7.4, 140 mmol/L KCl. Oregon Green 488-dextran (OG-dextran; 70 000 MW), FITC-dextran (150 000 MW), and Cy-3 conjugated mouse immunoglobulin (Cy-3 IgG) were adjusted to 0.15, 2.5, and 0.5 μg/mL, respectively. pGreen Lantern (5 kb; Gibco-BRL, Bethesda, MD) and linearized phosphoglycerate kinase Green Fluorescent Protein (pgkGFP) DNA (1.7 kb) were adjusted to 5 copies per fL. Both vectors contain the humanized, red shifted GFP from Aequorea victoria jellyfish. The linearized pgkGFP construct is based on pCMV-Beta (CLONTECH, Palo Alto, CA). The lacZ gene in pCMV-Beta was replaced with the GFP gene of pGreen Lantern, and the cytomegalovirus (CMV) enhancer/promoter sequences were replaced with the pgk promoter sequences.13 Linear pgkGFP sequences were obtained by digesting pgkGFP with Pac I and Asc I and isolating the resulting 1.7 kb fragment after agarose gel electrophoresis.
NOD/SCID engraftment assay
Nonobese diabetic and severe combined immmunodeficient NOD/LtSz-Prkc-scid/scid (NOD/SCID) mice were obtained from Jackson Laboratory (Bar Harbor, ME14) and were bred and maintained in microisolator cages on laminar flow racks. NOD/SCID mice of 6 to 8 weeks of age were sublethally irradiated with 350 or 400 rad (137Cs source) and injected intravenously (iv; tail vein) with purified CD34+ cells (2 × 104cells/mouse). CD34+ cells had either been maintained in suspension or first immobilized on RN or FN, then detached by pipetting or treatment with peptides. At 6 weeks inoculation, marrow cells from both femurs of the mice were analyzed for the presence of human cells by fluorescence-activated cell sorter (FACS) analysis using antihuman CD45FITC and antihuman CD34 PE (Becton Dickinson). Percent CD45+ cells represents the sum of the frequency of human CD45+/CD34− and CD45+/CD34+ cells. When the level of human cell engraftment was low (< 1% human cells), semiquantitative DNA polymerase chain reaction amplification for human Cart-1 sequences was performed to confirm engraftment.15 Statistical analysis was performed with the Student's t test (unless otherwise noted) using Statview 4.1 (Abacus Concepts, Inc, Berkeley, CA) and Microsoft Excel 8.0 (Microsoft, Redmond, WA); P values were considered to be statistically significant if less than .05.
Results
Immobilization of human blood stem/progenitor cells on matrix-coated dishes
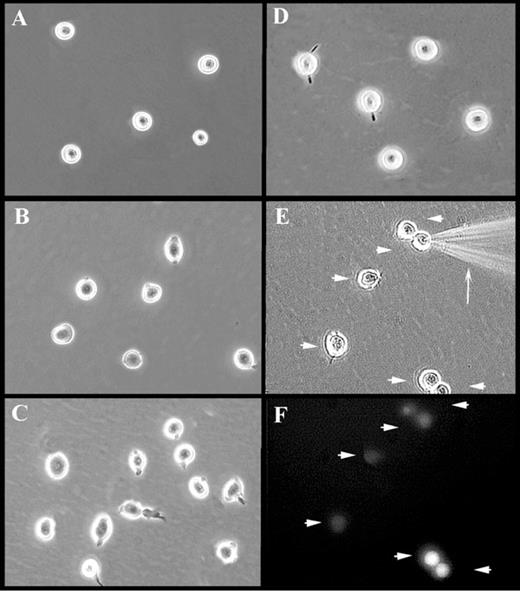
Hematopoietic stem/progenitor cells in the bone marrow express α4β1 and α5β1integrins, allowing them to interact with FN through the CS-1 region and RGDS sequence, respectively.16 We evaluated whether the attachment of blood stem/progenitor cells to FN-coated plates was sufficient for glass needle–mediated injection. Attachment of primary CD34+ cells to plates coated with FN resulted in only tethering of the cells rather than solid attachment (Figure1B; unattached CD34+ cells in suspension culture are shown in Figure 1A); the cells dislodged during injection. However, when CD34+ cells were treated with specific anti-β1 integrin mAb, they attached strongly to FN-coated plates (Figure 1C). Up to 100% of CD34+ cells were immobilized in this way and acquired a more flattened morphology and extended micropodia (Figure 1C). CD34+/CD38− and CD34+/CD38−/Thy-1lo cells, believed to represent increasingly enriched populations of blood stem cells, were also immobilized using this method (data not shown). Up to 100% of primary blood stem/progenitor cells also attached firmly to RN (Figure 1D), a recombinant derivative of FN. RN contains only the heparin-binding, CS-1, and RGDS-containing cell association regions of FN.17 Attachment to RN occurred even in the absence of activating anti-β1 mAb. For both methods of attachment, cells withstood both manual (Figure 1E) and semiautomatic injection without becoming dislodged.
Photomicrographs of attached and injected CD34+ cells.
Represented are CD34+ cells maintained in suspension (A) or attached to FN-coated dishes in the absence (B) or presence of TS2/16.2.1 mAb (C) or RN-coated dishes (D-F). Injection of attached CD34+ cells is shown in panel E. Note the injection needle (arrow) and cells injected with OG-dextran (arrowheads). A fluorescence micrograph (F) of the identical field shown in panel E demonstrates the successful delivery of fluorescent material (arrowheads). Images in panels E-F were captured from videotape during a live injection session and, therefore, display a decreased resolution, compared with images in panels A-D.
Photomicrographs of attached and injected CD34+ cells.
Represented are CD34+ cells maintained in suspension (A) or attached to FN-coated dishes in the absence (B) or presence of TS2/16.2.1 mAb (C) or RN-coated dishes (D-F). Injection of attached CD34+ cells is shown in panel E. Note the injection needle (arrow) and cells injected with OG-dextran (arrowheads). A fluorescence micrograph (F) of the identical field shown in panel E demonstrates the successful delivery of fluorescent material (arrowheads). Images in panels E-F were captured from videotape during a live injection session and, therefore, display a decreased resolution, compared with images in panels A-D.
Biologic activity of human blood stem/progenitor cells after immobilization on FN and RN
Despite their strong attachment to FN (in the presence of activating TS2/16.2.1 mAb) and RN, cells could be recovered with vigorous pipetting. However, this resulted in decreased viability (64% ± 12%) and incomplete recovery (≤ 85%) of cells. Virtually 100% of cells were recovered from FN and RN by competing for attachment with peptides corresponding to the CS-1 and RGDS regions of FN. In all cases, detached cells were > 95% viable and rapidly reacquired their normal rounded morphology and nonadherent behavior.
We evaluated the effects of in vitro attachment on stem/progenitor cell function. Immobilization of CD34+ and CD34+/CD38− cells to FN or RN for 2 hours and release with peptide had no effect on viability or proliferative capacity of cells in liquid culture. Doubling rates of 21.2 ± 1.1 hours and 23.2 ± 1.7 hours were obtained for untreated control CD34+ and TS2/16 mAb-treated treated CD34+cells grown in liquid culture, respectively. Similarly, doubling rates of 21.6 ± 2.5 hours and 21.6 ± 2.1 hours were obtained for CD34+ cells attached for 2 hours to either FN or RN, respectively, released by peptide and grown in liquid culture.
Because CD34+ cells are highly enriched in progenitor cells,18 the effect of temporary immobilization on colony-forming activity was evaluated. CD34+ cells were allowed to attach for 2 hours to either FN, RN, or Con A lectin, detached, then plated in methylcellulose with appropriate cytokines to compare their colony-forming activity (erythroid and myeloid) to nonattached suspension cells (Table 1). FN- or RN-immobilized cells demonstrated no significant alteration in either frequency (Table 1) or size (data not shown) of erythroid or myeloid colonies. In contrast, Con A immobilization lead to a significant reduction in myeloid colony formation (Table 1).
Immobilization on FN or RN and subsequent detachment does not affect the colony forming ability of CD34+cells
| Method of Attachment† . | Method of Release . | Colony Formation* . | |||
|---|---|---|---|---|---|
| Number of Colonies Per 2000 Cells . | Percent of Nonimmobilized Control . | ||||
| Erythroid . | Myeloid . | Erythroid . | Myeloid . | ||
| — | — | 70 ± 5 | 52 ± 6 | 100 ± 5 | 100 ± 6 |
| Con A | Pipetting | 75 ± 16 | 26 ± 2 | 108 ± 20 | 54 ± 4 |
| FN + TS2/16.2.1 mAb | Pipetting | 67 ± 4 | 43 ± 8 | 98 ± 6 | 84 ± 19 |
| RN | Peptide | 81 ± 18 | 53 ± 8 | 117 ± 23 | 102 ± 14 |
| Method of Attachment† . | Method of Release . | Colony Formation* . | |||
|---|---|---|---|---|---|
| Number of Colonies Per 2000 Cells . | Percent of Nonimmobilized Control . | ||||
| Erythroid . | Myeloid . | Erythroid . | Myeloid . | ||
| — | — | 70 ± 5 | 52 ± 6 | 100 ± 5 | 100 ± 6 |
| Con A | Pipetting | 75 ± 16 | 26 ± 2 | 108 ± 20 | 54 ± 4 |
| FN + TS2/16.2.1 mAb | Pipetting | 67 ± 4 | 43 ± 8 | 98 ± 6 | 84 ± 19 |
| RN | Peptide | 81 ± 18 | 53 ± 8 | 117 ± 23 | 102 ± 14 |
Colonies formed were evaluated and designated as erythroid- or myeloid-containing. Colony formation is represented as number of colonies formed per 2000 cells plated and percentage of the nonimmobilized control (number of colonies formed by treated cells per number of colonies formed by control suspension cells × 100) ± SD for 4 separate experiments.
CD34 cells were incubated in Stem Cell Media 48 hours before onset of assay and then attached to matrix coated plates for 45 minutes. Cells were detached, resuspended in MethoCult GF Kit (StemCell Technologies, Vancouver, BC, Canada) (1000 cells/mL/plate), and incubated for 14 days in a humidified incubator at 37°C, 6% CO2.
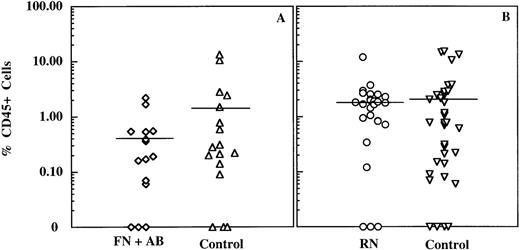
We also examined whether there was any adverse effect of immobilization on stem cell function using the NOD/SCID engraftment model. NOD/SCID mice demonstrate active human blood cell development in the marrow of transplanted mice after intravenous delivery of total marrow, total cord blood, or primitive hematopoietic subpopulations.15 19Sublethally irradiated mice were injected intravenously with CD34+ cells that had been immobilized on FN (with activating mAb) or RN and detached or with control CD34+cells. Control cells for the FN plus mAb experimental group consisted of CD34+ cells incubated on FN-coated dishes in the absence of activating mAb. Controls for the RN-attached cells consisted of CD34+ cells incubated on noncoated plastic dishes or FN-coated dishes without activating mAb. No statistical difference was detected when comparing the percentage of mice engrafting (P > .42) or the extent of engraftment (P > .35) with either control group. As shown in Table2, there was no evidence for any adverse effect on the engraftment ability of the human NOD/SCID reconstituting cells after attachment to RN. Although a slight decrease in engraftment ability was seen in the cells attached to FN with activating mAb (75% compared with the control value of 94% for suspension cells or 84% for cells incubated on FN in the absence of activating mAb), this difference was not statistically significant (P > .19 andP > .22, respectively; Student's paired t test). The extent of engraftment was determined for each mouse by quantitating via FACS analysis the percentage of human CD45+ cells present in the bone. As can be seen in Figure2, there was considerable variability in the range of engraftment levels obtained for each of the experimental conditions. Although not statistically significant (P > .13), there was a distinct trend toward lower levels of engraftment when cells were attached to FN in the presence of activating antibody (0.4% ± 0.6% human CD45+ cells; mean ± SD) as compared with control cells incubated on FN in the absence of activating mAb (1.8% ± 3.7%). Engraftment levels with RN-attached cells (1.9% ± 2.3%) were equivalent to those of control cells (2.4% ± 4.3%). Thus, primitive cord blood cells temporarily attached to RN are neither affected in their proliferation, colony-forming activity, nor NOD/SCID engrafting ability. In contrast, primitive cord blood cells attached to FN in the presence of activating mAb may have impaired NOD/SCID engrafting ability, while still retaining control levels of proliferative and colony-forming activity. In light of these observations, subsequent experiments used RN attachment.
NOD/SCID engrafting ability of CD34+ cells after attachment and detachment
| Matrix . | Presence of TS2/16 mAb . | Method of Detachment . | Engrafted per Injected Mice . | % of Mice Engrafted* . |
|---|---|---|---|---|
| —† | no | Pipetting | 16/17 | 94 |
| FN‡ | no | Pipetting | 16/19 | 84 |
| FN‡ | yes | Pipetting | 12/16 | 75 |
| RN‡ | no | Pipetting | 5/6 | 83 |
| RN‡ | no | Peptides | 9/11 | 82 |
| RN (16 h) | no | Peptides | 9/9 | 100 |
| Matrix . | Presence of TS2/16 mAb . | Method of Detachment . | Engrafted per Injected Mice . | % of Mice Engrafted* . |
|---|---|---|---|---|
| —† | no | Pipetting | 16/17 | 94 |
| FN‡ | no | Pipetting | 16/19 | 84 |
| FN‡ | yes | Pipetting | 12/16 | 75 |
| RN‡ | no | Pipetting | 5/6 | 83 |
| RN‡ | no | Peptides | 9/11 | 82 |
| RN (16 h) | no | Peptides | 9/9 | 100 |
Values represent total mice engrafted/total mice injected × 100.
Cells were incubated in suspension.
Cells were incubated on matrix for 75 to 120 minutes.
Extent of CD34+ cell engraftment in NOD/SCID mice after attachment and detachment.
Mouse bone marrow mononuclear cells were immunostained with antihuman CD45FITC and CD34PE mAbs and analyzed by FACS by using Cell Quest Software (Becton Dickinson). The percentage CD45+ cells was determined on ungated samples. (A) Plot of the percentage CD45+ cells in mice injected with cells plated on FN in the presence (diamonds) or absence (control; triangles) of TS2/16.2.1 activating mAb. (B) Scatter plot of the percentage of CD45+ cells in mice injected with RN-attached cells (circles) or control cells (ie, cells maintained in suspension or plated on FN in the absence of activating mAb; inverted triangles). Horizontal lines represent the mean percentage CD45+ cells in each group.
Extent of CD34+ cell engraftment in NOD/SCID mice after attachment and detachment.
Mouse bone marrow mononuclear cells were immunostained with antihuman CD45FITC and CD34PE mAbs and analyzed by FACS by using Cell Quest Software (Becton Dickinson). The percentage CD45+ cells was determined on ungated samples. (A) Plot of the percentage CD45+ cells in mice injected with cells plated on FN in the presence (diamonds) or absence (control; triangles) of TS2/16.2.1 activating mAb. (B) Scatter plot of the percentage of CD45+ cells in mice injected with RN-attached cells (circles) or control cells (ie, cells maintained in suspension or plated on FN in the absence of activating mAb; inverted triangles). Horizontal lines represent the mean percentage CD45+ cells in each group.
Microinjection-mediated delivery of fluorescencelabeled dextrans into CD34+, CD34+/CD38−, and CD34+/CD38−/Thy-1lo cells
To optimize injection conditions for primitive blood cells, it was critical to directly monitor the flow of material from the injection needle and the fate of individual cells after injection. Therefore, we used OG-dextran or FITC-dextran when optimizing blood stem/progenitor cell injections.20
CD34+ cells were immobilized on RN and manually injected with OG-dextran. Immediately after injection, only 5% to 10% of the injected cells exhibited damage visible by light microscopy. Examples of CD34+ cells during and after injection with OG-dextran are shown in Figure 1E and F, respectively. Approximately 67%, 55%, and 52% of cells were fluorescent 2, 24, and 48 hours, respectively, after injection (Table 3). Table 3 also shows the successful delivery of OG-dextran to CD34+ cells using semiautomatic injection.
Microinjection of fluorescent compounds into CD34+, CD34+/CD38−, and CD34+/CD38−/Thy-1lo cells results in high cell viability
| Cells . | Matrix . | Material Injected . | Mode of Injection . | Percent Viability3-150,3-151 . | ||
|---|---|---|---|---|---|---|
| 2-4 h . | 24 h . | 48 h . | ||||
| CD34+ | RN | OG-dextran | Manual | 67 ± 13 | 55 ± 8.5 | 52 ± 8.4 |
| (n = 63) | (n = 45) | (n = 45) | ||||
| CD34+ | RN | OG-dextran | Semiautomatic | n.d. | 56 ± 5.2 | 54 ± 5.2 |
| (n = 6) | (n = 6) | |||||
| CD34+ | RN | Cy-3 IgG | Manual | 70 ± 7.5 | 53 ± 6.2 | 44 ± 5.4 |
| (n = 3) | (n = 3) | (n = 3) | ||||
| CD34+/CD38− | RN | OG-dextran | Manual | 58 ± 23 | 41 ± 17 | 42 ± 14 |
| (n = 15) | (n = 9) | (n = 9) | ||||
| CD34+/CD38−/Thy-1lo | FN | FITC-dextran | Manual | 28, 54 | 9, 52 | 3, 32 |
| Cells . | Matrix . | Material Injected . | Mode of Injection . | Percent Viability3-150,3-151 . | ||
|---|---|---|---|---|---|---|
| 2-4 h . | 24 h . | 48 h . | ||||
| CD34+ | RN | OG-dextran | Manual | 67 ± 13 | 55 ± 8.5 | 52 ± 8.4 |
| (n = 63) | (n = 45) | (n = 45) | ||||
| CD34+ | RN | OG-dextran | Semiautomatic | n.d. | 56 ± 5.2 | 54 ± 5.2 |
| (n = 6) | (n = 6) | |||||
| CD34+ | RN | Cy-3 IgG | Manual | 70 ± 7.5 | 53 ± 6.2 | 44 ± 5.4 |
| (n = 3) | (n = 3) | (n = 3) | ||||
| CD34+/CD38− | RN | OG-dextran | Manual | 58 ± 23 | 41 ± 17 | 42 ± 14 |
| (n = 15) | (n = 9) | (n = 9) | ||||
| CD34+/CD38−/Thy-1lo | FN | FITC-dextran | Manual | 28, 54 | 9, 52 | 3, 32 |
Values represent mean ± SD for number of separate plates (n), each containing 50 injected cells per plate.
Values shown in last row are, respectively, for 2 separate experiments of 32 and 50 injected cells per plate.
Results for CD34+/CD38−and CD34+/CD38−/Thy-1lo cells injected with fluorescent dextrans are also shown in Table 3. The disparate viabilities observed between the 2 CD34+/CD38−/Thy-1loexperiments likely occurred because these experiments were performed before optimization of the cell attachment protocol and injection technology.
The particular field shown in Figure 1E and F shows the range of fluorescent intensities obtainable using the manual setting on the injector. The most optimal injections were those in which the resultant fluorescence intensity was low, indicating the delivery of a relatively small volume of material. In subsequent experiments, care was taken to ensure injections were consistent and delivery of material was minimal (ie, low levels of fluorescence). For example, in the last 5 experiments performed with CD34+ cells and CD34+/CD38− cells (using needles of 0.22 μ OTD), cell viabilities 2 hours after OG-dextran injections were 84% ± 3.3% and 87% ± 2.8%, respectively. These results demonstrate that macromolecules can be successfully delivered to various populations of immobilized primitive, human cord blood cells via glass needle–mediated injection.
Survival, proliferation, and hematopoietic activity of individual microinjected CD34+ and CD34+/CD38−cells
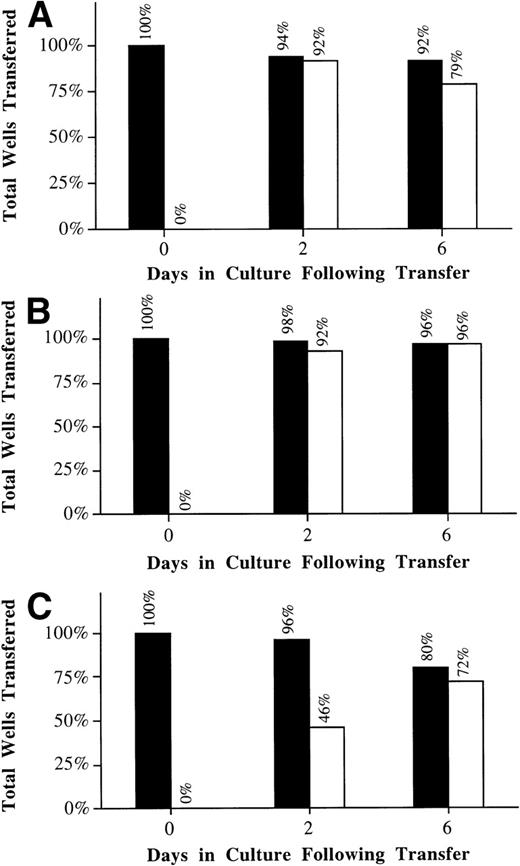
It was critical that we follow the fate of individual injected cells for subsequent survival and biologic activity. Therefore, 2 hours after injection with OG-dextran, CD34+ cells were detached with peptides and individual fluorescent cells were transferred, as single cells, into individual wells of 96-well plates. Individual wells were monitored for the number of surviving and proliferating cells. The frequency of surviving cells in the microinjected group (80%) was marginally lower than the attached/detached cells (96%) or suspension controls (92%) at day 6 (Figure 3, closed bars). Because the cells were transferred promptly after microinjection, the slight decrease (20%) in overall cell survival is likely due to the increased fragility of the cells immediately after microinjection. The frequency of proliferating microinjected and attached/detached cells was not obviously different from controls by day 6 (Figure 3, open bars). This was especially evident when the frequency of proliferating cells was calculated, based on the actual number of surviving cells (ie, frequency of proliferation/frequency of survival; control: 79%/92% = 86%; attached/detached: 96%/96% = 100%; injected: 72%/80% = 90%). However, it appeared that microinjection induced a delay in the average time before proliferation (Figure 3, open bars). The observed delay in proliferation was likely due to either the effect(s) of OG-dextran or the mechanical stress induced by the microinjection process. Finally, microinjected cells showed no significant difference in either range or distribution of values for the total number of progeny derived from each well compared with control cells (F test, P > .3).
Microinjected CD34+ cells are viable and retain their ability to proliferate after single cell transfer.
Cells were attached to RN and then microinjected with OG-dextran. Two hours after injection, cells were detached with peptide, and the fluorescent cells were transferred as single cells into individual wells of 96-well plates (C). Cells in suspension (A) and cells attached to RN and detached with peptide (B) were also transferred as single cells. Survival and proliferation of the cells was monitored at 0, 2, and 6 days after transfer. Values shown represent the percentage of total wells transferred that displayed cells surviving (closed bar) and proliferating (open bar) for 1 experiment in which 30 suspension, 30 RN attached/peptide detached, and 45 OG-dextran microinjected CD34+ cells were transferred.
Microinjected CD34+ cells are viable and retain their ability to proliferate after single cell transfer.
Cells were attached to RN and then microinjected with OG-dextran. Two hours after injection, cells were detached with peptide, and the fluorescent cells were transferred as single cells into individual wells of 96-well plates (C). Cells in suspension (A) and cells attached to RN and detached with peptide (B) were also transferred as single cells. Survival and proliferation of the cells was monitored at 0, 2, and 6 days after transfer. Values shown represent the percentage of total wells transferred that displayed cells surviving (closed bar) and proliferating (open bar) for 1 experiment in which 30 suspension, 30 RN attached/peptide detached, and 45 OG-dextran microinjected CD34+ cells were transferred.
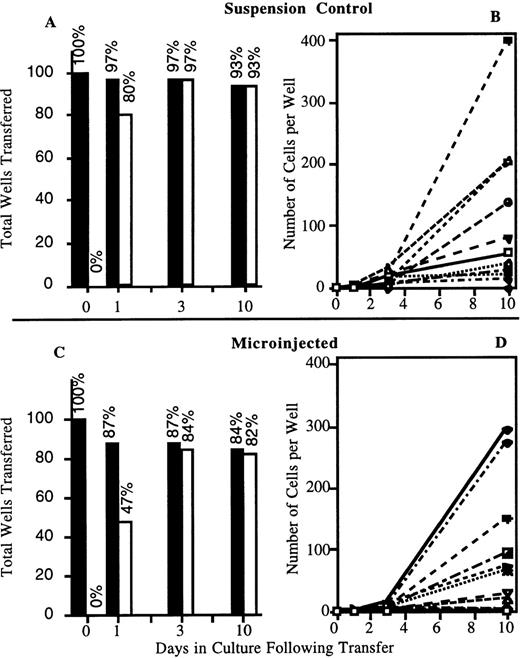
Similar results were obtained in CD34+/CD38− cell experiments (Figure4). The left panels (A and C) summarize the frequency of wells containing surviving or proliferating cells. The frequency of surviving cells in the injected group (84%) was marginally lower than the suspension controls (93%) at day 10. A slight delay in proliferation of injected CD34+/CD38− cells was also detected. However, by day 3 after transfer, the frequency of proliferating cells was similar to that of the suspension control. As described previously for CD34+ cells, this was especially evident when the values were corrected for the actual number of surviving cells (ie, frequency of proliferation/frequency of survival; control: 97%/97% = 100%; injected: 84%/87% = 97%). As can be seen in the right panels (Figure 4, B and D), there was no significant difference in either range or distribution of values for the total number of progeny derived from each well (F test, P > .3) (for ease of visualization, only 12 individual wells representing the full range of proliferation rates are presented). Therefore, attachment, injection, and detachment have no significant impact on the survival or proliferation of cells in culture.
Injected CD34+/CD38− cells are viable and retain their ability to proliferate after single cell transfer.
Cells were attached to RN and injected with OG-dextran. Two hours after injection, cells were detached with peptide and fluorescent cells transferred as single cells to individual wells of 96-well plates. In total, 46 suspension and 75 injected cells were analyzed after 2 injection sessions. (A, C) Percentage of total wells transferred that displayed cells surviving (closed bar) and proliferating (open bar) after transfer. (B, D) Representative sampling of the survival and proliferation of 12 individually transferred suspension (B) or injected (D) cells. Each symbol represents the progeny generated by an individually transferred cell.
Injected CD34+/CD38− cells are viable and retain their ability to proliferate after single cell transfer.
Cells were attached to RN and injected with OG-dextran. Two hours after injection, cells were detached with peptide and fluorescent cells transferred as single cells to individual wells of 96-well plates. In total, 46 suspension and 75 injected cells were analyzed after 2 injection sessions. (A, C) Percentage of total wells transferred that displayed cells surviving (closed bar) and proliferating (open bar) after transfer. (B, D) Representative sampling of the survival and proliferation of 12 individually transferred suspension (B) or injected (D) cells. Each symbol represents the progeny generated by an individually transferred cell.
To determine whether injection of blood stem cells had any impact on their hematopoietic activity, we evaluated whether injected CD34+/CD38− cells retained the ability to generate colony-forming cells (CFCs).21 Gross microscopic analysis revealed no apparent difference in either the number of wells giving rise to colonies, the total amount of hematopoietic progeny per well, or the distribution of erythroid versus myeloid lineages when comparing injected to control suspension cells (see Table 4). Microinjection of CD34+/CD38− cells does not adversely affect their ability to generate CFCs or their selection of differentiation program.
Immobilization on RN followed by microinjection and detachment does not affect the ability of CD34+/CD38− cells to produce colony forming cells
| Method of Attachment4-150 . | Method of Release . | Microinjection . | % Wells Producing Colony-Forming Cells4-150 . | Colony Formation (% of Nonimmobilized Control)4-151 . | |
|---|---|---|---|---|---|
| Erythroid . | Myeloid . | ||||
| — | — | − | 74 | 100 | 100 |
| n = 42 | |||||
| RN | Peptide | − | 68 | 79 | 86 |
| n = 40 | |||||
| RN | Peptide | + | 77 | 132 | 100 |
| n = 64 | |||||
| Method of Attachment4-150 . | Method of Release . | Microinjection . | % Wells Producing Colony-Forming Cells4-150 . | Colony Formation (% of Nonimmobilized Control)4-151 . | |
|---|---|---|---|---|---|
| Erythroid . | Myeloid . | ||||
| — | — | − | 74 | 100 | 100 |
| n = 42 | |||||
| RN | Peptide | − | 68 | 79 | 86 |
| n = 40 | |||||
| RN | Peptide | + | 77 | 132 | 100 |
| n = 64 | |||||
Individual cells were transferred to wells of 96-well plates using the Quixell Transfer unit. To allow for the development and expansion of colony forming cells, transferred cells were cultured in Stem Cell Medium for 10 days before the addition of MethoCult GF media. After 14 days incubation in methylcellulose, the individual wells were evaluated for colony growth and phenotype. The total number of wells containing either erythroid or myeloid growth was determined for each condition.
Colony formation is represented as the % of nonimmobilized control (number of wells giving rise to colony growth for treated cells ÷ number of wells giving rise to colony growth for suspension cells). Values represent combined results of 2 microinjection sessions in which the number of wells specified (n) were analyzed.
Delivery of fluorescently labeled protein to CD34+cells
Glass needle–mediated microinjection of other cell types (eg, fibroblasts) with proteins (eg, recombinant proteins or antibodies to specific intracellular proteins) has been a powerful method for assaying the function of specific proteins. The frequency of CD34+ cells surviving injection with Cy-3 IgG is similar to that for OG-dextran (Table 3), demonstrating efficient protein delivery to primitive CD34+ cord blood cells.
Transient expression of GFP by microinjected CD34+ and CD34+/CD38− cells
To examine transient reporter gene expression, CD34+ and CD34+/CD38− cells were injected with various expression constructs containing the humanized rsGFP protein under control of either CMV (pGreen Lantern plasmid DNA) or pgk regulatory sequences (linearized pgk-GFP). GFP expression at 5 hours after injection in CD34+ and CD34+/CD38− cells successfully injected with pGreen Lantern was 47% ± 16% and 76% ± 9%, respectively (Table 5). GFP expression was detected in 68% of the CD34+/CD38− cells successfully injected with the linearized pgkGFP DNA. OG-dextran injection results from experiments performed during the same period demonstrated that ∼ 84% and 87% of injected CD34+ and CD34+/CD38− cells, respectively, survived quantitative delivery of injected material at 2 hours after injection (n = 5 most recent experiments). Using these values, we have estimated that ∼56% (47%/84%) and ∼87% (76%/87%) of viable injected CD34+ or CD34+/CD38−cells transiently expressed GFP 5 hours after injection (as estimated for pGreen Lantern). Expression frequencies gradually decreased with increasing time of culture (data not shown); this was not unexpected, because almost all expression should be transient, due to unintegrated DNA copies. However, we have observed GFP-expressing cells (and a smaller number of dividing, expressing cells) as late as 4 to 5 days after injection. In 2 preliminary experiments, CD34+/CD38− cells were injected with a 19.6 kb GFP construct (5 copies/fL) 13.3% and 54.5% of injected cells expressed GFP at 5 hours after injection. Thus, injection yields transient transgene (GFP) expression in primitive blood cell populations with DNAs as large as 19.6 kb in size.
CD34+ and CD34+/CD38− cells express green fluorescent protein after injection with GFP constructs
| Cells5-150 . | DNA Construct Injected . | % Expression at 5 h5-151 . |
|---|---|---|
| CD34+ | pGreen Lantern | 47 ± 165-152 |
| CD34+/CD38− | pGreen Lantern | 76 ± 95-152 |
| CD34+/CD38− | Linearized pgkGFP | 685-153 |
| Cells5-150 . | DNA Construct Injected . | % Expression at 5 h5-151 . |
|---|---|---|
| CD34+ | pGreen Lantern | 47 ± 165-152 |
| CD34+/CD38− | pGreen Lantern | 76 ± 95-152 |
| CD34+/CD38− | Linearized pgkGFP | 685-153 |
Cells attached to RN-coated dishes.
Values represent number of expressing cells/number of injected cells × 100.
Values represent the mean ± SD of 3 experiments of 100 cells per experiment.
Value represents 1 experiment of 110 injected cells.
Discussion
To our knowledge, this is the first report of glass needle–mediated microinjection of DNA, protein, and/or dextran into primary human stem/progenitor cells. The difficulties in injecting hematopoietic cells, which are normally nonadherent and very small, were overcome by development of improved attachment protocols and ultrafine microinjection needles. We have identified conditions whereby primary human cord blood stem/progenitor cells may be temporarily immobilized in a manner sufficiently strong to withstand microinjection without altering cell function/biology.
CD34+ cells represent ∼ 0.5% to 1.0% of nucleated umbilical cord blood and bone marrow cells and comprise all measurable human progenitor activity.18 With the possible exception of a limited subset of CD34−cells,22,23 CD34+ cells also contain all measurable human stem cell activity.18 The CD34+/CD38− (∼ 5% to 10% of CD34+ cells) and CD34+/lineage−/Thy-1lophenotype characterize more primitive subpopulations.2, 24-27 CD34+ cells, and subsets thereof, attach strongly to plates coated with a recombinant derivative of FN, CH-296 known as RN. The RN attachment method had no effect on survival, proliferative potential, or NOD/SCID engrafting ability. In contrast, attachment of CD34+ cells to Con A adversely affected myeloid colony formation (see Table 1), which is consistent with previous reports suggesting that attachment of transformed hematopoietic cells via lectins (eg, Con A28-30) can have mitogenic or inhibitory effects on hematopoietic cells. Strong attachment to FN requires treatment with specific anti-β1integrin antibodies, which induce low avidity β1-containing integrin heterodimers (eg, α4β1, α5β1) to a high avidity state.16 31 Activation of the integrins with the TS2/16.2.1 activating antibody, followed by attachment to FN, may have an effect on NOD/SCID reconstituting activity in the cells. A decrease in the number of mice demonstrating human cell engraftment was noted with the cells attached to FN in the presence of activating mAb compared with control cells (FN with activating mAb). Although this difference was not statistically significant, it was consistent with a reduction in the number of human CD45+ cells in the mice injected with cells attached to FN with activating mAb. Because RN attachment of cells had no effect on cell function, we chose to use RN for cell immobilization in all subsequent studies.
Previous reports of the successful injection of transformed hematopoietic cells are limited.28,29 Attempted injection of small, normally nonadherent cells (eg, primary hematopoietic cells) has resulted in extremely low cell survival.30 Injection needles with a minimal OTD had to be developed to achieve high viability of blood stem/progenitor cells. We have demonstrated increased viability of injected fibroblasts when using needles with decreased OTD (Brown et al, unpublished data). Reducing the injection needle OTD to 0.2 μ did indeed yield a considerable increase in the viability of injected CD34+ cells (unpublished data). This increased viability using ultrafine needles can be attributed to reduced physical damage to the cell during needle insertion and retraction and minimized injection volumes due to finer control of sample flow from the injection needle.
Our technology has allowed for efficient macromolecule delivery and excellent postinjection viabilities of both CD34+ and CD34+/CD38− cells. Optimization of injection conditions (reflected in the last 5 experiments) and observation of individual injected cells resulted in calculated actual long-term survival frequencies at 10 days after injection of 73% and 67% for successfully injected CD34+ and CD34+/CD38− cells, respectively. Furthermore, there was no observed change in the frequency of surviving CD34+ or CD34+/CD38− cells undergoing proliferation at 6 to 10 days after transfer. Finally, injection had no impact on the ability of CD34+/CD38− cells to generate or expand progeny CFCs, an in vitro measure of primitive hematopoietic activity.
Excellent transgene delivery and transient expression was observed in injected CD34+and CD34+/CD38− cells. Interestingly, the more primitive CD34+/CD38− population displayed an increased rate of CMV-driven GFP expression. Although not statistically significant, this trend is consistent with an observed increase in frequency of pGreen Lantern expression in electroporated CD34+/CD38− versus CD34+/CD38+ cells (B.R.D., unpublished results). In preliminary experiments, transient GFP expression was also seen in injected CD34+/CD38−/Thy-1lo cells (data not shown). Future experiments will focus on assaying and optimizing long-term transgene maintenance in primitive hematopoietic stem/progenitor cells.
We have achieved delivery of DNAs 19.6 kb in length and subsequent expression of GFP in the injected cells demonstrating that glass needle–microinjection is a powerful tool for delivering large DNAs to cells. These DNAs can accommodate the regulatory elements and intron/exon structure necessary for long-term, cell type-specific, integration site-independent expression of transgenes. Thus, injection may be preferable for developmental or gene therapeutic applications requiring tightly regulated gene expression in progeny hematopoietic cells.
An evaluation of glass needle–mediated microinjection as a potential method for stem cell gene therapy must take into account the number of stem cells required for transplantation, the frequency of actual stem cells in the injected population, and the total time required to perform the injections. Children have been reconstituted with as little as 30 mL of transplanted cord blood32 containing approximately 1.5 to 3 × 108 nucleated cells. If the frequency of stem cells is 1 in 105 to 106, then successful engraftment could occur with as few as 150 to 3000 stem cells. Although enriched stem cell–containing populations of primitive human hematopoietic cells have already been described,11,23,26,27 further definition of the human stem cell phenotype(s) is necessary. Indeed, determination of the mouse stem cell phenotype has reached the point where delivery of as few as 10 cells,33 or even 1 marrow cell34 is sufficient for total hematopoietic reconstitution.
Both manual (100-200 injected cells/h) and semiautomated (200-400 cells/h) modes of injection were used in this study; rates likely insufficient for feasible therapeutic time constraints. Fully automated systems, capable of 1500 cell injections per hour,20 would likely be used to inject a sufficient number of stem cells for transplantation. Any significant in vitro or in vivo expansion of stem cells,35 perhaps together with selection for marked cells, would further decrease the number of injected stem cells required for engraftment.
In summary, we have demonstrated glass needle microinjection-mediated delivery of macromolecules (protein, DNA, and dextrans) into human CD34+, CD34+/CD38−, and CD34+/CD38−/Thy-1lo cord blood cells. Moreover, our immobilization and injection methods are applicable to quiescent blood stem cells because immunomagnetically isolated CD34+ cells are capable of quantitative attachment to RN immediately after isolation without cytokine prestimulation (data not shown). It has been demonstrated that 95% of cord blood CD34+/CD38− cells are quiescent at time of purification.24 36 The demonstration that these cells can be injected with high viability without any observable adverse effect on proliferation or biologic function strongly supports our intended application of this technology to experimental studies and gene therapeutic applications of hematopoietic stem/progenitor cells.
Acknowledgments
We thank Gina Barron, Mark Griffin, Aqing Yao, Jianming Lu, and Fuming Pan for expert technical assistance.
Partly supported by NIH R21DK53923 to B.R.D. and Sealy and Smith Endowment grants to J. Y.-B. and B.R.D.
Reprints:Brian R. Davis, Sealy Center for Oncology and Hematology, MRB 9.104, University of Texas Medical Branch, Galveston, TX 77555-1048; or David B. Brown, Department of Human Biological Chemistry and Genetics, BSB 506H, University of Texas Medical Branch, Galveston, TX 77555-0645.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal