The retinoic acid receptor (RAR) agonist, all-trans retinoic acid (ATRA), is a potent inducer of terminal differentiation of malignant promyelocytes, but its effects on more primitive hematopoietic progenitors and stem cells are less clear. We previously reported that pharmacologic levels (1 μmol) of ATRA enhanced the generation of colony-forming cell (CFC) and colony-forming unit-spleen (CFU-S) in liquid suspension cultures of lin− c-kit+ Sca-1+ murine hematopoietic precursors. In this study, we further investigated the effects of ATRA as well as an RAR antagonist, AGN 193109, on the generation of transplantable cells, including pre–CFU-S, short-term repopulating stem cells (STRCs), and long-term repopulating stem cells (LTRCs). ATRA enhanced the ex vivo maintenance and production of competitive repopulating STRCs and LTRCs from lin− c-kit+ Sca-1+ cells cultured in liquid suspension for 14 days. In addition, ATRA prevented the differentiation of these primitive stem cells into more mature pre–CFU-S during the 14 days of culture. In marked contrast, lin− c-kit+ Sca-1+ cells cultured with AGN 193109 for 7 days had virtually no short- or long-term repopulating ability, but displayed an approximately 6-fold increase in the pre–CFU-S population. The data suggest that the RAR agonist ATRA enhances the maintenance and self-renewal of short- and long-term repopulating stem cells. In contrast, the RAR antagonist AGN 193109 abrogates reconstituting ability, most likely by promoting the differentiation of the primitive stem cells. These results imply an important and unexpected role of retinoids in regulating hematopoietic stem cell differentiation.
Retinoic acid (RA) and RA receptors (RARs) play an important role in regulating the growth and differentiation of a variety of different cells types.1 In hematopoiesis, the RAR agonist, all-trans retinoic acid (ATRA) is predominantly known for its differentiating effects, being a potent inducer of terminal differentiation of malignant promyelocytes.2 In a recent study, however, we showed that ATRA has different effects on cultured hematopoietic cells depending on their maturational state.3Specifically, the addition of pharmacologic levels (1 μmol) of ATRA to liquid suspension cultures of lineage-negative, c-kit-positive, Sca-1-positive (lin− c-kit+ Sca-1+) hematopoietic precursors markedly enhanced the generation of cells with colony-forming cell (CFC) and colony-forming unit-spleen (CFU-S) potential. This effect of ATRA was restricted to a defined population of these precursors, as ATRA did not enhance the generation of cells with CFC potential from cultures of more mature, lin− c-kit+ Sca-1- progenitors. Furthermore, when the addition of ATRA to cultures of primitive hematopoietic precursors was delayed until the cultures had accumulated a significant number of committed granulocyte/monocyte progenitors, ATRA accelerated the terminal granulocytic maturation of these progenitors.3
The pleiotropic effects of ATRA are also evident in other developmental systems. For example, during embryonic limb development in the mouse, the application of pharmacological levels of ATRA had a self-renewal–like effect, inducing the formation of supernumerary limbs.4,5 When ATRA was applied between 1030 and 1200 hours on 5.5 days postcoitum (dpc), limb duplications occurred, whereas no duplications resulted when ATRA was administered after 1300 hours on 5.5 dpc.5 Moreover, when ATRA was given to the embryo at later stages of development, between 10 and 12.5 dpc, it induced the opposite effect of stunted limb development.6 7 Hence, ATRA induces different effects on embryonic limb development in the mouse, dependent upon the stage of embryonic development.
In a previous study, we observed that the addition of pharmacological levels of ATRA to serum-containing liquid suspension cultures of primitive hematopoietic precursors markedly enhanced the generation of CFCs and CFU-S.3 Such cultures already contain endogenous levels of ATRA, as ATRA is present both in serum-containing media and in serum-free media, bound to the albumin present in these media. Hence, in this study we were interested in further determining the effects of both pharmacological and endogenous levels of ATRA on cultured primitive hematopoietic precursors. To examine the effects of endogenous ATRA, we used an RAR pan-antagonist, AGN 193109. This antagonist binds to RARs with high affinity but does not activate transcription, and thus acts as a competitive inhibitor of RAR activation by endogenous levels of retinoic acid present in the cultures.8 9 Our results indicate that ATRA enhanced the maintenance and/or generation of short- and long-term repopulating stem cells from lin− c-kit+ Sca-1+ hematopoietic precursors cultured in liquid suspension for 14 days. ATRA also prevented the differentiation of these primitive stem cells into the more mature pre–CFU-S population during the 7 to 14 days of culture. In marked contrast, hematopoietic precursors lost their short- and long-term competitive repopulating ability after 7 days of culture with AGN 193109, accompanied by an increase in the more mature pre–CFU-S. These data suggest an unexpected role for ATRA in enhancing the in vitro maintenance and/or inducing self-renewal of in vivo repopulating hematopoietic stem cells.
Materials and methods
Mice
C57BL/6J (Ly5.2) female mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Congenic C57BL/6.SJL-Ly5.1-Pep3b (Ly5.1) mice were bred at the Fred Hutchinson Cancer Research Center (Seattle, WA). All animals were housed in specific pathogen-free conditions and maintained on acidified drinking water and autoclaved chow ad libitum. Mice were used at 8 to 12 weeks of age.
Enrichment of hematopoietic precursor cells
The lineage-negative, c-kit–positive, Sca-1–positive (lin− c-kit+ Sca-1+) hematopoietic precursor cells were FACS enriched as previously described.3
Liquid suspension cultures of hematopoietic cells
Two thousand lin− c-kit+ Sca-1+ precursor cells were deposited into a single well of 24-well plates (Corning, Corning, NY) containing Iscove's modified essential medium (IMDM) supplemented with 20% fetal bovine serum (FBS) and cytokines (murine stem cell factor [SCF], human Flt-3–ligand, human interleukin-6 [IL-6]; each at 100 ng/mL and human IL-11 [10 ng/mL]; PeproTech, Inc, Rocky Hill, NJ). All-trans retinoic acid (Sigma) or the RAR pan-antagonist, AGN 193109 (Allergan Pharmaceuticals, Irvine, CA) were added to a portion of the wells of cells in each experiment, to a final concentration of 1 μmol. Cultures were placed in incubation at 37°C with 5% CO2 in air atmosphere. Cultures were replenished with fresh media at periodic intervals, by removing half the media in each well and replacing it with an equal volume of media containing 2 × concentration of cytokines and ATRA or AGN 193109, where applicable. Different lots of FBS used in cultures were screened for their ability to support colony growth of lin− c-kit+ Sca-1+ cells in semisolid media. Initial studies showed that adding ATRA or AGN 193109 to the cultures more often than once weekly did not have any effect on the experimental outcome.
In vitro colony assay
The cultured cells were analyzed for colony formation in 35-mm culture dishes (Nalge Nunc International) containing methylcellulose-based semisolid medium as previously described.3
CFU-S assay
The spleen colony assay of Till and McCulloch10 was applied. Ly5.1 female recipients 8 to 12 weeks of age were exposed to a single dose of 10.0 Gy of γ radiation from dual opposed60Co sources at an exposure rate of 20 cGy/min on the day of transplantation. After 7 or 14 days of culture, all cells that grew from 500 or 1000 Ly5.2 lin− c-kit+ Sca-1+ cells, respectively, were injected into lethally irradiated female Ly5.1 mice. Transplanted mice were euthanized 8 or 12 days later, their spleens dissected, fixed in Bouin's fixative for 5 minutes, then transferred to 10% neutral buffered formalin (Sigma). Colony-forming unit-spleen (CFU-S) were counted under a dissecting microscope. The number of colonies in recipient spleens has been directly stated without correction for seeding (f) factor.
Pre–CFU-S assay
Pre–CFU-S was assayed as described by Hodgson et al.11Ly5.1 female recipients 8 to 12 weeks of age were exposed to a single dose of 10.0 Gy of γ radiation from dual opposed 60Co sources at an exposure rate of 20 cGy/min on the day of transplantation. After 7 or 14 days of culture, all cells that grew from 500 or 1000 Ly5.2 lin− c-kit+ Sca-1+ cells, respectively, were injected into lethally irradiated female Ly5.1 mice. Thirteen days after the transplant, the primary recipients were euthanized, and bone marrow from the femurs of 3 primary recipients were pooled. Fractions of the pooled marrow were then injected into new lethally irradiated recipients. Twelve days after transplant, the secondary recipients were euthanized, their spleens dissected and fixed for CFU-S evaluation as described previously. A total of 12 secondary recipients were used per treatment group. The values given are for pre–CFU-S generated from 1 femur and have been extrapolated from colonies arising from 0.3 femurs injected per secondary recipient (n = 4).
Short- and long-term competitive repopulation assay
Ly5.1 female recipients 8 to 12 weeks of age were exposed to a single dose of 10.0 Gy of γ radiation from dual opposed60Co sources at an exposure rate of 20 cGy/min on the day of transplantation. The primitive hematopoietic precursors (lin− c-kit+ Sca-1+) were deposited into 24-well plates at an initial density of 2000 cells per well in 1 mL of the culture medium described previously, supplemented with or without 1 μmol ATRA or 1 μmol AGN 193109. One thousand freshly sorted female Ly5.2 lin− c-kit+ Sca-1+ were injected into the tail vein of irradiated female Ly5.1 recipients, together with 1 × 105normal male Ly5.1 bone marrow. After 7 and 14 days of culture, all cells that grew in culture from 1000 Ly5.2 lin− c-kit+ Sca-1+ cells under each culture condition were injected into irradiated female Ly5.1 mice, together with 1 × 105 normal male Ly5.1 bone marrow.
Analysis of transplant recipients
Peripheral blood from each recipient was obtained from the retro-orbital sinus at monthly intervals. The red blood cells were lysed with ammonium chloride lysis buffer, and the remaining cells were washed in PBS/FBS, preincubated with FcγRII block for 10 minutes at 4°C, and distributed into 12 × 75 mm polypropylene tubes (Fisher Scientific) for immunofluorescent staining. Nucleated cells were stained with biotinylated monoclonal antibodies specific for Ly5.2 (clone 104) and Ly5.1 (clone A20) (the kind gifts of Dr G. Spangrude) or biotinylated mouse IgG2a (Pharmingen) for 30 minutes at 4°C. The cells were then washed with PBS/FBS and stained with streptavidin-phycoerythrin. At 3, 6, 9, and 12 months afer transplant, donor cells in T-lymphocyte, B-lymphocyte, granulocyte, and monocyte/macrophage lineages were analyzed by staining for donor (Ly5.2) positive cells, along with FITC-conjugated monoclonal antibodies: anti-Thy1.2, anti-B220, anti-Gr-1, anti-CD11b, or FITC-conjugated isotype-matched control antibodies (Pharmingen). Host (Ly5.1)-positive cells were also investigated in the B-lymphocyte lineage at these time points, to confirm accuracy of the donor cell (Ly5.2) staining. The stained cells were washed with PBS/FBS and resuspended in PBS/FBS containing 1 μg/mL PI and analyzed on a FACSCAN.
Statistical analysis
Data comparing the effects of ATRA versus No ATRA or AGN 193109 versus No AGN 193 109 in the short- and long-term competitive repopulation assays were analyzed with the Wilcoxon rank-sum test. The transplant data of the 7-day cultured cells treated with or without ATRA were further analyzed by fitting a regression model on the ranks of the data, with treatment group being the explanatory variable of interest and transplant number included as an additional explanatory variable.
Results
The RAR pan-antagonist, AGN 193109, enhances the production of CFU-S day 8 but not CFU-S day 12 in cultures of lin− c-kit+ Sca-1+ hematopoietic precursors
The current studies were designed to investigate the effects of retinoids on transplantable hematopoietic cells. In a previous study, we observed that ATRA (1 μmol) markedly enhanced CFU-S production in liquid suspension cultures of primitive hematopoietic precursors (lin− c-kit+ Sca-1+).3 We therefore further explored the role of retinoids in the production of CFU-S by determining the effects of the RAR pan-antagonist, AGN 193109, on the generation of CFU-S from cultured hematopoietic precursors.
Lethally irradiated recipients were injected with 500 freshly sorted lin− c-kit+ Sca-1+ hematopoietic precursors or all cells that grew from 500 or 1000 hematopoietic precursors after 7 or 14 days of culture, respectively, with or without 1 μmol AGN 193109. Mice were euthanized at day 8 and day 12 after transplant, their spleens removed, fixed, and counted for CFU-S. Endogenous CFU-S were also measured in mice that were lethally irradiated but injected with PBS/2% FBS only, and no spleen colonies were visible in these mice (data not shown).
The results of these experiments are shown in Table1. Hematopoietic precursors cultured for 7 days without AGN 193109 produced approximately 4- to 5-fold more CFU-S D8 than the freshly sorted, noncultured lin− c-kit+ Sca-1+ hematopoietic precursors (Table 1, experiment 2). This was accompanied, however, by a 4-fold decrease in CFU-S D12 compared with the noncultured precursors. The addition of 1 μmol AGN 193109 to the cultures of hematopoietic precursors resulted in a 1.5- to 2-fold increase in CFU-S D8 at 7 days of culture compared with the cells cultured without AGN 193109, but there was no change in CFU-S D12 production. Again, this represented an increase in CFU-S D8 but a decrease in CFU-S D12 when compared with freshly sorted lin− c-kit+ Sca-1+ precursors. After 14 days of culture, there were few CFU-S D8 or D12 produced from either treatment groups (Table 1).
The effect of AGN 193109 on CFU-S production from cultured lineage-negative, c-kit–positive, Sca-1–positive hematopoietic precursors
| Experiment 1 . | Experiment 2 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day of Culture . | AGN 193109 . | Number of Cells Injected . | CFU-S D8 . | CFU-S D12 . | Day of Culture . | AGN 193109 . | Number of Cells Injected . | CFU-S D8 . | CFU-S D12 . |
| 0 | ND | ND | ND | ND | 0 | − | 500 | 4.25 ± 0.85 | 30.0 ± 10.0 |
| 7 | − | 6.90 × 105 | 14.0 ± 2.55 | 9.75 ± 1.25 | 7 | − | 4.80 × 105 | 21.0 ± 2.65 | 7.00 ± 0.00 |
| 7 | + | 6.80 × 105 | 28.0 ± 2.00 | 10.0 ± 4.0 | 7 | + | 4.90 × 105 | 32.3 ± 0.33 | 8.00 ± 0.58 |
| 14 | − | 2.26 × 106 | 1.00 ± 0.32 | 0* | 14 | − | 3.92 × 106 | 2.00 ± 1.15 | N/A |
| 14 | + | 2.32 × 106 | 0.40 ± 0.40 | 0* | 14 | + | 2.36 × 106 | 6.00 ± 1.15 | 1* |
| Experiment 1 . | Experiment 2 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day of Culture . | AGN 193109 . | Number of Cells Injected . | CFU-S D8 . | CFU-S D12 . | Day of Culture . | AGN 193109 . | Number of Cells Injected . | CFU-S D8 . | CFU-S D12 . |
| 0 | ND | ND | ND | ND | 0 | − | 500 | 4.25 ± 0.85 | 30.0 ± 10.0 |
| 7 | − | 6.90 × 105 | 14.0 ± 2.55 | 9.75 ± 1.25 | 7 | − | 4.80 × 105 | 21.0 ± 2.65 | 7.00 ± 0.00 |
| 7 | + | 6.80 × 105 | 28.0 ± 2.00 | 10.0 ± 4.0 | 7 | + | 4.90 × 105 | 32.3 ± 0.33 | 8.00 ± 0.58 |
| 14 | − | 2.26 × 106 | 1.00 ± 0.32 | 0* | 14 | − | 3.92 × 106 | 2.00 ± 1.15 | N/A |
| 14 | + | 2.32 × 106 | 0.40 ± 0.40 | 0* | 14 | + | 2.36 × 106 | 6.00 ± 1.15 | 1* |
CFU-S are given as the total CFU-S counted per spleen at day 8 (D8) or day 12 (D12) posttransplant (mean ± sem; n = 5, experiment 1; n = 3, experiment 2). CFU-S after day 7 and 14 of culture is a measure from all cells that grew in liquid suspension culture from 500 or 1000 hematopoietic precursors, respectively. The total number of cells injected per mouse is given for each time point. The culture medium was supplemented with 100 ng/mL SCF, IL-6, Flt-3L and 10 ng/mL IL-11, without (−) or with (+) 1 μmol AGN 193109.
Only 1 mouse survived at this time point.
N/A = not available due to deaths of all mice before day 12. ND = not done.
Effect of RAR agonist, ATRA, and RAR antagonist, AGN 193109, on pre–CFU-S activity of cultured hematopoietic precursors
To further delineate the effects of both the RAR agonist, ATRA, and the RAR antagonist, AGN 193109, on cultured transplantable hematopoietic precursors, we determined their effects on cells within the lin− c-kit+ Sca-1+ population that are more primitive than CFU-S, the pre–CFU-S.12 13
The results of this experiment are shown in Table2. Hematopoietic precursors cultured without ATRA or AGN 193109 for 7 days had almost 2-fold the number of pre–CFU-S compared with the freshly isolated, noncultured lin− c-kit+ Sca-1+ cells (Table 2). The addition of 1 μmol ATRA to the cultures for 7 days resulted in fewer pre–CFU-S, at levels slightly lower than the noncultured hematopoietic precursors. By day 14 of liquid suspension culture, however, hematopoietic precursors cultured without ATRA could not rescue primary recipients to 13 days after transplant, hence no values could be obtained for pre–CFU-S for this time point. In contrast, hematopoietic precursors cultured with 1 μmol ATRA for 14 days maintained pre–CFU-S to levels similar to that present at 7 days of culture in ATRA (Table 2).
The effect of AGN 193109 and ATRA on pre-CFU-S production from cultured lineage-negative, c-kit–positive, Sca-1–positive hematopoietic precursors
| Day of Culture . | ATRA . | AGN 193109 . | Number of Cells Injected in Primary Recipients . | Pre-CFU-S . |
|---|---|---|---|---|
| 0 | − | − | 500 | 8.33 ± 5.0 |
| 7 | − | − | 6.30 × 105 | 16.7 ± 1.37 |
| 7 | + | − | 2.75 × 105 | 3.33 ± 1.37 |
| 7 | − | + | 5.80 × 105 | 46.7 ± 4.90 |
| 14 | − | − | 1.88 × 106 | N/A |
| 14 | + | − | 3.32 × 106 | 4.50 ± 0.96 |
| 14 | − | + | 2.06 × 106 | N/A |
| Day of Culture . | ATRA . | AGN 193109 . | Number of Cells Injected in Primary Recipients . | Pre-CFU-S . |
|---|---|---|---|---|
| 0 | − | − | 500 | 8.33 ± 5.0 |
| 7 | − | − | 6.30 × 105 | 16.7 ± 1.37 |
| 7 | + | − | 2.75 × 105 | 3.33 ± 1.37 |
| 7 | − | + | 5.80 × 105 | 46.7 ± 4.90 |
| 14 | − | − | 1.88 × 106 | N/A |
| 14 | + | − | 3.32 × 106 | 4.50 ± 0.96 |
| 14 | − | + | 2.06 × 106 | N/A |
The total number of cells injected per primary recipient is given, being 500 lin− c-kit+ Sca-1+ precursor cells (day 0 of culture) or all cells that grew in liquid suspension culture from 500 or 1000 hematopoietic precursors at day 7 and 14, respectively. The culture medium was supplemented with 100 ng/mL SCF, IL-6, Flt-3L, and 10 ng/mL IL-11, without (−) or with (+) 1 μmol ATRA or 1 μmol AGN 193109. Pre-CFU-S are given as the total CFU-S produced per femur of primary recipients at day 12 after transplant in all secondary recipients (mean ± SEM, n = 4).
Only 1 mouse survived at this time point.
N/A = not available due to deaths of all mice before time of analysis.
The addition of the RAR pan-antagonist, AGN 193109, to cultures of hematopoietic precursors for 7 days had a profound effect on the generation of pre–CFU-S, increasing this compartment by approximately 3-fold compared with that of hematopoietic precursors cultured without AGN 193109. This also represented approximately 6-fold and 14-fold increases in pre–CFU-S when compared with pre–CFU-S potential of the noncultured precursors and hematopoietic precursors cultured for 7 days with 1 μmol ATRA, respectively (Table 2). By day 14 of liquid suspension culture, however, hematopoietic precursors cultured with AGN 193109 could not rescue primary recipients to 13 days after transplant, hence no values could be obtained for pre–CFU-S at this time point.
ATRA enhances the ex vivo maintenance of in vivo long-term repopulating stem cells
Our previous observations that ATRA enhances the generation of CFC and CFU-S from cultured hematopoietic precursors,3 together with our current observation that pre–CFU-S levels are maintained, albeit at slightly lower levels, in such cultures (Table 2) suggest that ATRA may influence the production of even less mature hematopoietic precursors. We therefore wished to determine whether exogenous ATRA might enhance the production or maintenance of short- and long-term repopulating stem cells.
The FACS-enriched primitive hematopoietic precursors were deposited into 24-well plates at an initial density of 2000 cells per well in 1 mL of the culture medium described previously, with or without 1 μmol ATRA. At day 0, 1000 freshly sorted Ly5.2+ lin− c-kit+ Sca-1+ cells, together with 1 × 105 normal Ly5.1+ bone marrow cells, were injected into lethally irradiated Ly5.1+ recipients. Then, at days 7 and 14, all cells that grew in culture from 1000 of the initial lin− c-kit+ Sca-1+ cells, together with 1 × 105 normal Ly5.1+ bone marrow cells were injected into lethally irradiated Ly5.1+ recipients. FACS analysis, using antibodies specific for the Ly5.1 (host) and Ly5.2 (donor) epitopes, allowed us to quantitate the percentage of residual host and donor Ly5.1 (untreated) versus Ly5.2 donor (treated) cells in peripheral blood samples serially harvested from these animals after transplant. In all experiments, background staining of Ly5.2 in nontransplanted Ly5.1 mice was < 3.0%.
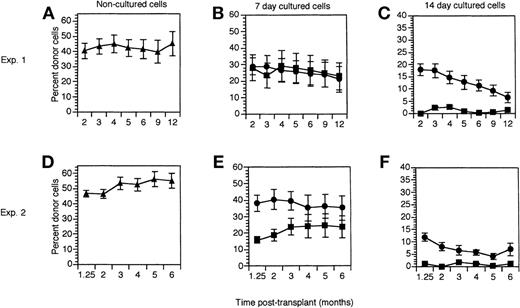
These in vivo repopulation studies showed that the addition of ATRA to cultures of hematopoietic precursors resulted in greater short- and long-term repopulating activity compared with cultures without ATRA (Figure 1). The number of cultured cells injected per mouse for each of 2 experiments are shown in Table3. In each experiment, mice transplanted with noncultured precursors showed short-term (≤ 4 months) and long-term (≥ 6 months) donor cell reconstitution (Figure 1A and D). Mice receiving all cells that grew from 1000 initial hematopoietic precursors cultured for 7 days with or without ATRA displayed similar levels of long-term donor cell reconstitution (Figure 1B and E). However, there were marked differences in the cell number injected into mice in the 2 treatment groups, with significantly fewer cells present in the ATRA-treated cultures at 7 days (Table 3). Therefore, we also calculated the donor cell reconstitution per 105 cells injected into the mice (Table 4). The results of 3 separate experiments were used in this analysis, where experiment 1 and experiment 2 are data from the respective experiments in Figure 1, and experiment 3 data are from an additional transplant of 7-day cultured cells analyzed at 6 months. Analysis of these data showed a statistically significant difference in 2 of 3 experiments (P ≤ .02). In addition, there was an overall statistically significant difference (P < .001) between the 2 groups, with ATRA-treated cells having significantly higher donor cell reconstitution per 105 cells injected into the mice. In these studies, this long-term reconstitution in both treatment groups was multilineage, as donor cells were detected in both myeloid (Gr-1 and CD11b) and lymphoid (B220 and Thy1.2) populations (Table5).
The effect of ATRA on liquid suspension cultures of short- and long-term repopulating hematopoietic stem cells.
2000 FACS-enriched hematopoietic precursors (lin− c-kit+ Sca-1+) were added to wells containing media and cytokines (SCF, IL-6, IL-11, and Flt-3-ligand), and cultured without (▪) or with (•) 1 μmol ATRA. Irradiated Ly5.1 recipients (10 mice per group) were transplanted with 1 × 105 normal Ly5.1 bone marrow cells together with 1000 noncultured (▴) Ly5.2 lin− c-kit+ Sca-1+ cells or with all cells that grew from 1000 of these precursors after 7 or 14 days in liquid suspension culture. Data are expressed as the mean ± SEM donor cell reconstitution in the peripheral blood of transplanted recipients analyzed between 5 weeks (1.25 mo) and 12 months after transplant. Results of noncultured cells (A, D), 7-day cultured cells (B, E) and 14-day cultured cells (C, F) are shown from 2 separate experiments (Exp. 1 and 2).
The effect of ATRA on liquid suspension cultures of short- and long-term repopulating hematopoietic stem cells.
2000 FACS-enriched hematopoietic precursors (lin− c-kit+ Sca-1+) were added to wells containing media and cytokines (SCF, IL-6, IL-11, and Flt-3-ligand), and cultured without (▪) or with (•) 1 μmol ATRA. Irradiated Ly5.1 recipients (10 mice per group) were transplanted with 1 × 105 normal Ly5.1 bone marrow cells together with 1000 noncultured (▴) Ly5.2 lin− c-kit+ Sca-1+ cells or with all cells that grew from 1000 of these precursors after 7 or 14 days in liquid suspension culture. Data are expressed as the mean ± SEM donor cell reconstitution in the peripheral blood of transplanted recipients analyzed between 5 weeks (1.25 mo) and 12 months after transplant. Results of noncultured cells (A, D), 7-day cultured cells (B, E) and 14-day cultured cells (C, F) are shown from 2 separate experiments (Exp. 1 and 2).
ATRA effects on cell number of cultured hematopoietic precursors
| Days in Culture . | ATRA . | Cell Counts . | |
|---|---|---|---|
| Experiment 1 . | Experiment 2 . | ||
| 7 | − | 0.77 × 106 | 1.18 × 106 |
| 7 | + | 0.45 × 106 | 0.37 × 106 |
| 14 | − | 2.88 × 106 | 1.96 × 106 |
| 14 | + | 2.58 × 106 | 1.42 × 106 |
| Days in Culture . | ATRA . | Cell Counts . | |
|---|---|---|---|
| Experiment 1 . | Experiment 2 . | ||
| 7 | − | 0.77 × 106 | 1.18 × 106 |
| 7 | + | 0.45 × 106 | 0.37 × 106 |
| 14 | − | 2.88 × 106 | 1.96 × 106 |
| 14 | + | 2.58 × 106 | 1.42 × 106 |
The indicated cell counts represent all cells that grew in culture from 1000 lin− c-kit+ Sca-1+ cells after 7 or 14 days in liquid suspension culture. These cells were injected into each lethally irradiated mouse in the competitive repopulation transplant. The culture medium was supplemented with 100 ng/mL SCF, IL-6, Flt-3L, and 10 ng/mL IL-11, without (−) or with (+) 1 μmol ATRA.
Donor cell reconstitution per 105 cells injected after 7 days of culture with or without 1 μmol ATRA
| Experiment Number . | Median Values for % Donor per 105 Cells Injected . | P-value . | |
|---|---|---|---|
| No ATRA . | ATRA . | ||
| 1 | 2.55 | 3.62 | 0.33 |
| 2 | 1.11 | 7.17 | 0.002 |
| 3 | 0.47 | 2.13 | 0.02 |
| Average of all 3 experiments | 0.78 | 4.09 | < 0.001 |
| Experiment Number . | Median Values for % Donor per 105 Cells Injected . | P-value . | |
|---|---|---|---|
| No ATRA . | ATRA . | ||
| 1 | 2.55 | 3.62 | 0.33 |
| 2 | 1.11 | 7.17 | 0.002 |
| 3 | 0.47 | 2.13 | 0.02 |
| Average of all 3 experiments | 0.78 | 4.09 | < 0.001 |
The indicated median values represent the median value of the percentage of donor cells in each mouse adjusted per 105cultured cells injected per treatment group after 7 days of culture with or without 1 μmol ATRA. Data were analyzed by the Wilcoxon rank-sum test, and the P-values are given for each experiment. To average the results of all 3 experiments and account for variability between transplants, the data were further analyzed by fitting a regression model on the ranks of the data, with treatment group (ATRA vs No ATRA) being the explanatory variable of interest and experiment number included as an additional explanatory variable.
Percentage of donor cells in myeloid and lymphoid lineages
| . | Summary Data5-150 . | |||||
|---|---|---|---|---|---|---|
| I. Short-term Reconstitution Analysis . | Percentage of Donor Cells in . | |||||
| Experiment # . | Months Post-BMT . | Culture Conditions . | Gr-1 . | CD11b . | B220 . | Thy-1.2 . |
| 1 | 3 | Fresh precursors | 36.3 ± 3.84 | 41.5 ± 4.39 | 51.0 ± 6.44 | 32.6 ± 5.20 |
| 1 | 3 | No ATRA D7 | 23.5 ± 7.72 | 23.8 ± 8.08 | 32.1 ± 9.89 | 25.5 ± 8.32 |
| 1 | 3 | ATRA D7 | 20.4 ± 5.85 | 22.1 ± 7.63 | 33.9 ± 6.38 | 24.9 ± 5.69 |
| 1 | 3 | No ATRA D14 | ND | ND | ND | ND |
| 1 | 3 | ATRA D14 | 8.77 ± 1.03 | 6.68 ± 1.26 | 28.6 ± 4.06 | 10.5 ± 2.59 |
| 2 | 3 | Fresh precursors | 43.5 ± 3.25 | 49.9 ± 6.13 | 54.2 ± 4.43 | 43.0 ± 3.78 |
| 2 | 3 | No ATRA D7 | 19.9 ± 5.08 | 20.0 ± 8.24 | 34.3 ± 7.43 | 20.9 ± 4.26 |
| 2 | 3 | ATRA D7 | 35.5 ± 6.29 | 30.0 ± 9.66 | 51.7 ± 6.81 | 32.5 ± 5.55 |
| 2 | 3 | No ATRA D14 | 2.77 ± 0.63 | 4.97 ± 1.05 | 3.29 ± 0.86 | 1.58 ± 0.44 |
| 2 | 3 | ATRA D14 | 8.12 ± 3.53 | 9.81 ± 5.22 | 9.72 ± 1.42 | 4.65 ± 1.05 |
| Summary Data5-150 | ||||||
| II. Long-term Reconstitution Analysis | Percentage of Donor Cells in | |||||
| Experiment # | Months Post-BMT | Culture Conditions | Gr-1 | CD11b | B220 | Thy-1.2 |
| 1 | 12 | Fresh precursors | 48.6 ± 3.65 | 61.9 ± 7.51 | 60.6 ± 6.06 | 52.6 ± 5.22 |
| 1 | 12 | No ATRA D7 | 35.8 ± 5.70 | 18.5 ± 7.99 | 32.6 ± 9.92 | 23.4 ± 8.59 |
| 1 | 12 | ATRA D7 | 33.2 ± 3.40 | 17.5 ± 9.26 | 30.9 ± 8.71 | 16.4 ± 6.67 |
| 1 | 12 | No ATRA D14 | 2.35 ± 0.51 | 1.42 ± 0.31 | 3.87 ± 0.38 | 0.35 ± 0.04 |
| 1 | 12 | ATRA D14 | 3.28 ± 0.88 | 4.20 ± 0.78 | 12.7 ± 2.65 | 4.58 ± 2.12 |
| 2 | 6 | Fresh precursors | 31.5 ± 5.46 | 40.7 ± 8.32 | 39.7 ± 6.71 | 28.7 ± 6.65 |
| 2 | 6 | No ATRA D7 | 20.0 ± 5.88 | 20.3 ± 7.72 | 30.2 ± 7.42 | 21.2 ± 7.24 |
| 2 | 6 | ATRA D7 | 28.5 ± 6.10 | 31.8 ± 8.85 | 40.5 ± 7.90 | 33.2 ± 8.05 |
| 2 | 6 | No ATRA D14 | 3.66 ± 1.25 | 0.98 ± 0.37 | 4.82 ± 2.25 | 0.42 ± 0.16 |
| 2 | 6 | ATRA D14 | 7.74 ± 5.09 | 7.73 ± 6.36 | 9.08 ± 3.09 | 3.66 ± 1.31 |
| . | Summary Data5-150 . | |||||
|---|---|---|---|---|---|---|
| I. Short-term Reconstitution Analysis . | Percentage of Donor Cells in . | |||||
| Experiment # . | Months Post-BMT . | Culture Conditions . | Gr-1 . | CD11b . | B220 . | Thy-1.2 . |
| 1 | 3 | Fresh precursors | 36.3 ± 3.84 | 41.5 ± 4.39 | 51.0 ± 6.44 | 32.6 ± 5.20 |
| 1 | 3 | No ATRA D7 | 23.5 ± 7.72 | 23.8 ± 8.08 | 32.1 ± 9.89 | 25.5 ± 8.32 |
| 1 | 3 | ATRA D7 | 20.4 ± 5.85 | 22.1 ± 7.63 | 33.9 ± 6.38 | 24.9 ± 5.69 |
| 1 | 3 | No ATRA D14 | ND | ND | ND | ND |
| 1 | 3 | ATRA D14 | 8.77 ± 1.03 | 6.68 ± 1.26 | 28.6 ± 4.06 | 10.5 ± 2.59 |
| 2 | 3 | Fresh precursors | 43.5 ± 3.25 | 49.9 ± 6.13 | 54.2 ± 4.43 | 43.0 ± 3.78 |
| 2 | 3 | No ATRA D7 | 19.9 ± 5.08 | 20.0 ± 8.24 | 34.3 ± 7.43 | 20.9 ± 4.26 |
| 2 | 3 | ATRA D7 | 35.5 ± 6.29 | 30.0 ± 9.66 | 51.7 ± 6.81 | 32.5 ± 5.55 |
| 2 | 3 | No ATRA D14 | 2.77 ± 0.63 | 4.97 ± 1.05 | 3.29 ± 0.86 | 1.58 ± 0.44 |
| 2 | 3 | ATRA D14 | 8.12 ± 3.53 | 9.81 ± 5.22 | 9.72 ± 1.42 | 4.65 ± 1.05 |
| Summary Data5-150 | ||||||
| II. Long-term Reconstitution Analysis | Percentage of Donor Cells in | |||||
| Experiment # | Months Post-BMT | Culture Conditions | Gr-1 | CD11b | B220 | Thy-1.2 |
| 1 | 12 | Fresh precursors | 48.6 ± 3.65 | 61.9 ± 7.51 | 60.6 ± 6.06 | 52.6 ± 5.22 |
| 1 | 12 | No ATRA D7 | 35.8 ± 5.70 | 18.5 ± 7.99 | 32.6 ± 9.92 | 23.4 ± 8.59 |
| 1 | 12 | ATRA D7 | 33.2 ± 3.40 | 17.5 ± 9.26 | 30.9 ± 8.71 | 16.4 ± 6.67 |
| 1 | 12 | No ATRA D14 | 2.35 ± 0.51 | 1.42 ± 0.31 | 3.87 ± 0.38 | 0.35 ± 0.04 |
| 1 | 12 | ATRA D14 | 3.28 ± 0.88 | 4.20 ± 0.78 | 12.7 ± 2.65 | 4.58 ± 2.12 |
| 2 | 6 | Fresh precursors | 31.5 ± 5.46 | 40.7 ± 8.32 | 39.7 ± 6.71 | 28.7 ± 6.65 |
| 2 | 6 | No ATRA D7 | 20.0 ± 5.88 | 20.3 ± 7.72 | 30.2 ± 7.42 | 21.2 ± 7.24 |
| 2 | 6 | ATRA D7 | 28.5 ± 6.10 | 31.8 ± 8.85 | 40.5 ± 7.90 | 33.2 ± 8.05 |
| 2 | 6 | No ATRA D14 | 3.66 ± 1.25 | 0.98 ± 0.37 | 4.82 ± 2.25 | 0.42 ± 0.16 |
| 2 | 6 | ATRA D14 | 7.74 ± 5.09 | 7.73 ± 6.36 | 9.08 ± 3.09 | 3.66 ± 1.31 |
Irradiated Ly5.1 recipients (10 mice per group) were transplanted with 1 × 105 normal Ly5.1 bone marrow cells together with 1000 fresh Ly5.2 lin− c-kit+ Sca-1+ cells (fresh precursors) or with all cells that grew from 1000 of these precursors after 7 (D7) or 14 (D14) days in liquid culture suspension. The culture medium was supplemented with 100 ng/mL SCF, IL-6, Flt-3L, and 10 ng/ml LIL-11, without (No ATRA) or with (ATRA) 1 μmol ATRA. Levels of reconstitution at 3, 6, and 12 months after transplant are shown as a summary of groups.
Mean ± SEM % donor derived nucleated peripheral blood cells identified by anti-Ly5.2. Background (peripheral blood cells of nontransplanted Ly5.1 female mice) staining was < 3.0%.
ND = Not done. Number of mice per group = 10, except No ATRA 2 weeks experiment #1 (n = 9) and No ATRA 2 weeks experiment #2 (n = 8).
After 14 days of culture, there was a significant difference between the repopulating ability of cells from the ATRA-treated and nontreated cultures (Figure 1C and F). Of note, the number of cultured cells injected into the mice in both treatment groups was similar at this time point (Table 3). However, none of the 10 mice receiving cells cultured for 14 days without ATRA showed any donor cell reconstitution, whereas mice receiving hematopoietic precursors cultured with ATRA for 14 days showed significant short- and long-term donor cell reconstitution (Figure 1C and F). Again, these reconstituting donor cells gave rise to multilineage progeny (Table 5). This difference in reconstituting ability of the ATRA-treated versus untreated day 14 cultured cells was statistically significant at all time points (P < .001, Wilcoxon rank-sum test).
The RAR antagonist, AGN 193109, abrogates the short- and long-term repopulating activity of cultured stem cells
The marked increase in pre-CFU-S activity of hematopoietic precursors cultured for 7 days with the RAR antagonist AGN 193109 (Table 2) may have resulted from a direct effect of the antagonist on the pre–CFU-S population. Alternatively, it may be a reflection of an effect of the antagonist on more primitive repopulating stem cells, such as enhancing the differentiation of these cells into the pre–CFU-S compartment. Given that the RAR agonist, ATRA, enhances the production and/or maintenance, and likely blocks the commitment/differentiation of short- and long-term repopulating stem cells in ex vivo liquid suspension cultures, the latter seemed to be the likely explanation. Therefore, we assessed the effects of the RAR pan-antagonist on short- and long-term repopulating stem cells in the liquid suspension cultures.
The short- and long-term competitive repopulation assays were performed as described for the ATRA-treated cultures, except that cells were cultured with or without 1 μmol AGN 193109 in liquid suspension culture.
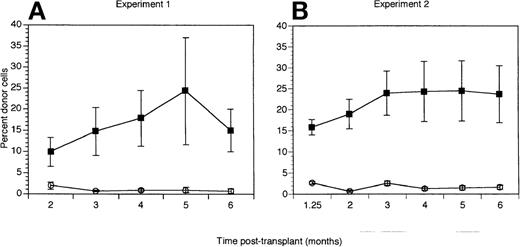
The number of cells and CFC produced from 1000 initial hematopoietic precursors after 7 days of culture with or without AGN 193109 was similar in each of 2 experiments (Table 6). In contrast, the AGN 193109-treated cells had significant differences in their repopulating activity compared with precursors cultured without the RAR pan-antagonist (Figure 2). In each of 2 experiments, mice receiving precursors cultured for 7 days without AGN 193109 showed short- and long-term donor cell reconstitution (Figure 2). In marked contrast, the addition of the RAR antagonist, AGN 193109, to cultures of hematopoietic precursors for 7 days significantly abrogated both short- and long-term repopulating ability of these cells, with donor cell levels below the background levels of 3% Ly5.2 positive cells in untransplanted Ly5.1 mice (P ≤ .002, Wilcoxon rank-sum test). Hematopoietic precursors cultured for 14 days with or without the RAR pan-antagonist did not contribute to short- or long-term reconstitution of the recipients (data not shown).
RAR antagonist effects on cell number and CFC output of cultured hematopoietic precursors
| AGN 193109 . | Cell Counts . | CFU-GM . | HPP-Mix . | |||
|---|---|---|---|---|---|---|
| Experiment 1 . | Experiment 2 . | Experiment 1 . | Experiment 2 . | Experiment 1 . | Experiment 2 . | |
| − | 0.77 × 106 | 1.18 × 106 | 6000 ± 764 | 4000 ± 1155 | 2333 ± 1202 | 523 ± 333 |
| + | 0.77 × 106 | 1.30 × 106 | 6750 ± 661 | 6333 ± 882 | 6333 ± 667 | 917 ± 300 |
| AGN 193109 . | Cell Counts . | CFU-GM . | HPP-Mix . | |||
|---|---|---|---|---|---|---|
| Experiment 1 . | Experiment 2 . | Experiment 1 . | Experiment 2 . | Experiment 1 . | Experiment 2 . | |
| − | 0.77 × 106 | 1.18 × 106 | 6000 ± 764 | 4000 ± 1155 | 2333 ± 1202 | 523 ± 333 |
| + | 0.77 × 106 | 1.30 × 106 | 6750 ± 661 | 6333 ± 882 | 6333 ± 667 | 917 ± 300 |
Counts and colony-forming cells (CFU-GM and HPP-mix) are estimated to be from all cells that grew in culture from 1000 lin− c-kit+ Sca-1+ cells after 7 days in liquid suspension culture, and are given as the total cell number and colony-forming cells (mean ± SEM, n = 3). The culture medium was supplemented with 100 ng/mL SCF, IL-6, Flt-3L, and 10 ng/mL IL-11, without (−) or with (+) 1 μmol RAR antagonist (AGN 193109).
The effect of the RAR antagonist, AGN 193 109, on liquid suspension cultures of short- and long-term repopulating hematopoietic stem cells.
2000 FACS-enriched hematopoietic precursors (lin− c-kit+ Sca-1+) were added to wells containing media and cytokines (SCF, IL-6, IL-11, and Flt-3-ligand), and cultured without (▪) or with (○) 1 μmol AGN 193109. Irradiated Ly5.1 recipients (5 mice per group) were transplanted with 1 × 105 normal Ly5.1 bone marrow cells together with all cells that grew from 1000 lin− c-kit+ Sca-1+ precursors after 7 days in liquid suspension culture. Data are expressed as the mean ± SEM donor cell reconstitution in the peripheral blood of transplanted recipients analyzed between 5 weeks (1.25 mo) and 6 months after transplant. Results of 7-day cultured cells (A, B) are shown from 2 separate experiments.
The effect of the RAR antagonist, AGN 193 109, on liquid suspension cultures of short- and long-term repopulating hematopoietic stem cells.
2000 FACS-enriched hematopoietic precursors (lin− c-kit+ Sca-1+) were added to wells containing media and cytokines (SCF, IL-6, IL-11, and Flt-3-ligand), and cultured without (▪) or with (○) 1 μmol AGN 193109. Irradiated Ly5.1 recipients (5 mice per group) were transplanted with 1 × 105 normal Ly5.1 bone marrow cells together with all cells that grew from 1000 lin− c-kit+ Sca-1+ precursors after 7 days in liquid suspension culture. Data are expressed as the mean ± SEM donor cell reconstitution in the peripheral blood of transplanted recipients analyzed between 5 weeks (1.25 mo) and 6 months after transplant. Results of 7-day cultured cells (A, B) are shown from 2 separate experiments.
Discussion
In recent years, an increasing number of investigators have been interested in ex vivo culture of hematopoietic precursor cells for purposes such as stem cell expansion and retroviral-mediated gene transduction. Invariably, the culture of these cells leads to a rapid decline in stem cell activity, resulting in markedly impaired transplantability of the cultured cell populations. The need to improve on such methods is obvious, and, for gene therapy purposes using retroviral vectors, it is essential for a hematopoietic stem cell to divide but be prevented from differentiating during the culture period in order to enhance the possibility of correcting a genetic deficiency in hematopoietic stem cells.
The experiments described here involved attempts to determine whether ATRA might alter the differentiation of lin− c-kit+ Sca-1+ hematopoietic precursors cultured in liquid suspension. In a previous study, we demonstrated that hematopoietic precursors cultured in liquid suspension in pharmacologic levels (1 μmol) of ATRA were maintained in a less differentiated state than those cultured without ATRA as assessed by cell surface phenotyping and enhanced CFC and CFU-S activity.3 The increase in CFC numbers was likely to be a result of enhanced generation of a cell population more primitive than CFC, as we also reported that ATRA did not enhance CFC production from the more mature population of lin− c-kit+ Sca-1-progenitors. The ATRA-induced increase in CFU-S and CFC production in these cultures may therefore have resulted from either a direct effect of ATRA on the self-renewal of CFU-S or from ATRA-induced effects on a hematopoietic precursor more primitive than CFU-S. To distinguish these possibilities, we determined the effects of ATRA on the generation of pre–CFU-S, short-term repopulating stem cells (STRCs) and long-term repopulating stem cells (LTRCs) in liquid suspension cultures of lin− c-kit+ Sca-1+ hematopoietic precursors.
The most striking observation involved the effect of the RAR agonist and antagonist on the generation of STRCs and LTRCs. In the absence of exogenous ATRA, the repopulating ability of the cultured hematopoietic precursors gradually declined and, by 14 days, such ex vivo cultures no longer harbored any stem cells as measured in an in vivo competitive repopulating assay. The addition of 1 μmol ATRA to cultures of these hematopoietic precursors, however, prolonged the maintenance of stem cells so that after 14 days of culture both short- and long-term competitive repopulating stem cells could still be detected in these cultures. In marked contrast, the addition of the RAR antagonist to these cultures had the opposite effect. Indeed, this compound completely abrogated the production of both STRCs and LTRCs after 7 days of culture, a time at which considerable competitive repopulating stem cell activity was readily detected in the ATRA-treated and nontreated culture systems. This RAR antagonist-induced loss of functional primitive hematopoietic stem cells does not appear to merely be the result of defective homing of these cultured cells, because the AGN 193 109-treated hematopoietic precursors were capable of homing to both marrow and spleen as demonstrated by their marrow repopulating ability (pre–CFU-S) and CFU-S activity.
Interestingly, the pre–CFU-S have been previously reported to resemble hematopoietic stem cells in that they share a pattern of low rhodamine-123 fluorescence staining intensity.12 13However, although the pre–CFU-S are relatively primitive hematopoietic precursors, our observed association of an increase in pre–CFU-S production in the RAR antagonist-treated cultures with a concomitant decrease in short- and long-term repopulating stem cell production indicates that the pre–CFU-S and competitive repopulating hematopoietic stem cells represent discrete hematopoietic precursor compartments.
Our observation that the RAR antagonist abrogates STRC and LTRC production in liquid suspension cultures strongly indicates that endogenous levels of RA that are normally present in serum, or bound to proteins present in serum-free media, can influence the generation and maintenance of primitive hematopoietic stem cells in liquid suspension culture, and underscores the importance of functioning RARs to the integrity of hematopoietic stem cells. Previous studies involving mice with “knockouts” of single and double RAR isoforms (α, β, γ) have indicated numerous abnormalities in various organs, particularly in double “knockouts,” including malformations of the head, vertebrae, limbs, neck, trunk, and abdominal regions.14-19 However, only mice null for both the α and γ isoforms have hematopoietic defects,19 with the RARγ and RARα1 double null mutants displaying impaired granulocytic differentiation in vitro.19Interestingly, these mice have no observable stem cell defects, and hematopoiesis, including granulopoiesis, is normal in vivo, suggesting compensatory mechanisms in these mice. To our knowledge, however, mutant mice null for all 3 isoforms have not been created, and, given that double null mutants are often embryonically lethal or die shortly after birth, the feasibility of creating such mutants is questionable. Our transplant results using the RAR antagonist, AGN 193109, which is a pan-(α, β, γ) antagonist and effectively “knocks out” all RAR activity,8 would suggest, however, that such triple null mutant mice would have severe hematologic defects arising at the primitive stem cell level.
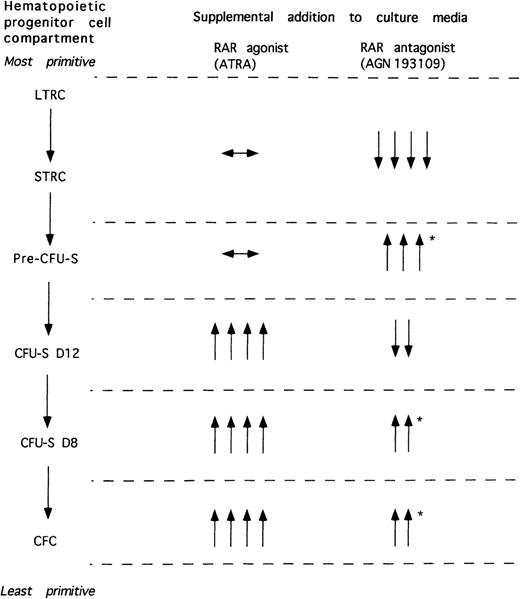
Our observations on hematopoietic precursor and progenitor production in RAR agonist and antagonist-treated cultures of lin− c-kit+ Sca-1+ hematopoietic precursors noted both in the current study and in our previous experimental efforts3 are summarized in Figure 3. It is clear that the RAR agonist and antagonist exert complex, pleiotropic effects in these cultures that are likely heavily dependent on the maturational state of the hematopoietic cell type being affected. ATRA appears to maintain both STRCs and LTRCs in these cultures, whereas the RAR antagonist depletes such stem cells most probably by encouraging their differentiation to a more committed precursor, the pre–CFU-S. In addition, although the LTRC, STRC and pre–CFU-S compartments are maintained in ATRA-treated cultures, the CFU-S compartment is dramatically increased, suggesting that ATRA most likely has a direct effect in enhancing the self-renewal of CFU-S in these cultures. The enhanced CFC production that we previously observed in the ATRA-treated cultures is also likely secondary to enhanced production and maintenance of more primitive CFU-S and marrow reconstituting stem cells rather than a direct effect of ATRA on the CFC progenitors.
Schematic depiction of the effects of RAR agonists and antagonists on cultured hematopoietic progenitor cells.
The effects of all-trans retinoic acid (ATRA) on various hematopoietic progenitor cell compartments during culture of lin− c-kit+ Sca-1+ hematopoietic precursors for 7 and 14 days with 1 μmol RAR agonist, ATRA, or 1 μmol RAR antagonist, AGN 193109, compared with the magnitude of the progenitor compartments of the noncultured hematopoietic precursors are shown. The magnitude of the effect is indicated by the number of arrows, with 1 arrow representing the smallest effect and 4 arrows representing the largest effect. Arrows indicate as follows: ↔, maintenance in potential; ↑, increase in potential; ↓, decrease in potential. *denotes effects seen only at 7 days of culture. Abbreviations: LTRC, long-term repopulating stem cell; STRC, short-term repopulating stem cell; pre–CFU-S, pre–colony-forming unit-spleen; CFU-S, colony-forming unit-spleen; D8, day 8; D12, day 12; CFC, colony-forming cell.
Schematic depiction of the effects of RAR agonists and antagonists on cultured hematopoietic progenitor cells.
The effects of all-trans retinoic acid (ATRA) on various hematopoietic progenitor cell compartments during culture of lin− c-kit+ Sca-1+ hematopoietic precursors for 7 and 14 days with 1 μmol RAR agonist, ATRA, or 1 μmol RAR antagonist, AGN 193109, compared with the magnitude of the progenitor compartments of the noncultured hematopoietic precursors are shown. The magnitude of the effect is indicated by the number of arrows, with 1 arrow representing the smallest effect and 4 arrows representing the largest effect. Arrows indicate as follows: ↔, maintenance in potential; ↑, increase in potential; ↓, decrease in potential. *denotes effects seen only at 7 days of culture. Abbreviations: LTRC, long-term repopulating stem cell; STRC, short-term repopulating stem cell; pre–CFU-S, pre–colony-forming unit-spleen; CFU-S, colony-forming unit-spleen; D8, day 8; D12, day 12; CFC, colony-forming cell.
We do not know whether our observed effect of ATRA in enhancing the maintenance or production of transplantable hematopoietic stem cells in liquid suspension culture results from a direct effect of ATRA on the stem cells or an indirect effect through “accessory” cells that may regulate stem cell behavior in these cultures. The ATRA-treated cultures at 7 days exhibited reduced cell density compared with the untreated cultures (Table 3), perhaps leading to changes in cellular cross talk at this time point that might account for differences in stem cell production/maintenance. However, cultures of the hematopoietic precursors treated with the RAR antagonist, which markedly abrogated transplantable stem cell activity at 7 days, displayed comparable cell densities to cultures without the antagonist (Table 6), suggesting that the differences in stem cell activity were a direct effect of the antagonist.
The molecular mechanisms involved in the observed effects of ATRA on the hematopoietic precursors have not yet been determined. ATRA regulates its biologic activities by triggering the activation of RAR-RXR heterodimers that serve as transcription factors to regulate the expression of specific target genes. Key genes regulated by ATRA in the mouse and chick during embryonic development include members of the homeobox (HOX) superfamily.5,20,21 Hox genes are expressed in hematopoietic cells and have been implicated in the regulation of various aspects of hematopoiesis.22-28 Interestingly, there are many parallels between our observations and those observed when the HOX gene, HOX B4 was overexpressed in mouse bone marrow.25 These observations included a maintenance of pre–CFU-S, an increase in CFU-S D12 and a marked increase in CFC.
Our studies indicate that pharmacological levels, as well as endogenous culture media-containing levels of ATRA influence the primitive hematopoietic stem cells, as shown by the enhanced ex vivo maintenance of repopulating stem cells in liquid suspension culture. Regardless of whether ATRA enhances self-renewal of primitive hematopoietic stem cells or slows their differentiation in liquid suspension cultures, these results suggest that ATRA may be a useful tool for aiding retroviral- or lentiviral-mediated gene transduction into primitive hematopoietic stem cells. We are currently investigating this possibility.
Acknowledgments
We thank Cynthia Nourigat for her excellent technical assistance and Dr Ted Gooley for statistical analysis.
Supported by National Institutes of Health (NIH) grants nos. HL54881 and CA58292. L.E.P. is a Leukemia Society of America Special Fellow. I.D.B. is supported as a Clinical Research Professor by the American Cancer Society.
Reprints:Louise E. Purton, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, C1-169, PO Box 19024, Seattle, WA 98109-1024.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal